Abstract
The molecular carbon allotropes have an enduring attraction to chemists and physicists for their elusive structures and extraordinary properties. Cyclo[16]carbon has been produced on the surface and is well characterized, while it is interesting that molecular carbon allotrope, like C16, referring to molecules composed of 16 carbon atoms, presents a fascinating realm of isomeric possibilities. Except for cyclo[16]carbon, C16 isomers with other structures have been predicted only by theory. Here, we report the synthesis and structural characterization of a graphene-shaped isomer, i.e., C16 flake on a bilayer NaCl surface grown on Au(111), using an atom-manipulation strategy by eliminating chlorine from a fully chlorinated pyrene molecule, C16Cl10. The sp- and sp2-hybridized structure of C16 flake is well characterized by bond-resolved atomic force microscopy. Theoretical calculations reveal an open-shell singlet ground state of C16 flake.
Similar content being viewed by others
Introduction
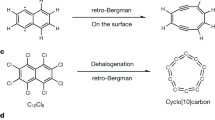
The diverse carbon allotropes have long captivated the curiosity of scientists with their enigmatic structures and remarkable properties. The discovery of fullerenes1, carbon nanotubes2,3, and graphene4 opened a new frontier in synthetic carbon allotropes, unlocking potential for novel materials and applications and spurring the exploration of unconventional carbon-based structures5. Lately, unconventional synthetic strategies like dynamic covalent chemistry and on-surface synthesis have been employed to produce new forms of carbon, such as linear carbons6,7,8, fullerene networks9,10, biphenylene networks11, and cyclocarbons12,13,14,15,16,17,18. Within the realm of molecular carbon allotropes, e.g., C16, representing molecules composed of 16 carbon atoms, has emerged as an intriguing area of investigation19,20,21. Many hypothetical isomers of C16 have been discussed19, including chains, bicyclic rings, flake, cage, bowl, and ring structures (cf. Fig. 1), but only the ring-shaped cyclo[16]carbon has been synthesized and well-characterized on the surface very recently14. C16 isomers of other shapes have been predicted to be less stable19, posing challenges for their synthesis and characterization.
The sp2-hybridized fullerenes and the sp-hybridized cyclocarbons are the only two kinds of molecular carbon allotropes that have been isolated1,12. The graphene-shaped C16 isomer, i.e., C16 flake, however, is a new type of molecular carbon allotrope containing both sp- and sp2-hybridized carbon atoms (Fig. 1). The distinct sp-sp2-hybridized structure of the C16 flake is highly appealing for both theoretical and experimental investigations into its intrinsic structure (see Supplementary Fig. 1 for possible resonance structures). Thus, it would be of great interest to experimentally characterize this specific carbon allotrope.
On-surface synthesis is emerging as a promising approach for atomically precise characterization of highly reactive carbon allotropes that could be hardly synthesized via conventional solution chemistry11,12,22. Developments in scanning tunneling microscopy (STM) and atomic force microscopy (AFM) have enabled the synthesis and in situ characterization of a single molecule with unprecedented resolution at the atomic scale and chemical-bond level23,24, and single-molecule reactions can be further triggered by atom manipulation25,26,27,28. Herein, we report the synthesis and characterization of a graphene-shaped molecular carbon allotrope, C16 flake, by using tip-induced dehalogenation of Perchloropyrene (C16Cl10) precursor on the bilayer NaCl/Au(111) surface at 4.7 K. A low-temperature STM-AFM was used to sequentially remove Cl atoms from the precursor C16Cl10 by atom manipulation (Fig. 2). The structure of the C16 flake was revealed by bond-resolved AFM with defined positions of triple bonds, and the electronic and magnetic properties of the C16 flake were investigated by theoretical calculations. Moreover, we also demonstrated that chlorine migration and skeleton isomerization inside a single molecule could be induced by atom manipulation.
Results
On-surface synthesis and characterization
The C16Cl10 precursor was synthesized in solution through a one-step sequence as shown in Fig. 2 (see Methods for synthetic details) and then deposited onto a Au(111) single-crystal surface partially covered with bilayer NaCl held at approximately 6 K. On-surface synthesis and characterization by STM and AFM with CO-functionalized tip23 were performed at 4.7 K. Figure 3b shows an AFM image of a precursor, and only two Cl atoms are imaged as two bright features, implying a nonplanar adsorption configuration, which is caused by the steric hindrance of Cl atoms in such a highly strained molecule (also see Supplementary Fig. 2). This is further confirmed by AFM simulation (Fig. 3c).
a–d Precursor. e–t The most frequently observed reaction intermediates. u–x C16 flake. Molecular structures are shown in line 1. AFM images (line 2) were recorded with a CO-functionalized tip, with respect to an STM set point of I = 3 pA, V = 0.3 V above the NaCl surface. Lines 3 and 4 show corresponding simulated and Laplace-filtered AFM images. The bright features in the right part of (l, x) correspond to detached Cl adatoms. The scale bars in (b) and (v) apply to experimental and simulated AFM images in columns 1–5 and column 6, respectively.
To remove Cl atoms from the molecule, the tip was initially positioned on a single molecule, and retracted by about 4 Å from the STM set point (I = 3 pA, V = 0.3 V), and the sample bias then gradually increased from 0.3 V to 4 V. This process typically resulted in yielding C16Cl5, C16Cl4 or C16Cl3 intermediates (Fig. 3e–l, and Supplementary Fig. 3a–f). For further dehalogenation, larger bias voltages were required, typically about 4.2–4.4 V. Voltage pulses were applied for a short time (500 ms) at constant tip height on one specific Cl atom of the C16Cl3 intermediate. This process dissociates the remaining Cl atoms one by one, resulting in C16Cl2 and C16Cl intermediates (Fig. 3m–t, and Supplementary Fig. 3g–i). The structures of these intermediates were structurally characterized by AFM imaging and further supported by AFM simulations (Fig. 3e–t). We speculate that the tip-induced dehalogenations are related to the anionic charge states of molecules and the applied electric field29,30. In addition, inelastic electron tunneling may also contribute to initiating the dissociation process12,22.
Further voltage sweeping at 4.5 V induced complete dehalogenation of the intermediates and generated the final product, C16 (Fig. 3u–x). The carbon backbone of the product was clearly resolved by AFM (Fig. 3v), exhibiting a graphene-shaped flake. We assigned this molecule as a new isomer of C16, called C16 flake. Notably, two distinct bright features are observed on both the upper and lower sides of the flake in the AFM images (Fig. 3v, x), corresponding to two triple bonds24,31,32. The AFM contrast provided evidence for the molecular structure of the C16 flake with the defined positions of triple bonds supported by AFM simulation (Fig. 3w). DFT calculations of different adsorption configurations of the C16 flake on a bilayer NaCl are shown in Supplementary Fig. 4. The yield for the on-surface synthesis of the C16 flake was approximately 23% (other C16 flakes see Supplementary Figs. 5 and 6), and in unsuccessful attempts, the molecules underwent migration or fused with each other during voltage sweeping (Supplementary Fig. 7).
Structural and properties analysis
The molecular structure of the C16 flake was further analyzed by density functional theory (DFT) calculations. As illustrated in Fig. 4a, the C16 flake contains both sp- and sp2-hybridized carbon atoms (denoted by cyan and pink dots). We calculated the bond length and Mayer bond order of the C16 flake at the ωB97XD/def2-TZVP level (Fig. 4b and Supplementary Fig. 8), suggesting quasi-cumulenic structures on both the left and right sides of the flake. The shortest bonds are calculated to be 1.22 Å between two sp-hybridized atoms 1 and 2 (8 and 9) as shown in Fig. 4b and Supplementary Fig. 8, exhibiting a bond order close to a triple bond, which is also visualized in our AFM results showing two characteristic bright features (Fig. 3v, x).
a–d Chemical structure, bond length, NICS(1)zz plot and spin density distribution for C16 flake (ωB97XD/def2-TZVP). The sp- and sp2-hybridized carbon atoms are indicated in cyan and red colors, respectively. Blue/red denote spin up/spin down density. e, f Calculated DOS and wave functions of SOMOs and SUMOs (UB3LYP/6-311 G**). Blue/green isosurfaces denote opposite signs of the wave function.
The delocalization degree of π electrons in the C16 flake is directly related to the characteristics of the induced ring current under an external magnetic field, reflecting its aromaticity32. This ring current can be reflected by the pattern of shielding and deshielding, which can be visualized using the nucleus-independent chemical shift (NICS)33. The ZZ component of the NICS calculated at 1 Å above the plane of the C16 flake, named NICS(1)ZZ, is presented in Fig. 4c. It can be seen that there are significant deshielding regions protruding in the flake, and a shielding region surrounded it, reflecting its aromatic nature. Additionally, the color of the six-membered rings on the left and right sides is notably darker than the ones on the top and bottom, suggesting a greater aromatic character (A comparison with pyrene is also shown in Supplementary Fig. 9). Another aromatic indicator called AV1245 provided similar results (see Supplementary Fig. 10). The localized orbital locator (LOL) function34 was further performed to visualize the delocalization of π electrons in the C16 flake, as illustrated in the LOL-π maps (Supplementary Fig. 11).
Due to the high mobility of C16 flake on the NaCl surface, the electronic properties were challenging to measure; thus, extended theoretical calculations were performed to gain more insight into the electronic structures (Fig. 4d–f). As predicted by theory, the C16 flake exhibits an open-shell singlet ground state, in which two unpaired electrons are antiferromagnetically coupled yet spatially localized on opposite edges of the carbon framework with a coupling strength J = 20 meV (Supplementary Fig. 12). The asymmetry between spin-up and spin-down electronic distributions, as evidenced by the spin density map (Fig. 4d) and frontier orbital analyses (Fig. 4f), underscores the presence of diradical character. Thus, despite the overall singlet multiplicity, the presence of distinct local magnetic moments reflects an open-shell electronic configuration35,36. The corresponding density of states (DOS) plot further supports this interpretation, displaying a spin-split electronic structure with a frontier gap of 2.47 eV between spin-up and spin-down frontier states (Fig. 4e). Interestingly, C16 flake also potentially exhibits a peculiar electronic configuration wherein the singly occupied molecular orbitals (SOMOs) are lower in energy than the highest occupied molecular orbital (HOMO), which is termed SOMO-HOMO inversion (Supplementary Fig. 13)37.
More importantly, similar to [n]-rhombenes (hydrogenated carbon flakes)36, larger Cn flakes exhibit progressively stronger spin polarization and the emergence of multiple unpaired electrons, indicating an enhancement of edge-localized magnetism with increasing molecular size as shown in Supplementary Fig. 14, which enables robust local magnetism in all-carbon systems, and may give rise to exotic many-body phenomena.
Chlorine migration and skeleton isomerization
During atom manipulations, it is interesting to observe that the chlorine atom could migrate. Such a migration was triggered by a short (500 ms) voltage pulse with a sample bias voltage of 4.2 V on the target C16Cl molecule as illustrated in Fig. 5a. The tip was positioned directly over a selected Cl atom (for migration) or over the ring junction region (for skeleton isomerization), retracted by about 4 Å from the STM set point (I = 3 pA, V = 0.3 V). After applying a voltage pulse, the chlorine atom was found to move from site-1 to site-2 of the carbon skeleton as revealed by AFM results (Fig. 5b–e). More interestingly, in another case, the carbon skeleton of the C16Cl molecule was observed to transform from 6–6 membered rings to 5–7 membered rings, also triggered by a voltage pulse of about 4.2 V (Fig. 5g–k). To better understand the molecular transformation processes, we calculated the reaction barriers for chlorine migration and skeleton isomerization (Fig. 5f, l), obtaining barriers of 2.24 eV and 1.39 eV, respectively. It is worth noting that both of these processes are endothermic, demonstrating that the chlorine migration and skeleton isomerization would not occur without applying a voltage pulse. The reaction barriers could generally be overcome by electronic excitation and the weakening of bonds by the antibonding orbital upon electron tunneling27,38,39.
a–f Chlorine migration. g–l Skeleton isomerization. a, g Reaction scheme for tip-induced chlorine migration and skeleton isomerization from 6–6 to 5–7 membered rings. AFM images (line 2) were recorded with a CO-functionalized tip, with respect to an STM set point of I = 3 pA, V = 0.3 V above the NaCl surface. Line 3 shows corresponding Laplace-filtered AFM images. The scale bar in (b) applies to all AFM and Laplace-filtered AFM images. f, l Calculated reaction barriers for chlorine migration and skeleton isomerization, respectively. The energy scale is not linear. The structural models are given for the initial, transition, and final states, respectively. Energies are given in units of eV.
In summary, we have generated a new isomer of C16, the C16 flake, by atom manipulation on the bilayer NaCl/Au(111) surface at 4.7 K. The sp-sp2-hybridized structure of the C16 flake was experimentally characterized using bond-resolved AFM, in good agreement with calculations. Theoretical calculations revealed an open-shell singlet ground state of C16 flake. Moreover, we demonstrated that atom manipulation could further induce chlorine migration and skeleton isomerization inside a single molecule. Our results provide direct experimental insights into the structure of the C16 flake, and the potentially polyradicaloid states in Cn flakes could present a gateway to rich spin physics and correlated electron behavior in all-carbon molecular systems.
Methods
STM and AFM measurements
The experiments were carried out in a low-temperature STM/nc-AFM (CreaTec) under ultra-high vacuum conditions (base pressure below 1 × 10−10 mbar). Au(111) single crystals purchased from MaTeck were used as substrates for the growth of NaCl. Preparation of clean Au(111) surfaces was achieved by cycles of Ar+ ion sputtering and annealing at 850 K. NaCl films were grown on Au(111) held at room temperature, resulting in islands of two- and three-monolayer thickness. C16Cl10 precursors were deposited on a cold NaCl/Au(111) surface held at 6 K by thermal sublimation from a molecular evaporator (evaporator temperature 390 K).
STM images were acquired in the constant-current mode at sample temperatures of 4.7 K. Non-contact AFM measurements were performed with a W tip attached to a tuning fork sensor. The tip was functionalized by controlled picking up of a CO molecule23. CO molecules for tip modification were dosed onto the cold sample via a leak valve. We used a qPlus sensor40 with a resonance frequency f0 = 29.49 kHz, quality factor Q ≈ 45,000, and a spring constant k ≈ 1800 N/m operated in frequency-modulation mode41. The bias voltage V was applied to the sample with respect to the tip. AFM images were acquired in constant-height mode at V = 0 V and an oscillation amplitude of A = 1 Å.
Theoretical calculations
DFT calculations were carried out in the gas phase using the Gaussian 16 program package42. ωB97XD exchange-correlation functional43 in conjunction with def2-TZVP44 basis sets was used for C16 flake-related calculations in the gas phase. The NICS33, LOL-π34, AV124545, and bond length calculations of C16 flake were performed at the ωB97XD/def2-TZVP level; the DOS, spin densities, and frontier orbitals of C16 flake were performed at the UB3LYP/6-311 G** level combined with the Multiwfn 3.8 code46.
The Vienna ab initio simulation package (VASP)47,48 was used to perform the DFT calculations on the NaCl surface. For describing the interaction between electrons and ions, the projector-augmented wave method49,50 was used, and the Perdew-Burke-Ernzerhof generalized gradient approximation exchange-correlation functional was employed51. Van der Waals corrections to the PBE density functional were also included using the DFT-D3 method of Grimme52. A bilayer NaCl(001) slab was used, separated by a vacuum thicker than 15 Å and the bottom layer of the NaCl was fixed.
For the chlorine migration and isomerization calculations, we used a p(20 × 20) unit cell with a 1 × 1 × 1 gamma-centered Monkhorst-Pack k-point grid. The kinetic energy cut-off was set to 420 eV. Transition states were searched by applying the climbing image nudged elastic band53 and Dimer methods54, and all local minima and saddle points were optimized until the forces on all unconstrained atoms were ≤0.03 eV/Å.
The AFM simulations were conducted by the PP-AFM code provided by Hapala et al.55. The detailed parameters are listed below. The lateral spring constant for the CO-tip was 0.2 N/m, and a quadrupole-like charge distribution at the tip apex was used to simulate the CO tip with q = − 0.1 e. In addition, e is the elementary charge and refers to |e | , and q is the magnitude of the quadrupole charge at the tip apex. The amplitude was set as 1 Å.
Synthesis of precursors
Perchloropyrene (C16Cl10) precursor was prepared using published procedures56. C16Cl10 was obtained by dissolving the Pyrene (C16H10) in the BMC reagent consisting of a mixture of S2Cl2 and AlCl3 in a Cl equivalent ratio of 1: 0.5 in 150 mL of SO2Cl2 and heating to 64 °C for 4 h. At the end of the reaction, the mixture was treated with icy water. After neutralization with NaHCO3, the product was filtered or extracted with CHCl3.
Data availability
All data that support the findings of this study are available from the corresponding authors upon request. Source data are provided in this paper.
References
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318, 162–163 (1985).
Iijima, S. Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991).
Iijima, S. & Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Hirsch, A. The era of carbon allotropes. Nat. Mater. 9, 868–871 (2010).
Shi, L. et al. Confined linear carbon chains as a route to bulk carbyne. Nat. Mater. 15, 634–639 (2016).
Patrick, C. W. et al. Masked alkynes for synthesis of threaded carbon chains. Nat. Chem. 16, 193–200 (2024).
Gao, W. et al. On-surface synthesis and characterization of polyynic carbon chains. Natl. Sci. Rev. 11, nwae031 (2024).
Hou, L. et al. Synthesis of a monolayer fullerene network. Nature 606, 507–510 (2022).
Meirzadeh, E. et al. A few-layer covalent network of fullerenes. Nature 613, 71–76 (2023).
Fan, Q. et al. Biphenylene network: A nonbenzenoid carbon allotrope. Science 372, 852–856 (2021).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365, 1299–1301 (2019).
Scriven, L. M. et al. Synthesis of cyclo[18]carbon via debromination of C18Br6. J. Am. Chem. Soc. 142, 12921–12924 (2020).
Gao, Y. et al. On-surface synthesis of a doubly anti-aromatic carbon allotrope. Nature 623, 977–981 (2023).
Sun, L. et al. On-surface synthesis of aromatic cyclo[10]carbon and cyclo[14]carbon. Nature 623, 972–976 (2023).
Albrecht, F. et al. The odd-number cyclo[13]carbon and its dimer, cyclo[26]carbon. Science 384, 677–682 (2024).
Sun, L. et al. On-surface synthesis and characterization of anti-aromatic cyclo[12]carbon and cyclo[20]carbon. Nat. Commun. 15, 7649 (2024).
Sun, L. et al. On-surface synthesis and characterization of linear and cyclic C6. Nat. Synth. 4, 940–946 (2025).
Jones, R. O. Density functional study of carbon clusters C2n (2⩽n⩽16). I. Structure and bonding in the neutral clusters. J. Chem. Phys. 110, 5189–5200 (1999).
Ohno, K. Quantum chemical exploration of conversion pathways and isomeric structures of C16 molecules. Chem. Phys. Lett. 711, 60–65 (2018).
Novoa, J. J., Myung-Hwan, W. & Williams, J. M. On the structures, stabilities and fragmentation patterns of carbon clusters including Buckminsterfullerene. Inorg. Chim. Acta 198–200, 133–138 (1992).
Pavliček, N. et al. Polyyne formation via skeletal rearrangement induced by atomic manipulation. Nat. Chem. 10, 853–858 (2018).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
De Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Pavliček, N. & Gross, L. Generation, manipulation and characterization of molecules by atomic force microscopy. Nat. Rev. Chem. 1, 0005 (2017).
Zhong, Q. et al. Constructing covalent organic nanoarchitectures molecule by molecule via scanning probe manipulation. Nat. Chem. 13, 1133–1139 (2021).
Hla, S.-W., Bartels, L., Meyer, G. & Rieder, K.-H. Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: Towards single molecule engineering. Phys. Rev. Lett. 85, 2777–2780 (2000).
Repp, J., Meyer, G., Paavilainen, S., Olsson, F. E. & Persson, M. Imaging bond formation between a gold atom and pentacene on an insulating surface. Science 312, 1196–1199 (2006).
Albrecht, F. et al. Selectivity in single-molecule reactions by tip-induced redox chemistry. Science 377, 298–301 (2022).
Suresh, R. et al. Cyclo[18]carbon formation from C18Br6 and C18(CO)6 precursors. J. Phys. Chem. Lett. 13, 10318–10325 (2022).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Liu, Z., Lu, T. & Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Bonding character, electron delocalization, and aromaticity. Carbon 165, 468–475 (2020).
Schleyer, P. V. R., Maerker, C., Dransfeld, A., Jiao, H. & Van Eikema Hommes, N. J. R. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 118, 6317–6318 (1996).
Schmider, H. L. & Becke, A. D. Chemical content of the kinetic energy density. J. Mol. Struct. 527, 51–61 (2000).
Hod, O., Barone, V. & Scuseria, G. E. Half-metallic graphene nanodots: A comprehensive first-principles theoretical study. Phys. Rev. B 77, 035411 (2008).
Mishra, S. et al. Large magnetic exchange coupling in rhombus-shaped nanographenes with zigzag periphery. Nat. Chem. 13, 581–586 (2021).
Mishra, S. et al. Observation of SOMO-HOMO inversion in a neutral polycyclic conjugated hydrocarbon. ACS Nano 18, 15898–15904 (2024).
Kawai, S. et al. Local probe-induced structural isomerization in a one-dimensional molecular array. Nat. Commun. 14, 7741 (2023).
Pavliček, N. et al. Generation and characterization of a meta-Aryne on Cu and NaCl surfaces. ACS Nano 11, 10768–10773 (2017).
Giessibl, F. J. High-speed force sensor for force microscopy and profilometry utilizing a quartz tuning fork. Appl. Phys. Lett. 73, 3956–3958 (1998).
Albrecht, T. R., Grütter, P., Horne, D. & Rugar, D. Frequency modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys. 69, 668–673 (1991).
Frisch, M. J. et al. Gaussian 16 Rev. C.01 (Gaussian, 2016).
Chai, J.-D. & Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 10, 6615 (2008).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297 (2005).
Matito, E. An electronic aromaticity index for large rings. Phys. Chem. Chem. Phys. 18, 11839–11846 (2016).
Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Kästner, J. & Sherwood, P. Superlinearly converging dimer method for transition state search. J. Chem. Phys. 128, 014106 (2008).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Sun, J., Gruetzmacher, H. F. & Lifshitz, C. Ion/molecule reactions of carbon cluster ions and acrylonitrile. J. Am. Chem. Soc. 115, 8382–8388 (1993).
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (22125203).
Author information
Authors and Affiliations
Contributions
W.X. conceived and supervised the experiments; W.G., L.S., and F.K. performed the SPM experiments; W.Z. and Z.Z. synthesized the precursor monomers; W.G., L.S., and F.K. carried out the DFT calculations; all authors contributed to writing the manuscript. W.G. and W.Z. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, W., Zheng, W., Sun, L. et al. An sp-sp2-hybridized molecular carbon allotrope C16 flake. Nat Commun 16, 8502 (2025). https://doi.org/10.1038/s41467-025-63525-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63525-9