Abstract
Enhancing solar evaporation performance while minimizing material consumption is essential for advancing the practical application of interfacial solar evaporation technologies. Although introducing external airflow can significantly boost evaporation rates, it requires additional components and electricity input, compromising the simplicity, passivity and sustainability of interfacial solar evaporation. To address this challenge, Dyson sphere-like evaporators (DSEs) capable of self-generating convective flow inside the evaporator are designed. This self-generated internal airflow facilitates the removal of generated vapor from both inner and outer evaporation surfaces, thus significantly improving the evaporation rate. Notably, despite sacrificing 36% of solar light energy to generate internal convection, the DSE still achieves a much higher evaporation rate (4.08 kg m−2 h−1) compared to a typical spherical evaporator (2.04 kg m−2 h−1) which utilizes all the solar light energy directly for water evaporation. This finding suggests that future evaporator design should consider the balance between the energy used for water evaporation and convection generation for vapor removal.
Similar content being viewed by others
Introduction
Interfacial solar evaporation (ISE) is a promising and environmentally friendly technology for many applications, including seawater desalination1,2,3,4,5,6,7,8,9,10, wastewater treatment11,12,13,14,15,16, sterilization17,18,19, power generation20,21,22,23,24, lithium extraction25,26,27,28,29, and soil remediation30,31,32,33. After a decade of intensive research, the light-to-vapor energy conversion efficiency and evaporation rate of ISE systems have been significantly enhanced by optimizing light-absorbing materials34,35,36,37,38,39, introducing extra energy sources15,40,41,42, and lowering evaporation enthalpy43,44,45,46,47. Rational design of photothermal material and evaporator configuration, together with elaborated energy management, are crucially important in achieving outstanding solar evaporation performance48,49. Recently, due to its ability to dramatically enhance solar evaporation rate, the role of airflow in ISE systems has also attracted intense research interest48,50,51. In the presence of external convective flow, vapor generated at the evaporation surface can be efficiently removed, inducing a transient low-humidity environment. This drives continuous and rapid water evaporation to replenish the removed vapor, as the evaporation rate is inversely proportional to the local humidity level52. However, applying external convective flow has some drawbacks. For example, additional components, such as electrical fans, are required to provide oriented airflow, making the ISE system more complicated with extra electrical power requirements. Hence, a solar-driven photothermal evaporator capable of delivering high evaporation efficiency in the absence of external convection is desirable yet challenging.
To realize this goal, it is required to create a new type of evaporator that can generate convection itself and take advantage of the self-generated convection to enhance solar evaporation. Inspired by the Dyson sphere, a hypothetical megastructure that circles a star to harvest its energy, a series of Dyson sphere-like evaporators (DSEs) are designed (Fig. 1a). The newly designed DSEs draw the special structural concept of the Dyson sphere and adopt a spherical configuration with a perforated outer shell and a small inner photothermal sphere. It is hypothesized that during ISE, due to the temperature difference between the inner sphere, outer porous shell, and the surrounding air, the DSEs would spontaneously generate convective flow both inside and outside of the evaporator, which passes through the perforations on the outer shell to effectively remove the vapor generated on both inside and outside evaporation surfaces (Fig. 1b, c). Therefore, in this design, all the evaporation surfaces are activated, significantly improving the solar evaporation performance. Surprisingly, our results find that during ISE, even when 36% of the input solar light energy is sacrificed to generate internal convection, the achieved evaporation rate of the DSE (4.08 kg m−2 h−1) is still much higher than that of a traditional spherical evaporator where all the input solar light energy is directly consumed for water evaporation. This counterintuitive result indicates that during ISE, instead of only focusing on direct light-to-vapor energy conversion, indirect light-to-heat-to-convection energy conversion for efficient removal of generated vapor from the evaporation system also needs to be considered as an effective strategy to improve overall solar evaporation performance.
Results
Design, preparation, and characterization of DSEs
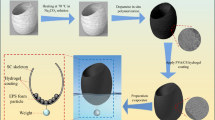
A typical spherical evaporator (SE) and various DSEs were designed and prepared by 3D printing of base structures (Fig. 2a and Supplementary Fig. 1), followed by coating of photothermal hydrogels. UV-curing resin was selected as the raw material to print the base structures for SE and DSEs due to its high precision in printing complex structures. Although the printed base structure showed good hydrophilicity (Supplementary Fig. 2), its water transportation capability is not sufficient to satisfy the demand for practical solar evaporation. In addition, solar evaporators require excellent light absorption performance to harvest sunlight as the energy source to drive ISE. Therefore, a photothermal hydrogel layer composed of reduced graphene oxide (rGO), cellulose fiber, and sodium alginate (SA) was coated on the surface of the base structures. To realize quick and homogenous hydrogel coating throughout the 3D printed structures, a modified in situ crosslinking assisted dip-coating (ICDC) method was applied (Fig. 2b)53. Since SA can be crosslinked in the presence of Ca2+, if a material/substrate hosting Ca2+ is inserted in an SA solution containing photothermal materials, the localized Ca2+ is able to induce in situ SA crosslinking, leading to the spontaneous formation of a photothermal hydrogel layer on the surface of the substrates. To demonstrate the effectiveness of the ICDC method, initially a 3D-printed slice was used as a model to assess hydrogel coating efficiency. Thanks to the good hydrophilicity (Supplementary Fig. 2) and existing micron-scale grooves of the 3D printed structures (Fig. 2c), Ca2+ ions can be localized on its surfaces after simple immersion in an aqueous CaCl2 solution (Supplementary Fig. 3). Thereafter, the Ca2+-containing slice was immersed in the SA solution containing dispersed reduced graphene oxide (rGO, Supplementary Figs. 4 and 5) and cellulose fibers (Supplementary Fig. 6). A thin layer of photothermal hydrogel (i.e., SA-cellulose fiber-rGO) was quickly formed on the slice (Supplementary Fig. 7a, b). In comparison, directly dipping a bare 3D printed slice into the same SA-cellulose fiber-rGO solution could not lead to the formation of any hydrogel layer (Supplementary Fig. 7c). The coated hydrogel was very stable and could resist 20 mins sonication treatment followed by immersing in water for 7 days (Supplementary Fig. 8). Thus, this ICDC method was readily applied to the bare 3D printed Dyson sphere-like base structures to obtain the DSEs (Fig. 2b). For the Dyson sphere-like structure, due to the presence of the shell wall perforations, the photothermal hydrogel can be coated both on the outside and inside surfaces, thus maximizing the available evaporation surface area. After photothermal hydrogel coating, the surface feature apparently changed (Fig. 2d–f), with wrinkled surfaces and 1D structures originating from rGO, SA, and cellulose fibers, respectively, clearly observed. The coated hydrogel layer also showed excellent hydrophilicity, as manifested by rapid water droplet absorption within 0.5 s (Supplementary Fig. 9). The vertical water transportation ability was also excellent (Supplementary Fig. 10), which ensured sufficient water supply to the evaporation surface during ISE. In addition, the photothermal hydrogel coating significantly improved the light absorption of the DSEs relative to the bare 3D printed structure (Supplementary Fig. 11), which rendered the evaporators an excellent photothermal effect for ISE.
a 3D printed spherical and Dyson sphere-like base structures. b Schematic diagram of the photothermal hydrogel coating processes. SEM images of the surface structure of the (c) 3D printed base, (d) High-magnification coated photothermal hydrogel surface, (e) Low-magnification coated photothermal hydrogel surface, and (f) SEM images of the cellulose in photothermal hydrogel.
Impact of self-generated convection on solar evaporation performance
The advantages of Dyson sphere-like structures for interfacial solar evaporation were initially investigated by assessing the evaporation performance of a traditional SE and a simple DSE without an inner sphere (denoted as DSE-I) (Fig. 3a and Supplementary Fig. 12). Compared to the evaporation surface area of the SE (2261.9 mm2, normalized as S0), DSE-I had a much larger evaporation surface of 1.6 S0 due to the exposure of both inner and outer surfaces (Supplementary Table 1 and Supplementary Note 1), while the 22 perforations on the outer shell could allow the generated vapor on the inner evaporation surfaces to escape more efficiently. Thus, intuitively, DSE-I should deliver a higher evaporation rate than SE. However, the measured dark evaporation rate of the DSE-I (0.58 kg m−2 h−1) was lower than that of SE (0.75 kg m−2 h−1) (Fig. 3b, c). The ratio of dark evaporation of DSE-I to SE (0.773) was very similar to the ratio of the outer surface area of DSE-I to ISE (0.755), which implied that only the external surfaces of the DSE-I contributed to the water evaporation while the inner surfaces did not. Previous studies have shown that evaporation rates are inversely proportional to the humidity near the evaporation surface52. Therefore, although DSE-I had a larger evaporation surface than the SE, its inner evaporation surface was not activated due to the accumulated vapor inside the DSE-I. A notable change was observed under one sun illumination. The solar evaporation rate of DSE-I increased to 2.00 kg m−2 h−1, similar to that of SE (2.04 kg m−2 h−1). It should be noted that, in DSE-I, due to the existence of the outer shell perforations, 22.5% of the light passed through the evaporator and was not harnessed for solar evaporation. Therefore, it is reasonable to infer that a part of the inner evaporation surface of DSE-I was activated under sunlight irradiation to contribute to water evaporation. Unlike dark conditions, where no noticeable internal air convection could be generated due to the relatively homogenous temperature distribution, during solar evaporation under 1.0 sun, due to the temperature difference across the evaporator, convection flow could be induced to enhance vapor escape from the evaporation surfaces. To verify this hypothesis, numerical simulations were conducted (Fig. 3d, Supplementary Figs. 13 and 14, and Supplementary Note 2) using the actual geometries and measured temperatures of the evaporators (Supplementary Table 2). Simulation results showed that, for SE, the outer upper surface absorbed the vertically incident light, thus has the highest temperature. The temperature difference between the top surface and the surrounding air induced a convective airflow of ~0.07 m s−1 (Fig. 3d). However, since SE was a solid spherical evaporator, the airflow only assisted the vapor removal on the outer surface. In contrast, during solar evaporation, DSE-I induced convection across the outer shell (0.05 m s−1) to activate the internal evaporation surface near the top (Fig. 3e). Although the induced internal convection flow (0.01 m s−1) was not as strong as the external one, it still can remarkably lower the humidity level near the inner evaporation surface (Supplementary Fig. 15), thus improving the overall evaporation rate. It should be pointed out that the generated convection flow did not activate all the inner evaporation surface since it did not reach the bottom of DSE-I (Fig. 3e). Therefore, the evaporation rate of DSE-I still did not reach the theoretical maximum, otherwise, the solar evaporation rate of DSE-I should be higher than that of SE.
The above results and discussion imply that in a solar evaporator, light harvesting, convection strength and distribution, and activated evaporation surface area are all important in enhancing evaporation rates. Therefore, a series of different DSEs with modified structures were designed and prepared to evaluate how Dyson sphere-like structures contribute to greater evaporation performance (Fig. 4a). For example, to fully capture the incident solar light that passes through the outer shell perforations and introduce more internal evaporation surface area, an additional inner sphere (DSE-II) and hemisphere (DSE-III) were added in DSE-I (Fig. 4a). The introduced inner sphere and hemisphere were also coated with a photothermal hydrogel layer to act as photothermal evaporation surfaces. During ISE, both the internal sphere/hemisphere should provide a relatively high-temperature surface (Supplementary Table 2) and therefore induce a stronger internal convection in the evaporator, which would be conducive to improving evaporation rates. However, the fact was that the DSE-II with the largest evaporation surface area (2.05 S0) delivered the lowest evaporation rate of 1.95 kg m−2 h−1, while DSE-III with a smaller evaporation surface area (1.82 S0) exhibited the highest evaporation rate of 3.2 kg m−2 h−1 (Fig. 4b). These results clearly demonstrate that simply increasing evaporation surface area and light absorption does not guarantee improved evaporation rates. Although the inner sphere of DSE-II absorbed light and induced convection, its large volume blocked effective airflow inside DSE-II, thus failed to activate the inner evaporation surfaces (Fig. 4b, c). In comparison, although DSE-III had a smaller internal hemisphere, stronger convective flow (~0.02 m s−1) was generated, which could freely pass through the interior of the evaporator to remove the generated vapor (Fig. 4c), thus delivering a much higher evaporation rate. As the configuration DSE-III has demonstrated superior ability to generate and take advantage of convection to enhance solar evaporation, we further modified the evaporator to enhance its self-generated internal convective flow. Since the photothermal hydrogel coating on the inner hemisphere of DSE-III also participated in water evaporation, its surface temperature was not very high due to the evaporative cooling effect. To achieve a higher surface temperature for generating stronger internal convection, the photothermal hydrogel coating was replaced by an acrylic black paint, followed by a hydrophobic treatment. Therefore, while the acrylic black paint still showed high light absorption (Supplementary Fig. 11), it was not involved in any water evaporation due to its hydrophobicity (Supplementary Fig. 16). As a result, the non-evaporative photothermal surface showed a much higher temperature under light irradiation than the photothermal hydrogel coated surface (Supplementary Fig. 17). Compared to DSE-III (1.82 S0), although this modified evaporator (DSE-IV, 1.6 S0) sacrificed a part of the evaporation surface (i.e., internal hemisphere), its solar evaporation rate (3.45 kg m−2 h−1) was higher than that of DSE-III (3.20 kg m−2 h−1). Numerical simulations revealed that DSE-IV had a much stronger self-generated internal airflow (~0.04 m s−1) compared to that of DSE-III (~0.02 ms−1), resulting in the higher evaporation rate. It should be noted that, for DSE-III, all the solar light energy was directly converted to heat for water evaporation, while for DSE-IV, about 22.5% of light energy was utilized for convective flow generation through multi-step energy conversion processes (Supplementary Note 3). Intuitively, when more energy is directly used for water evaporation, a higher evaporation rate should be obtained. However, this is not true for DSE-IV, which harnessed less solar energy for water evaporation but delivered a higher evaporation rate. This implies that consuming a part of the light energy to generate airflow to remove generated vapor is more effective than directly using all the light energy for water evaporation. To further test this hypothesis, the top part (18 cm in diameter, same as that of the size of the internal hemisphere) of the external sphere of DSE-IV was removed to allow more light energy to be absorbed by the non-evaporative inner hemisphere. In this case, the obtained evaporator (DSE-V) achieved a much higher surface temperature (~51.8 °C) of the inner hemisphere (Supplementary Table 2). Despite the evaporation surface area of the DSE-V being further reduced (1.46 S0) and more light energy was sacrificed (36%) to generate convective flow but not direct water evaporation (Supplementary Note 4), the overall evaporation rate of DSE-V was further remarkably increased to 4.08 kg m−2 h−1, which was the highest amongst all the evaporators tested. This dramatic increase in evaporation rate was attributed to the self-generation of a much stronger internal convection (~0.08 m s−1) (Fig. 4c), which maximized the performance of the reduced evaporation surfaces (Fig. 4b, c). To verify and visualize the self-generated convection, a smoke flow test was conducted (Supplementary Fig. 18). As shown in Supplementary Movie S1, under sunlight irradiation, smoke was seen entering the DSE-V through the side perforations and subsequently exiting through the top outlet, reflecting the pattern of the self-generated internal convection. Concurrently, the external smoke rose along the outer surfaces of DSE-V (Supplementary Movie S1). The overall observed smoke flow patterns were in good agreement with the simulated convective flow (Fig. 4c), verifying the existence of self-generated convection during solar evaporation. The convective flow can efficiently remove vapor from the evaporation surfaces via enhanced mass transfer. The Sherwood number (Sh) is a dimensionless parameter that quantifies the ratio of convective mass transfer to diffusive mass transfer54. A higher Sh indicates more efficient vapor removal. The Sh at the top open hole of DSE-V is calculated to be 7.6, which is much higher than that of static diffusion conditions (≈ 2) (Supplementary Note 5). Therefore, DSE-V has a higher vapor removal efficiency due to the self-generated convection, which greatly improves the evaporation performance. In addition, the strong self-generated air flow inside and outside of DSE-V could also improve the efficiency of energy harvesting from the environment and thermal energy recycling by the cold evaporation surface of DSE-V due to the improved convective heat transfer coefficient (Supplementary Fig. 19, Supplementary Movie S2, and Supplementary Note 6), further enhancing the evaporation performance.
a Schematic illustration of the structure of SE (left) and DSE-I (right). b Dark evaporation rates, solar evaporation rates, and outer evaporation surface area of SE and DSE-I. The error bar represents the standard deviation of three-time measurements. c Mass change of water with SE and DSE-I during solar evaporation. Simulated convection around the (d) SE and (e) DSE-I.
a Schematic illustration of the structures of DSE-II, DSE-III, DSE-IV, and DSE-V. b Comparative analysis of the performance of SE, DSE-I to DSE-V, including the metrics of solar evaporation rate (SER, kg m−2 h−1), dark evaporation rate (DER, kg m−2 h−1), solar energy input to the evaporation surface (SEI, %), internal convection flow rate (ICS, m s−1), evaporation surface area (EA mm2), and scaffold material consumption (SMC, mm3). c Simulated convection in the DSE-II to DSE-V from left to right, respectively.
Evaluation of cost, stability, and outdoor performance of DSE-V
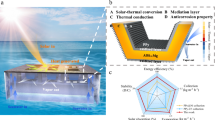
Considering materials consumption, direct solar light energy input for water evaporation, and solar evaporation rate (Fig. 4b), DSE-V delivered the highest evaporation performance with less materials consumption compared to typical SE and other DSEs. The overall materials cost for a single DSE-V was estimated to be approximately 0.027 USD (Supplementary Note 7 and Supplementary Table 3). The evaporation rate of DSE-V is also higher than that of many recently reported evaporators (Fig. 5a and Supplementary Table 4)9,39,55,56,57,58,59,60,61,62,63,64,65,66. Based on the structure of DSE-V, we adjusted the number and size of the perforations on the outer shell while maintaining the evaporation surface area similar (denoted as DSE-V1 and DSE-V2, Supplementary Figs. 20–22, and Supplementary Note 8). Under 1.0 sun irradiation, the obtained DSE-V1 and DSE-V2 all exhibited high surface temperature of the non-evaporative hemisphere (Supplementary Fig. 20). Simulation results confirmed that strong convective flow across the outer shell also occurred (Supplementary Fig. 21). As a result, these two evaporators also delivered a high evaporation rates of 3.96 and 4.09 kg m−2 h−1 respectively (Supplementary Fig. 22), further confirming the advantage of DSE-V based structure in generating and utilizing convection to enhance solar evaporation. To assess the stability and salt-resistance performance of DSE-V, 8 hours of continuous solar evaporation tests using real seawater were conducted, where stable evaporation rates of 3.5–3.8 kg m−2 h −1 were achieved (Fig. 5b). Throughout the test, the evaporation surfaces remained free of salt accumulation, demonstrating excellent salt-resistant performance (Supplementary Fig. 23). During a more extended test across 7 days (8 h per day), DSE constantly delivered stable and high evaporation rates without salt accumulation (Supplementary Figs. 24, 25), further confirming its excellent adaptability and reliability for seawater desalination. Outdoor tests under natural sunlight were also conducted to evaluate water evaporation and clean water collection performance (Fig. 5c–f). An enclosed device was fabricated to hold 6 DSE-Vs and a container filled with real seawater (Fig. 5c–e). Weather conditions during the tests were recorded (Fig. 5f). Over 10 hours of continuous outdoor solar evaporation (8:00 am to 6:00 pm), 89.98 g of water was evaporated, and 63.76 g of clean water was collected, corresponding to an average evaporation rate of 2.12 kg m−2 h−1 and an average water collection rate of 1.51 kg m−2 h−1, respectively. The concentrations of Na+, K+, Ca²+, and Mg²+ in the collected condensed water were significantly lower than the guideline salinity levels for WHO and EPA drinkable water (Fig. 5g), confirming the production of high-quality clean water and the applicability of the evaporators and device for practical seawater desalination.
a Comparison of solar evaporation rates between DSE-V and recently reported evaporators. b Mass change and evaporation rates of DSE-V during 8 h of solar evaporation tests. c Photograph of the water collection device (diameter: 20 cm, length: 20 cm) for outdoor solar evaporation of seawater. d Six DSE-V were placed in the device. e Condensed water on the inner wall of the device. f Light intensity, environmental temperature, and humidity during the outdoor tests. g Major ion concentrations of the original seawater and condensed water.
Discussion
This work revealed that enhancing ISE should not be approached solely from the perspective of energy conservation but rather needs to be fundamentally reconsidered ISE as a “chemical reaction” process. If evaluated solely through an energy aspect, enhancing light absorption and direct energy utilization for water evaporation would theoretically deliver the highest evaporation rates. However, experimental evidence contradicts this assumption. For example, although SE and DSE II-IV utilized all the incident light for direct water evaporation, they did not achieve the highest evaporation rates. From a “chemical reaction” perspective, where vapor is considered the reaction “product”, the removal of this “product” from the reaction system effectively continuous evaporation. This mechanism explains why strategically sacrificing partial light energy to generate convective flow for vapor removal can paradoxically yield higher evaporation rates (i.e., DSE-V) than complete light energy dedication to direct water evaporation. In addition, the self-generated air flow and convection loop not only effectively remove vapor from the evaporation surfaces but also enable thermal energy recycling to enhance solar evaporation. Our findings suggest that the future design of solar evaporators and systems requires a sophisticated balance between energy and “reaction equilibrium” aspects. The implementation of product (vapor) removal mechanisms, even at the cost of partial energy diversion, emerges as a critical strategy for maximizing evaporation performance. This conceptual shift opens new avenues for optimizing ISE designs beyond traditional energy accounting frameworks.
Methods
Materials and chemicals
Evaporator base models were printed by a 3D printer (LD-006, Creality). Alginic acid sodium salt from brown algae (SA) and α-cellulose were purchased from Sigma–Aldrich. Calcium chloride (94.0%) and ethanol (99.5%) were purchased from Chem-Supply. rGO was provided by Huasheng Graphite Co., Ltd, China. Black acrylic paint and Silicon hydrophobic spray were purchased from local retailers. Milli-Q water (resistance > 18.2 MΩcm−1) was used for all solution preparations.
Preparation of SA-cellulose fiber-rGO mixture
SA solution (20 mg mL−1) was prepared by dissolving 1 g SA in 50 mL Milli-Q water. rGO (0.5 g) was added into 50 mL of mixed solvent of Milli-Q water and ethanol (4:1 v/v%) and sonicated for 1 hour to form a dispersion. Thereafter, 5 mL of the prepared rGO dispersion and 1 g α-cellulose were mixed with 25 mL SA solution (20 mg mL−1). Then, Milli-Q water was added to adjust the final volume to 50 mL, so that the final concentrations of SA, rGO, and cellulose were 10, 1, and 20 mg mL−1, respectively. As the light absorption material, the optimized rGO content was identified as 1 mg mL−1 (Supplementary Note 9 and Supplementary Fig. 26). Finally, the mixture was sonicated for 30 minutes.
Preparation of photothermal evaporators
The 3D printed base models were first immersed in CaCl2 solution (5 wt%) for 5 s. Then, the models were immersed in the SA-cellulose fiber-rGO mixture for 15 s to form a thin layer of SA-cellulose fiber-rGO hydrogel on the surface of the model. The above steps were repeated twice to fabricate the evaporators. Finally, the fabricated evaporators were soaked in water for 12 h to remove any extra ions.
Preparation of non-evaporative photothermal surface
The inner hemisphere of DSE-V was initially coated with black acrylic, followed by further treatment with a commercial hydrophobic silicon spray. The obtained evaporator was then placed in a fume hood for 12 h for drying.
Solar steam generation performance test
Different evaporators were floated on the water surface assisted by an expanded polyethylene (EPE) foam. Simulated solar light at an intensity of 1.0 kW m−2 was provided by a Newport Oriel Solar Simulator (Class ABA, 450 W, 69920). An electronic balance was used to record the mass change of the water samples. The surface temperature of the evaporators was measured by an IR camera and thermocouples. All the indoor solar evaporation tests were proceeded under controlled environmental temperature (25.0–25.5 °C) and humidity (30–32%). Each sample was tested at least three times.
Outdoor evaporation performance test
The solar evaporation and water collection device was made of a horizontal acrylic cylinder (diameter: 20 cm, length: 20 cm). A water outlet was drilled at the bottom of the device for condensed water collection. During the evaporation tests, solar illumination intensity, environmental temperature, and humidity were recorded every 30 mins. The outdoor tests were conducted at Mawson Lakes Campus (34.9229°S, 138.5912°E), the University of South Australia, Adelaide, Australia on 15th November 2024.
Smoke flow visualization test
A DSE-V was placed inside a sealed glass chamber filled with smoke. The smoke was produced by an ultrasonic spray humidifier, using a solution composed of glycerol and water in a 1:4 volume ratio. After the smoke reached the static state, the evaporator was exposed to the solar simulator. A CCD camera was used to record the video of the smoke movement.
Characterizations
Morphology and structure of materials were characterized using scanning electron microscopy (SEM, Zeiss Merlin) and transmission electron microscopy (TEM, JEOL JEM-2100). The composition of materials was investigated by X-ray photoelectron spectroscopy (XPS, Kratos AxisUltra XPS). The hydrophilicity of the materials is assessed by water contact angle using Dataphysics OCA 20. Light absorption spectra of the samples were measured by a UV-3600 Spectrometer (Shimadzu).
Data availability
The data supporting the findings of the study are included in the main text, supplementary information and the source data files. The source data are available in the Figshare database under accession code [https://doi.org/10.6084/m9.figshare.29400170].
References
Xu, N. et al. Going beyond efficiency for solar evaporation. Nat. Water 1, 494–501 (2023).
Hao, X. et al. Multifunctional solar water harvester with high transport selectivity and fouling rejection capacity. Nat. Water 1, 982–991 (2023).
Liang, H. et al. Thermal efficiency of solar steam generation approaching 100% through capillary water transport. Angew. Chem. Int. Ed. 58, 19041–19046 (2019).
Kuang, Y. et al. A high-performance self-regenerating solar evaporator for continuous water desalination. Adv. Mater. 31, 1900498 (2019).
He, S. et al. Nature-inspired salt resistant bimodal porous solar evaporator for efficient and stable water desalination. Energy Environ. Sci. 12, 1558–1567 (2019).
Ni, G. et al. A salt-rejecting floating solar still for low-cost desalination. Energy Environ. Sci. 11, 1510–1519 (2018).
Yang, K., Pan, T., Dang, S., Gan, Q. & Han, Y. Three-dimensional open architecture enabling salt-rejection solar evaporators with boosted water production efficiency. Nat. Commun. 13, 6653 (2022).
Xu, Z. et al. Ultrahigh-efficiency desalination via a thermally-localized multistage solar still. Energy Environ. Sci. 13, 830–839 (2020).
Wang, X. et al. Self-rotating wood-based floating solar-driven interfacial evaporator for continuous and high-efficiency desalination. Chem. Eng. J. 509, 161363 (2025).
Sun, L. et al. Ultra-high solar steam generation based on regulated water management strategy in 3D biomass hydrogels inspired by the “binding effect” of cell walls. Desalination 587, 117954 (2024).
Shi, L. et al. Multi-functional 3D honeycomb ceramic plate for clean water production by heterogeneous photo-Fenton reaction and solar-driven water evaporation. Nano Energy 60, 222–230 (2019).
Guo, Y. et al. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 32, 1907061 (2020).
Zhang, P. et al. Super water-extracting gels for solar-powered volatile organic compounds management in the hydrological cycle. Adv. Mater. 34, 2110548 (2022).
Hu, J. et al. Photothermal fabrics for solar-driven seawater desalination. Prog. Mater Sci. 150, 101407 (2024).
Li, X. et al. Three-dimensional artificial transpiration for efficient solar waste-water treatment. Natl. Sci. Rev. 5, 70–77 (2018).
Gao, X. et al. Construction of black g-C3N4/loofah/chitosan hydrogel as an efficient solar evaporator for desalination coupled with antibiotic degradation. Sep. Purif. Technol. 355, 129615 (2025).
Zhao, L. et al. A passive high-temperature high-pressure solar steam generator for medical sterilization. Joule 4, 2733–2745 (2020).
Li, Y. et al. 3D-printed, all-in-one evaporator for high-efficiency solar steam generation under 1 sun illumination. Adv. Mater. 29, 1700981 (2017).
Neumann, O. et al. Compact solar autoclave based on steam generation using broadband light-harvesting nanoparticles. Proc. Natl. Acad. Sci. USA 110, 11677–11681 (2013).
Gao, M., Zhu, L., Peh, C. K. & Ho, G. W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 12, 841–864 (2019).
Zhu, L., Ding, T., Gao, M., Peh, C. K. N. & Ho, G. W. Shape conformal and thermal insulative organic solar absorber sponge for photothermal water evaporation and thermoelectric power generation. Adv. Energy Mater. 9, 1900250 (2019).
Zhang, Y. et al. Manipulating unidirectional fluid transportation to drive sustainable solar water extraction and brine-drenching induced energy generation. Energy Environ. Sci. 13, 4891–4902 (2020).
Zhang, Y., Xiong, T., Nandakumar, D. K. & Tan, S. C. Structure architecting for salt-rejecting solar interfacial desalination to achieve high-performance evaporation with in situ energy generation. Adv. Sci. 7, 1903478 (2020).
Wu, Y. et al. Asymmetric evaporation for efficient and simultaneous extraction of freshwater, salt, and electrical energy from seawater. Energy Environ. Sci. 17, 9303–9312 (2024).
Song, Y. et al. Solar transpiration–powered lithium extraction and storage. Science 385, 1444–1449 (2024).
Chen, X. et al. Spatially separated crystallization for selective lithium extraction from saline water. Nat. Water 1, 808–817 (2023).
Chen, X. et al. Solar-driven Lithium extraction by a floating felt. Adv. Funct. Mater. 34, 2316178 (2024).
Li, H.-N. et al. Design of photothermal “ion pumps” for achieving energy-efficient, augmented, and durable lithium extraction from seawater. ACS Nano 18, 2434–2445 (2024).
Zhang, S. et al. Solar-driven membrane separation for direct lithium extraction from artificial salt-lake brine. Nat. Commun. 15, 238 (2024).
Song, Y., Fang, S., Xu, N. & Zhu, J. Solar-driven interfacial evaporation technologies for food, energy and water. Nat. Rev. Clean. Technol. 1, 55–74 (2025).
Wu, P., Wu, X., Wang, Y. D., Xu, H. L. & Owens, G. Towards sustainable saline agriculture: Interfacial solar evaporation for simultaneous seawater desalination and saline soil remediation. Water Res. 212, 118099 (2022).
Shi, C. et al. A green and energy-supply‒free artificial plant for efficient and non-selective enrichment of heavy metal ions out of soil. Adv. Funct. Mater. 34, 2409445 (2024).
Yu, Z. et al. Microplastic detection and remediation through efficient interfacial solar evaporation for immaculate water production. Nat. Commun. 15, 6081 (2024).
Ghasemi, H. et al. Solar steam generation by heat localization. Nat. Commun. 5, 4449 (2014).
Ni, G. et al. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 1, 1–7 (2016).
Yang, B. et al. Flatband λ-Ti3O5 towards extraordinary solar steam generation. Nature 622, 499–506 (2023).
Shi, Y. et al. A 3D photothermal structure toward improved energy efficiency in solar steam generation. Joule 2, 1171–1186 (2018).
Zhang, X. et al. Hydrogel-based 3D evaporator with cross-linked fixation by carbon dots for ultra-high and stable solar steam generation. Chem. Eng. J. 497, 154793 (2024).
Liu, H. et al. Conformal microfluidic-blow-spun 3D photothermal catalytic spherical evaporator for omnidirectional enhanced solar steam generation and CO2 reduction. Adv. Sci. 8, e2101232 (2021).
Li, X. et al. Enhancement of interfacial solar vapor generation by environmental energy. Joule 2, 1331–1338 (2018).
Song, H. et al. Cold vapor generation beyond the input solar energy limit. Adv. Sci. 5, 1800222 (2018).
Wang, Y. D. et al. Boosting solar steam generation by structure enhanced energy management. Sci. Bull. 65, 1380–1388 (2020).
Zhao, F. et al. Highly efficient solar vapour generation via hierarchically nanostructured gels. Nat. Nanotechnol. 13, 489–495 (2018).
Zhao, F., Guo, Y., Zhou, X., Shi, W. & Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 5, 388–401 (2020).
Zhou, X., Zhao, F., Guo, Y., Rosenberger, B. & Yu, G. Architecting highly hydratable polymer networks to tune the water state for solar water purification. Sci. Adv. 5, eaaw5484 (2019).
Yu, H. et al. Making interfacial solar evaporation of seawater faster than fresh water. Adv. Mater. 36, 2414045 (2024).
Hu, A. et al. Highly efficient solar steam evaporation via elastic polymer covalent organic frameworks monolith. Nat. Commun. 15, 9484 (2024).
Wu, X. et al. Interfacial solar evaporation: from fundamental research to applications. Adv. Mater. 36, 2313090 (2024).
Liang, Y. et al. Recent innovations in 3D solar evaporators and their functionalities. Sci. Bull. 69, 3590–3617 (2024).
Wang, Y. et al. Same materials, bigger output: A reversibly transformable 2D–3D photothermal evaporator for highly efficient solar steam generation. Nano Energy 79, 105477 (2021).
Li, J. L. et al. Over 10 kg m−2 h−1 Evaporation Rate Enabled by a 3D Interconnected Porous Carbon Foam. Joule 4, 928–937 (2020).
Gao, T. et al. More from less: improving solar steam generation by selectively removing a portion of evaporation surface. Sci. Bull. 67, 1572–1580 (2022).
Shao, B. et al. A general method for selectively coating photothermal materials on 3D porous substrate surfaces towards cost-effective and highly efficient solar steam generation. J. Mater. Chem. A 8, 24703–24709 (2020).
Ranz, W. E. Evaporation from drops: Part II. Chem. Eng. Prog. 48, 173–180 (1952).
Ge, Y. M. et al. Self-rotating spherical evaporator based on hydrogel and black titanium oxide for continuous desalination of seawater. ACS Mater. Lett. 5, 2576–2583 (2023).
Wu, X. et al. Dual-zone photothermal evaporator for antisalt accumulation and highly efficient solar steam generation. Adv. Funct. Mater. 31, 2102618 (2021).
Xia, Q. C. et al. A floating integrated solar micro-evaporator for self-cleaning desalination and organic degradation. Adv. Funct. Mater. 33, 2214769 (2023).
Yuan, B. et al. A low-cost 3D spherical evaporator with unique surface topology and inner structure for solar water evaporation-assisted dye wastewater treatment. Adv. Sustain. Syst. 5, 2000245 (2021).
Geng, L. et al. Interfacial solar evaporator synergistic phase change energy storage for all-day steam generation. J. Mater. Chem. A 10, 15485–15496 (2022).
Wang, M. et al. Hierarchically structured bilayer Aerogel-based Salt-resistant solar interfacial evaporator for highly efficient seawater desalination. Sep. Purif. Technol. 287, 120534 (2022).
He, W. X. et al. A novel solar-driven interfacial evaporator with multi-stage tunable liquid supply for efficient adaptive evaporation inspired by human thermal sweating. Chem. Eng. J. 509, 161249 (2025).
Wan, Y. F. et al. Bird’s nest-shaped Sb2WO6/D-Fru composite for multi-stage evaporator and tandem solar light-heat-electricity generators. Small 20, 2302943 (2024).
Mustakeem, M. et al. MXene-coated membranes for autonomous solar-driven desalination. ACS Appl. Mater. Interfaces 14, 5265–5274 (2022).
Yu, X. et al. COF@ CNT based fiber gable roof-shaped solar evaporators for efficient salt-rejecting desalination and agricultural applications. Chem. Eng. J. 501, 157500 (2024).
Ku, B. J. et al. Solar-driven desalination using salt-rejecting plasmonic cellulose nanofiber membrane. J. Colloid Interface Sci. 634, 543–552 (2023).
Ying, P. et al. A bio-inspired nanocomposite membrane with improved light-trapping and salt-rejecting performance for solar-driven interfacial evaporation applications. Nano Energy 89, 106443 (2021).
Acknowledgements
This work was financially supported by the Australian Research Council (DP240101581 to H.X. and G.O., DP220100583 to H.X., FT190100485 to H.X.) and the Future Industries Institute, University of South Australia. This work was performed in part at the South Australian node of the Australian National Fabrication Facility under the National Collaborative Research Infrastructure Strategy.
Author information
Authors and Affiliations
Contributions
D.W. and H.X. conceived the project. H.X. and X.Y. supervised the project. D.W. designed and conducted the experiments. X.W., H.Y., Y.B., D.C., Y.L. and G.O. performed materials characterization and discussed the experiments. D.W., X.Y. and H.X. processed the data and interpreted the results. H.Y., G.O., X.Y. and H.X. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, D., Wu, X., Yu, H. et al. Dyson sphere-like evaporators enhanced interfacial solar evaporation via self-generated internal convection. Nat Commun 16, 7985 (2025). https://doi.org/10.1038/s41467-025-63268-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63268-7