Abstract
Clathrin-mediated endocytosis internalizes proteins and lipids from the cell surface. A flexible condensate of initiator proteins catalyzes assembly of clathrin-coated vesicles in diverse organisms. Here we reveal that an endocytic adaptor protein, Epsin1, conditionally stabilizes this network, creating a cargo-dependent endocytic checkpoint. Epsin1 recruits ubiquitylated cargo to endocytic sites. Using in vitro assays, we demonstrate that Epsin1 destabilizes condensation of initiator proteins in the absence of ubiquitin. However, when polyubiquitin is present, Epsin1 binds to both ubiquitin and initiator proteins, stabilizing condensation. Similarly, in mammalian cells, endocytosis is disrupted by removal of either ubiquitin or Epsin1. When both components are removed simultaneously, endocytic defects are largely rescued, although the ability to preferentially internalize ubiquitylated cargo is lost. These results suggest that Epsin1 tunes protein condensation to internalize ubiquitylated cargo. More broadly, these findings illustrate how a balance of attractive and repulsive molecular interactions can exert dynamic control over cellular events.
Similar content being viewed by others
Introduction
Clathrin-mediated endocytosis (CME) is a fundamental cellular process that internalizes proteins and lipids from the plasma membrane and supports numerous physiological functions including nutrient uptake, signal transduction, synaptic vesicle recycling, and intracellular trafficking1,2,3. In mammalian cells, CME begins when initiator proteins, including Eps15, Fcho, and Intersectin, assemble on the plasma membrane2,3 and subsequently recruit adapter proteins such as AP2, CALM/AP180, and Epsin14,5. These adapters then recruit clathrin and transmembrane cargo proteins5,6,7. The adapter proteins promote the assembly of clathrin into an icosahedral lattice and work together with clathrin to bend the membrane, leading to vesicle budding, scission, and ultimately the formation of clathrin-coated vesicles5,6,7,8. Notably, CME is a highly stochastic process. As initiators, adapters, and clathrin triskelia come together at the plasma membrane, some nascent assemblies mature productively into coated vesicles that encapsulate cargo, while others disassemble abortively or stall indefinitely9,10,11,12. Importantly, initiator proteins, which are some of the first components to arrive at nascent endocytic sites, play a key role in determining which assemblies develop into productive vesicles and which do not4,13,14,15.
Each of the initiator proteins contains substantial regions of intrinsic disorder. These regions contain multiple weak binding motifs for one another such that they facilitate dynamic multivalent binding, a key requirement for biomolecular condensation16,17,18. Recent work has suggested that a flexible network of endocytic initiator proteins is important for the efficient assembly of clathrin-coated vesicles16. Specifically, mammalian Eps15 and Fcho1/2, which form biomolecular condensates in vitro, assemble into a network which substantially influences the fate of endocytic sites14. Stable assembly of this network leads to productive vesicles, while unstable assembly results in abortive structures. Similarly, Ede1, the homolog of Eps15 in budding yeast, forms liquid-like complexes that recruit diverse endocytic adapter proteins19 and the AtEH1/2 subunits of the TPLATE complex, the key endocytic initiator proteins in plants, form liquid-like condensates in vitro and in live cells20. Together, these studies suggest a conserved propensity of endocytic initiator proteins to assemble into flexible networks that orchestrate the fate of nascent clathrin-coated vesicles.
If the assembly of endocytic initiator proteins controls the fate of endocytic vesicles, how are these assemblies regulated to ensure that productive vesicles contain transmembrane cargo proteins? Among the many types of cargo encapsulated by CME, ubiquitylated transmembrane proteins, marked for recycling and degradation by posttranslational modification, are a major class21,22,23. Eps15 and its homologs use ubiquitin interacting motifs (UIMs) at their C-termini to bind to ubiquitylated proteins24,25,26,27. These interactions are known to promote assembly of endocytic sites26 by promoting condensation of the Eps15 network15. However, as an initiator protein, Eps15 is not incorporated into productive coated vesicles. Instead, it remains at the plasma membrane28,29, where it catalyzes multiple rounds of endocytosis. Therefore, incorporation of ubiquitylated cargo into clathrin coated vesicles requires adapter proteins of the Epsin family8,30,31,32,33. Epsin1 in particular plays a critical role in the internalization of ubiquitylated cargo34,35.
Here we asked how Epsin1 works together with Eps15 to ensure the productive assembly of endocytic vesicles that are loaded with ubiquitylated cargo. Our in vitro studies revealed that the impact of Epsin1 on condensates of Eps15 is ubiquitin dependent, with Epsin 1 destabilizing condensates in the absence of ubiquitin, yet promoting condensate formation in the presence of polyubiquitin. In mammalian cells, removal of either Epsin134,35 or ubiquitin15,26 from endocytic sites disrupted receptor endocytosis, as expected. However, simultaneous removal of both components rescued endocytic dynamics to near wild-type levels, though cargo selectivity was no longer ubiquitin-dependent. Therefore, Epsin 1 enforces a checkpoint for ubiquitylated cargo during clathrin-mediated endocytosis by destabilizing nascent endocytic sites in the absence of ubiquitylated cargo, while promoting maturation of endocytic sites in the presence of ubiquitylated cargo.
Results
Epsin1 fails to form protein condensates
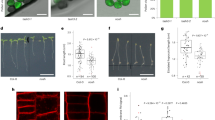
Eps15 consists of three N-terminal Eps15 Homology (EH) domains, a central coiled-coil region that facilitates oligomerization, a large intrinsically disordered region (IDR, ~400 amino acids) which contains multiple DPF (Asp-Pro-Phe) motifs, followed by two UIMs at its C-terminus36 (Fig. 1a). As previously reported, we found that Eps15 forms liquid-like condensates that fuse together rapidly upon contact (Fig. 1b, Supplementary Movie S1). Previous work showed that the coiled-coil domain and interactions between the EH and IDR domains are essential to this condensation14. In particular, the coiled-coil domain drives Eps15 dimerization, while EH-IDR interactions likely promote assembly of anti-parallel tetramers37. Epsin1 consists of an N-terminal ENTH (Epsin N-Terminal Homology) domain that binds PI(4,5)P2 membrane lipids, followed by two UIMs and an intrinsically disordered C-terminal domain. The disordered region contains three NPF (Asn-Pro-Phe) motifs, which bind EH domain-containing proteins such as Eps158. Given that both proteins have substantial regions of intrinsic disorder, a feature that is often associated with a propensity for condensation38,39,40,41, we began by asking whether Epsin1, similar to Eps15, formed protein condensates in vitro. To assess Epsin1’s potential for condensation, we added Epsin1 at increasing concentrations to a solution containing 3 weight% polyethylene glycol 8000 (PEG8K), which is often used to mimic the crowded cytosolic environment14,42,43. We did not observe any evidence of protein condensation for protein concentrations ranging from 20 to 80 μM (Fig. 1c), substantially above the concentration required for condensation of Eps15 (12 μM). Next, we held the protein concentration at 40 μM and increased the concentration of PEG8K from 3 weight% to 10 weight%, substantially exceeding the concentration required for condensation of Eps15 (3 weight%). Nonetheless, no condensation of Epsin1 was observed (Fig. 1d). These experiments suggest that Epsin1 is substantially less prone to condensation compared to Eps15, and that Epsin1 likely resists condensation under physiological conditions. This resistance to condensation may stem from the lack of self-interaction motifs in Epsin1 and electrostatic repulsion between negatively charged amino acids36,44. However, when mixed at a 10:1 molar ratio with 16 μM Eps15 (3 weight% PEG8K), Epsin1 (Atto 594 fluorophore) partitioned uniformly into Eps15 condensates (Fig. 1e), with a partition coefficient (Kapp) of 8 ± 1, (Fig. 1f). To determine if this partitioning was driven by binding between Epsin1’s NPF motifs and Eps15’s EH domains, we created an Epsin1 variant lacking all three NPF motifs (Epsin1ΔNPF), which exhibited significantly weaker partitioning, with a Kapp of 2 ± 0.3 (Fig. 1e, f). As a negative control, Amphiphysin, an endocytic protein that contains a long intrinsically disordered region (~400 amino acids) but no NPF motifs45, had a similarly low Kapp of 2 ± 0.3 (Supplementary Fig. S1 and Fig. 1f). Importantly, condensates composed of Eps15 and Epsin1 retained their liquid-like properties, fusing and re-rounding upon contact (Fig. 1g, Supplementary Movie S2). Collectively, these results suggest that while Epsin1 is unlikely to form condensates on its own, it uses its NPF motifs to interact with condensates composed of Eps15 (Fig. 1h).
a Schematic of Eps15 and Epsin1 functional domains. Eps15 consists of three EH domains at its N terminus followed by a coiled-coil domain and a long intrinsically disordered region followed by two ubiquitin interacting motifs (UIMs) at the C terminal end. Epsin1 consists of the N-terminus ENTH (Epsin1 N-Terminal Homology) domain followed by two UIMs. The central part includes the CLAP (Clathrin/AP2 binding) region and multiple DPW motifs for binding AP2 followed by the C-terminus containing 3 NPF (Asn-Pro-Phe) motifs that bind to EH domain-containing proteins like Eps15. The side cartoons depict domain organization of Eps15 in dimeric form and Epsin1 as monomer. b Time course of fusion and re-rounding of Eps15 (12 μM) condensates (green) on contact. n = 3 independent replicates. c Varying concentration of Epsin1 (20 μM to 80 μM) in 3 weight% PEG8K. d 40 μM Epsin1 in varying weight% of PEG8K (3 weight% to 10 weight%). n = 3 independent replicates. e Eps15 (16 μM) condensates (green) incubated with 1.6 μM of Epsin1 and Epsin1ΔNPF (red), respectively. Plots on the right depict the intensity profile of the Epsin1 / Epsin1ΔNPF channel along the white dashed line shown in the corresponding images. n = 3 independent replicates. A.U. represents arbitrary units. f The distribution of the Epsin1 and Epsin1ΔNPF intensity ratio (Kapp) between the intensity inside the condensates and the solution. Partition of Amphyphysin was used as a negative control. In total, 100 condensates were analyzed under each condition. Data are mean ± standard deviation, calculated from n = 3 independent replicates. Statistical significance was tested using an unpaired, two-tailed student’s t test on GraphPad Prism. g Representative time course of fusion events between condensates containing Eps15 and Epsin1. n = 3 independent replicates. Inset in (h) shows the interaction between Eps15 and Epsin1 (the NPF motif of Epsin1 binds to the EH domains of Eps15). All droplet experiments were performed in 20 mM Tris-HCl, 150 mM NaCl, 5 mM TCEP, 1 mM EDTA and 1 mM EGTA at pH 7.5 with PEG8K, at room temperature, 24 °C. All scale bars equal 5 μm.
Epsin1 destabilizes condensation of Eps15
We next asked what impact Epsin1 has on the stability of Eps15 condensates. We mixed unlabeled Epsin1 with Eps15 at molar ratios ranging from 8:1 to 1:1. For each ratio, we measured the partitioning of Eps15 between the solution and condensate phases (Fig. 2a–c), where higher Kapp values reflect denser, more stable condensates. We observed a steady decline in Eps15 partitioning to the condensate phase as the ratio decreased from 8:1 to 2:1 (Fig. 2a, b). At a ratio of 1:1, condensates no longer formed (Fig. 2a). These results suggest thatcondensation of Eps15 is destabilized by Epsin1 (Fig. 2a, b). We also measured the melting temperature (Tm) for condensates formed in the presence of increasing amounts of Epsin1, where a higher Tm indicates a more stable condensate. Condensates consisting of Eps15 alone (16 µM) dissolved at 32 °C, in agreement with prior studies (Fig. 2c, d)14,15. As the concentration of Epsin1 increased, Tm steadily decreased, reaching 28 °C for a 2:1 mixture of Eps15 and Epsin1 (Fig. 2c–e and Supplementary Fig. S2-S3). These results further confirm that Epsin1 reduces the thermodynamic stability of Eps15 condensates. This destabilizing effect relied on specific interactions between Eps15’s EH domains and Epsin1’s NPF motifs, as Epsin1ΔNPF failed to destabilize condensation of Eps15 when the two proteins were mixed at a 1:1 ratio (Fig. 2f). Overall, these results suggest that Epsin1 is capable of destabilizing Eps15 condensates by competing with interactions between Eps15 proteins (Fig. 2g).
a Representative images of Eps15 condensates with varying stoichiometry of Epsin1. 16 μM Eps15 was used for all the experiments and Epsin1 was mixed with Eps15 from 8:1 to 1:1 molar ratio. The line profiles depict the intensity profile of green (Eps15) channels along the white dashed line. n = 3 independent replicates. A.U. represents arbitrary units. b The distribution of the Eps15 intensity ratio (Kapp) between the intensity inside the condensates and the solution with varying molar ratio of Epsin1. In total 60–70 condensates were analyzed under each condition. Data are mean ± standard deviation, calculated from n = 3 independent replicates. Statistical significance was tested using an unpaired, two-tailed student’s t test on GraphPad Prism. c Bar graph shows the melting temperature (Tm) of Eps15 and Eps15:Epsin1 mixed condensates, n = 3 independent replicates. The error bar represents ± 1 °C (deviation) of the melting temperature, which was the resolution of the temperature control system. All replicates resulted in the same melting temperature within this error. A.U. represents arbitrary units. d,e Representative images of protein condensates at increasing temperatures. Plots show fluorescence intensity of Eps15 measured along dotted lines in each image. n = 3 independent replicates. Condensates are formed from (d) 16 μM Eps15, (e) 16 μM Eps15 with 8 μM Epsin1. f Eps15 (16 μM) condensates mixed with 16 μM (1:1) of Epsin1ΔNPF, the line profile shows the intensity of Eps15 across the white dashed line. n = 3 independent replicates. A.U. represents arbitrary units. g Shows the cartoon of the Eps15 multimerization through intermolecular interaction between DPF motif and EH domains and how the addition of Epsin1 can destabilize Eps15 condensates. Epsin1 binds to the EH domain of Eps15 and destabilizes the condensates by preventing Eps15 multimerization. All condensate experiments were performed in 20 mM Tris-HCl, 150 mM NaCl, 5 mM TCEP, 1 mM EDTA and 1 mM EGTA at pH 7.5 with PEG8K, at room temperature, 24 °C. All scale bars equal 5 μm.
When ubiquitin is present, Epsin1 stabilizes condensation of Eps15
Given that Eps15, Epsin1, and ubiquitin are all present simultaneously at endocytic sites26,27,34,35, we next investigated the impact of ubiquitin on the tendency of Eps15 and Epsin1 to form condensates. It was previously established that poly-ubiquitin, but not mono-ubiquitin, partitions favorably to Eps15 condensates and stabilizes their assembly15. Therefore, we first confirmed that K63-linked tetra ubiquitin (TetraUb), partitioned positively into liquid-like condensates composed of Eps15 and Epsin1 (Fig. 3a and Supplementary Movie S3). Next, we investigated the impact of Epsin1 on TetraUb partitioning. Specifically, we mixed Eps15 and Epsin1 in ratios from 8:1 to 2:1, and measured the partitioning of TetraUb (Atto 646 labeled) into the resulting condensates (Fig. 3b, c). Interestingly, addition of a small amount of Epsin1 to condensates composed of Eps15 (8:1 Eps15:Epsin1) increased the partitioning of TetraUb, suggesting that polyubiquitin may cross-link UIM domains within Eps15 and Epsin1 proteins. However, further increasing the relative concentration of Epsin1 (6:1 to 2:1 Eps15:Epsin1) led to a progressive decrease in TetraUb partitioning, likely owing to destabilization of the Eps15 network by increasing amounts of Epsin1 (Fig. 2a–c). Similarly, the presence of TetraUb, but not mono ubiquitin (MonoUb), resulted in stronger partitioning of Epsin1 to condensates (10:1 Eps15 and Epsin1), an effect which required the UIM domains of both Eps15 and Epsin1 (Supplementary Fig. S4). Further, while we showed in Fig. 2a that a 1:1 solution of Eps15 and Epsin1 does not form condensates, adding TetraUb at increasing concentrations to this solution caused protein condensates to progressively reemerge (Fig. 3d, e). Together, these results suggest that when polyubiquitin is present, Epsin1 loses its ability to destabilize protein condensates and can instead stabilize them. To test this idea, we measured the impact of Epsin1 (8:1 Eps15:Epsin1) on the melting temperature (Tm) of protein condensates in the presence and absence of TetraUb, Fig. 3f, (Supplementary Fig. S6). First, we established that condensates composed of Eps15 and Epsin1 in an 8:1 ratio had a similar melting temperature (32 °C) to condensates composed purely of Eps15 (Supplementary Fig. S2, S5), suggesting that small amounts of Epsin1 do not substantially destabilize Eps15 condensates. Previous work demonstrated that polyubiquitin stabilizes Eps15 condensates by cross-linking Eps15’s UIMs15. In line with these results, we found that addition of 0.12 µM TetraUb increased the Tm of Eps15 condensates to 36 °C in the absence of Epsin1 (Fig. 3f, Supplementary Fig. S6). However, addition of TetraUb at the same concentration to condensates consisting of both Eps15 and Epsin1 (8:1) led to an even higher Tm of 38 °C, (Fig. 3f, Supplementary Figs. S5 and S7). Epsin1 disrupted Eps15 condensates by interfering with EH-IDR interactions, but this effect was prevented by TetraUb, which crosslinked Eps15. To test if EH-IDR interactions were essential to Eps15 phase separation in the presence of TetraUb, we used an Eps15 mutant lacking all three EH domains (Eps15Δ3EH). In agreement with a previous report, 16 µM Eps15Δ3EH did not form condensates in 3 weight% PEG8K, when ubiquitin was absent14. However, upon addition of TetraUb at increasing molar ratios relative to Eps15Δ3EH (80:1, 40:1, 20:1, 10:1, 5:1), robust condensates emerged at a 5:1 (Eps15Δ3EH:TetraUb) ratio (Supplementary Fig. S8). These results suggest that, although EH-IDR interactions promote condensation of Eps15, they are not strictly required when polyubiquitin is present at sufficient concentration. Collectively, our in vitro results suggest that, while Epsin1 destabilizes condensation of Eps15 alone (Fig. 2), in the presence of polyubiquitin, Epsin1 loses its destabilizing capacity. Presence of polyubiquitin enables Epsin1 to further stabilize the protein condensates (Fig. 3g).
a Representative time course of fusion events between Eps15 (16 μM) condensates (green) with 1.6 μM of Epsin1 (red) and 0.1 μM TetraUb (magenta), respectively. n = 3 independent replicates. b Representative images of Eps15 condensates with varying molar ratio of Epsin1 (8:1 to 2:1) in presence of TetraUb (magenta). The corresponding line profiles depict the intensity profile of the TetraUb (magenta) channel along the white dashed line. n = 3 independent replicates. A.U. represents arbitrary units. c The distribution of the TetraUb (magenta) intensity ratio (Kapp) between the intensity inside the condensates and the solution with varying molar ratio of Epsin1. 60–70 condensates were analyzed under each condition. Data are mean ± standard deviation, calculated from n = 3 independent replicates. Statistical significance was tested using an unpaired, two-tailed student’s t test on GraphPad Prism. d Representative images of the condensates containing 16 µM of Eps15 (green) and 16 µM of Epsin1 (1:1 ratio) in addition to 0.12 µM and 0.25 µM of TetraUb, respectively. The corresponding line profile depicts intensity profile of green (Eps15) channel along the white dashed line, n = 3 independent replicates. A.U. represents arbitrary units. e The size distribution histogram in Gaussian fit of the condensates containing 16 µM of Eps15 and Epsin1 in presence of different (0.12 and 0.25 µM) TetraUb concentration. In total 500 condensates were analyzed under each condition from n = 3 independent replicates. f The bar plot depicts the melting temperature (Tm) of condensates composed of Eps15, Eps15 mixed with Epsin1 (8:1), Eps15 with TetraUb and when all three present together, n = 3 independent replicates. The error bar represents ± 1 °C (deviation) of the melting temperature, which was the resolution of the temperature control system. All replicates resulted in the same melting temperature within this error. g Shows the cartoon of UIM mediated crosslinking between Eps15 and Epsin1 that can stabilize the condensates. All condensate experiments were performed in 20 mM Tris-HCl, 150 mM NaCl, 5 mM TCEP, 1 mM EDTA and 1 mM EGTA at pH 7.5 with PEG8K, at room temperature, 24 °C. All scale bars equal 5 μm.
Epsin1 and ubiquitylation play compensatory roles in endocytic dynamics
We next sought to determine how interactions between Epsin1 and ubiquitin influence the dynamics of endocytic events in living cells. Prior research has established ubiquitin as a crucial stabilizer of the early endocytic protein complex, of which Eps15 is a major component15. The assembly of endocytic structures at the plasma membrane is highly stochastic and dynamic46,47. Early in the process, endocytic initiator proteins, including Eps15, arrive at the plasma membrane, where they subsequently recruit multiple endocytic adapter proteins, including Epsin1 and AP2, which in turn recruit clathrin1,2,3. The majority of these nascent assemblies mature into productive endocytic vesicles47. However, a substantial minority, approximately 30%, disassemble prematurely leading to “short-lived” endocytic events that typically reside at the plasma membrane less than 20 s and fail to internalize cargo47. Conversely, more stable assemblies, which result in vesicle formation and cargo internalization, remain at the plasma membrane for periods ranging from 20 s to several minutes41. At the opposite end of the spectrum, endocytic structures that persist for longer than a few minutes are considered “long-lived”47,48. These overly stable assemblies are often larger than productive endocytic structures and may be removed from the plasma membrane by autophagy rather than endocytosis49. Shifts in the dynamics of endocytic structures can serve as an indicator of endocytic efficiency14,46,47,48. An increase in short-lived structures suggests reduced stability, while an increase in intermediate-lived fraction or long-lived structures indicates enhanced stability. We examined the impact of Eps15, Epsin1, and ubiquitin on the distribution of short-lived, intermediate-lived, and long-lived endocytic structures in mammalian cells.
Our studies employed a human breast cancer-derived epithelial cell line (SUM159), which was gene-edited to fuse a halo-tag to the C-terminus of the σ2 subunit of adapter protein 2 (AP2)50 for the purpose of visualizing the assembly and dynamics of endocytic sites. A second cell line was created by further gene editing these cells to knock out Eps15 for the purpose of replacing it with modified variants, as described below14. These cell lines will be henceforth referred to as wild type cells and Eps15 knock out (Eps15 KO) cells, respectively. We have previously established that defects in endocytic dynamics resulting from deletion of Eps15 are fully rescued by transient expression of a version of Eps15 with an mCherry tag fused to its C-terminus14,15. AP2 is a critical adapter protein that is thought to be present at endocytic sites in a nearly 1:1 stoichiometric ratio with clathrin51. Fluorescent tagging of AP2 (JF646 bound to halotag52) allowed us to visualize and track endocytic structures in real-time during live-cell imaging. Notably, it is preferable to track AP2 rather than clathrin because AP2 localizes exclusively to the plasma membrane51. We conducted live cell imaging experiments using total internal reflection fluorescence (TIRF) microscopy to quantify endocytic dynamics. In TIRF images, endocytic structures appeared as diffraction-limited fluorescent puncta in the AP2 channel (JF646, Fig. 4a). Based on established classifications46,48,53 we defined endocytic structures with lifetimes shorter than 20 s as “short-lived”, those lasting between 20 and 180 seconds as “intermediate-lived fraction,” and those persisting longer than 180 s as “long-lived” (Fig. 4b).
a Representative image of a SUM cell expressing gene-edited AP2 σ2-HaloTag: JF646 (cyan) and Eps15-mCherry (red). The scale bars 10 μm. Large inset highlights three representative clathrin-coated structures shown in smaller insets. The scale bars 5 μm. b Representative image of short-lived (blue), intermediate-lived fraction (yellow) and long-lived (green) structures lasting <20 s, > 20 s and > 5 min, respectively. c Histograms of lifetime distributions of clathrin-coated structures under different experimental groups. Lifetime shorter than 20 s is considered short-lived, lifetime between 20 and 180 s is labeled as intermediate-lived fraction and structures lasting longer than 180 s are long-lived. Data are mean ± S.E.M. WT represents SUM cells that have endogenous Eps15 and Epsin1 expression. Eps15 KO represents SUM cells that were CRISPR modified to knock out alleles of endogenous Eps15. Epsin1 KD represents Epsin1 knock down in WT cells using siRNA. Eps15-DUB represents Eps15 knock out cells transfected with Eps15 fused with DUB (to C terminal end of Eps15). Eps15-DUB Epsin1 KD represents Eps15 knock out cells transfected with Eps15 fused with DUB and knocked down for Epsin1. All three constructs have mCherry at their C terminus for visualization. for WT, Epsin1 KD, Eps-DUB and Eps-DUB Epsin1 KD, n = 19, 19, 18 and 15 biologically independent cells were imaged. d Bar plot summarizing percentage of short-lived, intermediate-lived fraction and long-lived fraction for the four conditions. Data are mean ± S.E.M. e Bar plot of the intermediate-lived fraction for all four groups. Data are mean ± S.E.M. f Bar plot of the short-lived fraction for all four groups, zoomed-in representation of the short-lived fraction from panel c. Data are mean ± S.E.M. In panel (d), (e), and (f), for WT, Epsin1 KD, Eps-DUB and Eps-DUB Epsin1 KD, n = 19, 19, 18 and 15 biologically independent cells were imaged. In total > 10000 pits were analyzed for each group. An unpaired, two-tailed student’s t test was used for statistical significance using GraphPad prism. p < 0.05 is considered significantly different. All cell images were collected at 37 °C.
In wild type cells, we observed endocytic structures falling into each of these categories (Fig. 4c, d). We first compared the dynamics of endocytic structures in wild type cells to those in wild type cells in which Epsin1 was knocked down using siRNA (Supplementary Fig. S9). Consistent with previous reports54, the fraction of intermediate-lived structures decreased significantly (60 ± 4% to 52 ± 6%) upon Epsin1 knock down, mirrored by an increase in short-lived structures (27 ± 3 % to 35 ± 6%), while the long-lived fraction changed only slightly (5.5 ± 2 to 6 ± 2); Fig. 4d–f. Next, to probe the effect of removal of ubiquitin from endocytic sites, we transiently expressed a version of Eps15 linked to a deubiquitylating enzyme (DUB) at its C-terminus (Eps15-DUB) in Eps15 KO cells. Here we used a “broad-spectrum” DUB, UL36, which is expected to disassemble both K63 and K48-linked polyubiquitin chains55,56,57. In a recent study, expression of Eps15-DUB resulted in a significant increase in short-lived endocytic structures15, highlighting the role of ubiquitin in the assembly of clathrin-coated vesicles. Similarly, in our experiments, the expression of Eps15-DUB resulted in a significant decrease in the fraction of intermediate-lived fraction (47 ± 6%) and long-lived (1.5 ± 1%) endocytic events, coupled with an increase in the fraction of short-lived endocytic events (42 ± 6%).
We next asked what happens when ubiquitin and Epsin1 are simultaneously removed from endocytic sites. Because Epsin132,34,35,54 and ubiquitin15,24,26,58 have each been shown to play important roles in endocytosis, the conventional expectation would be that loss of both components simultaneously would lead to an even larger endocytic defect than loss of either component individually. However, our in vitro results showed that Epsin1 destabilizes assembly of Eps15 in the absence of polyubiquitin (Fig. 2), such that removal of Epsin1 in the absence of ubiquitin might be expected to increase the stability of nascent endocytic sites. Interestingly, upon simultaneously removing Epsin1 and ubiquitin (Epsin1 knock down and transient expression of Eps15-DUB in Eps15 KO cells), endocytic dynamics were largely rescued, such that the fraction of intermediate-lived fraction increased relative to the single knock out experiments (55 ± 7%), while the fraction of short-lived structures decreased (33 ± 8%; Fig. 4e, f). In control experiments, we observed that scrambled siRNA had no impact on endocytic dynamics and that transient expression of Eps15 fused to a catalytically inactive DUB (Eps15-DUB-dead) rescued the Eps15 knock out phenotype, similar to transient expression of wild type Eps15 (Supplementary Figs. S10-S11). Taken together, our results suggest that Epsin1 serves as a checkpoint for the presence of ubiquitin at nascent endocytic sites. When ubiquitin is present, it overcomes the inhibitory effect of Epsin1 on Eps15, leading to formation of more stable endocytic structures as indicated by increase in intermediate-lived fraction, while an absence of ubiquitin results in Epsin1-dependent destabilization of nascent sites. This prompted us to examine endocytic recycling of endogenous receptor to determine if the increase of the intermediate-lived fraction is associated with productive endocytosis.
Loss of Epsin1 and ubiquitylation from endocytic sites rescues transferrin uptake
Having established that simultaneous removal of Epsin1 and ubiquitin from endocytic sites can rescue endocytic dynamics (Fig. 4e, f), we next evaluated the impact of these modifications on endocytic recycling. Specifically, we used flow cytometry to quantify uptake of fluorescent-labeled transferrin (Atto 488) by the cell’s endogenous population of transferrin receptors (TfRs, Fig. 5a). Notably, ubiquitylation does not play a role in uptake of transferrin, as mutation of TfR to remove lysine residues in its cytosolic domain was found to have no impact on iron uptake59. While ubiquitylation of TfR is known to play an important role in its degradation60,61, this process is distinct from transferrin uptake.
a Illustration showing TfR uptake at the plasma membrane. b Flow cytometry histogram of the transferrin fluorescence intensity (green fluorescence) of cells in each condition. n = 3 biological replicates. An unpaired, two tailed student’s t test was used for statistical significance using GraphPad Prism. P < 0.05 is considered significantly different. WT represents SUM cells that have endogenous Eps15 and Epsin1 expressions. Eps15 KO represents SUM cells that were CRISPR modified to knock out alleles of endogenous Eps15. Epsin1 KD represents Epsin1 knock down in WT cells using siRNA. Eps15-DUB represents Eps15 knock out cells transfected with wild type Eps15 fused with DUB (deubiquitylase fused to C terminal end of Eps15). Eps15-DUB Epsin1 KD represents Eps15 knock out cells transfected with wild type Eps15 fused with DUB and knocked down for Epsin1. All three constructs have mCherry at their C terminus for visualization. c Bar graph represents transferrin fluorescence intensity measured by flow cytometry. Data are mean ± standard deviation, calculated from n = 3 biological replicates. A.U. represents arbitrary units. Flow cytometry runs were carried out at 24 °C. d Histograms of the AP2 intensity distribution tracked in endocytic pits under different experimental groups. The black line indicates the median of the wild-type (WT) cells. For WT, Epsin1 KD, Eps-DUB and Eps-DUB Epsin1 KD, n = 20, 17, 16 and 16 biologically independent cells were used. Total of 6745 and 6034 pits were analyzed for WT, Epsin1 KD. For Eps-DUB and Eps-DUB Epsin1 KD, > 10000 pits were analyzed. e Plots the fraction of endocytic structures that have an AP2 intensity greater or equal to that of a median intensity structure in wild-type (WT) cells. Data are mean ± S.E.M. For WT, Epsin1 KD, Eps-DUB and Eps-DUB Epsin1 KD, n = 20, 17, 16 and 16 biologically independent cells were used. An unpaired, two tailed student’s t test was used for statistical significance using GraphPad Prism. P < 0.05 is considered significantly different. f Shows the density (number per area) of endocytic tracks (lifetime between 20 − 180 sec) over 10 minutes for different experimental groups. For WT, Epsin1 KD, Eps-DUB and Eps-DUB Epsin1 KD, n = 19, 19, 16 and 16 biologically independent cells were imaged. An unpaired, two tailed student’s t test was used for statistical significance using GraphPad Prism. P < 0.05 is considered significantly different. g–h Schematic showing how polyubiquitin stabilizes the endocytic protein network by interacting with Eps15 and Epsin1 and how Epsin1 functions as a checkpoint for ubiquitylated cargo. g In presence of polyubiquitin Eps15 and Epsin1 forms a liquid-like initiator network resulting in more stable endocytic structures thereby more productive clathrin-mediated endocytosis. h In absence of ubiquitylated cargo Epsin1 interacts with Eps15 and prevents the assembly of the liquid-like network of Eps15 and thereby acting as a checkpoint for ubiquitylated cargo to prevent unproductive endocytic events.
Owing to constitutive uptake of TfR by the clathrin pathway via binding interactions with the adapter protein, AP262,63, transferrin uptake has frequently been used to evaluate the overall productivity of CME64. Cells were exposed to labeled transferrin for 30 minutes, followed by trypsinization and washing to remove unbound and surface bound ligands. In wild type cells we observed a substantial fluorescence shift upon exposure to transferrin (compare Fig. 5b, c to Supplementary Fig. S12), indicating uptake. Epsin1 knock down led to a significant decrease in transferrin uptake (Fig. 5b, c and Supplementary Figs. S14-S15), as expected65,66. Removal of ubiquitin from endocytic sites via transient expression of Eps15-DUB also impaired transferrin uptake, consistent with previous work15. In agreement with our data on endocytic dynamics (Fig. 4), simultaneous removal of Epsin1 and ubiquitin (Epsin1 knock down and transient expression of Eps15-DUB) restored transferrin uptake to levels similar to wild type cells (Fig. 5b, c).
How can endocytic flux be preserved after removing both ubiquitin and Epsin1 from endocytic sites? To understand these data better, we looked more closely at the endocytic structures themselves. First, we asked whether the endocytic structures in cells lacking Epsin1 and ubiquitin assembled to the same extent as those in wildtype cells. To assess the development of endocytic structures, we quantified the intensity of AP2, which is thought to be in approximately 1:1 stoichiometric ratio with clathrin, for each endocytic structure11. Figure 5d plots the distribution of AP2 intensities for all conditions. Comparing the distributions for wildtype cells and Epsin1 knockdown cells, the median intensity falls by 10%, likely owing to decreased recruitment of ubiquitylated cargo, which helps to stabilize endocytic structures67,68. Comparing the wildtype cells to cells lacking ubiquitin at endocytic sites (Eps15-DUB), we observed a more substantial reduction of 25%, likely owing to the loss of stability of endocytic structures in the absence of ubiquitylation of both cargo and the endocytic proteins, themselves15,26. Knockdown of Epsin1 simultaneously with loss of ubiquitin at endocytic sites (Epsin1KD + Eps15-DUB) led to the largest reduction in median AP2 intensity, 33%, suggesting that the cumulative loss of ubiquitylated cargo and Epsin1 substantially hindered the development of endocytic structures.
Another way to look at these results is to calculate the fraction of endocytic structures that have an AP2 intensity greater or equal to that of a median intensity structure in wild type cells. Figure 5e plots this fraction, which is 50 ± 7% for wildtype cells, by definition, but falls to 41 ± 9% upon removal of Epsin1, 23 ± 7% upon removal of ubiquitin, and 15 ± 7% upon removal of both Epsin1 and ubiquitin. These results suggest that many of the endocytic structures formed in the absence of Epsin1 and ubiquitin, despite having similar lifetimes to those in wild type cells (Fig. 5d, e and Supplementary Figs. S16), likely do not develop sufficiently to become productive endocytic vesicles.
How can these substantial losses in the development of endocytic structures be sustained without a reduction in endocytic flux? One possibility is that the loss of continuity in the endocytic protein network arising from removal of ubiquitin and Epsin1 corresponds to a more rapid release of endocytic proteins from unsuccessful sites, such that they can be incorporated into new endocytic sites more efficiently. If so, then we would expect that the overall density of new endocytic structures might increase, even as their development (AP2 intensity) decreases. To evaluate this idea, we quantified changes in the density (number per area) of endocytic tracks (lifetime between 20 – 180 sec) per time upon successive removal of Epsin1 and ubiquitin. As shown in Fig. 5f, the density of endocytic structures decreased (37 ± 25 %) significantly upon knockdown of Epsin1 compared to WT. Loss of ubiquitin (Eps15-DUB) led to a sizable increase in pit density (86 ± 57%), which further increased (146 ± 124%) when Epsin1 and ubiquitin were removed simultaneously.
Comparing the number of endocytic structures that exceed median wildtype intensity (Fig. 5e) to the overall density of endocytic structures (Fig. 5f), we observed opposite effects for the case when ubiquitin and Epsin1 were removed simultaneously. Specifically, the decrease in the development of individual endocytic structures (AP2 intensity, Fig. 5c, d) corresponded to an increase in the overall density of endocytic structures per time (Fig. 5f). This comparison suggests that when both Epsin1 and ubiquitylation are removed, the reduced stability of endocytic sites is compensated by an increase in the overall number of endocytic events. In contrast, when Epsin1 is removed but ubiquitin remains, this compensatory mechanism fails, likely because ubiquitin keeps the protein network stable, preventing unsuccessful endocytic structures from being rapidly repurposed. Similarly, when ubiquitin is removed but Epsin1 remains, the compensatory mechanism again fails, likely because Epsin1 destabilizes nascent endocytic sites rich in Eps15, such that their density is insufficient to rescue their loss of stability. Taken together, these results help to explain how a dynamic balance between stability and initiation of nascent endocytic structures maintains endocytic flux in the absence of Epsin1 and ubiquitin.
Taken together, these results are consistent with the idea that Epsin1 acts as a checkpoint for ubiquitylated cargo during endocytosis. Specifically, ubiquitin reinforces endocytic assemblies, driving endocytosis forward. In contrast, when ubiquitin is removed from endocytic sites, Epsin1 destabilizes assembly of the Eps15 network, reducing the probability that nascent endocytic sites will mature into productive vesicles (Fig. 4e, f and Fig. 5d, f). This switch-like mechanism is consistent with the findings of our in vitro experiments, which showed that Epsin1 changes from dissolving protein condensates in the absence of ubiquitin (Fig. 2) to reinforcing them in its presence (Fig. 3). Interestingly, while removal of Epsin1 creates an endocytic defect (Figs. 4e, f and 5b, c), when Epsin1 and ubiquitylation are absent simultaneously, endocytic function and transferrin uptake are rescued (Figs. 4e, f and 5b, c), consistent with the counterbalancing effects of Epsin1 and ubiquitin on Eps15 condensates in vitro (Figs. 2a–c and 3d). From a cellular perspective, this result suggested that in the absence of both Epsin1 and ubiquitylation, endocytic sites prioritize other cargos and adapters, which continue to drive endocytosis forward. Therefore, we next asked how the simultaneous loss of ubiquitin and Epsin1 impacts the ability of endocytic sites to detect and prioritize ubiquitylated cargo.
Loss of Epsin1 and ubiquitin from endocytic sites disrupts selective internalization of ubiquitylated cargo
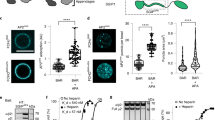
How can CME accommodate the loss of both Epsin1 and ubiquitin while maintaining near wild type dynamics and transferrin uptake? Surely some loss of function must result from the removal of these two important components from endocytic sites. If Epsin1 functions as a checkpoint for ubiquitylated cargo, as we have hypothesized, then we might expect its removal to result in endocytic sites losing their ability to selectively incorporate such cargo. To test this hypothesis, we compared the recruitment of permanently ubiquitylated and non-ubiquitylated model transmembrane proteins into endocytic sites of wild type cells and cells for which endocytic sites lacked both Epsin1 and ubiquitin (Eps15-DUB Epsin1 KD). The non-ubiquitylated model protein (0-Ub) consisted of the intracellular and transmembrane domains of the transferrin receptor, followed by an extracellular GFP domain. The ubiquitylated model protein (1-Ub) was identical to its non-ubiquitylated counterpart except for the fusion of one ubiquitin to its intracellular N-terminus (Fig. 6a). To prevent endogenous ubiquitylation and deubiquitylation by cellular enzymes, we mutated all lysine residues in the intracellular domain and ubiquitin domain to arginine and replaced the C-terminal Gly-Gly residues of ubiquitin with Ala-Ala, respectively. Here we chose a mono-ubiquitylated model cargo, rather than a poly-ubiquitylated version, because we wanted to measure the ability of endocytic sites to sense ubiquitylated cargo, without further cross-linking the endocytic network, as might occur if a poly-ubiquitylated cargo were introduced. Importantly, monoubiquitylation is known to be sufficient for selective cargo uptake by CME58,69.
a Schematic representation of model proteins with defined ubiquitin modifications; the 0-Ub model protein with no attached ubiquitin and the 1-Ub model protein with a single attached ubiquitin. All lysine residues in the cytoplasmic domain were mutated to arginines to prevent ubiquitylation. To further stabilize the attached ubiquitin moieties, all lysines within ubiquitin were mutated to arginines, and the C-terminal GG residues were altered to prevent intracellular deubiquitylation. b Shows the illustration and recruitment of ubiquitylated and non-ubiquitylated model cargo proteins in the endocytic structure. c,d Spinning disk confocal images of the plasma membrane of cells transiently expressing the 0-Ub and 1-Ub model proteins. Green fluorescence (GFP) highlights the model proteins, while red fluorescence (AP2-JF647) marks endocytic structures. The scale bars are 500 nm (insets). e,f The relative number of model proteins localized in clathrin-coated structures is shown against the relative concentration of model proteins on the plasma membrane around each structure. Data were collected from over 5000 endocytic sites in n = 20 independent cells, cells expressing 0-Ub and 1-Ub model protein. Each point reflects the average value from clathrin-coated structures binned by the local membrane concentration of the proteins. Data are mean ± SEM, while dashed lines represent model predictions based on the best-fit values. A.U. represents arbitrary units. g Bar plot shows the relative Csaturation (maximum local concentration of fusion proteins in clathrin-coated structures) values for the 0-Ub and 1-Ub in WT and Eps15-DUB Epsin1 KD cells. Data = mean ± standard deviation, form n = 3 different replicates (best-fits). An unpaired, two tailed student’s t test was used for statistical significance using GraphPad Prism. p < 0.05 is considered significantly different. h Shows the schematic illustration of cargo (model receptor) sorting at endocytic sites, in WT cells, ubiquitylated cargo (1-Ub) gets selectively enriched at the site over non-ubiquitylated cargo; in Eps15-DUB Epsin1 KD cells, the selective enrichment of 1-Ub at the endocytic site is lost.
Confocal imaging revealed significant colocalization of both 0-Ub and 1-Ub proteins with endocytic structures in both groups of cells, consistent with the known interaction of the intracellular domain of TfR with AP262,63,64 (Fig. 6c, d and Supplementary Fig. S17). However, in wild type cells, the 1-Ub protein appeared to colocalize more strongly with endocytic sites in comparison to the 0-Ub protein (Fig. 6c), while 1-Ub and 0-Ub appeared to colocalize equally with endocytic sites in Eps15-DUB, Epsin1 knock down cells (Fig. 6d). Using CMEAnalysis9, we quantified the partitioning of these model proteins at endocytic sites. The algorithm identified clathrin-coated structures (diffraction limited puncta in AP2 channel) and measured the intensity of model proteins (GFP channel) colocalized with them, relative to their intensity on the surrounding plasma membrane9,70. In wild type cells, both 0-Ub and 1-Ub proteins showed an initial increase in recruitment to endocytic sites with increasing plasma membrane concentration, followed by saturation at higher concentrations (Fig. 6e). The saturation value for 1-Ub (104 ± 4) was nearly double that of 0-Ub (56 ± 3), indicating that endocytic sites in wild type cells selectively concentrated ubiquitylated cargo (Fig. 6e–g). In contrast, experiments in Eps15-DUB Epsin1 knock down cells revealed no significant difference in saturation value between 0-Ub and 1-Ub proteins (Fig. 6f, g), suggesting an inability of endocytic sites in these cells to distinguish between ubiquitylated and non-ubiquitylated cargo. Notably, the 0-Ub protein was internalized about equally by both wild type and Eps15-DUB Epsin1 knock down cells, further confirming that simultaneous removal of Epsin1 and ubiquitin from endocytic sites rescues endocytic uptake of non-ubiquitylated transmembrane proteins. Taken together, these results suggest that, while endocytic dynamics (Fig. 4) and receptor recycling (Fig. 5) are largely normal when ubiquitin and Epsin1 are removed from endocytic sites, the loss of these components renders cells incapable of prioritizing ubiquitylated transmembrane proteins for internalization.
Discussion
Significant efforts have focused on elucidating the key requirements or “checkpoints” which govern which endocytic assemblies mature into productive clathrin-coated vesicles and which do not2,10,12,53,71. Mettlen et al. reported checkpoints that monitor clathrin-coated pit assembly, membrane curvature, and the presence of cargo, suggesting that abortive endocytic structures are indicative of a fidelity-monitoring process12. Conversely, Pedersen et al. challenged the notion that cargo primarily influences the initiation of endocytic sites, proposing instead a regulatory transition point that governs the shift from the initiation phase to the internalization phase10. This transition was thought to be influenced by cargo, which accelerated the maturation, rather than initiation, of endocytic sites10. In both models, checkpoints are thought to be regulated by complex interactions among endocytic proteins, adapters, and cargo, where post translational modifications are hypothesized to play a significant role2,10,12,15,53. However, the specific mechanisms and molecular interactions that encode these checkpoints have remained elusive.
In this study, we demonstrate that Epsin1 functions as a checkpoint for ubiquitylated cargo. Specifically, our results suggest that Epsin1 acts as a biomolecular sensor, detecting ubiquitin as a cargo marker, and thereby regulating the formation of productive endocytic structures in a condensation-dependent manner. In vitro experiments showed that Epsin1 does not form condensates independently but integrates into Eps15 condensates through its NPF motifs (Fig. 1c–g). In the absence of ubiquitin, Epsin1 destabilizes Eps15 condensates (Fig. 2a–e). However, the addition of poly-ubiquitin promotes multivalent interactions between Eps15 and Epsin1, such that Epsin1 switches from destabilizing protein condensation to stabilizing it (Fig. 3).
Cell dynamics and transferrin uptake assays revealed that removing Epsin1, or ubiquitin separately leads to defects in productive endocytosis (Fig. 4d–f), consistent with previous reports14,15,54. Interestingly, however, simultaneous removal of both Epsin1 and ubiquitin rescued endocytic dynamics and transferrin uptake to near wild type levels (Fig. 4d–f and Fig. 5a, b). This counterintuitive finding is consistent with our in vitro observation that Epsin1 and ubiquitin have opposite effects on the stability of Eps15 condensates. Nonetheless, removal of Epsin1 and ubiquitin was not without effect, as it resulted in endocytic sites losing the ability to selectively concentrate ubiquitylated cargo (Fig. 6), a key requirement for maintaining the integrity of the plasma membrane25,72,73. Together these findings suggest that Epsin1 serves as a biomolecular sensor that recognizes ubiquitylated cargo and facilitates their internalization by selectively stabilizing or destabilizing the assembly of endocytic vesicles (Fig. 5f–g).
Our study provides new insights into the ubiquitin-dependent role of Epsin1 during CME. Epsin1 ability to switch from destabilizing Eps15 condensation in ubiquitin’s absence to stabilizing condensation in ubiquitin’s presence reveals a regulatory mechanism that is rooted in the recently discovered ability of endocytic proteins to form multi-valent, condensate-like assemblies in diverse systems including mammalian, yeast, and plant cells14,19,20. Epsin1 ability to sense the presence of ubiquitin within condensates and selectively modulate their stability suggests that condensates could play important roles in endocytic dynamics. More broadly, while the study of protein condensation has focused primarily on biomolecular interactions that promote condensation74,75,76,77,78, our results suggest that interactions that destabilize condensation, while little explored, could be equally important to cell physiology and may facilitate environmental sensitivity through switch-like mechanisms.
Methods
Reagents
Tris-HCl (Tris hydrochloride), HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), IPTG (isopropyl-β-D-thiogalactopyranoside), NaCl, β-mercaptoethanol, Triton X-100, Sodium bicarbonate, sodium tetraborate, EDTA (Ethylene diamine tetraacetic acid), EGTA (Ethylene glycol tetraacetic acid), glycerol, TCEP (tris(2-carboxyethyl) phosphine), DTT (Dithiothreitol), thrombin, imidazole, sodium bicarbonate, PLL (poly-l-lysine), Atto640 NHS ester, and Atto488 NHS ester were sourced from Sigma-Aldrich. Human Holo-Transferrin protein, Monoubiquitin, and K63 linked Tetraubiquitin (TetraUb) were procured from Boston Biochem (Catalog #: 2914-HT-100MG, U-100H, and UC-310). PEG8K (Polyethylene glycol 8000) was acquired from Promega (Catalog #: V3011). Amine-reactive PEG (mPEG-succinimidyl valerate MW 5000) and PEG-biotin (Biotin-PEG SVA, MW 5000) were purchased from Laysan Bio. DP-EG10-biotin (dipalmitoyl-decaethylene glycol-biotin) was supplied by D. Sasaki (Sandia National Laboratories). K63 Tetra-Ubiquitin was sourced from South Bay Bio (SBB-UP0073). siRNA against Epsin1 was sourced from ThermoFisher scientific (HSS121071). All reagents were used without further purification.
Plasmids and cloning
The pGex4T2 plasmid containing the rat Epsin1 was a gift from H. McMahon79. To generate the Epsin1ΔUIM (pGEX4T2-GST-Epsin1ΔUIM) amino acid residues 183-227 were deleted. For Epsin1ΔNPF (pGEX4T2-GST-Epsin1ΔNPF) mutant acid residues 477-551 were deleted. Both constructs were custom-designed and constructed by GenScript (GenScript). The plasmid used for purifying Eps15 was pET28a-6 × His-Eps15 encoding H. sapiens Eps15, kindly provided by T. Kirchhausen80. Eps15Δ3EH (pET28a-6×His-Eps15-Δ3×EH) was generated deleting residues 15–314 as previously described14. Plasmids used for mammalian cell expression of Eps15 variants were derived from Eps15-mCherryN1 (Addgene plasmid #27696, a gift from C. Merrifield3, which encodes Eps15-mCherry. All Eps15 variants contain mCherry at their C-terminal end for visualization even though mCherry is not mentioned in their names. Plasmids encoding the broad-spectrum deubiquitylase (DUB), UL36, and the catalytically inert mutant (C56S, DUB-dead), were generously provided by J. A. MacGurn56. Plasmids encoding the Eps15-DUB and Eps15-DUB-dead were generated by restriction cloning as previously described15.
Protein expression and purification
Epsin1 and its constructs were purified as previously described81. In short Epsin1 and its constructs were expressed overnight at 18 °C and purified from bacterial extracts by incubation with glutathione–Sepharose beads, followed by extensive washing with 25 mM HEPES, at pH 7.4, 150–300 mM NaCl, 2 mM EDTA and 2 mM dithiothreitol (final buffer composition: 25 mM HEPES at pH 7.4, 150 mM NaCl, 2 mM EDTA and 2 mM dithiothreitol). The protein was cleaved by incubation for 2 h at room temperature and overnight at 4 °C with thrombin (Novagen). Thrombin was removed by incubation with benzamidine Sepharose (GE Healthcare) for 30 min at room temperature. Eluted proteins were concentrated and dialyzed for 2 h at room temperature in 1 L and overnight in 2 L of 25 mM HEPES, at pH 7.4, 150 mM NaCl, 1 mM EDTA and 1 mM β-mercaptoethanol.
Eps15 and Eps15Δ3EH were purified based on a previously reported protocol14. Briefly, Eps15 was expressed as N-terminal 6×His-tagged constructs in BL21 (DE3) cells. Protein expression was induced with 1 mM IPTG at 30 °C for 6–8 h. Cells were lysed in a lysis buffer using homogenization and probe sonication on ice. Lysis buffer consisted of 20 mM Tris-HCl, pH 8.0, 300 mM NaCl, 5% glycerol, 10 mM imidazole, 1 mM TCEP, 1 mM PMSF, 0.5% Triton X-100. Eps15 was first purified using Ni-NTA Agarose (Qiagen, Cat#30230) resin. Then it was further purified by gel filtration chromatography using a Superose 6 column run in 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, and 5 mM DTT. For condensate experiments, prior to running the gel filtration column, the 6×His tag on the proteins was further cleaved with Thrombin CleanCleave kit (Sigma-Aldrich, Cat# RECMT) overnight at 4 °C on the rocking table after desalting in 50 mM Tris-HCl pH 8.0, 10 mM CaCl2, 150 mM NaCl and 1 mM EDTA using a Zeba Spin desalting column (Thermo Scientific, Cat#89894).
Protein labeling
Eps15 was labeled with amine-reactive NHS ester dyes Atto488 in phosphate-buffered saline (PBS, Hyclone) containing 10 mM sodium bicarbonate, pH 8. Epsin1 and its constructs were labeled with amine-reactive NHS ester dyes Atto594 in PBS containing 10 mM sodium bicarbonate, pH 8. TetraUb was labeled with Atto640 in HEPES, pH 7.5. The concentration of dye was adjusted experimentally to obtain a labeling ratio of 0.5–1 dye molecule per protein, typically using 1.5 to 2-fold molar excess of dye. Reactions were performed for 30 min on ice. Then labeled proteins were buffer exchanged into 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM TCEP, 1 mM EDTA, 1 mM EGTA and separated from unconjugated dye using dialysis (overnight at 4 °C). The labeled TetraUb was buffer exchanged to 20 mM Tris-HCl, 150 mM NaCl, pH 7.5 and separated from unconjugated dye as well using 3 K Amicon columns. Labeled proteins were dispensed into small aliquots, flash frozen in liquid nitrogen and stored at −80 °C. For all condensate experiments a mix of 95% unlabeled and 5% labeled protein was used. 100% labeled TetraUb were directly used due to their small fraction compared to Eps15 variants.
PLL-PEG preparation
PLL-PEG and biotinylated PLL-PEG were prepared as described previously15. Briefly, for PLL-PEG, amine-reactive mPEG-succinimidyl valerate was mixed with poly-L-lysine (15–30 kD) at a molar ratio of 1:5 PEG to poly-L-lysine. For biotinylated PLL-PEG, amine reactive PEG and PEG-biotin was first mixed at a molar ratio of 98–2%, respectively, and then mixed with PLL at 1:5 PEG to PLL molar ratio. The conjugation reaction was performed in 50 mM sodium tetraborate pH 8.5 solution and allowed to react overnight at room temperature with continued stirring. The products were buffer exchanged into 5 mM HEPES, 150 mM NaCl pH 7.4 using Zeba spin desalting columns (7 K MWCO, ThermoFisher) and stored at 4 °C.
Protein condensates
Eps15 condensates were formed by mixing proteins with 3 weight% PEG8K in 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM TCEP 1 mM EDTA, 1 mM EGTA. 16 μM Eps15 or was used to form the condensates, with addition of Epsin1, and its constructs. For imaging 2% PLL-PEG were used to passivate coverslips (incubated for 20 min) before adding protein-containing solutions. Imaging wells consisted of 5 mm diameter holes in 0.8 mm thick silicone gaskets (Grace Bio-Labs). Gaskets were placed directly onto no.1.5 glass coverslips (VWR International), creating a temporary water-proof seal. Prior to well assembly, gaskets and cover slips were cleaned in 2% v/v Hellmanex III (Hellma Analytics) solution, rinsed thoroughly with water, and dried under a nitrogen stream. The imaging well was washed 6-8 times with 20 mM Tris-HCl, 150 mM NaCl and 5 mM TCEP buffer before adding solutions that contained proteins.
Condensate melting temperatures (Tm)
Eps15 condensates (Eps15 alone or with Epsin1, TetraUb, or both) were formed in 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM TCEP, 1 mM EDTA, 1 mM EGTA, and 3 weight% PEG 8 K, with 16 μM Eps15 and specified binding partners. Solutions were heated incrementally (1 °C steps, 1 min equilibration) from 24 °C to maximum 45 °C in a temperature-controlled chamber. Atto488-labeled Eps15 fluorescence was monitored at each temperature to monitor condensate stability. Relative fluorescence intensities of condensates (CD) and surrounding solution (CS) were quantified to estimate phase-separated protein concentrations. Horizontal tie lines connecting CS and CD at each temperature were used to construct phase diagrams, with critical point identified where CS and CD converged. Melting temperatures (Tm) were derived from curve derivatives, where higher Tm indicated greater network stability. N = 3 independent replicates. 10–12 condensates are analyzed under each temperature.
Cell culture
Human-derived SUM159 cells gene-edited to add a HaloTag to both alleles of AP2 σ2 were a gift from T. Kirchhausen50. Cells were further gene-edited to knock out both alleles of endogenous Eps15 using CRISPR-associated protein 9 (Cas9) to produce the Eps15 knock out cells developed previously14. Cells were grown in 1:1 DMEM high glucose: Ham’s F-12 (Hyclone, GE Healthcare) supplemented with 5% fetal bovine serum (Hyclone), Penicillin/Streptomycin/l-glutamine (Hyclone), 1 μg ml−1 hydrocortisone (H4001; Sigma-Aldrich), 5 μg ml−1 insulin (I6634; Sigma-Aldrich) and 10 mM HEPES, pH 7.4 and incubated at 37 °C with 5% CO2. Cells were seeded onto acid-washed coverslips. Plasmid transfection was carried out by mixing 1 μg of plasmid DNA with 3 μl Fugene HD transfection reagent (Promega). HaloTagged AP2 σ2 was visualized by adding Janelia Fluor 646-HaloTag ligand (Promega).
siRNA transfection and Epsin1 knock down
Approximately 60,000 cells were seeded per well in a 6-well plate and incubated for 24 hours prior to transfection. siRNA knock down was performed using Lipofectamine RNAiMAX (ThermoFisher Scientific, catalog no. 13778075). For the transfection mixture, 6.5 µl of Lipofectamine RNAiMAX and 5.5 µl of 20 µM siRNA were each diluted in 100 µl of OptiMEM and incubated separately at room temperature for 5 min. The siRNA solution was then combined with the Lipofectamine RNAiMAX solution and incubated for an additional 8–10 min at room temperature before being added dropwise to the cells in fresh medium. Transfections were conducted twice, with a 24 h interval between rounds, and measurements were taken on the fourth day after cell plating. Western blot analysis confirmed knock down efficiencies exceeding 82% for all targeted proteins. Control cells were transfected concurrently with control siRNA obtained from QIAGEN (Germantown, MD).
Western blot analysis
Knock down of Epsin1 was verified through Western blot analysis. siRNA-treated SUM cells were washed with ice-cold Dulbecco’s Phosphate Buffered Saline (Sigma) and lysed in 1% NP-40 lysis buffer (100 mM Tris, pH 7.5, 100 mM NaCl, 1% NP-40) supplemented with 170 mg/mL PMSF, 2 mg/mL leupeptin, 2 mg/mL aprotinin, and 10 mM DTT. Protein concentration in the lysates was determined using the Coomassie Protein Assay Reagent (ThermoScientific), and 30 µg of protein per sample was standardized for loading. The lysates were mixed with 4x SDS-PAGE loading buffer (250 mM Tris, pH 6.8, 400 mM DTT, 40% glycerol, 8% SDS, 60 µM bromophenol blue), boiled at 100 °C for 5 min, and resolved on a 4–12% BOLT Bis-Tris Plus polyacrylamide gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane (BioRad), blocked for 1 hour at room temperature with LI-COR Block Buffer, and probed with an anti-Epsin1 antibody (mouse monoclonal, Santa Cruz, Product no. sc-55556)) diluted 1:1000 in 1x TNET Wash Buffer (10 mM Tris, pH 7.5, 2.5 mM EDTA, pH 8.0, 50 mM NaCl, 0.1% Tween-20). After three washes in TNET Wash Buffer (5 min each), the membrane was incubated for 1 hour at room temperature with secondary Goat anti-Mouse IgG (H + L) Alexa Fluor™ Plus 680 antibody (Invitrogen, Product # A32729) diluted 1:15,000 in Intercept Antibody Diluent Buffer (LI-COR). Following another three washes in TNET Wash Buffer, the blot was imaged using a LI-COR Odyssey Imaging System. The membrane was subsequently reprobed with anti-β Actin antibody (Invitrogen, Product no. 2856849) and the same secondary antibody before imaging again.
Fluorescence microscopy
Images of protein condensates were collected on a spinning disc confocal super resolution microscope (SpinSR10, Olympus, USA) equipped with a 1.49 NA/100X oil immersion objective. Condensate imaging was performed, 5 min after adding proteins, providing sufficient time to achieve protein binding and reach a steady state level of binding. For experiments used to construct phase diagrams, temperature was monitored by a thermistor placed in the protein solution in a sealed chamber to prevent evaporation. Samples were heated from room temperature through an aluminum plate fixed to the top of the chamber. Temperature was increased in steps of 1 °C (and was held onto the temperature for 1 minute) until the critical temperature was reached. Live cell plasma membrane imaging was conducted using the same Olympus spinning disk confocal microscope. Fluorescence emission was captured using a Hamamatsu ORCA C13440-20CU CMOS camera. Excitation was achieved with lasers at 488 nm for GFP, and 640 nm for JF646.
Live-cell plasma membrane images were collected on a TIRF microscope consisting of an Olympus IX73 microscope body, a Photometrics Evolve Delta EMCCD camera, and an Olympus 1.4 NA ×100 Plan-Apo oil objective, using MicroManager version 1.4.23. All live-cell imaging was conducted in TIRF mode at the plasma membrane 24–48 h after transfection. Imaging media was supplemented with 1 μL OxyFluor (Oxyrase, Mansfield, OH) per 33 μL media to decrease photobleaching during live-cell fluorescence imaging. The coverslip was heated to produce a sample temperature of 37 °C using an aluminum plate fixed to the back of the sample. 532 and 640 nm lasers were used for excitation of mCherry and JF646-HaloTag ligands of AP2, respectively. Cell movies were collected over 10 min at 2 s intervals between frames.
Flow cytometry
The internalization of transferrin (Tf) through clathrin-mediated endocytosis was measured using flow cytometry based on the previous report61. First, holo-transferrin was labeled with Atto-488. SUM cells were plated on a 6-well plate at a density of 100,000 cells per well and a total volume of 2 mL per well. After 24 h, media was aspirated from the cells and they were rinsed with PBS 3 times. The cells were starved for 30 min in 1 mL pre-warmed serum-free media and were then incubated with 50 μg/mL Tf-Atto 488 for 25 min. Subsequently, the cells were incubated with 500 μL trypsin for about 2 min at 37 °C, and then the trypsin was removed and replaced with fresh trypsin. This process was repeated twice to wash away excess Tf. Then cells were fully detached from the wells by incubation with trypsin for 5 min. The trypsin was then quenched with 1 mL of media, and cells from each well were collected and resuspended in 300 μL of cold PBS in preparation for flow cytometry analysis.
A Guava easyCyte Flow Cytometer (Millipore Sigma) with 488 and 532 nm excitation lasers was used to analyze fluorescence of cells after Tf-Atto 488 incubation. All data were collected at 35 μL/min and flow cytometry data were analyzed using FlowJo (Treestar). A gate was drawn in forward scattering versus side scattering plots to exclude debris. Within this gate, cell populations were further analyzed by plotting histograms of FITC fluorescence intensity to determine shifts in Tf-Atto 488 uptake for each group. Mean fluorescence intensity was used to indicate the level of transferrin internalization.
Image analysis
Fluorescence images were analyzed in ImageJ (http://rsbweb.nih.gov/ij/). Intensity values along line scans were measured in unprocessed images using ImageJ. For phase diagrams, fluorescence intensity was measured in the center square of a 3 × 3 grid for each image where illumination was even. The background intensity was subtracted in all images. Clathrin-coated structures were detected and tracked using CME analysis in MATLAB9. The point spread function of the data was used to determine the standard deviation of the Gaussian function. AP2 σ2 signal was used as the master channel to track clathrin-coated structures. Detected structures were analyzed if they persisted in at least three consecutive frames. The lifetimes of clathrin-coated pits that met these criteria were recorded for lifetime distribution analysis under different conditions.
Statistics and Reproducibility
For all experiments yielding micrographs, each experiment was repeated independently on different days at least three times (n = 3), with similar conditions. Collection of cell image data for CME analysis was performed independently on at least two different days for each cell type and n = 15 to 20 independent cells were used for each group. More than 10000 pits were analyzed for each condition. Statistical analysis was carried out using a two-tailed student’s t-test (unpaired, unequal variance) on GraphPad Prism to probe for statistical significance (p < 0.05).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting this work are available on request from the corresponding author. Source data are provided with this paper.
Code availability
Cell data analysis was conducted using CMEanalysis software (https://www.utsouthwestern.edu/labs/danuser/software/). No custom code was generated for this study.
References
McMahon, H. T. & Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 (2011).
Mettlen, M., Chen, P.-H., Srinivasan, S., Danuser, G. & Schmid, S. L. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 87, 871–896 (2018).
Taylor, M. J., Perrais, D. & Merrifield, C. J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Wang, L., Johnson, A., Hanna, M. & Audhya, A. Eps15 membrane-binding and -bending activity acts redundantly with Fcho1 during clathrin-mediated endocytosis. Mol. Biol. Cell 27, 2675–2687 (2016).
Sengar, A. S. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 18, 1159–1171 (1999).
Morgan, J. R., Prasad, K., Jin, S., Augustine, G. J. & Lafer, E. M. Eps15 homology domain-NPF motif interactions regulate clathrin coat assembly during synaptic vesicle recycling. J. Biol. Chem. 278, 33583–33592 (2003).
Ma, L. et al. Transient fcho1/2⋅Eps15/R⋅AP-2 nanoclusters prime the AP-2 clathrin adaptor for cargo binding. Dev. Cell 37, 428–443 (2016).
Chen, H. et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394, 793–797 (1998).
Aguet, F., Antonescu, C. N., Mettlen, M., Schmid, S. L. & Danuser, G. Advances in analysis of low signal-to-noise images link dynamin and AP2 to the functions of an endocytic checkpoint. Dev. Cell 26, 279–291 (2013).
Pedersen, R. T. A., Hassinger, J. E., Marchando, P. & Drubin, D. G. Spatial regulation of clathrin-mediated endocytosis through position-dependent site maturation. J. Cell Biol. 219, e202002160 (2020).
Kadlecova, Z. et al. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 216, 167–179 (2017).
Mettlen, M., Loerke, D., Yarar, D., Danuser, G. & Schmid, S. L. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J. Cell Biol. 188, 919–933 (2010).
Henne, W. M. et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328, 1281–1284 (2010).
Day, K. J. et al. Liquid-like protein interactions catalyse assembly of endocytic vesicles. Nat. Cell Biol. 23, 366–376 (2021).
Yuan, F. et al. Ubiquitin-driven protein condensation stabilizes clathrin-mediated endocytosis. PNAS Nexus 3, pgae342 (2024).
Schiano Lomoriello, I., Sigismund, S. & Day, K. J. Biophysics of endocytic vesicle formation: a focus on liquid–liquid phase separation. Curr. Opin. Cell Biol. 75, 102068 (2022).
Zarin, T. et al. Proteome-wide signatures of function in highly diverged intrinsically disordered regions. eLife 8, e46883 (2019).
Naudi-Fabra, S. et al. An extended interaction site determines binding between AP180 and AP2 in clathrin mediated endocytosis. Nat. Commun. 15, 5884 (2024).
Kozak, M. & Kaksonen, M. Condensation of Ede1 promotes the initiation of endocytosis. eLife 11, e72865 (2022).
Dragwidge, J. M. et al. Biomolecular condensation orchestrates clathrin-mediated endocytosis in plants. Nat. Cell Biol. 26, 438–449 (2024).
Mosesson, Y. et al. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278, 21323–21326 (2003).
Voss, P. & Grune, T. The nuclear proteasome and the degradation of oxidatively damaged proteins. Amino Acids 32, 527–534 (2007).
Pajares, M. et al. Redox control of protein degradation. Redox Biol. 6, 409–420 (2015).
Piper, R. C., Dikic, I. & Lukacs, G. L. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb. Perspect. Biol. 6, a016808–a016808 (2014).
Sigismund, S. et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl Acad. Sci. 102, 2760–2765 (2005).
Weinberg, J. S. & Drubin, D. G. Regulation of clathrin-mediated endocytosis by dynamic ubiquitination and deubiquitination. Curr. Biol. 24, 951–959 (2014).
Polo, S. et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416, 451–455 (2002).
Tebar, F., Sorkina, T., Sorkin, A., Ericsson, M. & Kirchhausen, T. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271, 28727–28730 (1996).
Sochacki, K. A., Dickey, A. M., Strub, M.-P. & Taraska, J. W. Endocytic proteins are partitioned at the edge of the clathrin lattice in mammalian cells. Nat. Cell Biol. 19, 352–361 (2017).
Maldonado-Báez, L. et al. Interaction between Epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol. Biol. Cell 19, 2936–2948 (2008).
Shih, S. C. et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4, 389–393 (2002).
Sen, A., Madhivanan, K., Mukherjee, D. & Aguilar, R. C. The epsin protein family: coordinators of endocytosis and signaling. Biomol. Concepts 3, 117–126 (2012).
Wendland, B. Epsins: adaptors in endocytosis? Nat. Rev. Mol. Cell Biol. 3, 971–977 (2002).
Hawryluk, M. J. et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic 7, 262–281 (2006).
Kazazic, M. et al. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic 10, 235–245 (2009).
Jagannathan, N. S., Hogue, C. W. V. & Tucker-Kellogg, L. Computational modeling suggests binding-induced expansion of Epsin disordered regions upon association with AP2. PLOS Comput. Biol. 17, e1008474 (2021).
Tebar, F., Confalonieri, S., Carter, R. E., Di Fiore, P. P. & Sorkin, A. Eps15 Is Constitutively Oligomerized Due to Homophilic Interaction of Its Coiled-coil Region. J. Biol. Chem. 272, 15413–15418 (1997).
Lotthammer, J. M., Ginell, G. M., Griffith, D., Emenecker, R. J. & Holehouse, A. S. Direct prediction of intrinsically disordered protein conformational properties from sequence. Nat. Methods 21, 465–476 (2024).
Ibrahim, A. Y. et al. Intrinsically disordered regions that drive phase separation form a robustly distinct protein class. J. Biol. Chem. 299, 102801 (2023).
Posey, A. E., Holehouse, A. S. & Pappu, R. V. Phase Separation of Intrinsically Disordered Proteins. in Methods in Enzymology vol. 611 1–30 (Elsevier, 2018).
Martin, E. W. et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367, 694–699 (2020).
Mehta, N., Mondal, S., Watson, E. T., Cui, Q. & Chapman, E. R. The juxtamembrane linker of synaptotagmin 1 regulates Ca2+ binding via liquid-liquid phase separation. Nat. Commun. 15, 262 (2024).
Zhang, M. et al. SKAP interacts with Aurora B to guide end-on capture of spindle microtubules via phase separation. J. Mol. Cell Biol. 13, 841–852 (2022).
Busch, D. J. et al. Intrinsically disordered proteins drive membrane curvature. Nat. Commun. 6, 7875 (2015).
Zeno, W. F., Snead, W. T., Thatte, A. S. & Stachowiak, J. C. Structured and intrinsically disordered domains within Amphiphysin1 work together to sense and drive membrane curvature. Soft Matter 15, 8706–8717 (2019).
Ehrlich, M. et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004).
Nawara, T. J. et al. Imaging vesicle formation dynamics supports the flexible model of clathrin-mediated endocytosis. Nat. Commun. 13, 1732 (2022).
Boulant, S., Kural, C., Zeeh, J.-C., Ubelmann, F. & Kirchhausen, T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat. Cell Biol. 13, 1124–1131 (2011).
Wilfling, F. et al. A selective autophagy pathway for phase-separated endocytic protein deposits. Mol. Cell 80, 764–778.e7 (2020).
Aguet, F. et al. Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418–3435 (2016).
Blondeau, F. et al. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl Acad. Sci. 101, 3833–3838 (2004).
Grimm, J. B. et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250 (2015).
Loerke, D. et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 7, e1000057 (2009).
Wang, X. et al. DASC, a sensitive classifier for measuring discrete early stages in clathrin-mediated endocytosis. eLife 9, e53686 (2020).
Bolstad, M., Abaitua, F., Crump, C. M. & O’Hare, P. Autocatalytic activity of the ubiquitin-specific protease domain of herpes simplex virus 1 VP1-2. J. Virol. 85, 8738–8751 (2011).
Xu, P. et al. COPI mediates recycling of an exocytic SNARE by recognition of a ubiquitin sorting signal. eLife 6, e28342 (2017).
Kattenhorn, L. M., Korbel, G. A., Kessler, B. M., Spooner, E. & Ploegh, H. L. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family herpesviridae. Mol. Cell 19, 547–557 (2005).
Barriere, H. et al. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in mammalian cells. Traffic 7, 282–297 (2006).
Tachiyama, R. et al. Proteome of ubiquitin/MVB pathway: possible involvement of iron-induced ubiquitylation of transferrin receptor in lysosomal degradation. Genes Cells 16.4, 448–466 (2011).
Fujita, H. et al. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J. Cell Sci. 126.13, 2798–2809 (2013).
Shao, M. et al. E3 ubiquitin ligase CHIP interacts with transferrin receptor 1 for degradation and promotes cell proliferation through inhibiting ferroptosis in hepatocellular carcinoma. Cell. Signal. 118, 111148 (2024).
Mayle, K. M., Le, A. M. & Kamei, D. T. The intracellular trafficking pathway of transferrin. Biochim. Biophys. Acta BBA - Gen. Subj. 1820, 264–281 (2012).
Kelly, B. T. et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456, 976–979 (2008).
Géminard, C., De Gassart, A., Blanc, L. & Vidal, M. Degradation of AP2 during reticulocyte maturation enhances binding of Hsc70 and alix to a common site on TfR for sorting into exosomes. Traffic 5, 181–193 (2004).
Messa, M. et al. Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. eLife 3, e03311 (2014).
Vanden Broeck, D. & De Wolf, M. J. S. Selective blocking of clathrin-mediated endocytosis by RNA interference: epsin as target protein. BioTechniques 41, 475–484 (2006).
MacDonald, C. et al. Cargo ubiquitination is essential for multivesicular body intralumenal vesicle formation. EMBO Rep. 13.4, 331–338 (2012).
Dores, M. R. et al. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic 11.1, 151–160 (2010).
Belouzard, S. and Yves R. Ubiquitylation of leptin receptor OB-Ra regulates its clathrin-mediated endocytosis. EMBO J. 25.5, 932–942 (2006).
DeGroot, A. C. M. et al. Entropic control of receptor recycling using engineered ligands. Biophys. J. 114, 1377–1388 (2018).
Chen, Y. et al. Dynamic instability of clathrin assembly provides proofreading control for endocytosis. J. Cell Biol. 218, 3200–3211 (2019).
Haglund, K. & Dikic, I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 125, 265–275 (2012).
Marmor, M. D. & Yarden, Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 23, 2057–2070 (2004).
Jiang, S., Fagman, J. B., Chen, C., Alberti, S. & Liu, B. Protein phase separation and its role in tumorigenesis. eLife 9, e60264 (2020).
Zeigler, T. M., Chung, M. C., Narayan, O. P. & Guan, J. Protein phase separation: physical models and phase-separation- mediated cancer signaling. Adv. Phys. X 6, 1936638 (2021).
Dutagaci, B. et al. Charge-driven condensation of RNA and proteins suggests broad role of phase separation in cytoplasmic environments. eLife 10, e64004 (2021).
Wen, Y. & Ma, J. Phase separation drives the formation of biomolecular condensates in the immune system. Front. Immunol. 13, 986589 (2022).
Velichko, A. K. et al. Treacle’s ability to form liquid phase condensates is essential for nucleolar fibrillar center assembly, efficient rRNA transcription and processing, and rRNA gene repair. Elife 14, 96722 (2024).
Ford, M. G. J. et al. Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366 (2002).
Cupers, P., Ter Haar, E., Boll, W. & Kirchhausen, T. Parallel dimers and anti-parallel tetramers formed by epidermal growth factor receptor pathway substrate clone 15 (EPS15). J. Biol. Chem. 272, 33430–33434 (1997).
Zeno, W. F. et al. Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing. Nat. Commun. 9, 4152 (2018).
Acknowledgements
We thank T. Kirchhausen (Harvard U) for the gift of SUM159/AP2σ2-HaloTag cells and J. MacGurn (Vanderbilt U) for the gift of plasmids encoding deubiquitylase UL36 and its catalytically inactive mutant. We acknowledge funding from NIH to J. C. Stachowiak (NIGMS: R35GM139531) and to J. Huibregtse (NIAID: AI096090), NSF BIO (2327244, 2529782) to J. C. Stachowiak, and by the Welch Foundation (F-2257) to J. C. Stachowiak. We thank Advika Kamatar for her valuable suggestions in cell data analysis.
Author information
Authors and Affiliations
Contributions
S.S., E.M.L., J.M.H. and J.C.S. designed experiments. L.W. and E.M.L. purified Eps15, Epsin1 and their variants for in vitro experiments; S.S., J.M.H., E.M.L. and J.C.S. wrote and edited the manuscript. S.S., HY.L., J.P., B.T.M., F.Y., L.W., E.M.L., J. M. H. and J.C.S. performed experiments and analyzed data. All authors consulted on manuscript preparation and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Yuan Ren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sarkar, S., Liu, HY., Yuan, F. et al. Epsin1 enforces a condensation-dependent checkpoint for ubiquitylated cargo during clathrin-mediated endocytosis. Nat Commun 16, 7929 (2025). https://doi.org/10.1038/s41467-025-63238-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63238-z