Abstract
Gaps in knowledge about the epididymal mucosa contribute to the prevalent classification of male idiopathic infertility. Inflammatory triggers, such as infections and autoimmunity, can breach immune privilege, induce anti-sperm antibody (ASA) production, and impair fertility. However, the mechanisms governing ASA production are poorly characterized. Here, using a murine model of epididymitis induced by regulatory T cell (Treg) depletion, we show that the disruption of immunotolerance leads to chronic autoimmunity characterized by the presence of ASA, and distinct testicular and epididymal immune landscapes. These inflammatory features impair sperm function, contribute to epididymal damage, and drive subfertility. Treg depletion induces the formation of tertiary lymphoid structures (TLS) within the epididymis, as indicated by the presence of B and T cell clusters, fibroblasts, and high endothelial venules. Similar autoantibody responses were detected in the seminal plasma of infertile patients, suggesting conserved mechanisms. Thus, we provide an in-depth analysis of immune cell dynamics and TLS during epididymitis, offering insights for the development of precision-targeted therapies for fertility disorders, as well as the identification of new contraceptive strategies.
Similar content being viewed by others
Introduction
Chronic inflammation and autoimmune conditions in the male reproductive system can impair sperm production, function, and transport, leading to infertility. Male infertility accounts for roughly 50% of 17% of infertility cases worldwide1. Immunological factors are believed to contribute to about 15% of male infertility cases, though this may be underestimated because immunological aspects in infertile patients are still underexplored. The limited understanding of epididymal immunity is a major reason why many cases of male infertility remain classified as “idiopathic.” Immunological infertility is characterized by elevated anti-sperm antibody (ASA) levels that interfere with reproductive function. Vasectomy, infections, or trauma can expose sperm to the immune system, resulting in increased ASA production2,3,4. However, the immune mechanisms behind the presence of ASAs are not fully understood1.
The recognition of sperm antigens as foreign by immune cells is linked to their delayed development, which occurs long after the process of establishing self-tolerance, during puberty5,6. When protective epithelial barriers are damaged, autoantigens that are usually confined become exposed to the immune system, potentially leading B lymphocytes to produce autoantibodies. The proliferation and maturation of autoreactive B cells are typical features of autoimmune disorders and generally happen in secondary lymphoid organs7,8. However, B cells in follicles and germinal centers (GC) can also mature in ectopic sites known as tertiary lymphoid structures (TLS), which form in response to chronic inflammation and tumors7,8. TLSs resemble lymphoid organs by containing distinct T and B cells, follicular dendritic cells (DC), high endothelial venules (HEV), and specialized immunofibroblasts9. For effective antibody production, B cells need to receive signals from other cells, such as mononuclear phagocytes (MP), epithelial cells, and T lymphocytes.
Regulatory T cells (Treg) prevent immune responses against sperm in the testis, where spermatozoa are produced, and the epididymis, where they mature and are stored5,6,10. In a murine model, autoimmune orchitis and epididymitis developed within 2 weeks following Treg depletion5,10.
In this study, we enhance our understanding of chronic epididymitis by offering a region- and time-specific analysis of its immune environment, emphasizing its clinical importance. Unlike acute inflammation, mainly driven by myeloid cell responses, chronic epididymitis involves ongoing activation of T and B cells that impair epididymal function, leading to persistent male subfertility. A key finding is the discovery of TLSs in the distal epididymis. Absent in acute inflammation, these structures represent a mechanism that sustains immune activation, fuels chronic inflammation, and worsens fertility issues. We identify antigens targeted by ASAs, providing potential insights into their functional impacts. Similar autoantibody responses are found in the seminal plasma of infertile men, indicating shared underlying mechanisms in mice and humans. Understanding these immune regulation processes broadens our knowledge of the largely unexplored post-testicular environment and its vital role in ongoing infertility. These findings could inspire new therapeutic strategies for male fertility problems and help develop novel targets for male contraception.
Results
Treg depletion induced early epididymal epithelial changes
Using transgenic mice that express the diphtheria toxin (DT) receptor (DTR) under the control of the Foxp3 promoter (Foxp3-DTR/EGFP mice), we disrupted immunotolerance by Treg ablation using a DT-based depletion protocol5,10 (Fig. 1A). The total Treg number decreased in the proximal (IS, and caput) and distal (corpus and cauda) epididymis and the testis 1-day post-DT (Fig. 1B–F). The Treg depletion within the epididymis was further confirmed by confocal microscopy (Fig. 1G). Note that in a naïve epididymis, Tregs are relatively sparse in the interstitium. To discriminate circulating Tregs from tissue-resident cells, in vivo intravascular CD45+cell staining was performed11 (Fig. 1H). The number of resident Tregs within the proximal and distal epididymis and testis was lower in the DT-treated Foxp3-DTR mice (Fig. 1H–K). The local-circulating Tregs in both organs showed no changes following DT (Supplementary Fig. 1).
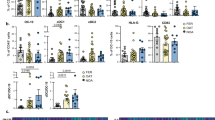
A Diagram of Treg depletion protocol and subsequent analysis 1 day after DT. The figure was created with BioRender.com. B Flow cytometry gating strategy for identifying Tregs (Foxp3+CD4+CD45+) in the proximal (prox.) and distal (dist.) epididymides. Absolute count (#) of total Tregs per gram of proximal (C) and distal (D) epididymis. Proximal epididymis comprises initial segments (IS) and caput; Distal comprises corpus and cauda. n = 9 (WT prox.), n = 7 (Foxp3-DTR prox. and WT dist.), n = 8 (Foxp3-DTR dist.). E Flow cytometry gating strategy for identifying testicular Tregs. F Total Tregs per gram of testis. n = 9 (WT), n = 8 (Foxp3-DTR). G Confocal microscopy images showing Foxp3-EGFP+Tregs (arrows) in naïve and 1-day DT-injected Foxp3-DTR epididymides. n = 3 epididymides. H Flow cytometry gating strategy for identifying tissue-resident Tregs (CD45-PE−Foxp3+CD4+CD45-BV711+). Tissue-resident Tregs per gram of epididymis (I, J) and testes (K). n = 9 (WT prox. and testis), n = 7 (Foxp3-DTR prox.), n = 8 (WT, Foxp3-DTR dist. and testis). L Confocal microscopy images showing B1V-ATPase+ cells (clear cells (CC); green), AQP9+ cells (principal cells; red), and F4/80+ mononuclear phagocytes (MP, magenta) in all regions of 1-day DT-injected WT and Foxp3-DTR mice. Arrows: CC blebs. Asterisks: AQP9 morphological alteration in epithelia. Double-headed arrows: F4/80+ luminal-reaching projections. n = 4 epididymides. M Imaging and quantification of luminal-reaching projections (arrows) in the WT and Treg-depleted epididymides per tissue area (110,540 µm2). Each image is represented as a dot. n = 20 images from 4 epididymides. N Imaging and quantification of the number of CC-MP contacts (arrows) in the WT and Treg-depleted epididymides per area (110,540 µm2). Asterisk: CC nanotube reaching a sperm. Each image is represented as a dot. n = 8 (WT prox.); n = 10 (Foxp3-DTR prox.), n = 11 (WT dist.), n = 14 (Foxp3-DTR dist.) images from 3 epididymides. DAPI (blue, cell nuclei). Bars: 20 μm. L: Lumen. Data are means ± SEM, and two-sided Student’s t-test (D, J, N) or Mann–Whitney test (C, F, I, K, M) were performed. C,D, F, I–K Each dot represents a pool of 2 organs. n = number of samples from different mice.
Hematoxylin and eosin (H&E) staining revealed a higher incidence of damaged sites in the Treg-depleted epididymis (Supplementary Fig. 2A, B). The Treg-depleted epididymal epithelium presented injury features: clear cells (CC) changed their morphology, increased their membrane protrusions, and shed several of them into the lumen; and principal cells (PC) showed epithelial discontinuity and thicker microvilli (Fig. 1L). Treg-depleted cauda presented multiple MP luminal-reaching projections like those detected in the IS. Quantification of these projections was higher in the Treg-depleted epididymis (Fig. 1L, M). The luminal-reaching projections interacted with CCs (Fig. 1L, N). The number of CC-MP contacts was amplified in the distal but not in the proximal regions, suggesting increased cell-to-cell crosstalk in this area12,13,14. CCs extend nanotubes13 that touch the sperm head (Fig. 1N). We didn’t detect spermatogenesis impairment at this early time point (Supplementary Fig. 2C).
Systemic and local autoantibody production 2 weeks after DT
The isotypification of autoantibodies showed distinct profiles based on the antigen tested. In Treg-depleted mouse serum, increases were observed in IgM, IgG2b, IgG2c, and IgA against testicular antigens (Supplementary Fig. 3A–F). IgM, IgG1, IgG2b, and IgG2c were elevated against epididymal antigens (Supplementary Fig. 3G–L). Notably, IgM and IgG2c levels rose against proximal sperm antigens, and IgM, IgG1, IgG2b, IgG2c, IgG3, and IgA were higher against distal sperm antigens (Supplementary Fig. 3M–X).
Regarding the local presence of tissue autoantibodies, we revealed that in testicular homogenates IgG1, IgG2b, IgG2c, and IgG3 levels were increased against testicular antigens 2 weeks after depletion (Fig. 2A–F). Additionally, epididymal homogenates displayed elevated levels of IgM, IgG1, IgG2b, IgG2c, IgG3, and IgA against epididymal antigens (Fig. 2G–L). We confirmed the accumulation of these isotypes in the Treg-depleted testicular and epididymal interstitium (Fig. 2M–R and Supplementary Figs. 4–9). The different isotype deposition patterns in both organs might be due to distinctive antigen-specificities among the autoantibodies. In the epididymal lumen of Treg-depleted mice, we observed agglutinated sperm containing IgG1+ and IgG2c+ ASAs (Fig. 2S). Some of these ASA-bound sperm clusters were surrounded by F4/80+MPs (Fig. 2S), potentially indicating phagocytosis of antibody-bound sperm. Concomitantly, anti-sperm IgG2c antibodies were attached to sperm from Treg-depleted mice using sperm suspension immunostaining (Fig. 3A). Antibody-secreting B-lymphocytes congregated in the epididymal interstitium (Fig. 2P–R and Supplementary Figs. 7–10).
Isotypification of autoantibody levels (IgM, IgG1, IgG2b, IgG2c, IgG3, and IgA) in testicular homogenates against testicular antigens (A–F), and epididymal homogenates against epididymal antigens (G–L). A–F: n = 8 (WT: IgM, IgG1, IgG2b, IgG2c, IgG3, IgA; Foxp3-DTR: IgM, IgG3), n = 6 (Foxp3-DTR: IgG1, IgG2b, IgG2c, IgA). G–L: n = 7 (WT: IgM, IgG1, IgG2c, IgA), n = 6 (WT: IgG2b; Foxp3-DTR: IgM, IgG1, IgG2b, IgG2c, IgG3, IgA), n = 5 (WT: IgG3). Each dot represents a pool of 4 organs from 2 mice. n = number of samples. Immunolabeling of CD19+ B cells (red, arrows) and IgG1 (M), IgG2b (N), or IgG2c (green) (O), in the DT-injected WT and Foxp3-DTR caput. n = 3 epididymides from different mice. Immunolabeling of CD19+ B cells (red) and IgG1 (green, P) in the caput, IgG2b (green, Q) in the cauda, and IgG2c (green, R) in the caput of DT-injected WT and Foxp3-DTR mice. A dashed circle indicates cell aggregation in the interstitium. Antibody deposition can be observed in the epididymal interstitium of the different regions. See images of testes and other epididymal regions in Supplementary Figs. 4–9. n = 3 epididymides from different mice. S Immunolabeling of F4/80+ MPs (red) and IgG1 (green, upper panels) and IgG2c (green, bottom panels) in the DT-injected WT and Foxp3-DTR caput. Arrowheads show agglutination of sperm positive for IgG1 and IgG2c (green), surrounded by F4/80+ MPs (red, asterisks) in the DT-injected Foxp3-DTR mice. Note that these images were taken using higher laser intensity. Nuclei are labeled with DAPI (blue). n = 4 epididymides from different mice. Bars: 20 μm. L: Lumen. Data were analyzed using two-sided Student’s t-test (A–F, H–K) or Mann–Whitney test (G, L). Data are shown as means ± SEM.
A Immunolabeling of IgG2c (red) on distal sperm from DT-injected WT and Foxp3-DTR mice. n = 3 number of samples from different mice. B Total IgG autoantibodies against proximal and distal sperm antigens were detected in proximal and distal epididymal fluids of Treg-depleted mice by ELISA. n = 9 (WT); n = 11 (Foxp3-DTR). C Flow cytometry gating strategy for identifying relative abundance of pro-inflammatory-like macrophages (CD11b+F4/80+CD64+CD11c+) in the proximal and distal epididymal fluids of DT-treated WT and Foxp3-DTR mice. n = 5 number of samples from different mice. D Sperm protein immunoprecipitation strategy with serum, proximal, and distal epididymal fluids protocol diagram. The figure was created with BioRender.com. E Venn diagram showing the serum/epididymal fluid-immunoprecipitated sperm proteins detected in the DT-treated Foxp3-DTR samples by proteomics. In bold are the proteins reported to be present in sperm cells. See also Supplementary Data 3. n = 2 independent experiments. Each experiment contained pools of at least 3 samples from different mice. Levels of total IgG (F) and IgA (G) ASAs in seminal plasma from healthy (normozoospermic, normo) and pathological (asthenozoospermia, astheno) patients. n = 26 (NORMO IgG), n = 12 (ASTHENO IgG, IgA), n = 24 (NORMO IgA). Total IgG (H) and IgA (I) ASA levels in seminal plasma of patients with low (no RC) versus high (RC) percentages of round cells. n = 16 (NO RC IgG), n = 14 (RC IgG, IgA), n = 22 (NO RC IgA). J Total IgG (green) immunofluorescence imaging in the sperm fraction from normozoospermic and pathological (astheno) patients. n = 3 number of human samples. Nuclei are labeled with DAPI (blue). Bars: 20 μm. Data were analyzed using two-sided Student’s t-test (C, I), or Mann–Whitney test (B, F, G, H). Data are shown as means ± SEM.
Sperm antigens detected by ASAs
Two weeks after Treg depletion, IgG ASAs were elevated in the Treg-depleted epididymal fluids (Fig. 3B). We demonstrated an increase in the pro-inflammatory macrophage abundance in the Treg-ablated distal fluid (Fig. 3C). Immunoprecipitation of sperm proteins using serum, along with proximal and distal epididymal fluids from DT-treated WT and Foxp3-DTR mice (Fig. 3D), confirmed the presence of ASAs following depletion (Supplementary Data 1–3). A total of 87 proteins were commonly identified after immunoprecipitation using serum and fluids post-Treg depletion (Fig. 3E). In Treg-ablated mice, we detected 134 immunoglobulin (Ig)-related proteins in serum, along with 91 and 102 Ig-related proteins in the proximal and distal fluids, respectively (Supplementary Data 3, red). These confirmed the presence of IgG1 and IgG2c ASAs in the epididymal lumen. We identified 8 complement proteins in serum, whereas only 1 was detected in the Treg-depleted distal fluid (Supplementary Data 3).
The 220 proteins in Fig. 3E include those with a ≥ twofold increase or uniquely detected in Foxp3-DTR samples versus WT after immunoprecipitation. Proteomic data identified proteins involved in sperm maturation, motility, capacitation, and sperm-egg interaction, such as MDH1, GADPHS, KRT1, PSMA4, PDE4D, ZP3R, Rab2A, NUCB2, and CALML315,16,17,18,19,20. Other identified proteins are crucial for embryo development: H3-521, SMCHD122, HSPA1B23, and ARMCX424. Some detected proteins, like PBDC1, are known to be present in sperm, but their role in fertility remains unclear. We revealed novel sperm proteins, such as NALCN, which might interact with the CatSper25, key to sperm motility and fertility26. Serum ASAs recognized complement-related proteins, some of which have already been described in sperm and semen27. However, their direct impact on sperm function and fertility is not well-described. The serum and fluid ASAs recognize sperm proteins functioning as immune modulators, such as IFIT1, MUG1, ICOSLG, and CLU.
Human ASAs were detected in patients with fertility challenges
Given that Treg depletion in mice leads to an autoimmune response with ASA production, we investigated whether similar ASAs are present in patients with compromised sperm function, particularly asthenozoospermic individuals facing fertility challenges. We selected this population based on our prior observations that sperm motility is reduced in mice 2 weeks post-Treg depletion10. We conducted ELISA on seminal plasma from patients from assisted reproduction units at Cruces and Galdakao Hospital, Spain (Supplementary Tables 1 and 2). Our findings revealed elevated ASA levels in 20% of the samples. Asthenozoospermic patients exhibited higher IgG ASAs than normozoospermic patients (Fig. 3F). However, no significant differences in IgA ASAs were observed between these groups (Fig. 3G). Conversely, samples with a high content of round cells (RC) showed an increase in IgA ASAs (Fig. 3H, I). We detected IgG ASAs on sperm heads and tails of patients with high ASA levels in their seminal plasma (Fig. 3J).
B cell dynamics 2 weeks after Treg depletion
Two weeks after Treg ablation, B cells localized in the proximal and, to a lesser extent, in the distal interstitium (Fig. 4A and Supplementary Fig. 10). t-SNE (t-distributed Stochastic Neighbor Embedding) and FlowSOM (clustering algorithm) analysis of CD45+cell node, identified 12 cell subtypes and a shift in the immune landscape in the different epididymal regions (Fig. 4B, C) and the testis (Supplementary Fig. 11A). Consistent with previous findings10, we observed an increase in the CD4+T cells in both organs (Fig. 4B, C and Supplementary Fig. 11A). The analysis revealed a rise in different CD8+T and CD20+B cells across both tissues (Fig. 4B, C and Supplementary Fig. 11A). t-SNE and FlowSOM analysis of the B-lymphocyte node (B220+/CD19+/CD20+) and B cell markers, identified 8 distinct proximal and testicular B cell clusters 2-week post-depletion (Supplementary Figs. 11B, C and 12A, B), highlighting the B cell population diversity in these organs. Moreover, we observed higher counts of total and tissue-resident testicular and proximal B cells (Supplementary Fig. 11D–J and Fig. 4D–P). Regarding the local-circulating cells, testicular CD19+/CD20+B cells (Supplementary Fig. 11K–M), proximal B220+/CD19+/CD20+B cells, and distal CD20+B cells were elevated after depletion (Supplementary Fig. 12C–H). Total epididymal and testicular plasma B cells (CD3−CD19⁺IgD−CD138⁺) were raised, whereas local-circulating cells were amplified only in the epididymis (Fig. 4Q–S and Supplementary Figs. 11N–Q and 12I, J). Tissue-resident plasma B cell expansion occurred in the corpus/cauda (Fig. 4T, U) and the testis (Supplementary Fig. 11P).
A Immunolabeling of CD19 (red) and CD20 (green) in the IS, caput, and cauda of DT-injected WT and Foxp3-DTR mice. n = 3. t-SNE and FlowSOM analysis of proximal (B) and distal (C) from WT and Foxp3-DTR epididymides. The most relevant populations that relatively increased in the Foxp3-DTR samples are highlighted: i. Cyan:CD20; magenta:CD8; purple:CD4; green:CD20/CD8. ii. Light green:CD8; brown:CD4; dark green:CD20/CD62L/CD23; magenta:CD27/FAS/CD21/CD38. n = 3. D Flow cytometry gating strategy and absolute count (#) of total B cells (CD3−B220+, CD3−CD19+, and CD3−CD20+ (E–J) per gram of proximal/distal epididymis. n = 12 (WT), n = 10 (Foxp3-DTR). K–P Tissue-resident B cells per gram of proximal and distal epididymis. n = 10 (WT and Foxp3-DTR: B220, CD20, CD19 prox.), n = 9 (WT and Foxp3-DTR: CD19 dist.). Q Flow cytometry gating strategy and relative abundance of total (R, S) and tissue-resident (T, U) Plasma B cells (CD3⁻CD19⁺IgD⁻CD138⁺) in the proximal/distal epididymides. n = 12 (WT total), n = 10 (Foxp3-DTR total and resident). V Flow cytometry gating strategy and relative abundance of total (W, X) and tissue-resident (Y, Z) Germinal center (GC) B cells (CD3⁻B220⁺IgD⁻GL7⁺) in the proximal/distal epididymides. n = 13 (WT total), n = 10 (Foxp3-DTR total and resident), n = 11 (WT resident). Aa Flow cytometry gating strategy and relative abundance of total (Bb, Cc) and tissue-resident (Dd, Ee) Regulatory B cells (CD3−CD19+CD5+CD62L−CD1dhi) in the proximal/distal epididymides. n = 13 (WT prox. total), n = 10 (Foxp3-DTR total and resident), n = 11 (WT dist. total, WT prox. resident), n = 9 (WT dist. resident). Ff–Hh Flow cytometry gating strategy and absolute count (#) of total follicular dendritic cells (DC, CD4−CD19−CD21+) in the proximal/distal epididymides. n = 10 (WT prox.), n = 7 (Foxp3-DTR), n = 8 (WT dist.). Flow cytometry gating strategy and absolute count (#) of total CD8+T cells (CD4−CD8+) (Ii–Kk) and activated CD8+T cells (CD4−CD8+CD44+) (Ll–Nn) per gram of proximal/distal epididymis. n = 13 (WT), n = 10 (Foxp3-DTR). DAPI (blue). Bars: 20 μm. Data are means ± SEM, and two-sided Student’s t-test (E, K, U, W, Gg), or Mann–Whitney test (F–J, L–P, R–T, X–Z, Bb–Ee, Hh–Nn) were performed. Each dot represents a pool of 2 organs. n = number of samples from different mice.
We revealed a higher abundance of total, tissue-resident, and local-circulating CD3-B220+IgD−GL7+B cells, indicative of GC, in the Treg-depleted proximal epididymis, but not in the total, tissue-resident, and local-circulating cells of the distal, nor the testis (Fig. 4V–Z and Supplementary Figs. 11R–U and 12K, L). Regulatory B cells (Breg, CD3−CD19+CD5+CD62L−CD1dhi) increased in the epididymis (Fig. 4Aa–Ee and Supplementary Fig. 12M, N), while only total and tissue-resident Bregs were elevated in the testis (Supplementary Fig. 11V–Y). Alongside the B cell expansion, we noted infiltration of proximal and testicular follicular DCs, with no changes in the distal epididymis (Fig. 4Ff–Hh and Supplementary Figs. 11Z–Cc and 12O–R). Remarkably, CD8+ and activated CD8+CD44+T lymphocytes were also raised in both organs (Fig. 4Ii–Nn and Supplementary Figs. 11Dd–Kk and 12S–Z).
Chronic inflammation in the testis and epididymis 8 weeks after Treg ablation
The immune response was assessed in the epididymis and testis 8 weeks post-DT to examine the long-term effects of tolerance disruption using in vivo staining of circulating CD45+cells (Fig. 5A). Total CD45+cells were prominent in the distal epididymis and testis (Fig. 5B–D and Supplementary Fig. 13A, B). Local-circulating CD45+cells were elevated in the epididymis/testis (Supplementary Figs. 13D and 14C, D). Tissue-resident CD45+cells were amplified in the Treg-ablated testis (Supplementary Fig. 13C). t-SNE and FlowSOM analysis identified 12 distinct epididymal and testicular immunocyte subtypes (Fig. 5E, F and Supplementary Fig. 13E). While the most dramatic immune cell landscape changes were observed in the proximal 2 weeks post-DT, by 8 weeks, these changes were more pronounced in the corpus/cauda. Interestingly, 8 weeks after the initial ablation, we observed Treg expansion in both organs (Fig. 5G–I and Supplementary Figs. 13F–K and 14F–H). The epididymal Treg rebound was also evident by confocal microscopy (Fig. 5L). Additionally, total and circulating IS/caput and testicular CD4+Foxp3−cells were greater, and an important surge was observed only in tissue-resident distal CD4+Foxp3−cells (Fig. 5J, K and Supplementary Figs. 13H–L and 14I–L).
A Diagram of Treg depletion protocol, in vivo staining of CD45+ circulatory cells, and subsequent analysis 8 weeks after DT treatment. The panel was created with BioRender.com. Flow cytometry gating strategy (B) and absolute count (#) of total CD45+ cells per gram of proximal (C) and distal (D) epididymis. n = 13 (WT); n = 16 (Foxp3-DTR). t-SNE and FlowSOM analysis of proximal (E): Magenta: CD20; blue: CD8/PD-1; purple: CD4) and distal (F): Cyan:Foxp3; green:B220/CD19/CD20/IgM; purple:CD4; brown: B220/CD19/CD21/CD62L/CD38; yellow:CD62L/CD44) epididymides from WT and Foxp3-DTR mice n = 3. The most relevant populations that relatively increased in the Foxp3-DTR samples are highlighted. Flow cytometry gating strategy (G) and absolute count (#) of total Tregs (CD4+Foxp3+ and T CD4+ cells (CD4+Foxp3−) per gram of proximal (H, J) and distal (I, K) epididymis. n = 12 (WT prox. Tregs), n = 14 (Foxp3-DTR prox. Tregs and T CD4+), n = 11 (WT dist. Treg, WT T CD4+), n = 13 (Foxp3-DTR dist. Tregs and T CD4+). L Confocal microscopy of epididymal distal regions of Foxp3-DTR-eGFP mice. Notice the Treg Foxp3+ (green, arrows) cell accumulation in the interstitium of the DT-treated Foxp3-DTR mice. n = 3 epididymides from different mice. Flow cytometry gating strategy (M) and relative abundance of T follicular helper cells (Tfh, CD4+CD19−CD8−CXCR5+) in the proximal (N) and distal (O) epididymides. n = 9 (WT), n = 11 (Foxp3-DTR prox.), n = 10 (Foxp3-DTR dist.). Flow cytometry gating strategy (P) and absolute count (#) of total follicular DCs (CD4−CD19−CD21+) per gram of proximal (Q) and distal (R) epididymides. n = 9 (WT), n = 11 (Foxp3-DTR). Flow cytometry gating strategy (S), absolute count (#) of total CD8+ T cells (CD4−CD8+) and activated CD8+ T cells (CD4−CD8+CD44+) per gram of proximal (T, V) and distal (U–W) epididymis. n = 12 (WT), n = 14 (Foxp3-DTR prox. CD8+ and CD8+CD44+), n = 13 (Foxp3-DTR dist. CD8+ and CD8+CD44+). Nuclei are labeled with DAPI (blue). L: Lumen. Bars: 20 μm. Data were analyzed using the two-sided Mann–Whitney test. Data are shown as means ± SEM. Each dot represents a pool of 2 organs from each mouse. n = number of samples from different mice.
Strikingly, total, tissue-resident, and -circulating follicular helper T cells (CD4+CD19−CD8−CXCR5+) were higher only in the corpus/cauda (Fig. 5M–O and Supplementary Fig. 14M–P) and in the testicular circulation 8 weeks after DT (Supplementary Fig. 13M–P). Total and tissue-circulating follicular DCs were also increased in the distal and testis (Fig. 5P–R and Supplementary Figs. 13Q–T and 14Q–T). Additionally, epididymal and testicular CD8+ and activated CD8+CD44+T lymphocytes were elevated (Fig. 5S–W and Supplementary Figs. 13U–Aa and 14U–Bb). Monocyte and macrophage infiltration previously noted after 2-week depletion10 had been resolved in the epididymis (Supplementary Fig. 15A, B). In the testis, monocyte infiltration remained elevated (Supplementary Fig. 15C), whereas macrophage infiltration was resolved (Supplementary Fig. 15D–H). Conversely, MP projections persisted higher (Supplementary Fig. 15G), indicating sustained antigen-sensing activity28.
Analyzing the B cell diversity of the 8-week Treg-ablated corpus/cauda, 8 clusters with different phenotypes from the ones defined at 2 weeks were found (Fig. 6A and Supplementary Fig. 16A). Total and local-circulating B220+/CD19+/CD20+cells and the resident B220+cells were higher in the distal (Fig. 6B–E and Supplementary Fig. 16B–R). However, no differences were observed in B cell counts in the proximal (Supplementary Fig. 16B–D, G, I, K, M, O, Q). In the testis, besides identifying 8 B cell populations (Supplementary Fig. 17A, B), we found a higher incidence of CD19+/CD20+cells (Supplementary Fig. 17C–N). Total and circulating plasma B cells were amplified in the distal regions, with circulating B cells being the only ones found in the testis (Fig. 6F–L and Supplementary Fig. 17O–R). GC B cells exhibited an elevation in the corpus/cauda, not in the testis or IS/caput (Fig. 6M–S and Supplementary Fig. 17S–V). Bregs rose in the epididymis, as well as the total and local-resident testicular Bregs (Fig. 6T–Z and Supplementary Fig. 17W–Z). Memory-like B cells, both total and resident, expanded in the testis (Supplementary Fig. 17Aa–Dd) and epididymis, except for the circulating cells in the proximal region (Fig. 6Aa–Gg). Together, region-specific B cell analysis at 2 and 8 weeks after Treg ablation revealed a shift of naïve B cells to memory and GC B cells, suggesting local B cell maturation in the distal regions 8 weeks after DT.
A t-SNE and FlowSOM analysis of distal epididymides from Foxp3-DTR mice. The most relevant populations that relatively increased in the Foxp3-DTR samples are highlighted: 0 red: CD20/CD62L/IgM/CD21/CD38/CD19/B220. 1 purple:CD20/IgM/B220. 2 violet:CD20/CD27/CD62L. 3 blue:CD20/CD138/CD62L/CD27. 4 cyan: B220/CD19/CD62L/CD21. 5 green:B220/CD19/IgM/CD62L. 6 yellow:CD20/CD27/CD62L/IgM/CD21. 7 orange:CD20/CD23/CD27/CD62L/IgM/CD21 (Supplementary Fig. 15A). n = 3. B Flow cytometry gating strategy and absolute count (#) of total (C), tissue-resident (D), and local-circulating (E) B cells (CD3−B220+) per gram of distal epididymis. n = 13 (WT total, Foxp3-DTR resident/circulating), n = 15 (Foxp3-DTR total), n = 8 (WT resident), n = 9 (WT circulating). F Flow cytometry gating strategy and relative abundance of total (G, H), tissue-resident (I, J), and local-circulating (K, L) plasma B cells (CD3−CD19⁺IgD−CD138⁺) in the proximal and distal epididymides. n = 10 (WT total), n = 12 (Foxp3-DTR prox.), n = 11 (Foxp3-DTR dist.), n = 7 (WT pro. and dist. resident/circulating). M Flow cytometry gating strategy and relative abundance of total (N, O), tissue-resident (P, Q), and local-circulating (R, S) Germinal center (GC) B cells (CD3−B220⁺IgD−GL7⁺) in the proximal and distal epididymides n = 9 (WT total), n = 11 (Foxp3-DTR prox.), n = 10 (Foxp3-DTR dist.), n = 6 (WT resident/circulating). T Flow cytometry gating strategy and relative abundance of total (U, V), tissue-resident (W, X), and local-circulating (Y, Z) regulatory B cells (B regs, CD3-CD19+CD5+CD62L⁻CD1dhi) in the proximal and distal epididymides. n = 10 (WT total), n = 12 (Foxp3-DTR total/circulating and Foxp3-DTR prox. resident); n = 11 (Foxp3-DTR dist. resident), n = 7 (WT resident/circulating). Aa Flow cytometry gating strategy and relative abundance of total (Bb, Cc), tissue-resident (Dd, Ee), and local-circulating (Ff, Gg), memory B cells (CD3−CD19+CD27+) in the proximal and distal epididymides. n = 9 (WT total); n = 11 (Foxp3-DTR prox.), n = 10 (Foxp3-DTR dist.), n = 6 (WT resident/circulating). Data were analyzed using two-sided Student’s t-test (N, Y, Dd) or Mann–Whitney test (C–E, G–L, O–S, U–X, Z; Bb, Cc, Ee–Gg). Data are shown as means ± SEM. Each dot represents a pool of 2 organs from each mouse. n = number of samples from different mice.
Treg depletion triggered TLS formation in the distal epididymis
Confocal microscopy revealed B cell clusters resembling TLSs in the corpus and cauda 8 weeks after depletion (Fig. 7A, B, G–M and Supplementary Fig. 18A–F). Within these TLSs, CD21+ follicular B and dendritic-like cells were present (Fig. 7A and Supplementary Movie 1). Several TLS B cells were IgD-positive, indicating the presence of mature naïve B cells (Fig. 7B, Supplementary Fig. 18A, and Supplementary Movies 2–5). Total and tissue-resident IgD+B cells were higher in the corpus/cauda (Fig. 7C–F). The epididymal TLSs contained T cells (Fig. 7G, Supplementary Fig. 18B, C, and Supplementary Movies 6 and 7) surrounding B cell zones. Few TLS B cells were positive for activation-induced cytidine deaminase (AID), B-cell lymphoma 6 (BCL6), and the proliferation marker Ki67 (Fig. 7H–J and Supplementary Fig. 18D–F). AID and BCL6 play crucial roles in regulating somatic hypermutation, isotype switching, and the ultimate effector function of Igs7,8,29. These markers suggest local B cell activation, which might reflect GC-like activity. AID was also detected in non-B cells, consistent with previous findings showing that chronic inflammation can induce aberrant AID expression in any cell type30. Follicular T cells could also express BCL6 in TLSs29. Additionally, we observed other Ki67-positive cells, particularly within the epididymal epithelia. This likely reflects ongoing tissue repair/regenerative processes, which might occur 8 weeks after depletion. Epididymal TLSs were observed surrounding endothelial cells like CD31+ and PNAD+ cells (Fig. 7K, Supplementary Fig. 19A, B, and Supplementary Movie 8), and fibroblasts (α-SMA+ and podoplanin+ (PDPN+) (Fig. 7L, M, Supplementary Fig. 19C, D, and Supplementary Movies 9 and 10). These findings confirm the presence of TLSs within the distal epididymis, identifying various stages of TLS development.
A Immunolabeling of CD19 (red), CD20 (green), and CD21 (magenta, arrows) in the DT-injected Foxp3-DTR distal epididymis. Arrows: double-positive cells. See also Supplementary Movie 1. n = 3. B Immunolabeling of IgD (red), CD20 (green), and CD3 (magenta, arrows) in the DT-injected Foxp3-DTR distal epididymis. See also Supplementary Movies 2–5. n = 5. Flow cytometry gating strategy (C) and relative abundance of total (D), tissue-resident (E), and local-circulating (F) IgD+CD20+B cells in the distal epididymides. n = 9 (WT total), n = 11 (Foxp3-DTR), n = 6 (WT resident/circulating). Each dot represents a pool of 2 organs from each mouse. G Immunolabeling of CD20 (green) and CD3 (magenta) in the cauda of DT-injected Foxp3-DTR mice. Arrows: T cells. See also Supplementary Movies 6 and 7. n = 5. H Immunolabeling of CD19 (green) and AID (red) in the DT-injected Foxp3-DTR cauda. Arrows: double-positive cells. n = 4. I Immunolabeling of CD20 (green) and BCL6 (magenta) in the DT-injected Foxp3-DTR cauda. Arrows: double-positive cells. n = 3. J Immunolabeling of CD20 (green) and Ki-67 (red) in the DT-injected Foxp3-DTR cauda. Arrows: double-positive cells. n = 5. K Immunolabeling of CD20 (red), PNAD (green), and CD31 (magenta) in the cauda of DT-injected Foxp3-DTR mice. Arrows: possible HEVs. See also Supplementary Movie 8. n = 5. L Immunolabeling of CD20 (green) and PDPN (magenta, see also Supplementary Movies 9 and 10) in the DT-injected Foxp3-DTR cauda. Arrows: fibroblasts. n = 5. M Immunolabeling of CD20 (green) and α-SMA (red) in the DT-injected Foxp3-DTR cauda. Arrows: fibroblasts, asterisks: muscle cells. n = 5. N Venn diagram of the soluble mediators measured in the proximal and distal epididymides at 2 and 8 weeks after DT. Molecules were grouped by general functions or inflammatory abilities such as TLS formation (&, cell recruitment, tissue remodeling, angiogenesis capacity) and pro- (*) and anti-inflammatory (#) mediators. See also Supplementary Fig. 20. n = 2 independent experiments. Each sample was a pool of 4 proximal or distal epididymides from different mice. DAPI (blue). L: Lumen. Bars: 20 μm. Data were analyzed using two-sided Student’s t-test (F), or Mann–Whitney test (D, E). Data are shown as means ± SEM. A, B, D–I: n = number of epididymides from different mice.
Multiplex proteome profiling assay revealed differences in soluble mediator abundances between the proximal and distal epididymis at 2 and 8 weeks following Treg depletion (Fig. 7N and Supplementary Fig. 20A, B). At 2 weeks, 19 molecules were twofold increase in the proximal and 9 in the distal, while 28 mediators were found upregulated in both regions. The 8-week Treg-depleted corpus/cauda exhibited 26 molecules with a twofold increase, whereas only 7 mediators were identified in the IS/caput at the same time point. Furthermore, 30 mediators were found in both areas at this time (Fig. 7N). Many secreted factors play critical roles in immune cell recruitment, displaying pro- and anti-inflammatory profiles, and contributing to TLS formation.
CXCL13, detected in both regions at 2- and 8-week post-DT, is crucial for TLS assembly. It recruits CXCR5-expressing B cells, organizing them into TLSs and maintaining the B cell zone31,32. BAFF, upregulated in the proximal at 2 weeks and in both regions at 8 weeks post-treatment, is also associated with TLS formation and maintenance by promoting the proliferation of B cells33. Notably, the highest number of upregulated molecules was observed in the distal epididymis at 8 weeks, correlating with the presence of TLSs. Chemokines such as CXCL9, CCL5/RANTES, CCL20, CCL22, among others, are immune cell attractants and activators that are important to initiate signals that trigger TLS establishment8. Together with pro-inflammatory cytokines such as IL-1β, IL-6, TNF, IFNγ, IL-23, and costimulatory molecules such as CD40/TNFRSF5 that activate DCs and B cells, enhance immune activation and B cell differentiation. Anti-inflammatory molecules, particularly IL-10, FGF-21, and IL-1ra, were observed in both regions and evaluated at various time points after depletion. These molecules counteract the inflammatory surge to limit excessive inflammation. Amphiregulin, Angiopoietin-1 and -2, MMP-9, Chitinase-3-like protein-1, IL-22, and GDF-15, involved in wound healing/tissue repair/angiogenesis, were consistently detected throughout the epididymis at both time points, actively remodeling the extracellular matrix, promoting blood vessel formation, and facilitating TLS formation7,8,9,31,32,34,35. These molecules collectively orchestrated the recruitment of different immune cells, promoting functionality of TLSs in response to chronic epididymitis.
Chronic inflammation resulted in severe immunological male subfertility
Regarding the humoral response 8 weeks after DT, increased serum and tissue Ig levels against sperm, testicular, and epididymal antigens were detected in the Treg-ablated mice. Isotyping of serum autoantibodies revealed distinct profiles compared to 2 weeks after depletion, depending on the antigen tested. In 8-week Treg-depleted serum, increases were noted in IgM, IgG1, IgG2c, IgG3, and IgA against testicular antigens, and elevated IgM, IgG1, and IgG2c levels were displayed against epididymal, proximal sperm, and distal sperm antigens (Supplementary Fig. 21A–X). At 8 weeks post-depletion, we revealed an accumulation of IgG2b in the testis and of IgM, IgG1, IgG2b, and IgG2c in the epididymis (Fig. 8A–N and Supplementary Fig. 22A–C). Supplementary Fig. 22D shows a summary of the humoral responses. Notably, 8-week serum IgG ASA titers decreased compared to serum from 2-week Treg-depleted mice (Fig. 8O, P). However, no changes were observed with the autoantibody titers against epididymal antigens (Supplementary Fig. 22E). No antibody deposition nor sperm conglomeration were seen in the epididymal lumen 8 weeks after ablation. Interestingly, the Foxp3-DTR proximal epithelium exhibited areas of injury 8 weeks post-DT, but such lesions were not observed in the distal region (Fig. 9A, B). At the same time, CCs and PCs did not show major defects (Supplementary Fig. 23). Importantly, spermatogenesis was not chronically affected (Supplementary Fig. 24A–C). Still, we observed reduced distal sperm counts (Fig. 9C, D). The decline in sperm motility observed 2 weeks after Treg depletion10 was restored by 8 weeks (Fig. 9E). Fertility was severely impacted, as shown by a decreased litter size (Fig. 9F). These results unveiled that immunotolerance disruption triggered an early and persistent pro-inflammatory response, adversely impacting the epididymis and testis, culminating in chronic subfertility.
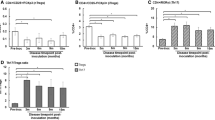
Isotypification of autoantibody levels (IgM, IgG1, IgG2b, IgG2c, IgG3, and IgA) in testicular homogenates against testicular antigens (A–F) and in epididymal homogenates against epididymal antigens (G–L). A–F: n = 6 (IgM), n = 9 (WT: IgG1, IgG2b, IgG2c), n = 8 (Foxp3-DTR: IgG1, IgG2b, IgG2c, IgA); n = 3 (WT: IgG3), n = 4 (Foxp3-DTR: IgG3), n = 7 (WT: IgA). G–L: n = 6 (WT: IgM, IgG3), n = 5 (Foxp3-DTR: IgM, IgG3), n = 9 (WT: IgG1, IgG2b, IgG2c, IgA), n = 7 (Foxp3-DTR: IgG1), n = 8 (Foxp3-DTR: IgG2b, IgG2c, IgA). M Immunolabeling of CD19+ B cells (red) and IgG2c (green) in the corpus region of DT-injected WT and Foxp3-DTR mice. n = 3 epididymides from different mice. N IgG2b (green) immunolabeling in the testis of DT-injected WT and Foxp3-DTR mice. n = 3 epididymides from different mice. O Foxp3-DTR serum total IgG titration at 2- and 8-weeks post-Treg ablation against distal sperm antigens by ELISA. Dose-response curves of optical density vs. serial dilutions of sera. P IgG titers are calculated as the inverse of the EC50 dilution. n = 5 (2 wks.); n = 6 (8 wks.). n = number of samples from different mice. Nuclei are labeled with DAPI (blue). Bars: 20 μm. L: Lumen. Data were analyzed using two-sided Student’s t-test (A–C, E, G–K), or Mann–Whitney test (D, F, L, P). Data are shown as means ± SEM.
H&E staining of the proximal (IS and caput) (A) and distal (corpus and cauda) (B) epididymal regions of DT-injected WT and Foxp3-DTR mice and quantification of the number of damaged sites normalized per tissue area (µm2). Any morphological alteration in epididymal epithelia (arrows) was counted as a damaged site. Each area quantified is represented as a dot. A: n = 9 pictures from 3 prox. epididymides from different mice; B: n = 10 (WT); n = 8 (Foxp3-DTR) images from 4 dist. epididymides from different mice. Computer-assisted sperm analysis (CASA) analysis of proximal sperm cells (C) and distal sperm (D) counts. n = 6 (WT), n = 11 (Foxp3-DTR). E Percentage of total sperm motility analyzed by CASA of distal sperm. n = 6 (WT), n = 11 (Foxp3-DTR). F Fertility assessment by natural mating as the number of litter size (pups/litter). n = 7 (WT), n = 10 (Foxp3-DTR). G Graphical representation of the key findings at 2 and 8 weeks after Treg depletion in the epididymis. The figure was created with BioRender.com. L: Lumen. Bars: 250 μm. Data were analyzed using two-sided Student’s t-test (C–E) or Mann–Whitney test (A, B, F). Data are shown as means ± SEM. n = number of samples from different mice.
Chronic inflammation in the epididymis triggers TLS formation, perpetuating impaired male fertility
In our working model (Fig. 9G), within 24 h of Treg depletion, epithelial damage initiates early responses from MPs and epithelial cells. By 2 weeks, the epididymis shows infiltration of T- and B-lymphocytes, alongside monocytes, neutrophils, and MPs, particularly concentrated in the proximal regions10. This autoimmune response induces the production of autoantibody isotypes in the epididymis and testis and the pro-inflammatory mediator release, including TNF and IFNγ. Crucially, both epididymal regions secrete factors, such as CXCL13 and BAFF, that facilitate B cell recruitment/maturation and promote TLS formation. At 8 weeks, persistent distal epididymal inflammation, characterized by differentiated B and T cell accumulation, supports the establishment of TLSs. The secretion of mediators, like CXCL9/10/11, IL-23, CCL22, and IL-1β, maintains these ectopic structures, perpetuating a chronic inflammatory environment. At this time, the Treg rebound occurs to counteract the inflammation. As a result, the prolonged epithelial injury, along with the presence of ASAs, severely impairs sperm counts, ultimately causing sustained male subfertility.
Discussion
Here, we report that chronic epididymal inflammation causes sustained immune responses that result in tissue damage and reduced male fertility. Treg dysfunction leads to autoimmune subfertility, characterized by unique T and B cell infiltrating profiles and in-situ production of autoantibodies targeting sperm and reproductive tissues. In chronic epididymitis, TLSs form in distal regions, serving as hubs for local lymphocyte activation and autoantibody production. The discovery of these ectopic lymphoid structures in the epididymis offers a new perspective on how immune system dysregulation impacts reproductive health. We identified a dynamic communication network among epithelial cells, lymphocytes, and myeloid cells. We also found multiple sperm antigens recognized by serum and epididymal fluid ASAs, providing valuable insights into their potential functional effects on sperm. Our analysis of autoantibodies in the seminal plasma of infertile patients revealed shared mechanisms with murine models, shedding light on reproductive immune disorders.
The epididymis is key for sperm maturation, and inflammation in this organ disrupts sperm function12,36. Our group showed that despite Tregs comprising only a small fraction of the epididymal cell population10, they are crucial in preventing autoimmune damage and maintaining fertility function. A similar amount of Tregs has been observed in non-lymphoid tissues under physiological conditions, including adipose tissue37 and muscle38. Improper management of epididymitis may lead to chronic inflammation and long-term complications, including infertility36. Mouse models of epididymitis typically use induced inflammation via infection, lipopolysaccharide, or immune stimulants. However, TLS formation has never been reported in any of these models13,36,39,40. Our study focused on understanding how epididymitis influences the composition and function of the B- and T cell populations. We revealed that chronic autoimmune epididymitis stimulates the expansion of specific subsets of B cells, such as GC, memory, and plasma B cells. These cells may continuously produce antibodies locally, contributing to sustained inflammation and injury. It is known that B cells undergo class switching and affinity maturation in response to prolonged antigen exposure7,8,9,31,33,41, as could be the case with damaged spermatozoa in the epididymal duct during inflammation. Interestingly, we found Breg expansion, which has immunosuppressive functions, that may counteract excessive inflammation.
Heterogeneity is a defining characteristic of epididymal segments, underscoring differences in immune responses across regions10,12,13,28,39. Immune cell diversity and soluble mediator profiles varied markedly between proximal and distal areas, and across 2 and 8 weeks following Treg depletion. The exclusive development of TLSs in distal areas highlights region-specific epididymal cues. This diversity reflects how the local niche shapes immune landscapes over time, similar to the gut42. The proximal epididymis exhibited a unique immunocyte composition and soluble mediator profile compared to the 2-week Treg-depleted distal, including robust B and T cell infiltration, and elevated pro-inflammatory mediator levels such as Chitinase3-like 1, and CXCL13. These molecules facilitate TLS formation by promoting lymphocyte recruitment and stimulating tissue remodeling and angiogenesis8,9,31,32,33,35. Also, CD40 is upregulated, which promotes GC formation and the generation of plasma and memory B cells43. The distal epididymis displayed a delayed yet intensified immune response, with a broader array of mediators and stronger TLS-associated signatures emerging prominently at 8 weeks post-DT. This spatially and temporally compartmentalized response showed concomitant upregulation of anti-inflammatory mediators, underscoring the nuanced balance between pro- and anti-inflammatory processes. These findings emphasize the importance of spatial and temporal context in understanding tissue-specific immune landscapes.
Local players—such as epithelial and resident immune cells—might drive the recruitment and activation of specific immune populations12,28,39,44,45. Even within the IS, caput, corpus, and cauda, distinct immune cell signatures are present under steady-state conditions. In this regard, the distal epididymal regions are populated by distinct pro-inflammatory MPs and innate γδ-T cells28,39,45, which may contribute to TLS induction8. We found several mediators, including IL-23, that might activate γδ-T cells46. The distal epididymis is where mature sperm are stored; thus, sperm load and antigenicity are distinct from the proximal regions47. These features may contribute to the promotion/maintenance of TLSs in the distal epididymis. They remain absent in the proximal region, despite an early increase in GC B cells. Characterizing the immunological region-specific landscape of the epididymal microenvironment is essential to advancing patient outcomes in various infertility contexts.
The TLS composition varies depending on the tissue type and the specific immune responses involved8,31. TLSs are generally classified as fully mature when GCs are present and immature in their absence8,31. Eight weeks after depletion, we observed multiple stages of TLS development in the distal epididymis. Some structures exhibited hallmarks of GC-like activity, including AID⁺, BCL6+, IgD⁺, or KI67⁺ B cells, as well as follicular DCs, HEVs, and fibroblasts. Flow cytometry further confirmed these data. At the evaluated time point (8 weeks post-DT), the T cell zones and GCs did not display the highly organized architecture typically seen in fully mature TLSs from other organs7,8,9,31,33. This variability likely reflects the dynamic nature of TLS formation and the influence of local microenvironmental factors. TLSs in the epididymis were located in perivascular regions, common sites for TLS formation8,9,33. We observed HEVs surrounding B cell clusters, elevated pro-angiogenic mediator levels, and a marked increase in circulating B cells, all indicative of enhanced TLS vascularization. PDPN-positive cells around the TLSs also suggest the presence of immunofibroblasts8,9,33. It is plausible that these structures have tissue-specific features distinct from TLSs found in other organs, further highlighting the diversity of TLSs across different anatomical locations and contexts8,31.
While TLSs have been identified in testicular tumors33,48, no data exists regarding these structures in epididymal cancer. Although testicular tumors are relatively common malignancies in young men, epididymal cancers are rare, typically arising as secondary tumors from testicular origins49. The testis (especially the Sertoli cells) can protect allografts from rejection. This phenomenon’s key contributors include Tregs, and anti-inflammatory MPs50. Further studies are needed to understand why TLSs form in the epididymis but not in the testis. Comparative analyses of TLSs induced by different conditions will help uncover shared and unique mechanisms across different reproductive tissues.
Tregs play a context-specific role in TLS formation8. In a mouse lung adenocarcinoma model, Treg absence increases T cell infiltration, particularly in TLSs51, while in pemphigus skin TLSs, Tregs drive CXCL13 production, which in turn promotes TLS development7,8,31,33. Breg’s involvement in TLS remains understudied8. Our findings suggest that CD8+cells may contribute to epididymitis-related damage, potentially causing reduced fertility52. CD8+cell prevalence also shifts in males with ASAs53. Memory-like B cell phenotypes have been identified in TLSs, with IgG1+ B cells enriched in pancreatic cancer8. Notably, IgG1, IgG2b, IgG2c, and IgM persist in the 8-week Treg-depleted epididymis, indicating ongoing local antibody production. The distinct autoantibody profiles detected may be linked to specific stages of autoimmunity progression. These autoantibodies could mediate sperm opsonophagocytosis, antibody-dependent cellular cytotoxicity, immune complex formation, and complement activation, ultimately leading to sperm lysis/removal2,3,54. These defects likely contribute to the reduced sperm counts and smaller litter sizes observed after mating, 8 weeks post-Treg depletion. Additionally, ASAs may exacerbate the condition by promoting inflammatory mediator release, further impairing sperm fertilizing ability3,55.
Sertoli cells in the testis are essential for maintaining the blood-testicular barrier56. Anti-Sertoli cell antibodies are linked to infertility57, and antibodies targeting the seminiferous tubule basement membrane are associated with autoimmune orchitis58,59. Notably, in our model, autoantibody deposition in the testis had no impact on spermatogenesis. However, epididymal damage occurs within the first day and persists for 8 weeks after depletion, priming B cell maturation, ASAs production, and TLS formation. Infertile patients, as well as most individuals who have undergone vasectomy, present ASAs3,60. ASAs are associated with reduced sperm motility and concentration, impairing the ability of sperm to penetrate cervical mucus, interact with the egg, and potentially affect early embryonic development2,3,60. The presence of IgG and IgA ASAs in the seminal plasma of patients with fertility challenges, along with ASA recognition of the human sperm, suggests shared mechanistic pathways between murine and human pathological processes.
Identifying the targets of ASAs remains a critical area of research, as doing so could lead to better diagnosis and treatment of ASA-mediated immune infertility and the development of new contraceptive methods. An IgG1 anti-sperm monoclonal antibody is currently under development as a non-hormonal contraceptive61. Several sperm antigens have been identified in ASAs from patients suffering from immune infertility3. Our work focused on pinpointing targets of ASAs and expanded the list of sperm antigens recognized by autoantibodies in the epididymal fluids and serum. ASAs recognize proteins functioning as immune modulators. IFIT1 has been detected in spermatozoa, with expression levels differing between semen samples that achieved pregnancy and those that did not62. MUG1 regulates immune responses by facilitating the internalization of viral proteins63; its role in spermatozoa is unknown. ICOSLG is essential for T cell activation and cytokine production, enabling B cell antibody secretion. The interaction between ICOSLG and its receptor is crucial for TLS formation8,9,31,33. Although ICOSLG is expressed in the male reproductive system13,28, its role in fertility has not yet been described. CCs, which are involved in immune responses induced by epididymitis, show increased Icolg expression in the distal region of the organ13,28. They may also transfer this ligand to sperm during maturation13. CLU is a crucial seminal plasma glycoprotein involved in sperm maturation and female immunotolerance64. CLU regulates complement activity. The fact that some of the sperm proteins detected by the ASAs are immune regulators may indicate that, after injury, accessible sperm antigens may be triggering signals of immune activation.
Autoimmune diseases are associated with male infertility due to localized inflammation, as seen in rheumatoid arthritis and lupus65. In the polyendocrine syndrome type 1, mutations in the AIRE gene, which maintains Treg function, are also linked to male autoimmune infertility66. AIRE-deficient male mice exhibit reduced fertility67. IPEX syndrome, caused by FOXP3 mutations, leads to Treg loss and severe autoimmunity68, but its impact on male fertility remains unclear due to patients’ reduced lifespan and quality of life. Fertility-related outcomes in these populations remain largely understudied68.
Immunotolerance breakdown in the epididymis and testis triggers chronic inflammation and local autoimmune responses, marked by autoantibody deposition, region-specific lymphocyte signatures, and soluble mediator secretion. This immune disruption impairs sperm function and drives prolonged male subfertility. Epididymal TLSs may exacerbate inflammation by driving local autoantibody production. Our study identifies key molecular mediators involved in TLS formation and highlights that unexplained male fertility disorders may be linked to immune dysregulation, characterized by pro-inflammatory cell recruitment, autoantibodies, and elevated soluble mediators. Overall, we provide an in-depth understanding of B and T cell diversity dynamics and TLS formation during epididymitis to develop precision-targeted therapies for reproductive dysfunction and chronic inflammation.
Methods
Animal procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Massachusetts General Hospital (MGH) Subcommittee on Research Animal Care (National Academies Press, 2011; protocol 2003N000216). The human study adhered to the ethical principles outlined in the Declaration of Helsinki (1975, revised in 1983) and was approved by the Medicine Research Ethics Committee of the Basque Country, Spain (protocol: PI2019184).
Mouse model
Female C57BL/6-Tg(Foxp3-DTR/EGFP)23.2Spar/Mmjax (032050-JAX, Foxp3-DTR mice) and male C57BL/6 WT mice were acquired from Jackson Laboratory and bred at the MGH animal facility (specific pathogen-free) to produce Foxp3-DTR or WT male littermates10. Experimental and control animals were housed together. Foxp3-DTR mice express the DTR fusion protein, which is controlled by the endogenous forkhead box P3 (Foxp3) promoter/enhancer regions. Male mice aged between six and forty weeks were used for all experiments. Similar to our previous studies10, six-week-old male littermates, both Foxp3-DTR and WT, received intraperitoneal injections of DT from Corynebacterium diphtheriae (D0564, Sigma-Aldrich, St. Louis, MO) dissolved in phosphate-buffered solution (PBS) at a dosage of 40 μg/kg of body weight. DT injections were administered on days 0, 2, and 4, and mice were assessed 2 and 8 weeks after the final DT injection. DT-injected WT mice served as controls. Some Foxp3-DTR and WT mice received a single injection to verify the Treg depletion after 1 day. Sex as a biological variable: This study exclusively utilized male subjects due to their direct relevance to investigating male reproductive health.
Mouse in vivo staining
The in vivo staining of circulating CD45+cells was performed following a reported protocol11. Briefly, mice were intravenously injected with anti-mouse (3 µg/100 µL saline) CD45-PE (Clone 30-F11, 103177, BioLegend, San Diego, CA) or CD45 PE-Firetm700 (Clone 30-F11, 103177, BioLegend) 10 min before euthanasia (a mixture of isoflurane (2%) and air (Baxter, Deerfield, IL)). As detailed below, tissue was collected for flow cytometry staining and fixation.
Mouse testis and epididymis collection
Mice were anesthetized using a mixture of isoflurane (2%) and air and then perfused through the left cardiac ventricle with PBS until the organs were free of blood (for ELISA and mediator arrays). Subsequently, the epididymis and testes were dissected for further analysis. For tissues fixation, perfusion continued with a 4% paraformaldehyde (PFA; 15714-S, Paraformaldehyde 32% Solution EM grade, Electron Microscopy Sciences) fixative for 7 min. Following harvesting, the organs were immersed in 4% PFA for 4 h. For in vivo CD45 staining, animals were not subjected to PBS perfusion. Instead, the organs were excised after euthanasia using a mixture of isoflurane (2%) and air.
Mouse sperm collection
Sperm samples were retrieved from the proximal (initial segments (IS) and caput) and distal (cauda) regions of the epididymis in Foxp3-DTR and WT littermates following DT injections. Sperm samples were obtained by excising the respective areas in modified Human Tubal Fluid (HTF) medium (#90126, FUJIFILM Irvine Scientific, Inc., Santa Ana, CA) enriched with 0.3% of Bovine Serum Albumin (BSA; A0281, Sigma-Aldrich). After a 15-min incubation at 37 °C, sperm were examined using CASA (computer analysis system assay) and/or fixed with 4% PFA for 10 min at room temperature.
Mouse serum collection
Blood was drawn from the left ventricle of the heart into BD Microtainer blood collection tubes (365967, Becton, Dickinson and Company, Franklin Lakes, NJ). After allowing the samples to sit at room temperature for 30 min, they were centrifuged at 4000 × g for 10 min to isolate the serum.
Mouse tissue processing
Cleaned, perfused, and frozen tissues were thawed in PBS supplemented with a protease inhibitor cocktail (#04693159001, cOmplete Mini EDTA-free Protease Inhibitor Cocktail, Roche, Basel, Switzerland). Tissues were minced with scissors, homogenized with a pestle, and incubated on ice for 30 min. The supernatant was collected after centrifuging for 20 min at 10,000 × g. Samples were either used directly for ELISA or stored at −80 °C until further use.
For cytokine/chemokine kit measurements, the same procedure was followed. Tissues (4 proximal and 4 distal epididymides from DT-treated WT and Foxp3-DTR mice) were thawed and processed in PBS with Triton ×-100 (Sigma-Aldrich) as stated in the manufacturer’s protocol (ARY028, Proteome Profiler Mouse XL Cytokine Array R&D Systems). Luminescence measurement was performed using Syngene G:BOX Mini 9). Heat maps were generated using the web-based software Morpheus (https://software.broadinstitute.org/morpheus).
Mouse epididymal fluid collection
Epididymal fluids were retrieved from the proximal (IS and caput) and distal (cauda) of the epididymis in Foxp3-DTR and WT post-DT. Fluids were obtained by excising the respective areas in PBS. After a 15-min incubation at 37 °C, tissues were discarded, and samples were centrifuged for 5 min at 500 × g to discard sperm. Supernatants were stored at −80 °C until use.
Confocal microscopy
Similar to previous studies10,44, the fixed organs were preserved in 30% sucrose in PBS (with 0.02% NaAzide) for at least 48 h at 4 °C and then embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA). Tissue sections, either 5 or 25 µm in width, were prepared using a Reichert Frigocut microtome and mounted onto Fisherbrand Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Alternatively, sperm samples were fixed for 10 min in 2% PFA at room temperature and then washed twice with PBS before being smeared onto slides.
Primary antibodies utilized were rat anti-mouse-F4/8010, chicken anti-V-ATPase B1 subunit10, rabbit anti-AQP910, goat anti-mouse-IgA (1 mg/mL, 62-6720, Invitrogen), goat anti-mouse-IgG1 (1 mg/mL, A10551, Invitrogen), rat anti-mouse/human PNAD (0.5 mg/mL, 120801, BioLegend), Syrian hamster anti-mouse PDPN (0.5 mg/mL, ab11936, Abcam, Waltham, MA), goat anti-mouse-IgG2b (1 mg/mL, M32407, Invitrogen, Waltham, MA), goat anti-mouse-IgG2c (0.8 mg/mL, 115-035-208, Jackson ImmunoResearch, West Grove, PA), goat anti-mouse-IgG3 (M32707, Invitrogen), donkey anti-mouse-IgM (0.8 mg/mL, 715-035-020, Jackson ImmunoResearch), rabbit anti-mouse-CD19 (0.2 mg/mL: sc-8500-R, Santa Cruz), rat anti-mouse-CD21/CD35 (0.5 mg/mL, 14-0211-81, Invitrogen), Armenian Hamster anti-mouse-CD3 (0.5 mg/mL, 100303, BioLegend), rat anti-human/mouse-AID (0.5 mg/mL, 14-5959-80, Invitrogen), rabbit anti-alpha smooth muscle actin (α-SMA, 0.2 mg/mL, ab5694, Abcam), rabbit anti-mouse-CD31 (ab28364, Abcam), rabbit anti-Ki6769 (0.031 mg/mL, MA5-14520, Thermofisher Scientific), rat anti-mouse-BLC6 (25ug/mL, 358512, BioLegend), rat anti-mouse-IgD (0.5 mg/mL, 405702, BioLegend), goat anti-mouse-CD20 (0.5 mg/mL, sc-7735, Santa Cruz), and donkey anti-human-IgG (1.5 mg/mL, 709-545-149, Jackson ImmunoResearch). The secondary antibodies used were Alexa Fluor 488 goat anti-mouse IgG (20 μg/ml; A-11001, Invitrogen), Alexa Fluor 647 donkey anti-rat IgG (3 μg/ml; 712-606-150, Jackson ImmunoResearch), Alexa Fluor 488 donkey anti-mouse IgG (20 μg/ml; A21202, Invitrogen), Alexa Fluor 488 donkey anti-chicken IgY (15 μg/ml; 703-545-155, Jackson ImmunoResearch), Alexa Fluor 647 donkey anti-rabbit IgG (1.5 μg/ml; 711-606-152, Jackson ImmunoResearch), Alexa Fluor 488 goat anti-rabbit IgG (1:800 dilution; 111-545-144, Jackson ImmunoResearch), Alexa Fluor 488 donkey anti-goat IgG (1:100 dilution; 705-545-147, Jackson ImmunoResearch), Cy3 donkey anti-rat IgG (1:800 dilution; 712-166-153, Jackson ImmunoResearch), Cy3 donkey anti-goat IgG (1:800 dilution; 705-165-1470, Jackson ImmunoResearch), DyLight 649 goat anti-hamster (STAR104D649, AbD Serotec), FITC donkey anti-human IgG (15 μg/ml; 709-545-149, Jackson ImmunoResearch), Cy3 donkey anti-rabbit IgG (7.5 μg/ml; 711-165-152, Jackson ImmunoResearch), and DyLight 405 Donkey anti-rat IgG (1.4 mg/ml; 712-475-150, Jackson ImmunoResearch).
Different antigen retrieval methods were employed based on the antibody used: a 4-min treatment with PBS containing 1% SDS (for V-ATPase, CD19, CD20, CD3, PNAD, PDPN, IgD, and AQP9 antibodies), or PBS containing 1% SDS and 0.1% Triton (for F4/80, IgG, and IgA antibodies). Slides were then blocked for 30 min at room temperature in PBS containing 1% BSA and incubated with primary antibodies overnight at 4 °C. All antibodies were diluted in DAKO medium (DAKO, Carpinteria, CA). Mounted slides were treated with SlowFade Diamond Antifade Mounting medium (Thermo Fisher Scientific, S36963) containing the DNA marker DAPI. Negative control slides underwent incubation with secondary antibodies alone for immunostaining. Positive controls on lymph nodes are shown in Supplementary Fig. 25.
H&E evaluation
Sections were stained with Hematoxylin Solution, Harris Modified (HHS32, Sigma-Aldrich), and subsequently counterstained with Eosin Y Solution (HT110132, Sigma-Aldrich). The stained slides were digitally scanned using a NanoZoomer 2.0RS digital scanner (Hamamatsu, Japan).
To assess spermatogenesis, we used the NDP.View2 software, which allows high-resolution zooming to single-cell detail. The quantification of spermatogenesis is based on the classification of germ cells according to their cellular and nuclear morphology, as well as their specific location within the seminiferous tubules. This includes distinguishing spermatogonia, primary and secondary spermatocytes, round and elongated spermatids, and spermatozoa70. The arrangement and proportion of these cells at different stages of the seminiferous epithelium cycle are also evaluated to assess the efficiency and completeness of spermatogenesis.
Flow cytometry analysis
The proximal and distal segments of the epididymis and testis were mechanically minced and enzymatically digested for 30 min at 37 °C in RPMI 1640 medium containing collagenase type I (0.5 mg/mL) and collagenase type II (0.5 mg/mL), following established protocols10. The resulting suspensions were filtered through a 70 μm nylon mesh strainer, rinsed with 2% fetal bovine serum (FBS) in PBS with 2 mM EDTA, and centrifuged for 5 min at 400 × g. The pellet was treated with ACK lysing buffer (#A10492-01, Gibco, Grand Island, NY) for 1 min, followed by centrifugation for 5 min at 400 × g. Cell suspensions with FcR II and III blocker (TruStain FcX™ (anti-mouse CD16/32), 101320, BioLegend) and BD Horizon™ Brilliant Stain Buffer, 563794 (BD Biosciences) were then incubated for 30 min with anti-mouse antibodies (1:500 dilution) against CD45 Brilliant Violet 711 (0.2 mg/ml; Clone 30-F11, 563709, BD Biosciences, San Jose, CA), CD45 PE-Fire700 (0.2 mg/ml; Clone 30-F11, 103177, BioLegend), CD45 PE (0.5 mg/ml; Clone 30-F11, 103105, BioLegend), CD45 BUV395 (0.2 mg/ml; Clone 30-F11; 565967, BD Biosciences), Ly6G PE (0.2 mg/ml; Clone 1A8, 551461, BD Biosciences), F4/80 PE/Cyanine7 (0.2 mg/ml; Clone BM8, 123113, BioLegend), Ly6C FITC (0.5 mg/ml; Clone AL-21; 553104, BD Biosciences), MHC Class II (I-A/I-E) Alexa Fluor 700 (0.2 mg/ml; Clone M5/114.15.2, 56-5321-82, Thermo Fisher Scientific), CD4 PE (0.2 mg/ml; Clone RM4-5, 100511, BioLegend), CD4 BV480 (0.2 mg/ml; Clone RM4-5; 565634, BD Biosciences), CD44 BUV737 (0.2 mg/ml; Clone IM7; 612799, BD Biosciences), CD11b APC/Cyanine7 (0.2 mg/ml; Clone M1/70, 101225, BioLegend), CD1d BUV805 (0.2 mg/ml; Clone IB1; 741965, BD Biosciences), CD19 BV750 (0.2 mg/ml; Clone 1D3; 747332, BD Biosciences), IgD BUV615 (0.2 mg/ml; Clone 217-170, 751474, BD Biosciences), CD138 BV510 (0.2 mg/ml; Clone 281-2, 563192, BD Biosciences), CD3 BV421 (0.2 mg/ml; Clone 17A2, 100227, BioLegend), CD20 PE (0.2 mg/ml; Clone SA275A11, 150409, BioLegend), B220 Pacific Blue (0.5 mg/ml; Clone RA3-6B2, 103230, BioLegend), FAS APC/Fire810 (0.2 mg/ml; Clone SA367H8, 152623, BioLegend), CD5 Alexa Fluor594 (0.5 mg/ml; Clone 53-7.3, 100632, BioLegend), GL7 PerCP/Cyanine5.5 (0.2 mg/ml; Clone GL7, 144609, BioLegend), CD8 FITC (0.5 mg/ml; Clone 53-6.7, 100706, BioLegend), CXCR5 BV605 (0.1 mg/ml; Clone L138D7, 145513, BioLegend), CD62L PE/Cyanine5 (0.2 mg/ml; Clone MEL-14, 104410, BioLegend), CD21/CD35 Alexa fluor647 (0.5 mg/ml; Clone 7E9, 123423, BioLegend), CD38 Alexa Fluor 700 (0.5 mg/ml; Clone 90, 102741, BioLegend), PD-1 APC/Cyanine7 (0.2 mg/ml; Clone 29 F.1A12, 135223, BioLegend), CD23 PerCP (0.1 mg/ml; Clone #011, 50695-R011, SinoBiological Inc.), CD27 PerCP-eFluor710 (0.2 mg/ml; Clone LG.7F9, 46-0271-80, Invitrogen), diluted in 2% FBS in PBS (with BD Horizon Brilliant Stain buffer (BD Biosciences). DAPI (62248, ThermoScientific), LIVE/DEAD Fixable Blue Dead Cell Stain Kit, for UV excitation (L23105, Invitrogen), or LIVE/DEAD Fixable Yellow Dead Cell Stain Kit, for 405 nm excitation (L34967, Invitrogen) were used as viability markers.
For intranuclear staining of the Foxp3 and intracellular staining of IgM and IgG, suspensions were incubated for 20 min with 1× Fixation/Permeabilization working solution and washed with 1× Permeabilization Buffer, following the manufacturer’s instructions (#00-5523-00, Thermo Fisher Scientific). Fixed cells were then incubated overnight with 1/100 dilution of anti-Foxp3-APC (0.2 mg/ml; Clone FJK-16s, 17-5773-82, ThermoFisher Scientific), IgM BV786 (0.2 mg/ml; Clone R6-60.2, 564028, BD Biosciences) IgG PE/Cyanine7 (0.2 mg/ml; Clone Poly4053, 405315, BioLegend) diluted in 1× Permeabilization Buffer (00-5523-00, Thermo Fisher Scientific) containing BD Horizon Brilliant Stain buffer (563794, BD Biosciences). After incubation, cells were washed with 2% FBS in PBS and filtered through a 40 μm cell strainer.
Flow cytometry data were collected using the BD FACSAria II flow cytometer (BD Biosciences), BD LSRFortessa ×-20 Cell Analyzer (BD Biosciences), or AURORA flow cytometer (Cytek Aurora System) and interpreted using FlowJo software version 10.8.1 (BD Biosciences). Negative (unstained cells from each organ) and fluorescence minus one controls were conducted to establish gating strategies, following established procedures. The gating strategies used are illustrated in the corresponding Figures and Supplementary Figs. 26 and 27.
t-distributed Stochastic Neighbor Embedding (t-SNE) and FlowSOM analysis were applied to flow data, enabling detailed characterization of cell populations. t-SNE was used for dimensionality reduction to visualize high-dimensional flow cytometry data projected into a lower-dimensional space, and FlowSOM self-organizing mapping clusters cells based on their phenotypic similarities, for predicting distinct cell populations.
Enzyme-linked immunosorbent assay (ELISA)
Proteins were extracted from the testis, epididymis, and sperm of WT mice and healthy human sperm donors, using a buffer consisting of RIPA buffer (R26200-125.0, RPI Research Products International, Mt. Prospect, IL) supplemented with a protease inhibitor cocktail (#04693159001, cOmpleteTM Mini EDTA-free Protease Inhibitor Cocktail, Roche, Basel, Switzerland) and a phosphatase inhibitor cocktail (#4906837001, PhosSTOP, Roche) for 20 min at 4 °C. Subsequently, the samples were centrifuged at 10,000 × g for 10 min, and the resulting supernatants containing soluble proteins were collected. 96-well plates were coated with 6 µg/mL tissue/cell proteins (testicular, epididymal, sperm) in PBS, and left overnight at 4 °C. The plates were then blocked with 3% BSA in PBS for 1 h at 37 °C before incubating with mouse serum diluted 1/25 (and 1/25-1/25600 serial dilutions for the titration experiments), tissue homogenates diluted 1/3, epididymal fluids diluted ½ or human seminal fluid diluted 1/5 in 1% BSA in PBS for 2 h at 37 °C. The different isotypes bound to the antigen-coated wells were detected following a 1-h incubation with horseradish peroxidase (HRP)-anti-mouse IgG (1:5000; 115-035-205, Jackson ImmunoResearch), IgA (1:3000; 62-6720, Invitrogen), IgG1 (1:2000; A10551, Invitrogen), IgG2b (1:5000; M32407, Invitrogen), IgG2c (1:5000; 115-035-208, Jackson ImmunoResearch), IgG3 (1:2000; M32707, Invitrogen), IgM (1:5000; 715-035-020, Jackson ImmunoResearch) and human totIgG (1:10000, 709-035-149, Jackson ImmunoResearch) and IgA (1:10000; 109-035-011, Jackson ImmunoResearch). The enzymatic reaction was initiated with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (TMB substrate kit, 34021, Thermo Fisher Scientific) and stopped with H2SO4 2 N. Optical density readings were measured using a microplate reader (Promega GloMax Discover, Madison, WI) at 450 nm.
Computer assisted sperm analysis (CASA)
Sperm were harvested from the proximal/distal epididymis as described above. After a 15-min incubation at 37 °C, proximal/distal sperm were collected and analyzed. Alternatively, distal sperm were obtained and then diluted (1:5 ratio) in HTF medium with 0.3% BSA, followed by a 60-min incubation at 37 °C to induce sperm capacitation. Sperm analysis was conducted using Hamilton Thorne’s CASA version 14 (Hamilton Thorne Inc., Beverly, MA). Each sample underwent a minimum of two analyses.
Assessment of fertility
Male littermates of both Foxp3-DTR and WT mice (8-week post-DT injections) were singly housed with a WT adult female for 5 days. The presence of a copulatory plug confirmed successful mating. Fertility assessment was determined based on litter size, representing the number of pups per litter.
Immunoprecipitation using anti-sperm antibodies
Serum and proximal and distal epididymal fluids were collected from WT and Foxp3-DTR mice 2 weeks following DT treatment. We pooled at least three samples per condition (3 serum, 6 proximal, and 6 distal epididymal fluids from WT and Foxp3-DTR) and performed two independent experiments. These samples were incubated with Dynabeads Protein G, washed, and then incubated with protein extracts (as previously described) derived from proximal or distal sperm cells of WT naïve mice, following the manufacturer’s protocol (Immunoprecipitation Kit—Dynabeads Protein G, 10006D, Invitrogen). Eluates were run on a 10% bis-acrylamide gel (NuPAGE 10% Bis-Tris Gel, NP0302BOX, Invitrogen), then stained with Coomassie dye R-250 (Imperial Protein Stain, 24615, Thermo Scientific), bands were cut and submitted to the Taplin Biological Mass Spectrometry Facility, Harvard Medical School, Building C, Room 523, 240 Longwood Ave. Boston, MA 02115, similar to previously reported10. Results are available in the Supplementary Data 1–3.
Proteomic data analysis
Proteomic datasets from each experimental group were compared using the VENNY 2.1 Venn Diagram online tool (https://bioinfogp.cnb.csic.es/tools/venny/). Protein abundance was quantified by summing intensity values, calculated as the sum of intensities of all peaks from the same protein. The proteins shown in Fig. 3 are those identified using the Foxp3-DTR samples with a twofold or greater increase compared to the WT samples. Supplementary Data 3 lists these proteins and the immunoglobulins detected in the serum and epididymal fluid from the Foxp3-DTR samples.
Human sample collection
Human semen samples were obtained from patients undergoing routine semen analysis at the assisted reproduction unit of the Cruces and Galdakao University Hospital of Bizkaia (Spain). Ejaculates were collected by masturbation after 3–5 days of sexual abstinence. All patients provided signed informed consent to the Declaration of Helsinki. Semen parameters and the presence of round cells (RC) were evaluated using the Sperm Class Analyzer CASA System (Microptics). After semen analysis, all samples were classified as normozoospermic or asthenozoospermic according to the World Health Organization guidelines1. Samples with RC levels ≥1 M/mL were classified into the “RC” group, while those with RC concentrations <1 M/mL were placed in the “no RC” group. Supplementary Tables 1 and 2 provide the descriptive parameters of the semen samples used in this study. Our study focuses on the analysis of semen samples, which are biologically specific to male individuals. As a result, only participants who were assigned male at birth and confirmed their ability to provide semen samples were included in the study. A limitation of our human study is the lack of additional clinical data regarding participants’ histories of epididymal or other urogenital infections. Furthermore, microbial cultures or molecular diagnostic tests were not performed to assess the presence of subclinical infections.
Human sample processing
Human semen samples were centrifuged at 300 × g for 15 min to separate the seminal plasma and sperm fraction. Then, isolated sperm cells were washed with 2 mL of PBS. Finally, sperm pellets and seminal plasma fractions were stored at −80 °C until usage.
Statistical analysis
Data analysis was conducted using GraphPad Prism 10 version 10.3.1 (464) (GraphPad Software, La Jolla, CA). The Shapiro–Wilk test was employed to assess normality. Analysis of variance using the F test was employed to compare the two groups. Parametric tests, including Student’s t-test (two-tailed), were applied. For non-parametric analyses, the Mann–Whitney test (two-tailed) was used. Statistical significance was set at p-values < 0.05. Data were presented as means ± SEM.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data are included in the Supplementary Information or available from the authors, as are unique reagents used in this Article. The raw numbers for charts and graphs are available in the Source Data file whenever possible. Further requests should be addressed to the corresponding author. The proteomic data generated is deposited in the ProteomeXchange Consortium (https://www.ebi.ac.uk/pride/archive/projects/PXD058247; https://www.ebi.ac.uk/pride/archive/projects/PXD062944) via the PRIDE partner repository with the dataset identifiers PXD058247 and PXD062944. Source data are provided with this paper.
References
World Health Organization. Infertility Prevalence Estimates, 1990–2021 (World Health Organization, 2023).
Chen, Y., Hasegawa, A., Wakimoto, Y. & Shibahara, H. Update on the research on the antigens of anti-sperm antibodies over the last decade. J. Reprod. Immunol. 164, 104292 (2024).
Mukherjee, A. G. & Gopalakrishnan, A. V. Anti-sperm antibodies as an increasing threat to male fertility: immunological insights, diagnostic and therapeutic strategies. Reprod. Sci. 31, 3303–3322 (2024).
Gupta, S. et al. Antisperm antibody testing: a comprehensive review of its role in the management of immunological male infertility and results of a global survey of clinical practices. World J. Mens. Health 40, 380–398 (2022).
Tung, K. S. et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Invest. 127, 1046–1060 (2017).
Wheeler, K. et al. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc. Natl. Acad. Sci. USA 108, 7511–7516 (2011).
Pipi, E. et al. Tertiary lymphoid structures: autoimmunity goes local. Front Immunol. 9, 1952 (2018).
Zhao, L. et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Signal Transduct. Target Ther. 9, 225 (2024).
Sato, Y., Silina, K., van den Broek, M., Hirahara, K. & Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 19, 525–537 (2023).
Barrachina, F. et al. Regulatory T cells play a crucial role in maintaining sperm tolerance and male fertility. Proc. Natl. Acad. Sci. USA 120, e2306797120 (2023).
Anderson, K. G. et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat. Protoc. 9, 209–222 (2014).
Battistone, M. A. et al. Immunoregulatory mechanisms between epithelial clear cells and mononuclear phagocytes in the epididymis. Andrology 12, 949–963 (2024).
Battiston, M. A. et al. Novel role of proton-secreting epithelial cells in sperm maturation and mucosal immunity. J. Cell Sci. 133, jcs233239 (2019).
Da Silva, A. A. S. et al. Proton-secreting cells as drivers of inflammation and sperm dysfunction in LPS-induced epididymiti. Function 6, zqaf023 (2025).
Cao, X. et al. Proteomic profile of human spermatozoa in healthy and asthenozoospermic individuals. Reprod. Biol. Endocrinol. 16, 16 (2018).
Tumova, L., Zigo, M., Sutovsky, P., Sedmikova, M. & Postlerova, P. Ligands and receptors involved in the sperm-zona pellucida interactions in mammals. Cells 10, 133 (2021).
Bae, J. W., Hwang, J. M. & Kwon, W. S. Prediction of male fertility using Ras-related proteins. J. Anim. Sci. Technol. 64, 1024–1034 (2022).
Andrews, R. E., Galileo, D. S. & Martin-DeLeon, P. A. Plasma membrane Ca2+-ATPase 4: interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase. Mol. Hum. Reprod. 21, 832–843 (2015).
Kim, S. et al. NUCB2/nesfatin-1 suppresses the acrosome reaction in sperm within the mouse epididymis. Anim. Cells Syst. 27, 120–128 (2023).
Leclerc, P. et al. Study on the role of calmodulin in sperm function through the enrichment and identification of calmodulin-binding proteins in bovine ejaculated spermatozoa. J. Cell Physiol. 235, 5340–5352 (2020).
Shiraishi, K. et al. Roles of histone H3.5 in human spermatogenesis and spermatogenic disorders. Andrology 6, 158–165 (2018).
Wanigasuriya, I. et al. Smchd1 is a maternal effect gene required for genomic imprinting. Elife 9, e55529 (2020).
Wilkerson, D. C. & Sarge, K. D. RNA polymerase II interacts with the Hspa1b promoter in mouse epididymal spermatozoa. Reproduction 137, 923–929 (2009).
Huang, Y., Jiang, Z., Gao, X., Luo, P. & Jiang, X. ARMC subfamily: structures, functions, evolutions, interactions, and diseases. Front. Mol. Biosci. 8, 791597 (2021).
Ren, D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 72, 899–911 (2011).
Chung, J. J., Navarro, B., Krapivinsky, G., Krapivinsky, L. & Clapham, D. E. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2, 153 (2011).
Harris, C. L., Mizuno, M. & Morgan, B. P. Complement and complement regulators in the male reproductive system. Mol. Immunol. 43, 57–67 (2006).
Battistone, M. A. et al. Region-specific transcriptomic and functional signatures of mononuclear phagocytes in the epididymis. Mol. Hum. Reprod. 26, 14–29 (2020).
Hatzi, K. & Melnick, A. Breaking bad in the germinal center: how deregulation of BCL6 contributes to lymphomagenesis. Trends Mol. Med. 20, 343–352 (2014).
Mechtcheriakova, D., Svoboda, M., Meshcheryakova, A. & Jensen-Jarolim, E. Activation-induced cytidine deaminase (AID) linking immunity, chronic inflammation, and cancer. Cancer Immunol. Immunother. 61, 1591–1598 (2012).
Gago da Graca, C., van Baarsen, L. G. M. & Mebius, R. E. Tertiary lymphoid structures: diversity in their development, composition, and role. J. Immunol. 206, 273–281 (2021).
Nayar, S. et al. Immunofibroblasts regulate LTalpha3 expression in tertiary lymphoid structures in a pathway dependent on ICOS/ICOSL interaction. Commun. Biol. 5, 413 (2022).
Esparcia-Pinedo, L., Romero-Laorden, N. & Alfranca, A. Tertiary lymphoid structures and B lymphocytes: a promising therapeutic strategy to fight cancer. Front. Immunol. 14, 1231315 (2023).
Berasain, C. & Avila, M. A. Amphiregulin. Semin. Cell Dev. Biol. 28, 31–41 (2014).
Zhao, T., Su, Z., Li, Y., Zhang, X. & You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target Ther. 5, 201 (2020).
Fijak, M. et al. Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice?. Hum. Reprod. Update 24, 416–441 (2018).
Spallanzani, R. G. et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 4, eaaw3658 (2019).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Pleuger, C. et al. The regional distribution of resident immune cells shapes distinct immunological environments along the murine epididymis. Elife 11, e82193 (2022).
Andrade, A. D. et al. Lipopolysaccharide-induced epididymitis modifies the transcriptional profile of Wfdc genes in mice†. Biol. Reprod. 104, 144–158 (2021).
Bod, L. Finding the right friends: stromal composition influences TLS formation in ovarian cancer. Cancer Cell 42, 1811–1812 (2024).
Hickey, J. W. et al. Organization of the human intestine at single-cell resolution. Nature 619, 572–584 (2023).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Barrachina, F. et al. CX3CR1 deficiency leads to impairment of immune surveillance in the epididymis. Cell Mol. Life Sci. 80, 15 (2022).
Mendelsohn, A. C. et al. From initial segment to cauda: a regional characterization of mouse epididymal CD11c(+) mononuclear phagocytes based on immune phenotype and function. Am. J. Physiol. Cell Physiol. 319, C997–C1010 (2020).
Hu, Y. et al. gammadelta T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct. Target Ther. 8, 434 (2023).
Skerrett-Byrne, D. A. et al. Global profiling of the proteomic changes associated with the post-testicular maturation of mouse spermatozoa. Cell Rep. 41, 111655 (2022).
Sakai, Y., Hoshino, H., Kitazawa, R. & Kobayashi, M. High endothelial venule-like vessels and lymphocyte recruitment in testicular seminoma. Andrology 2, 282–289 (2014).
Almohaya, N., Almansori, M., Sammour, M., Ajjaj, A. B. & Yacoubi, M. T. Leiomyoadenomatoid tumors: a type of rare benign epididymal tumor. Urol. Case Rep. 38, 101700 (2021).
Washburn, R. L., Hibler, T., Kaur, G. & Dufour, J. M. Sertoli cell immune regulation: a double-edged sword. Front. Immunol. 13, 913502 (2022).
Joshi, N. S. et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43, 579–590 (2015).
Guiton, R., Voisin, A., Henry-Berger, J., Saez, F. & Drevet, J. R. Of vessels and cells: the spatial organization of the epididymal immune system. Andrology 7, 712–718 (2019).
Marshburn, P. B. & Kutteh, W. H. The role of antisperm antibodies in infertility. Fertil. Steril. 61, 799–811 (1994).
Pisetsky, D. S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 19, 509–524 (2023).
Lombardo, F., Gandini, L., Dondero, F. & Lenzi, A. Antisperm immunity in natural and assisted reproduction. Hum. Reprod. Update 7, 450–456 (2001).
Washburn, R. L. & Dufour, J. M. Regulation of complement by Sertoli cells may contribute to the immune protective environment within the blood-testis barrier. Tissue Barriers 12, 2233385 (2024).
Wall, J. R., Stedronska, J. & Lessof, M. H. Antibodies against Sertoli cells in human infertility. Clin. Endocrinol.3, 187–194 (1974).
Qu, N. et al. Immunological microenvironment in the testis. Reprod. Med. Biol. 19, 24–31 (2020).
Siu, M. K. & Cheng, C. Y. Extracellular matrix and its role in spermatogenesis. Adv. Exp. Med. Biol. 636, 74–91 (2008).
Restrepo, B. & Cardona-Maya, W. Antisperm antibodies and fertility association. Actas Urol. Esp. 37, 571–578 (2013).
Nador, E. et al. Platform-specific Fc N-glycan profiles of an antisperm antibody. Antibodies (Basel) 13, 17 (2024).
Garcia-Herrero, S. et al. The transcriptome of spermatozoa used in homologous intrauterine insemination varies considerably between samples that achieve pregnancy and those that do not. Fertil. Steril. 94, 1360–1373 (2010).
Krause, K., Azouz, F., Nakano, E., Nerurkar, V. R. & Kumar, M. Deletion of pregnancy zone protein and murinoglobulin-1 restricts the pathogenesis of West Nile virus infection in mice. Front. Microbiol 10, 259 (2019).
Janiszewska, E. & Kratz, E. M. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker?. Mol. Reprod. Dev. 87, 515–524 (2020).
Chereshnev, V. A. et al. Pathogenesis of autoimmune male infertility: juxtacrine, paracrine, and endocrine dysregulation. Pathophysiology 28, 471–488 (2021).
Kekalainen, E. et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Immunol. 178, 1208–1215 (2007).
Warren, B. D. et al. Multiple lesions contribute to infertility in males lacking autoimmune regulator. Am. J. Pathol. 191, 1592–1609 (2021).
Bacchetta, R. & Roncarolo, M. G. IPEX syndrome from diagnosis to cure, learning along the way. J. Allergy Clin. Immunol. 153, 595–605 (2024).
Battistone, M. A. et al. Proinflammatory P2Y14 receptor inhibition protects against ischemic acute kidney injury in mice. J. Clin. Invest. 130, 3734–3749 (2020).
Meistrich, M. L. & Hess, R. A. Assessment of spermatogenesis through staging of seminiferous tubules. Methods Mol. Biol. 927, 299–307 (2013).
Acknowledgements
The authors thank the Microscopy Core of the Program in Membrane Biology (PMB) (MGH, Boston, MA) and the MGB Molecular Imaging Core (MGH, Boston, MA), the Taplin Mass Spectrometry Facility (Cell Biology Department, Harvard Medical School, Boston, MA). The authors thank Dr. Lloyd Bod for his valuable insights on B cell function. This work was supported by the National Institutes of Health (grant HD104672 to M.A.B.), the MGH Physician/Scientist Development and Claflin Distinguished Scholar Awards (to M.A.B), and the Lalor Foundation (to F.B. and M.L.E), the IBSA Foundation for Scientific Research (to F.B.), and the Ministry of Science and Innovation, Spain, grant numbers PID2020-119949RB-100 and TED2021-132681B-I00, CPP2021-008458, co-founded by the European Union and by Instituto de Salud Carlos III, co-funded by European Union (ERDF/ESF, “Investing in your future”, grant number PI20/01131) to N.S.
Author information
Authors and Affiliations
Contributions
M.L.E., F.B. and M.A.B. were involved in the study design and conceptualization. M.L.E., F.B., K.O., M.C.A., I.B., A.O., V.D.R., O.U. and A.C. performed the experiments and data analysis. M.L.E., F.B., M.C.A., N.S. and M.A.B. were involved in data interpretation. M.L.E. and M.A.B. wrote the original manuscript. All authors contributed to the writing of the manuscript, made critical comments, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Kristin Fenton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Elizagaray, M.L., Barrachina, F., Avenatti, M.C. et al. Chronic inflammation drives epididymal tertiary lymphoid structure formation and autoimmune fertility disorders in mice. Nat Commun 16, 8742 (2025). https://doi.org/10.1038/s41467-025-63514-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63514-y