Abstract
Few population-based studies have evaluated the importance of pre-existing respiratory syncytial virus (RSV) antibody on RSV susceptibility among children and adults. We conducted a prospective, community-based cohort study among individuals aged 6 months-50 years in Oregon and Washington State, USA (June 2022-May 2023), with weekly symptom surveys and swab collection regardless of symptoms. Swabs were tested for RSV using RT-qPCR. Enrollment sera were tested for RSV prefusion F IgG binding (all participants) and neutralizing antibodies (pediatric participants). We detected 305 RSV illnesses among 3237 participants from 1188 households. Using proportional hazards regression, higher RSV binding antibody titers were associated with a lower estimated hazard of RSV among pediatric participants (hazard ratio=0.66 per 1-unit difference in log10-RSV antibody titer; 95% CI: 0.56, 0.78). In a post-pandemic period, pre-existing RSV antibody titers were associated with a lower risk of RSV illness in children aged 6 months-17 years, which could inform vaccine development for this age group.
Similar content being viewed by others
Introduction
Respiratory syncytial virus (RSV) causes severe respiratory illness in infants, children <5 years, and adults >65 years1,2,3,4,5,6. Multiple prevention products7, including vaccines for pregnant persons and older adults as well as monoclonal antibodies for infants, are now available to reduce RSV morbidity and mortality8,9,10,11. At scale, these interventions will likely reduce disease burden among high-risk populations, including young children, and potentially shift the incidence and distribution of RSV in other age groups7.
RSV circulation was interrupted by COVID-19 mitigation measures12. Although infants have historically been disproportionately impacted by RSV, RSV-associated hospitalizations increased in children aged 12–23 months since these measures were lifted, which could be due to reduced exposure while mitigations measures were in place leading to lower levels of protective antibody4,12,13,14. To inform the implementation of RSV prevention products, age-specific incidence estimates from the community are needed to better characterize RSV burden since the onset of the COVID-19 pandemic12 and prior to full-scale implementation of RSV prevention products in these age groups. Updated incidence estimates could also facilitate assessing how RSV prevention products impact disease burden across the age spectrum15.
Moreover, the impact of pre-existing RSV antibodies on RSV susceptibility is incompletely defined. While previous studies have reported that RSV-specific antibodies protect against symptomatic and lower respiratory tract disease in infants16,17,18,19,20,21,22,23, there is not a consensus on whether the same level of pre-existing RSV antibody is also protective in older children and adults. Additional research23 could provide insight into clinical and immunologic factors protective against infection and disease, which could inform the design and implementation of RSV prevention products intended for multiple age groups. For instance, since the approval of RSV prevention products that protect infants and older adults, RSV vaccine research and development efforts are now focused on products intended for other age groups including older children, adolescents, and adults. As such, antibody data from these age groups are needed to inform the development of next-generation prevention products. In particular, vaccine responses in children aged 5–12 years who have likely been exposed to RSV multiple times may differ from RSV-naïve infants entering their first season as well as older adults due to immunosenescence.
Using data from the CASCADIA study24, we aimed to prospectively evaluate the association between baseline RSV antibody titers and risk of RSV illness among household members in a community setting after COVID-19 mitigation measures were lifted and prior to the approval of RSV prevention products to protect high-risk infants and older adults. We hypothesized that higher RSV antibody titers would be protective against incident RSV illness in children.
Results
Participants
Overall, 3237 individuals in 1188 households (Table 1; Supplementary Fig. 1) were enrolled on a rolling basis beginning in June 2022 through May 2023, with self-collection of weekly swabs. The median follow-up duration from the baseline blood draw to when a participant subsequently tested positive for RSV, withdrew from the study, or the end of the analytic period (May 31, 2023) was 189 days. See Supplementary Fig. 2 for individual participant timelines illustrating when participants enrolled and collected swabs throughout the analytic period among participants with RSV A and B illnesses, respectively. Weekly swabbing compliance was high; participants missed a median of 2 weekly swab instances (range: 0–46 weeks). Participant retention was also high, with 96.42% remaining in the study at the end of the analytic period. A total of 79,779 swabs were collected during the analytic period, of which 0.44% (n = 345 positive; n = 4 inconclusive) and 0.09% (n = 72 positive; n = 1 inconclusive) tested positive/inconclusive for RSV subtypes A and B, respectively (Supplementary Fig. 2). During RSV illness episodes, 29.84% of participants had RSV co-detected with one or more other pathogens (n = 91/305). RSV A/B was most frequently co-detected with rhinovirus (70.33%; n = 64/91); adenovirus (16.48%; n = 15/91); and influenza (12.09%; n = 11/91). Overall, most participants/guardians collected their nasal swabs in a valid manner with sufficient Ribonuclease P (RNase P) detection, which indicates adequate mucosal sampling by measuring nasal epithelial cells, with only 0.72% and 0.93% of the swabs tested for RSV A (n = 549/76284) and B (n = 171/18415), respectively, failing due to insufficient RNase P detection.
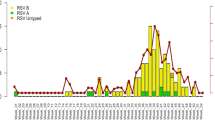
Overall, 328 participants experienced an RSV infection, regardless of symptom status, during the study period. Of these infections, 92.99% (n = 305/328) were symptomatic, defined as ≥1 respiratory illness symptom (e.g., fever, cough, sore throat, shortness of breath, myalgia, rhinorrhea). RSV illnesses (n = 305) were detected between September 2022 and May 2023, including 247 RSV A (September-May) and 63 RSV B (October-May) illnesses. Five participants had unique RSV A and B illness episodes at different timepoints; only the first illness episode was included in the analysis of incident RSV illness. The proportion of participants with RSV by age group was as follows: 6 months to 1 year (24.00%; n = 24/100); 2–4 years (24.46%; n = 57/233); 5–12 years (11.36%; n = 106/933); 13–17 years (5.41%; n = 19/351); 18–50 years (6.11%; n = 99/1620). Demographic characteristics were generally similar between individuals with and without RSV (Table 1). However, the median age was lower among participants with RSV compared to those without RSV (median age of 9 versus 30 years). Among participants <5 years, a greater proportion with RSV attended daycare compared to those without RSV (71.6% versus 61.9%). Of the adults with RSV (n = 99), 82.83% lived with children <18 years; 42.42% lived with a child <5 years of age attending daycare. Overall, study participants were healthy and few reported comorbidities. See Supplementary Tables 1, 2 for baseline characteristics by study site and RSV prefusion F (pre-F) binding antibody category.
RSV illness incidence
Among participants <5 years, we observed an earlier rise in incidence and higher incidence rates throughout the season compared to other age groups (Supplementary Fig. 3). Similarly, estimated RSV A/B peak monthly incidence rates per 1,000 person-days were higher among children <5 years (7.74 cases [95% confidence interval [CI]: 3.42, 17.52] for 6 months–1 year; 6.34 [95% CI: 3.60, 11.18] for 2–4 years) compared to children 5–17 years of age (2.65 [95% CI: 1.92, 3.68] for 5–12 years; 0.46 [95% CI: 0.15, 1.43] for 13–17 years) and adults (1.04 [95% CI: 0.69, 1.55]). During the months in which each RSV subtype was detected, the average monthly incidence rates were 2.07 × 10−3 (95 CI: 1.54 × 10−3, 2.77 × 10−3) and 0.39 × 10−3 (95% CI: 0.29 × 10−3, 0.53 × 10−3) per 1000 person-days for RSV A and B, respectively.
RSV illness characteristics
Among all participants, the most frequently reported symptoms were rhinorrhea, cough, and sore throat (Table 2). A higher proportion of participants 6 months–1 year reported shortness of breath (29.17%) compared to participants 2–17 years of age (5.26–6.60%) and adults 18–50 years of age (15.15%). Compared to pediatric participants, adults reported fever (17.17% versus 27.18%), rhinorrhea (86.87% versus 94.17%), and cough (63.64% versus 86.41%) less frequently, but reported sore throat (58.59% versus 35.44%), fatigue (50.51% versus 23.79%), headache (42.42% versus 19.42%), and myalgia (18.18% versus 8.25%) more frequently. See Supplementary Tables 3–6 for illness characteristics by RSV subtype and symptom reporting frequency by illness week.
During RSV illness, absenteeism (n = 24/81; 29.63%) and care-seeking (n = 15/81; 18.52%) were most common among children <5 years (Table 2). Among adults, 15.15% (n = 15/99) reported being absent from work/school. Both adult and pediatric participants sought care at a doctor’s office or urgent care facility most frequently (70.27%; n = 26/37) followed by telehealth services (32.43%; n = 12/37) with two participants seeking care at both locations during their illness episodes. One hospitalization was reported among a participant 6 months–1 year with RSV B; no adults were hospitalized. See Supplementary Table 7 for symptom reporting frequency by care-seeking status.
As age increased, relative cycle threshold (Crt) measured on Day 0 of an illness episode increased (Table 2). Although adult participants tended to experience more mild illness, the Crt distribution was similar between children and adults (Supplementary Fig. 4). We also observed a similar Crt distribution stratified by co-detection status (Supplementary Fig. 5). During an illness episode, Crt values typically increased over time corresponding to a decrease in viral load; measurable Crt values on multiple swabs after the first positive swab, suggesting longer time to viral clearance, was more common among children (Supplementary Fig. 6).
Distribution of baseline RSV antibody titers
We assessed RSV binding antibody in all serum samples, and RSV neutralizing antibody in pediatric serum samples (Fig. 1). Overall, median log10-binding antibody titers (Fig. 1A) and log10-neutralizing antibody titers (Fig. 1B) increased with age but titer variability, represented by the standard deviation (SD), decreased. Based on a nonparametric coefficient of determination (R2), 69.06% of the variability in neutralizing antibody titers could be predicted from binding antibody titers among pediatric participants (95% CI: 0.63, 0.74) (Fig. 2; Supplementary Table 8) suggesting that binding and neutralizing antibody titers were moderately correlated. Antibody titers stratified by age group and RSV illness status are shown in Fig. 2C, D. See Supplementary Fig. 7 for antibody titers stratified by continuous age.
Plots show participant-level antibody titers represented by the dots, which are overlaid by box plots and violin plots to illustrate the distribution and density of the antibody data. Box-plot elements are defined as follows: center line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range. Baseline antibody titers are stratified by age group only for (A) binding antibody titers (log10-AU/mL) (n = 2996) and (B) neutralizing antibody titers (log10-IU/mL) (n = 1373). These plots are further stratified by RSV A/B illness status for (C) binding antibody titers (log10-AU/mL) (n = 2996) and (D) neutralizing antibody titers (log10-IU/mL) (n = 1373). Abbreviations: log10-AU/mL log10-transformed arbitrary units per milliliter, log10-IU/mL log10-transformed international units per milliliter, RSV respiratory syncytial virus, SD standard deviation.
Correlation between binding and neutralizing antibody titers measured at baseline among (A) all pediatric participants 6 months–17 years (n = 1355) and (B) pediatric participants 6 months–4 years (n = 323). Data are presented as a scatter plot of individual participants’ binding and neutralizing antibody titer measurements overlaid with a blue correlation trendline ± the standard error of the smooth as a 95% confidence interval shown in gray. Abbreviations: log10-AU/mL log10-transformed arbitrary units per milliliter, log10-IU/mL log10-transformed international units per milliliter, RSV respiratory syncytial virus, R2 nonparametric coefficient of determination.
Risk of RSV illness
The overall estimated hazard ratio (HR) of RSV illness was 0.66 (95% CI: 0.56, 0.78) for any two groups differing by 1-unit in log10-binding antibody titers, with the higher titer group having a lower estimated hazard (Table 3). This association was statistically significant among pediatric participants (HR = 0.66; 95% CI: 0.56, 0.78), but not adults (HR = 0.62; 95% CI: 0.32, 1.20). When further stratifying by pediatric age group, the estimated association was strongest among participants aged 5–12 years (HR = 0.41; 95% CI: 0.27, 0.61). Among pediatric participants, higher log10-neutralizing antibody titers were also associated with a lower estimated hazard of subsequent RSV illness (HR = 0.36; 95% CI: 0.25, 0.53).
Standardized antibody titers
We observed similar HRs for binding and neutralizing antibody titers using SD-standardized antibody titers among pediatric participants. For any two groups differing by one SD in log10-antibody titers, the estimated HRs were 0.69 (95% CI: 0.60, 0.80) and 0.59 (95% CI: 0.48, 0.71) for binding and neutralizing antibody titers, respectively, with the higher titer group estimated to have a lower hazard of RSV (Supplementary Table 9).
To explore if there was a threshold effect of antibody titers on the risk of RSV illness, we estimated HRs for this association using antibody titer categories (Fig. 3). Compared to the lowest antibody titer category, we observed a dose-response relationship, with higher antibody titers associated with a lower estimated hazard of RSV for both binding and neutralizing antibody titers. Similarly, the slope of the estimated covariate-adjusted cumulative incidence curve (Fig. 4) was steepest for participants with lower antibody levels for both binding and neutralizing antibodies. At 180 days since baseline blood collection, the cumulative incidence in the low antibody category was estimated to be 21.70% and 19.77% for binding and neutralizing antibodies, respectively. For the very high antibody category, the cumulative incidence was estimated to be 3.50% and 3.95% for binding and neutralizing antibodies, respectively. See Supplementary Tables 10, 11 for additional proportional hazards (PH) sensitivity analyses conducted among all participants regardless of symptom status.
The x-axis shows the low, moderate, high, and very high antibody titer categories for neutralizing and binding antibody. The y-axis shows the hazard ratio of the association between categorical baseline antibody titers and RSV A/B illness for the low, moderate, high, and very high antibody titer categories using the very low antibody category as the reference group. Data are presented as the hazard ratio (measure of center) ± the upper and lower bound of the 95% Wald-type confidence interval for the hazard ratio estimate using robust standard errors. The proportional hazards analysis was conducted among pediatric participants in whom both antibodies were measured (n = 1355). Abbreviations: CI confidence interval, log10-AU/mL log10-transformed arbitrary units per milliliter, log10-IU/mL log10-transformed international units per milliliter, RSV respiratory syncytial virus.
Cumulative incidence curves by 180 days since the baseline blood draw for A binding and B neutralizing antibody among pediatric participants in whom both antibodies were measured. The x-axis shows days since a participant’s baseline blood draw and the y-axis shows the cumulative incidence. Cumulative incidence curves are averaged over covariates and stratified by low, moderate, high, and very high antibody titer categories. The curves for the lowest antibody titer category where titers were below the lower limit of quantitative are not shown. The cumulative incidence estimates were obtained from the proportional hazards regression analysis. Abbreviation: RSV respiratory syncytial virus.
Variable importance analysis
To more directly compare binding and neutralizing antibody titers measured in different units as well as their combination, we also conducted a variable importance analysis to quantify the ability of these antibodies to predict RSV illness at 180 days since enrollment. Using PH regression with baseline covariates alone (age, school/daycare attendance, and immunocompromised status), we estimated the achievable area under the receiver operating curve (AUC) with these models to be 0.70 (Fig. 5). When using antibody titers in addition to baseline covariates, the ability to predict RSV illness beyond random chance (AUC = 0.50) increased by 27.06% (estimated AUC = 0.76) and 29.48% (estimated AUC = 0.76) for binding and neutralizing antibodies, respectively. Using both binding and neutralizing antibody titers combined further increased the ability to predict RSV illness (estimated AUC = 0.77) resulting in a relative increase of 32.72%.
Among pediatric participants, in whom both antibodies were measured (n = 1355), estimated gain in achievable AUC is shown on the y-axis for time to RSV illness by 180 days from enrollment based on adding each RSV antibody to a prediction model containing only baseline covariates (age; school/daycare attendance; immunocompromised status). The AUC is measured on a scale of 0-1 with the y-axis starting at 0.50 to represent the ability to predict RSV illness beyond random chance (AUC = 0.50). The measure of center of the error bars represents the estimated difference in AUC comparing the model with the baseline covariates only to the model with the baseline covariates and the RSV antibody. The error bars represent the 2.5 and 97.5 percentile of the bootstrap replicate estimates for this AUC difference. The AUC estimates are the average of all the bootstrap replicate estimates, which are themselves averages within bootstrap datasets of sample splitting over 10 different seeds. Confidence intervals for the variable importance estimates were constructed using 500 bootstrap replicates with sampling accounting for within-household correlation. Abbreviations: AUC area under the receiver operating curve, RSV respiratory syncytial virus.
Discussion
We conducted a prospective community-based cohort study among household members in understudied age groups including school-aged children (5–12 years), teenagers (13–17 years), and adults (18–50 years) following a period of suppressed viral circulation due to COVID-19 mitigation measures. Using weekly symptom surveillance and home-based swabbing, we detected RSV illnesses at the community level including among individuals who did not seek medical care, a population not typically studied. Young children had the highest incidence of RSV illness, particularly those attending daycare. Adult RSV illness episodes were less severe than pediatric cases, with a lower proportion reporting fever and/or shortness of breath, missing work/school, and seeking care. Among all participants, higher baseline RSV-specific binding antibodies were associated with a decreased hazard of RSV. Among pediatric participants, both baseline binding and neutralizing antibodies were associated with a decreased hazard of RSV. Using antibody titer categories, we observed a potential dose-response relationship with higher antibody titers associated with a lower estimated hazard of RSV for both binding and neutralizing antibody. Furthermore, based on a variable importance analysis among pediatric participants, we found that binding and neutralizing antibody titers had a similar ability to predict RSV illness occurring by 180 days of follow-up after accounting for baseline sociodemographic and clinical risk factors.
We observed similar monthly incidence rates among children aged 6 months–1 year and 2–4 years. Although prior studies have typically documented the highest burden of disease among infants1,4,5,6, many of these studies only enrolled young children presenting for medical attention or hospital care. Notably, our study population did not include infants <6 months who are known to be at high risk of RSV morbidity and mortality1,4,5,6. However, our finding of increased RSV incidence rates in participants 2–4 years may be due to suppressed viral circulation and lower population-level immunity as a result of COVID-19 mitigation measures, leading to a greater pool of susceptible children in this post-pandemic period, which has been observed in other studies4,12,13,14. In our study population, we found that RSV illness was more likely to be first detected in the youngest age groups (6 months–1 year; 2–4 years) earlier in the respiratory virus season, followed by detection in older children (5–12 years; 12–17 years) and adults (18–50 years). This seasonality suggests that the predominant site of transmission occurs in settings with young children such as daycares and schools. This finding aligns with previous household-based studies conducted with different study designs, which found that school-aged children frequently introduce RSV into households25,26,27,28.
Fever, shortness of breath, care-seeking, and absenteeism were less frequently reported among symptomatic adult compared to pediatric participants. We also found that while 29.07% of adults in our study lived in households with children <5 years attending daycare (n = 471/1620), 42.24% of adults with RSV lived in those households (n = 42/99). Although RSV burden is not well-characterized among healthy adults, our findings are consistent with prior studies. For instance, a household transmission study among families with young children in Rochester, New York reported that 45.88% of members from positive households became infected with RSV25. A key difference in our study population is that we only observed 15.15% absenteeism among adults compared to previous studies reporting up to 38.0% work-absenteeism during RSV illness29,30. We may have observed lower absenteeism rates due to changes in telecommuting policies since the COVID-19 pandemic and individuals continuing to work from home during illness.
Higher levels of RSV neutralizing antibody have been shown to protect against disease in both observational studies20 and clinical trials8,21,22 of pre-F subunit RSV vaccines administered to maternal and older adult populations and monoclonal antibody prophylaxis administered to infants. Among our pediatric population of children 6 months–17 years, in whom both antibodies were measured, we found that binding and neutralizing antibody were moderately correlated. We also evaluated RSV pre-F binding antibody in serum collected prior to illness and found that (1) antibody titers increased with age and (2) higher antibody titers were associated with a decreased risk of RSV illness in our overall study population and among pediatric participants. Since previous studies have reported that RSV-specific antibodies from both natural infection and vaccination protect against RSV disease in infants, our findings suggest that vaccine-induced RSV antibodies may also provide protection against RSV illness in future clinical trials conducted among children >6 months of age.
Although higher binding antibody titers protected against RSV illness in our overall study population, when further stratifying by adult versus pediatric participants, this association was not statistically significant among adults; this may be because our adult participants had baseline binding antibody titers in a narrow range of high values, consistent with adults being exposed to RSV many times and building up higher antibody titer levels on average compared to young children. Moreover, our adult participants only experienced mild illnesses. As such, we may have observed a stronger protective association of binding antibody titers in a hospital- or outpatient-setting compared to our community-based cohort or if our study had included higher-risk adults.
We observed wider HR CIs using neutralizing compared to binding antibody, which was observed previously23. This could be due to a narrower distribution of neutralizing antibody titers, more participants with titers below the lower limit of quantitation for the microneutralization assay, and/or differences in assay measurement error. While these findings should be validated using post-vaccination sera as part of upcoming RSV vaccine clinical trials, our results suggest that binding antibody titers could be a viable RSV correlate of protection and alternative to neutralizing antibody, which is considerably more time- and resource-intensive to measure. The relevance of binding antibody as a potential RSV correlate of protection was further supported by the variable importance analysis, which suggested that binding and neutralizing antibody titers have a similar ability to predict RSV illness beyond baseline covariates when the predictive ability of these antibody titers was directly quantified to account for differences in their measurement scales/units. Moreover, since using binding and neutralizing antibody titers together resulted in the highest estimated AUC, our findings suggest that these biomarkers may partly provide complementary information for predicting RSV illness. Future studies should further explore binding antibodies as a correlate of risk against RSV illness, the role of binding versus neutralizing antibodies in protection against different RSV disease outcomes, as well as a composite biomarker leveraging both binding and neutralizing antibodies23. Researchers should also consider incorporating variable importance analyses into future studies to facilitate comparing RSV antibodies reported in different scales/units across studies.
Since our study population was relatively homogeneous with most individuals reporting few comorbidities and from households with higher socioeconomic status, our results may not be generalizable to the overall United States population or specific high-risk groups such as infants <6 months, older adults, or individuals with comorbidities. Moreover, we did not evaluate serology from the timepoint immediately prior to illness, which may have provided more precise estimates of the association of interest compared to baseline serology. In addition to pre-F antibody, there may be other RSV antibodies that protect against illness not captured in this analysis. Neutralizing antibody testing was not performed on adult sera, and it is possible that we may have observed different trends among adults using neutralizing compared to binding antibody. Moreover, our ability to capture asymptomatic disease was partially limited by our protocol, whereby all swabs were tested for RSV A, but only a subset were tested for RSV B, particularly for symptomatic participants. There is also a potential risk for misclassification of RSV illness status if a false-negative RT-qPCR result was reported and/or due to missing swabs. Furthermore, although RSV is often the driver of the illness when co-detected with other pathogens, co-detections may have impacted our observed association between RSV antibodies and RSV illness. However, we expect the impact of co-detections to be minimal since RSV was most frequently co-detected with rhinovirus (70.33%; n = 64/91) and Crt values were similar by co-detection status. Although severe morbidity and mortality were not observed in this study, preventing mild to moderate RSV illness in the community is of public health importance to prevent community transmission, protect vulnerable populations, and alleviate the burden on strained health systems.
This study represents a unique approach to the evaluation of RSV in children >6 months of age and adults, and provides an evaluation of RSV antibodies within a large, community-based cohort. In a period after COVID-19 mitigation measures were removed, we estimated RSV incidence across a broad age range finding that higher antibody levels were associated with a lower risk of mild to moderate RSV illness overall (6 months–50 years) and in children 6 months–17 years of age. Both our approach to tracking disease in the community and the correlation of clinical and laboratory findings are innovative approaches that will facilitate evaluation of RSV prevention products across the age spectrum. These data provide important baseline evidence for immunization policy decision-making and implementation of RSV prevention products, particularly next-generation RSV vaccines targeting young children 2–4 years of age as well as school-aged children 5–12 years of age.
Methods
Data source
We used data from the first year of the CASCADIA study (June 2022-May 2023), a community-based prospective cohort study of households in the states of Washington and Oregon in the United States24,31. The study protocol was reviewed and approved by the Kaiser Permanente Inter-regional Institutional Review Board, with reliance from University of Washington (UW) and Seattle Children’s Research Institute (See 45 C.F.R. part 46.114; 21 C.F.R. part 56.114). Informed consent was obtained from all adult participants. For pediatric participants, informed consent was obtained from the minor’s parent or legal guardian. We also obtained assent from children 7–17 years of age. If a participant turned 18 years old while participating in the study, we then consented these individuals as adults.
Data collection
Upon enrollment, participants (or their parent/guardian) completed an enrollment survey on demographic characteristics and health status, and attended a baseline blood draw appointment. Nasal swab kits were mailed to participants’ homes with instructions for self- and parent/guardian-collection. Participants completed weekly online symptom surveys and home nasal swabs regardless of symptoms. Participants could also report new or worsening symptoms at any time; if this occurred >72 h after their last swab, they were prompted to collect another swab. Participants with a reported illness or a positive test for SARS-CoV-2, influenza A, or RSV A completed additional surveys about symptoms, care-seeking, and absenteeism. For participants <13 years of age, symptoms were reported by the participant’s parent/guardian; symptoms were not evaluated by a clinician. All data were collected using REDCap32,33.
Nasal swab PCR testing
Mid-turbinate nasal swabs (RHINOsticTM) were collected and shipped dry without preservative or media to UW. RSV A, influenza A, and SARS-CoV-2 testing was performed on all samples via RT-qPCR34,35,36. Additionally, multipathogen testing was performed using a multiplex RT-qPCR assay including RSV A/B on swabs from participants who reported symptoms within 72 h of swab collection, and swabs with a positive/inconclusive result for any virus during the first phase of testing. We defined an RSV-positive swab as a cycle threshold (Ct) value ≤ 37 for RSV RT-qPCR testing and a Crt < 28 for multipathogen testing. We considered all inconclusive results as positive for the analysis. Crt values were used as a proxy for semiquantitative viral load.
RSV antibody assays
RSV pre-F IgG levels were measured in serum samples using a commercial electrochemiluminescence immunoassay (ECLIA) with plates pre-coated with RSV pre-F antigen (Mesoscale Diagnostics). Pediatric serum samples were also tested using an RSV subtype A microneutralization assay and reported in standardized units based on the World Health Organization’s first international standard for antiserum to RSV (16/284)37. Additional laboratory methods are detailed in the Supplementary Information.
Study population
In the 2022–2023 respiratory season prior to the introduction of novel RSV vaccines and monoclonal antibodies, we included participants who completed an enrollment survey, attended a baseline blood draw appointment (regardless of blood draw success), and provided at least one nasal swab by May 31, 2023. To complement other studies focused on the highest-risk groups and align with the target populations of other RSV vaccines in the pipeline, we recruited individuals 6 months–50 years who are underrepresented in studies of RSV burden.
Variables
Exposures
We evaluated log10-transformed baseline RSV pre-F IgG binding (arbitrary units per milliliter [AU/mL]) and neutralizing antibody (international units per milliliter [IU/mL]) titers measured at enrollment as primary and secondary exposures, respectively. To increase comparability of binding and neutralizing antibody titers among pediatric participants, in whom both antibodies were measured, titers were divided by their sample SD and discretized empirically into five categories of equal range to generate standardized and discretized38,39 antibody variables, respectively. See Supplementary Table 12 for antibody category ranges.
Outcome
The primary outcome was an incident RSV illness defined as ≥1 respiratory symptom (e.g., fever, cough, sore throat, shortness of breath, myalgia, rhinorrhea) ±7 days from a participant’s first RSV-positive swab via RT-qPCR.
Covariates
Potential confounders included age at enrollment (6 months–1 year; 2–4 years; 5–12 years; 13–17 years; 18–50 years); school/daycare attendance (yes/no); and self-reported immunocompromised status (yes/no). To describe RSV illness characteristics, we assessed symptoms reported on Days −7 to +14; work, school, or daycare absenteeism; and medical care-seeking reported within the 30 days of any reported symptom or positive test result. Sex assigned at birth and gender were self-reported or reported by a parent/guardian as part of the enrollment survey. These variables were included as part of the descriptive statistics, but no analyses were adjusted for sex or gender. Sex and gender were not considered in the study design.
Statistical methods
We estimated overall and age-specific RSV illness incidence rates by month using an intercept-only Poisson regression model with log-number of days at risk of RSV as offset. Participants contributed person-time at risk during the weeks they self-collected a nasal swab beginning on the date of their first nasal swab collection. We used generalized estimating equations with an independence working correlation structure to account for within-household correlation. Wald-type CIs were constructed using distribution- and correlation structure-robust standard errors.
We assessed the association between baseline RSV log10-antibody levels (binding for all participants and neutralizing for pediatric participants) and time to RSV illness using PH regression. Time at risk was calculated as the time elapsed between baseline blood draw and when a participant subsequently tested positive for RSV, withdrew from the study, or the end of the analytic period (May 31, 2023). We fit stratified PH models to estimate overall and age-specific HRs adjusting for potential confounders identified a priori (Supplementary Table 13; Supplementary Fig. 8). Model fits accounted for possible within-household correlation and included a baseline hazard stratified by 2-week enrollment intervals to account for seasonality. Wald-type CIs were constructed using robust standard errors. Additionally, all models were run using SD-standardized and discretized38 (very low; low; moderate; high; very high) antibody exposure variables. Based on the PH analysis using the discretized antibody variables, we also estimated covariate-adjusted cumulative incidence curves, which were plotted across time since baseline blood draw, to characterize absolute incidence. We conducted a complete-case PH analysis under the assumption that participants with missing RSV serologic measurements, due to unsuccessful blood draws and contaminated samples, were similar in outcome, exposure, and potential confounders as observed participants (Supplementary Table 14).
To complement the PH analysis, we conducted a variable importance analysis to assess the extent to which baseline binding and neutralizing antibody titers improve illness prediction within a time horizon of 180-days since enrollment compared to prediction based on adjustment variables alone (age; school/daycare attendance; immunocompromised status). This approach enables a direct comparison of antibodies that are quantified differently. AUC was used as a unit-independent measure of predictive ability and presented on a scale of 0–140,41. We restricted the variable importance analysis to our pediatric study population in whom both antibodies were measured. Confidence intervals for the variable importance estimates were constructed using 500 bootstrap replicates with sampling accounting for within-household correlation. Repeated sample splitting across 10 different seeds was used to ensure valid testing of null importance while limiting variance inflation40,42.
All hypothesis tests were two-sided and conducted at significance level 0.05. CIs were constructed to achieve a nominal coverage of 95%. All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing) in RStudio 2023.12.1 + 402 (RStudio, Inc; Boston, MA)43. The variable importance analysis was implemented using the R package survML with Cox PH regressions40. Additional statistical methods are detailed in the Supplementary Information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data underlying the figures in the main text are available in the Supplementary Datasets. Due to ethical restrictions and to protect participant confidentiality, the complete datasets are available under restricted access. Deidentified individual participant data (including data dictionaries), study protocols, the statistical analysis plan, and the informed consent form can be obtained by submitting a request to the corresponding author (cfrivold@uw.edu), subject to approval by the Institutional Review Board of the University of Washington and the CASCADIA Steering Committee. Access will be granted for a period of 1 year, with the option to renew, to researchers with a methodologically sound proposal for use in achieving the goals outlined in the approved proposal. Requests will be reviewed within 30 days. Source data are provided with this paper.
References
Li, Y. et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 399, 2047–2064 (2022).
Kenmoe, S. & Nair, H. The disease burden of respiratory syncytial virus in older adults. Curr. Opin. Infect. Dis. 37, 129–136 (2024).
O’Brien, K. L. et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 394, 757–779 (2019).
Suss, R. J. & Simões, E. A. F. Respiratory Syncytial virus hospital-based burden of disease in children younger than 5 years, 2015–2022. JAMA Netw. Open 7, e247125 (2024).
Hall, C. B. et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360, 588–598 (2009).
Curns, A. T. et al. Respiratory syncytial virus-associated hospitalizations among children <5 years old: 2016 to 2020. Pediatrics 153, e2023062574 (2024).
Fleming, J. A. et al. Value profile for respiratory syncytial virus vaccines and monoclonal antibodies. Vaccine 41, S7–S40 (2023).
Walsh Edward, E. et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 388, 1465–1477 (2023).
Moline, H. L. et al. Early estimate of nirsevimab effectiveness for prevention of respiratory syncytial virus-associated hospitalization among infants entering their first respiratory syncytial virus season—new vaccine surveillance network, October 2023-February 2024. MMWR Morb. Mortal. Wkly. Rep. 73, 209–214 (2024).
Kampmann, B. et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 388, 1451–1464 (2023).
Fleming-Dutra, K. E. et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus-associated lower respiratory tract disease in infants: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 72, 1115–1122 (2023).
Abu-Raya, B., Paramo, M. V., Reicherz, F. Lavoie, P. M. Why has the epidemiology of RSV changed during the COVID-19 pandemic? eClinicalMedicine 61, 102089 (2023).
Yeoh, D. K. et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 72, 2199–2202 (2021).
Fourgeaud, J. et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur. J. Clin. Microbiol. Infect. Dis. 40, 2389–2395 (2021).
Teoh, Z. et al. Burden of respiratory viruses in children less than two years in a community-based longitudinal U.S. birth cohort. Clin. Infect. Dis. ciad289. https://doi.org/10.1093/cid/ciad289 (2023).
Buchwald, A. G. et al. Respiratory syncytial virus (RSV) neutralizing antibodies at birth predict protection from RSV illness in infants in the first 3 months of life. Clin. Infect. Dis. 73, e4421–e4427 (2021).
Stensballe, L. G. et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 123, 398–403 (2009).
Kulkarni, P. S., Hurwitz, J. L., Simões, E. A. F. & Piedra, P. A. Establishing correlates of protection for vaccine development: considerations for the respiratory syncytial virus vaccine field. Viral Immunol. 31, 195–203 (2018).
Abu-Raya, B., Reicherz, F. & Lavoie, P. M. Correlates of protection against respiratory syncytial virus infection in infancy. Clin. Rev. Allergy Immunol. 63, 371–380 (2022).
Glezen, W. P., Paredes, A., Allison, J. E., Taber, L. H. & Frank, A. L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 98, 708–715 (1981).
Wilkins, D. et al. Durability of neutralizing RSV antibodies following nirsevimab administration and elicitation of the natural immune response to RSV infection in infants. Nat. Med. 29, 1172–1179 (2023).
Hammitt Laura, L. et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 386, 837–846 (2022).
Fong, Y. et al. Antibody correlates of protection from severe respiratory syncytial virus disease in a vaccine efficacy trial. Open Forum Infect. Dis. 10, ofac693 (2023).
Babu, T. M. et al. CASCADIA: a prospective community-based study protocol for assessing SARS-CoV-2 vaccine effectiveness in children and adults utilizing a remote nasal swab collection and web-based survey design. BMJ Open 13, e071446 (2023).
Hall, C. B. et al. Respiratory syncytial virus infections within families. N. Engl. J. Med. 294, 414–419 (1976).
Cohen, C. et al. Incidence and transmission of respiratory syncytial virus in urban and rural South Africa, 2017–2018. Nat. Commun. 15, 116 (2024).
Munywoki, P. K. et al. Frequent asymptomatic respiratory syncytial virus infections during an epidemic in a rural Kenyan household cohort. J. Infect. Dis. 212, 1711–1718 (2015).
Hetrich, M. K. et al. Epidemiology of human parainfluenza virus type 3 and respiratory syncytial virus infections in the time of coronavirus disease 2019: findings from a household cohort in Maryland. Clin. Infect. Dis. 76, 1349–1357 (2023).
Hall, C. B., Long, C. E. & Schnabel, K. C. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 33, 792–796 (2001).
Azziz-Baumgartner, E. et al. Incidence of laboratory-confirmed influenza and RSV and associated presenteeism and absenteeism among healthcare personnel, Israel, influenza seasons 2016 to 2019. Eurosurveillance 29, 2300580 (2024).
Feldstein, L. R. et al. Effectiveness of bivalent mRNA vaccines in preventing SARS-CoV-2 Infection among children aged 5–17 years: an evaluation of multicenter prospective cohorts, United States, September 2022—January 2023. Open Forum Infect. Dis. 10, ofad500.152 (2023).
Harris, P. A. et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).
Harris, P. A. et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
Kim, A. E. et al. Evaluating specimen quality and results from a community-wide, home-based respiratory surveillance study. J. Clin. Microbiol. 59, https://doi.org/10.1128/jcm.02934-20 (2021).
Pfau, B. et al. Tiny swabs: nasal swabs integrated into tube caps facilitate large-scale self-collected SARS-CoV-2 testing. J. Clin. Microbiol. 62, e0128523 (2024).
Srivatsan, S. et al. SwabExpress: an end-to-end protocol for extraction-free COVID-19 testing. Clin. Chem. 68, 143–152 (2021).
Piliper, E. A., Reed, J. C. & Greninger, A. L. Clinical validation of an RSV neutralization assay and analysis of cross-sectional sera associated with 2021–2023 RSV outbreaks to investigate the immunity debt hypothesis. Microbiol. Spectr. 12, e0211524 (2024).
Westling, T., van der Laan, M. J. & Carone, M. Correcting an estimator of a multivariate monotone function with isotonic regression. Electron. J. Stat. 14, 3032–3069 (2020).
Benkeser, D. et al. Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial. Sci. Transl. Med. 15, eade9078 (2023).
Wolock, C. & Kenny, A. survML: Tools for Flexible Survival Analysis Using Machine Learning. (2024).
Wolock, C. J., Gilbert, P. B., Simon, N. & Carone, M. Assessing variable importance in survival analysis using machine learning. Biometrika asae061. https://doi.org/10.1093/biomet/asae061 (2024).
Wolock, C. J., Gilbert, P. B., Simon, N. & Carone, M. A framework for leveraging machine learning tools to estimate personalized survival curves. J. Comput. Graph. Stat. 33, 1098–1108 (2024).
RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC (2021).
Acknowledgements
We would like to thank all CASCADIA study households for their time and active participation. We would like to acknowledge the following individuals for their contribution to this work: Kaiser Permanente Center for Health Research: Michael Allison, Deralyn Almaguer, David Amy, Britt Ash, Kristi Bays, Tara Beatty, Kristin Bialobok, Allison Bianchi, Cathleen Bourdoin, Delanie Brown, Stacy Bunnell, Joseph Cerizo, Evelin Coto, Phil Crawford, Robin Daily, Lantoria Davis, Kendall Frimodig, Stephen Fortmann, Lisa Fox, Kenni Graham, Tarika Holness, Matt Hornbrook, Emily Jubitz, Terry Kimes, Keelee Kloer, Dorothy Kurdyla, Isaiah Lankham, Teri Lawer, Caroline Lee, Max Lin, Richard Martin, Bryony Melcher, John Ogden, Aaron Piepert, Joanne Price, Sacha Reich, Angela Reyes-Ochoa, Jennifer Rivelli, Sperry Robinson, Katrina Schell, Emily Schield, Meagan Shaw, Anna Shivinsky, Nina Shockman, Ellen Sullivan, Martin Simer, Valencia Smith, Senait Tadesse, Britta Torgrimson-Ojerio, Meredith Vandermeer, Brooke Wainwright, Mica Werner, Danika Whitcomb. Kaiser Permanente Washington Heath Research Institute: Serah Kimachia, Rebecca Ziebell, Chester Pabiniak, Kristin Delaney. Vanderbilt University Medical Center: Sydney (Afan) Swan, Onika Abrams, Theresa (Terri) Scott. Seattle Children’s Hospital: Dallas Haws, Hanna Grioni, Josh Sanders, Irem Onalan, Laura Ostrina Restrepo. University of Washington: Ariana Magedson, Denise McCulloch, Kyle Luiten, Devon McDonald, Jenni Logue, Jean Mernaugh, Melissa MacMillan, Mark Drummond, Anna Elias-Warren, Pavitra Roychoudhury, Jean Mernaugh, Daniel Nguyen, Zarna Marfatia, Amanda Casto, Chidozie Iwu, Julia Bennett, Jordan Opsahl, Kathryn McCaffrey, Luis Gamboa, David Reinhart, Ben Cappodano, Sarah Heidl, Alex Harteloo, Zack Acker, Lani Regelbrugge, Brenna Ehmen, Madison Hollcroft, Leslie Rodriguez-Salas, Ailyn Perez, Hanna Edgar. The U.S. Centers for Disease Control and Prevention collaborated with partner sites to design and conduct the study; managed, analyzed, and interpreted the data; prepared, reviewed, and approved the manuscript; and had a role in the decision to submit the manuscript for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention. All phases of this study were supported by the U.S. Centers for Disease Control and Prevention contract 75D30121C12297 to Kaiser Foundation Hospitals (PI: ALN).
Author information
Authors and Affiliations
Contributions
Collrane Frivold conceptualized and designed the study, performed data analysis, contributed to the Electrochemiluminescence immunoassay testing, drafted the initial manuscript, and critically reviewed and revised the manuscript. Helen Y. Chu conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. Ana A. Weil conceptualized and designed the study, contributed to the RT-qPCR testing, and critically reviewed and revised the manuscript for important intellectual content. Tara M. Babu conceptualized and designed the study, contributed to the Electrochemiluminescence immunoassay testing, and critically reviewed and revised the manuscript for important intellectual content. Janet A. Englund, Allison L. Naleway, and Jennifer L. Kuntz conceptualized and designed the study, and critically reviewed and revised the manuscript for important intellectual content. Marco Carone conceptualized and designed the study, supervised data analysis, and critically reviewed and revised the manuscript for important intellectual content. Sarah N. Cox, Katherine L. Hoffman, Alexandra Varga, and Charles J. Wolock performed data analysis, and critically reviewed and revised the manuscript for important intellectual content. Lea Starita, Christina M. Lockwood, Peter Han, Jeremy Stone, Sally Grindstaff, contributed to the RT-qPCR testing, and critically reviewed and revised the manuscript for important intellectual content. Erica Clark and Grace Marshall contributed to the Electrochemiluminescence immunoassay testing and critically reviewed and revised the manuscript for important intellectual content. Jonathan Reed, Eli A. Piliper, Shah A K Mohamed Bakhash, and Alexander L. Greninger contributed to the RSV A microneutralization assay testing, and critically reviewed and revised the manuscript for important intellectual content. Richard A. Mularski, Cassandra L. Boisvert, Neil Yetz, Natalie K. Lo, Tara L. Hatchie, and Leora R. Feldstein critically reviewed and revised the manuscript for important intellectual content. All authors contributed to the acquisition, analysis, and/or interpretation of data; and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
S.N.C. reported consulting with Pfizer, Inc. outside of the submitted work. C.M.L. reported being on the Scientific Advisory Board for LGC group. J.L.K. reported research funding not related to the submitted work from Pfizer, Novartis, and Vir Biotechnology. R.A.M. reported research funding not related to the submitted work from PCORI, CDC, NHLBI, NIAID, Glaxo Smith Kline, Merck, and Sanofi; his institution is funded by Pfizer, Inc. for RSV vaccine development as site PI. A.L.G. reported central lab contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen and Hologic, research support from Gilead, outside of the described work. A.A.W. reported research funding unrelated to this work from Pfizer, Inc. J.A.E. reported consulting with Ark Biopharmaceuticals, Sanofi Pasteur, Moderna, Meissa Vaccines, Astra Zeneca, and Pfizer, Inc. outside of the submitted work, and has received research funding from AstraZeneca, Merck, GlaxoSmithKline, and Pfizer. H.Y.C. reported consulting with Ellume, Pfizer, and the Bill and Melinda Gates Foundation; she has served on advisory boards for Vir, Merck and Abbvie; she has conducted CME teaching with Medscape, Vindico, and Clinical Care Options; she has received research funding from Gates Ventures, and support and reagents from Ellume and Cepheid outside of the submitted work. The other authors have no conflicts of interest to disclose.
Peer review
Peer review information
Nature Communications thanks Koos Korsten and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Frivold, C., Cox, S.N., Starita, L. et al. Correlates of risk of respiratory syncytial virus disease: a prospective cohort study. Nat Commun 16, 8490 (2025). https://doi.org/10.1038/s41467-025-63434-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63434-x