Abstract
The separation of amino acids from complex mixtures remains an essential yet multi-step, energy-intensive process. Membrane separation technology offers a more energy-efficient alternative, but its effectiveness relies on achieving highly precise molecular recognition. Here, we report a homochiral covalent organic framework (COF) membrane with ordered ultra-microporous pore structures for targeted extraction of specific enantiomer from amino acid mixtures. Benefiting from its high crystallinity and ultra-microporous chiral channels, the membrane exhibits both excellent permeability and enantioselectivity. A combination of experimental results, density functional theory calculations, and molecular dynamics simulations reveal a retarded transport mechanism, wherein stronger interactions between L-enantiomers and the homochiral pores hinder their transmembrane diffusion. We further demonstrate a two-stage cascade membrane process to simultaneously fractionate and enantioseparate amino acid mixtures, achieving near pure (99.5%) D-threonine from an eight-component protein hydrolysis complex. This study offers a promising and sustainable membrane-based solution for efficient amino acid purification.
Similar content being viewed by others
Introduction
Chirality represents an intrinsic property for nature and biological systems, with many essential biomolecules – including enzymes, proteins, and DNA – exhibiting a specific handedness1. These systems are inherently chiral-selective, and enantiomers of a given compound can exhibit profoundly different biological effects. For instance, R-thalidomide acts as a sedative, whereas its S-teratogenic, leading to severe birth defects2. Therefore, the efficient resolution of enantiomers is not only essential for the synthesis of fine chemicals and pharmaceuticals but also critical for ensuring drug efficacy and biosafety3.
Amino acids are integral biomolecules related to peptides and proteins, which have been widely produced and utilized in the food and pharmaceutical sectors4,5,6. The global amino acids market is expected to grow at a compound annual growth rate of ~8.7% from 2025 to 2035 and reach US$75,000 million by 20357. The methods for the production of amino acids include protein hydrolysis, fermentation, and chemical synthesis, typically yielding mixtures of various amino acids. The separation process constitutes a critical step in amino acids production, accounting for approximately 50% of the total manufacturing costs8,9,10. This process primarily involves the effective fractionation of amino acid mixtures and the precise resolution of enantiomers, ensuring not only the high purity of amino acids but also maintaining their specific biological or physiological activities (Supplementary Fig. 3). The utilization of precipitation or ion-exchange resins to separate amino acid from mixtures depends on significant differences in the isoelectric points of the amino acid molecules11, and these methods presents significant challenges in separating amino acids with similar physicochemical properties. The resolution of a racemic mixture into pure enantiomers (L- or D- forms) employs chiral chromatography, displaying high selectivity. However, it often suffers from low capacity and the requirement for substantial eluent volumes12.
Membrane-based separation techniques has shown promise in efficient amino acid separation10,13,14. Conventional chiral polymeric membranes often suffer from a selectivity-permeability trade-off, originating from the ill-defined pore structure of amorphous polymer networks15. Crystalline metal-organic framework membranes are characterized by their highly well-defined pore structures, which significantly enhances their separation performance16,17,18,19. Nevertheless, intrinsic structural stability issues in an aqueous environment limit their application under real conditions. Covalent organic frameworks (COFs) represent an emerging class of porous crystalline materials that covalently link organic building blocks to periodically extended porous network structures20,21,22,23,24,25,26,27,28. COF membranes have shown potential in chiral separation due to their tunable pore structure and tailored functionalities29,30. However, leveraging COF membranes to extract targeted pure enantiomers from amino acid mixtures in practical applications presents a significant challenge, primarily due to the mismatch between large pore sizes and small amino acid molecules31,32,33, as well as inefficient chiral functionalities within the COF channels34.
Herein, we present the fabrication of a homochiral COF membrane featuring ultra-microporous structures for enantioseparation and fractionation of amino acids. The chiral ligand employed in the synthesis forms hydrogen bonds between adjacent COF layers, thereby inducing an offset stacking arrangement that leads to a well-organized ABC stacking configuration. This structural feature imparts the membrane with uniform sub-nanometer pore sizes around 0.6 nm. Due to its intrinsic ultra-microporosity and chirality, this COF membrane can effectively resolve amino acid enantiomers with excellent permeability and enantioselectivity.
Results
Fabrication of homochiral COF membranes with ultra-microporosity and high crystallinity
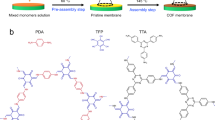
The homochiral COF membranes were synthesized on the PAN support through interfacial polymerization, a widely used method for industrial-scale production of polymeric membranes (e.g., polyamide membranes)35. As shown in Fig. 1a, the PAN support was kept flat and immersed in an aqueous solution containing tartaric acid dihydrazide monomers (L-TAH or D-TAH), which acted as both the chiral inducer and linker for the COF framework36. Despite the presence of multiple proton-accepting sites in TAH, no protonation occurred during the synthesis process (Supplementary Fig. 2). After 30 min, the surface was gently dried using an air blower, followed by deposition of a uniform layer of high-concentration 1,3,5-triformylphloroglucinol (TP) in octanoic acid solution. The entire system was subsequently placed in an oven to initialize the interfacial reaction, ultimately forming an ultrathin COF selective layer (~500 nm) on the PAN support and denoted as L-TAH-COF and D-TAH-COF membranes, respectively (Supplementary Fig. 4). One advantage of the interfacial polymerization method is the potential to produce large-scale membranes. Here, using the interfacial polymerization synthesis method, large-area chiral membranes (30 cm × 10 cm) were fabricated (Fig. 1b). These membranes were directly used for the following basic membrane structure characterization and separation evaluation. Surface SEM imaging result indicates that the COF membranes are uniform, compact and defect-free (Fig. 1c and Supplementary Fig. 5). The membrane chemical structures were confirmed using Fourier-transform infrared spectroscopy (Supplementary Fig. 6) and solid-state 13C cross polarization magic-angle-spinning nuclear magnetic resonance (Supplementary Fig. 7)36. We further performed solid-state 2D NMR analyses to elucidate the atomic connectivity within the TAH-COF framework (Supplementary Fig. 8). The 2D ¹³C–¹H correlation spectra offer detailed insight into the β-ketoenamine linkage (–C = CH, = 8.28 ppm, 150.04 ppm) and the aliphatic chain (–CH–OH, = 4.83 ppm, 99.34 ppm) present in the framework backbone.
a Schematic illustration of interfacial polymerization for preparing crystalline COF membranes with ABC stacking. b Digital photo of a large-scale D-TAH-COF membrane on the polyacrylonitrile (PAN) support. c SEM image of the membrane surface. d symmetric solid-state CD spectra of a pair of chiral membranes. e N2 adsorption isotherms of D-TAH-COF membrane measuring at 77 K. The inset shows the corresponding pore size distribution. f XRD patterns of D-TAH-COF membrane: experimental (red); Pawley refined (black); and the corresponding difference (blue) and Bragg position (green); simulated patterns for ABC stacking (purple).
Solid-state Circular Dichroism (CD) spectroscopy revealed that the L-TAH-COF and D-TAH-COF membranes exhibited perfect mirror-symmetric Cotton effects regarding optical activity at 200–700 nm, indicative of their enantiomeric nature (Fig. 1d)37. The maximum absorption wavelengths are located at ~500 nm, corresponding with the data from the solid-state UV absorption of the membranes (Supplementary Fig. 9). The absorption of chiral monomers L-TAH and D-TAH are significantly shorter, exhibiting maximum Cotton effects at ~200 nm, consistent with the corresponding adsorptions in UV-vis spectra (Supplementary Fig. 10). These results confirm that the chiral signals of the monomers are stably encoded into the COF membrane via covalent bonding.
The permanent porosities of the COF membrane were assessed by measuring N2 adsorption isotherms at 77 K, and the Brunauer−Emmett−Teller (BET) surface area was evaluated to be 303 m2 g−1. The average pore size was determined to be 6 Å using the nonlocal DFT model to fit the N2 adsorption isotherms, indicating its ultramicroporosity nature (Fig. 1e). The crystallinity of the chiral COF membrane was confirmed by the presence of diffraction peaks in X-ray diffraction patterns (XRD) (Fig. 1f and Supplementary Fig. 11). Three prominent peaks at 7.3°, 12.2° and 27.3° were identified in the XRD pattern of the TAH-COF membrane36. Three types of possible structure models of eclipsed stacking (AA) and staggered stacking (AB and ABC) models were built, and their geometries were optimized using the Forcite module. Structural simulation and Pawley refinement confirm that the experimental XRD curve closely aligns with those simulated based on the staggered stacking (ABC) model within the trigonal R−3 space group, Rwp, Rp values of 3.94%, 2.68%. The lattice parameters for the unit cell were determined as a = b = 26.67 Å, and c = 6.68 Å, α = β = 90°, and γ = 120°. Under the ABC stacking model, the simulated pore sizes centered at around 0.6 nm, which are consistent with the measurement results.
Enantioseparation of amino acids
The separation of chiral amino acids is crucial for investigating variations in biological activities among enantiomers, which is essential for designing enantiomer-specific downstream products. Given the homochiral pores of the COF membrane, there is significant potential for advancements in enantioselective separation. To evaluate this capability, three amino acids – threonine, serine, and leucine – with dimensions matching the pore size were selected, all of which are highly soluble in water (Supplementary Fig. 12). A custom diffusion apparatus was employed to conduct enantiomeric resolution experiments, where the chiral TAH-COF membrane was vertically positioned between two glass chambers (Supplementary Fig. 13). The diameter of the COF membrane utilized here is 47 mm, which corresponds to the inner diameter of standard membrane testing devices. One chamber contained a racemic amino acid aqueous solution at a specific concentration, while the other chamber was filled with Milli-Q water. The permeate collected from the pure water chamber was analyzed using high-performance liquid chromatography (HPLC). The concentrations of the amino acids in the permeate were quantified via a standard calibration curve (Supplementary Fig. 14). The results shown in Supplementary Fig. 15 confirm that HPLC effectively separated the three racemic amino acid solutions.
L- amino acids are naturally abundant and easy to obtain, while D- amino acids usually exist in trace amounts38. D- amino acids have been found to play vital roles in central nervous system function, serve as biomarkers for various pathophysiological processes, and hold potential as active components in next-generation therapeutic agents. Since they are mainly produced by chemical synthesis, efficient separation and purification methods are essential for their practical use. Considering the significant importance of the D-amino acid, we focused primarily on the separation of D-amino acid in the current study. We examined the effect of feed concentration and time on enantioselective separation using the D-TAH-COF membrane. After 2 h of permeation at a feed concentration of 0.002 mol L−1, HPLC analysis of the permeate showed a significant difference in peak intensities between D-threonine and L-threonine (Supplementary Fig. 16). The calculated fluxes of D-threonine and L-threonine through the membrane were 1.42 and 0.03 mmol m−2 h−1, respectively, yielding an enantiomeric excess (e.e.) value of 94.3% (Fig. 2a). These results indicate that the homochiral D-TAH-COF membrane can effectively retain L-threonine. In addition, an increase in feed concentration correspondingly enhances the flux, reaching 3.62 mmol m−² h−¹ at an initial concentration of 0.02 mol L−1 for D-threonine. The e.e. values slightly decrease yet stabilize at 85%, which can be primarily attributed to the intrinsic homochiral channels. Similar results are observed in gradient concentration experiments for the amino acids of serine and leucine (Supplementary Fig. 17). Specifically, given the similar sizes of the three amino acids, the e.e. values remain above 90% at low concentrations. A tenfold increase in feed concentration to 0.02 mol L−1 led to a reduction in the e.e. values to 80% for serine and 72% for leucine, respectively. During the initial 12 h of separation, the D-form membrane achieved enantiomeric selectivity above 95% for threonine and serine, and the enantioselective for leucine was 85% (Fig. 2b). Remarkably, the homochiral TAH-COF membrane exhibits high-performance enantioseparation, including selectivity and flux, surpassing the performance of state-of-the-art enantioselective membranes (Fig. 2c and Supplementary Table S1). This superior separation performance is contributed to the highly ordered chiral channels in the crystalline TAH-COF membranes, as verified by the non-selective separation performance of the amorphous counterpart (Fig. 2d). The separation performance of the L-form COF membrane was also preliminarily evaluated (Supplementary Fig. 18). In contrast to the D-form membrane, showed that the L-form COF exhibited higher permeation fluxes for L-amino acids. At a feed concentration of 0.002 mol L−1, the fluxes of threonine, serine, and leucine were 1.57, 1.02, and 0.63 mmol·m−2·h−1, respectively, with e.e. values exceeding 80%. Taking threonine as an example, when the feed concentration was increased to 0.02 mol L−1, the flux rose to 3.71 mmol·m−2·h−1, while the e.e. remained around 85%.
a Chiral separation results of D-TAH-COF membrane for the racemic threonine solution with concentrations of 0.002 mol L−1, 0.005 mol L−1, 0.01 mol L−1, 0.02 mol L−1 at room temperature. b Enantioseparation of racemic threonine, serine, and leucine under concentration diffusion through the D-TAH-COF membrane after 12 h. c Comparison of enantioselective separation performance of TAH-COF membrane with the reported representative state-of-the-art crystalline membrane. d Chiral separation performance of crystalline COF and polymer membranes. The COF membrane achieves a high e.e.% of 94.3% with a flux of 1.42 mmol m−2 h−1, whereas the polymer membrane counterpart exhibits a lower flux of 0.27 mmol m−2 h−1 and an e.e.% of 58.5%. Error bars represent the mean ± SD, n = 3.
Building on the demonstrated excellent separation performance, we further designed a scale-up experiment using a large-area membrane in a dialysis-like setup (Supplementary Fig. 19). The inner compartment contained a racemic solution, while the outer compartment was filled with pure water. HPLC results showed that the flux of D-threonine is 3.44 mmol m−2 h−1, with an e.e. of 95% at the initial 2 h (Supplementary Fig. 20). This result indicates the exceptional uniformity of the membrane fabricated through interfacial polymerization, as well as its ability to maintain excellent separation performance and reproducibility upon scaling up. Based on the advantage of an intrinsic homochiral pore, the TAH-COF membrane maintains its structural integrity after undergoing chiral separation experiments, as evidenced by SEM and XRD analyses (Supplementary Fig. 21).
Enantioseparation mechanism
The exceptional separation performance intrigues us to propose a resolution mechanism for the enantioselective membrane. We first conducted dynamic breakthrough experiments to evaluate the adsorption feasibility of D-TAH-COF for amino acid (Supplementary Fig. 22). Experimental results revealed that both D- and L-threonine rapidly reached equilibrium in columns packed with D-TAH-COF powder, suggesting that the material exhibits negligible adsorption capacity (Supplementary Fig. 23). This indicates that adsorption is not the dominant mechanism during the membrane separation process.
We then investigate the selective transport behaviors of enantiomers across the membrane. Specifically, we calculated the potential of the mean force (PMF) profile for the D- and L- threonine migration along the 1D nanochannels (z-axis) within D-TAH-COF membrane (Supplementary Fig. 24). The energy barrier for the L-threonine passing through the D-TAH-COF membrane is 0.8 eV, significantly higher than that of the D- threonine (0.4 eV). This finding implies that chiral nanopores result in a higher energy barrier for L-threonine upon entering the COF membrane pores, thereby impacting its transmembrane transport. Subsequently, we investigated the transmembrane differences between L- and D-threonine through ¹H NMR titration experiments by using the D-model compound (D-MC). In the 1H NMR titration system of D-MC and L-threonine, a significant shift was observed in the proton resonance Ha of the hydroxyl group within the chiral chain (Fig. 3a and Supplementary Fig. 25). In contrast, D-threonine could only cause a relatively subtle shift at the same position Ha for D-MC (Supplementary Fig. 26). The binding constants for L- and D-threonine were calculated to be 372 M−1 and 137 M−1, respectively, indicating that L-threonine has a higher binding energy (Fig. 3b)39. In addition, the binding energy between the model molecule of COF and the enantiomers was further studied by DFT calculations (Supplementary Fig. 27). The results show that the binding energy for L-threonine with D-MC is − 3.46 eV, which is much higher than that of D-threonine with D-MC (-1.75 eV). These results collectively indicate that L-form amino acids exhibit stronger interactions with the COF pores, leading to a reduced transmembrane transport rate. Thus, when the racemic mixture passes through the membrane, L-threonine exhibits preferential retention in the membrane phase.
a Partial 1H NMR spectra of the D-model compound (400 MHz, 293 K, DMSO-d6) upon the stepwise addition of L-threonine (proton Ha in the hydroxyl group of the chiral chain). b Comparison of the binding isotherms for threonine diastereomeric host–guest combinations. c Snapshot images of the MD simulation process, focusing on the D-TAH-COF membrane separating amino acids (white: H; blue: N; gray: C; yellow: L-threonine; light blue: D-threonine). d Time dependence of MSD curves of threonine diastereomers for D-TAH COF membrane. Density distribution profiles of L-threonine (e) and D-threonine (f) in XY-plane for the D-TAH COF membrane.
Molecular Dynamics (MD) simulations were further performed to understand the transport behavior of amino acids within the homochiral channels of the membrane. A simulation box was constructed comprising six layers of D-TAH-COF membrane, along with D- and L- threonine and water molecules (Fig. 3c and Supplementary Fig. 28). On the feed side contained, 16 molecules each of D- and L-threonine were introduced. Upon reaching dynamic equilibrium at 100 ns, all D-threonine molecules were observed to diffuse along the homochiral channel toward the permeate side. In addition, the mean square displacement (MSD) and self-diffusion coefficients of D- and L-threonine molecules along the z-direction were calculated (Fig. 3d). The diffusion coefficient for D-threonine in the homochiral channel was 2.31 × 10−3, notably higher than 2.47 × 10−4 for L-threonine, indicating its more efficient transmembrane capability. Furthermore, the XY-plane average density distributions of L- and D-threonine within the D-TAH-COF membrane channels revealed distinct top-view molecular density patterns (Fig. 3e, f). Collectively, these findings support a retarded transport mechanism in which the chiral nanochannels selectively hinder the permeation of L-enantiomers due to stronger interactions, enabling high enantioselectivity in chiral resolution.
Simultaneous fractionation and enantioseparation of amino acid mixtures
In the production of amino acids, protein hydrolysis typically yields complex mixtures of amino acids. Achieving efficient and selective separation of individual components from these mixtures is essential to improve overall process efficiency and reduce energy consumption40,41. The intrinsic chirality and small molecular sizes of amino acids pose significant challenges for conventional membrane-based separation technologies, particularly in systems containing structurally similar species. To address this challenge, we selected four representative amino acid molecules – threonine, leucine, tyrosine, and phenylalanine—to simulate the hydrolysate mixture (Fig. 4a). Beyond molecular size, the selection of these amino acids was informed by several key factors, including (i) structural diversity—ranging from small polar amino acids (threonine) to larger hydrophobic ones (leucine, tyrosine, phenylalanine); (ii) industrial relevance—as these amino acids are commonly found in protein hydrolysates and widely used in food, pharmaceutical, and biotech industries; and (iii) solubility in aqueous media, ensuring compatibility with membrane-based systems, making them ideal candidates to assess both the fractionation and enantioseparation performance of the homochiral COF membrane.
a Schematic of the cascade membrane process for enriching D-threonine from a model of protein hydrolysis system containing threonine, leucine, tyrosine and phenylalanine. Stage 1 separates amino acids with larger molecular dimensions and L-form, while Stage 2 enriches D-threonine. Figure 4a was created using BioRender.com. b The composition percentages of the model protein hydrolysis system used as feed solution. Separation performance of the D-TAH COF membranes for the model feed with Stage 1 (c) and Stage 2 (d). Error bars represent the SDs based on three independent tests.
A two-stage cascade membrane process was designed to achieve high-purity extraction of D-threonine from the model mixture. In the feed solution, threonine and leucine each constituted 40%, while tyrosine and phenylalanine accounted for 8% and 12%, respectively, with equal proportions of D- and L-enantiomers in each case (Fig. 4b and Supplementary Fig. 29). In the first stage, the membrane selectively permeated the smaller aliphatic amino acids, threonine and leucine, while effectively retaining the bulkier aromatic counterparts, tyrosine and phenylalanine. Following this initial stage, the proportions of tyrosine and phenylalanine are reduced to below the HPLC detection limit, demonstrating the efficient separation of amino acids with different molecular sizes by the homochiral COF membrane. In addition, no L-form amino acids were detected following the first stage, indicating effective enantioselective separation. As a result, the proportion of D-threonine increased from 20% to 85%, while D-leucine remained at about 15% of the permeate solution (Fig. 4c and Supplementary Fig. 30). Subsequently, a second membrane separation process was implemented. This stage further enhanced the purity of D-threonine to 99.5% (Fig. 4d and Supplementary Fig. 31), meeting the requirements for high purity of amino acids.
These results highlight the effectiveness of the homochiral, ultra-microporous COF membrane in the simultaneous fractionation and enantioseparation of multicomponent amino acid systems. Due to the well-defined sub-nanometer pores of the COF membrane, it can effectively sieve aromatic and aliphatic amino acids, and also selectively separates amino acids with similar molecular sizes. Moreover, this COF membrane effectively achieves the resolution of enantiomers by utilizing the differences in membrane transport between D- and L- form amino acid.
Discussion
In summary, this work establishes the significant potential of membrane-based strategies in addressing the long-standing challenge of separating complex amino acid mixtures. To this end, we have developed a homochiral covalent organic framework (COF) membrane featuring well-defined ultra-microporous channels via a scalable interfacial polymerization method. Due to its intrinsic sub-nanometer pores and chirality, the membrane exhibits superior permeability and selectivity for enantioseparation. Combining experimental results with theoretical calculations, we elucidate a retarded transport mechanism driven by enantiomer-specific interactions within the homochiral pores. Notably, this study represents a demonstration of a COF membrane achieving simultaneous fractionation and enantioseparation of amino acid mixtures, enabling the precise extraction of target enantiomers from complex feeds. We believe that this COF membrane technology is expected to be integrated into amino acid production processes, thereby enhancing the refinement and purification of amino acids.
Methods
Materials
Solvents, reagents and chemicals were commercially available and used without further purification. 1,3,5-triformylphloroglucinol (Tp, 99%), and Octanoic acid (99%) were purchased from Tokyo Chemical Industry Shanghai Co. Ltd. L/D-tartaric acid precursor was purchased from Sigma and subsequently modified (99%). Polyacrylonitrile (PAN) ultrafiltration membrane support with a molecular weight cut-off of 20 k PEG was supplied by Sepro Membranes Inc. (USA). N-octanoic, ethyl acetate, ethanol was purchased from Sigma Aldrich and used without further purification. The deionized water (18.2 MΩ·cm) was produced through a Millipore Milli-Q water purification system.
Fabrication of homochiral COF membranes on PAN support
The homochiral TAH-COF membrane was prepared using low-cost tartaric acid dihydrazide as the chiral origin during the interfacial polymerization synthesis method. Briefly, Tp (0.025 mmol) and TAH (0.017 mmol) were dissolved in 1 mL n-octanoic and 1 mL water, respectively. Then, fully stirred, sonicated and filtered to obtain clear, transparent solutions. TAH aqueous solution was applied to a clamped PAN substrate (~4 cm²) and maintained in a wetted state for 10 min. Blow with air for 1 min to remove surface moisture, and an n-octanoic solution (1 mL) containing Tp was poured onto the TAH-containing nanoporous substrate for the interfacial reaction at 80 °C for 12 h. The resulting COF membrane was sequentially washed with ethyl acetate, ethanol and deionized water to eliminate residual monomers and organic solvent. For the preparation of large-area membranes (~300 cm²), the same procedure was followed using proportionally scaled-up volumes of the monomer solutions—approximately 75 mL each of aqueous TAH and n-octanoic Tp solution—to ensure uniform membrane formation over the expanded surface.
Density functional theory (DFT) calculations
The density functional theory (DFT) calculations were carried out with the Material Studio code42. The Perdew–Burke–Ernzerhof (PBE) functional within generalized gradient approximation (GGA)43 was used to process the exchange–correlation, while the projector-augmented-wave pseudopotential (PAW)44 was applied with a kinetic energy cut-off of 500 eV, which was utilized to describe the expansion of the electronic eigenfunctions. The Brillouin-zone integration was sampled by a Γ-centered 1 × 1 × 1 Monkhorst–Pack k-point. All atomic positions were fully relaxed until energy and force reached a tolerance of 1 × 10−6 eV and 0.01 eV/Å, respectively. The dispersion-corrected DFT-D method was employed to consider the long-range interactions45. During the molecular dynamics simulation, the NVT ensemble is used to relax the system, with a time step set to 1 ps and a total duration of 100 ns. We used the implicit solvent model H2O dielectric constant to simulate its solvation in the environment.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. The data that support the findings of this study are available from the corresponding author upon request. Source data are provided in this paper.
References
Horvath, J. D., Koritnik, A., Kamakoti, P., Sholl, D. S. & Gellman, A. J. Enantioselective separation on a naturally chiral surface. J. Am. Chem. Soc. 126, 14988–14994 (2004).
Chen, W. et al. Fabrication and application of chiral separation membranes: a review. Sep. Purif. Technol. 327, 124898 (2023).
Maier, N. M., Franco, P. & Lindner, W. Separation of enantiomers: needs, challenges, perspectives. J. Chromatogr. A 906, 3–33 (2001).
Yokota, A. & Ikeda, M. Amino Acid Fermentation. (Springer, 2017).
Liu, Z. et al. A single-molecule electrical approach for amino acid detection and chirality recognition. Sci. Adv. 7, eabe4365 (2021).
Li, M. et al. Electrosynthesis of amino acids from NO and α-keto acids using two decoupled flow reactors. Nat. Catal. 6, 906–915 (2023).
Insights, F. M. Amino Acids Market Outlook (2025 to 2035)(2025).
Eyal, A. M. & Bressler, E. Industrial separation of carboxylic and amino acids by liquid membranes: Applicability, process considerations, and potential advantage. Biotechnol. Bioeng. 41, 287–295 (1993).
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Lee, H. D. et al. Separation and purification of lactic acid from fermentation broth using membrane-integrated separation processes. Ind. Eng. Chem. Res. 56, 8301–8310 (2017).
Jonckheere, D. et al. Adsorption and separation of aromatic amino acids from aqueous solutions using metal–organic frameworks. ACS Appl. Mat. Interfaces 9, 30064–30073 (2017).
Shen, J. & Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 116, 1094–1138 (2016).
Lakshmi, B. B. & Martin, C. R. Enantioseparation using apoenzymes immobilized in a porous polymeric membrane. Nature 388, 758–760 (1997).
Xie, R., Chu, L.-Y. & Deng, J.-G. Membranes and membrane processes for chiral resolution. Chem. Soc. Rev. 37, 1243–1263 (2008).
Vedovello, P., Paranhos, C. M., Fernandes, C. & Tiritan, M. E. Chiral polymeric membranes: Recent applications and trends. Sep. Purif. Technol. 280, 119800 (2022).
Chen, T., Li, H., Shi, X., Imbrogno, J. & Zhao, D. Robust homochiral polycrystalline metal–organic framework membranes for high-performance enantioselective separation. J. Am. Chem. Soc. 146, 14433–14438 (2024).
Lu, Y. et al. Homochiral MOF–polymer mixed matrix membranes for efficient separation of chiral molecules. Angew. Chem. Int. Ed. 131, 17084–17091 (2019).
Huang, K., Dong, X., Ren, R. & Jin, W. Fabrication of homochiral metal-organic framework membrane for enantioseparation of racemic diols. AlChE J. 59, 4364–4372 (2013).
Das, S., Xu, S., Ben, T. & Qiu, S. Chiral recognition and separation by chirality-enriched metal–organic frameworks. Angew. Chem. Int. Ed. 57, 8629–8633 (2018).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Wang, Z., Zhang, S., Chen, Y., Zhang, Z. & Ma, S. Covalent organic frameworks for separation applications. Chem. Soc. Rev. 49, 708–735 (2020).
Haase, F. & Lotsch, B. V. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 49, 8469–8500 (2020).
Zhang, W. et al. Reconstructed covalent organic frameworks. Nature 604, 72–79 (2022).
Yang, J. et al. Advancing osmotic power generation by covalent organic framework monolayer. Nat. Nanotechnol. 17, 622–628 (2022).
Zhao, S. et al. Hydrophilicity gradient in covalent organic frameworks for membrane distillation. Nat. Mater. 20, 1551–1558 (2021).
Kandambeth, S. et al. Selective molecular sieving in self-standing porous covalent-organic-framework membranes. Adv. Mater. 29, 1603945 (2017).
Sasmal, H. S. et al. Superprotonic conductivity in flexible porous covalent organic framework membranes. Angew. Chem. Int. Ed. 130, 11060–11064 (2018).
Biswal, B. P., Chaudhari, H. D., Banerjee, R. & Kharul, U. K. Chemically stable covalent organic framework (COF)-polybenzimidazole hybrid membranes: enhanced gas separation through pore modulation. Chem. Eur. J. 22, 4695–4699 (2016).
Yuan, C. et al. Nanochannels of covalent organic frameworks for chiral selective transmembrane transport of amino acids. J. Am. Chem. Soc. 141, 20187–20197 (2019).
Zhang, S., Zhou, J. & Li, H. Chiral covalent organic framework packed nanochannel membrane for enantioseparation. Angew. Chem. Int. Ed. 134, e202204012 (2022).
Cao, L. et al. Covalent Organic Framework Membranes with Patterned High-Density Through-Pores for Ultrafast Molecular Sieving. J. Am. Chem. Soc. 146, 21989–21998 (2024).
He, G., Zhang, R. & Jiang, Z. Engineering covalent organic framework membranes. Acc. Mater. Res. 2, 630–643 (2021).
Shinde, D. B. et al. Crystalline 2D covalent organic framework membranes for high-flux organic solvent nanofiltration. J. Am. Chem. Soc. 140, 14342–14349 (2018).
Cao, L. et al. Switchable Na+ and K+ selectivity in an amino acid functionalized 2D covalent organic framework membrane. Nat. Commun. 13, 7894 (2022).
Zhao, G., Gao, H., Qu, Z., Fan, H. & Meng, H. Anhydrous interfacial polymerization of sub-1 Å sieving polyamide membrane. Nat. Commun. 14, 7624 (2023).
Xu, T. et al. Constructing photocatalytic covalent organic frameworks with aliphatic linkers. J. Am. Chem. Soc. 146, 20107–20115 (2024).
Tang, X. et al. Self-standing chiral covalent organic framework thin films with full-color tunable guest-induced circularly polarized luminescence. Angew. Chem. Int. Ed. 136, e202413171 (2024).
Wan, H. & Blomberg, L. G. Chiral separation of amino acids and peptides by capillary electrophoresis. J. Chromatogr. A 875, 43–88 (2000).
Schulte, T. R., Holstein, J. J. & Clever, G. H. Chiral self-discrimination and guest recognition in helicene-based coordination cages. Angew. Chem. Int. Ed. 58, 5562–5566 (2019).
Marchetti, P., Peeva, L. & Livingston, A. The selectivity challenge in organic solvent nanofiltration: membrane and process solutions. Annu. Rev. Chem. Biomol. Eng. 8, 473–497 (2017).
Jiang, Z. et al. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 609, 58–64 (2022).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Acknowledgements
We gratefully acknowledge support from the KAUST Competitive Grant URF/1/4713-01-01 (Z.L.) and the Center of Excellence Grant FCC/1/5937-04-01 (Z.L.). We thank the Core Laboratories of KAUST for their support with characterizations.
Author information
Authors and Affiliations
Contributions
Z.L., L.C. and T.X. conceived the idea and designed the research plan. T.X., L.C. and S.A. carried out the experiment. T. X. and X.L. conducted the HPLC experiments. Z.Li. provided constructive suggestions for results and discussion. All authors participated in the discussion. Z.L., L.C. and T.X. co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Rahul Banerjee, Fusheng Pan and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, T., Cao, L., An, S. et al. A homochiral covalent organic framework membrane for the enantioseparation and fractionation of amino acids. Nat Commun 16, 7803 (2025). https://doi.org/10.1038/s41467-025-63247-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63247-y