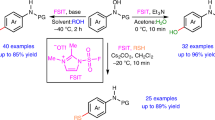
Abstract
Due to the universality of arylamine and arylazide building blocks in pharmaceuticals, natural products and functional materials, the formation of aryl C-N bonds from readily available materials appears to be highly appealing, especially for the exclusive regioselective C-H amination. Despite substantial advancements in the synthesis of aryl C-N bonds, achieving a general, efficient, and para-selective C-H amination under mild conditions remains a significant challenge. Here we showcase a fluorosulfuryl imidazolium triflate-mediated para-selective C-H amination of N-arylhydroxylamines employing primary and secondary amines, diphenylmethanimine and azides as nitrogen source, affording structurally diverse 1,4-diaminoarenes or para-azidoanilides in the absence of oxidants and transition-metal catalysts. This effective protocol exhibits exclusive para-selectivity and good functional group tolerance for both N-arylhydroxylamines and N-nucleophiles (>90 examples). DFT calculations have been performed to elucidate the high chemoselectivity observed amidst multiple nucleophilic and electrophilic species.
Similar content being viewed by others
Introduction
The aryl amine moiety represents privileged structural motif due to its widespread applications and presence in pharmaceuticals, natural products, dye industry, and material science1,2,3,4,5,6. Examples include Binimetinib7, Murrastifoline B8, Erdafitinib9 and P2Y12 receptor antagonist10 (Fig. 1). Additionally, aryl amines serve as highly versatile intermediates or synthons in organic synthesis, readily convertible into a wide range of structurally diverse functional groups11. Therefore, the construction of C-N bonds towards the synthesis of aryl amines has emerged as a significant research area in organic chemistry. Conventional methods for the synthesis of aryl amines majorly rely on the nitration of arenes followed by metal-catalyzed nitro reduction, and transition-metal-catalyzed cross-coupling reactions of amines with prefunctionalized aryl substrates (e.g., Ullman-Goldberg12, Buchwald-Hartwig13,14,15, Chan-Lam16,17 C-N bond formation reactions) (Fig. 2a). In addition, the employment of NaN3 or hydroxylamine as amination reagents to achieve C (sp2) amination through C-C bond cleavage18,19 or direct C(sp2)-H-amination20,21,22,23,24,25 are also representative strategies. Over the past two decades, remarkable advances have been made in the transition-metal-catalyzed C-H amination of arenes, in particular, the directed ortho-C-H amination has been relatively well studied for the construction of aryl C-N bond in the presence of a variety of metal-catalysts, such as Pd26,27,28,29,30, Rh31,32,33,34,35,36,37, Ir38,39,40,41, Ru42,43,44,45,46,47,48,49,50, Cu51,52, Co53,54,55,56, Fe23,57 et al. (Fig. 2b). Despite the remarkable success of these strategies, challenges remain, including the employment of transition metals or harsh reagents, the need for specific directing groups, and limitations in substrate scope.
Compared to the well-established directed ortho-C-H amination methods, the meta58,59,60,61 and para-selective C-H amination of arenes are considerably less explored and only limited examples have been reported across this field in recent years. In 2011, Zhang and co-workers62 disclosed a palladium-catalyzed, highly selective para-C-H amination of arenes by using N-fluorobenzenesulfonimide (NFSI) as nitrogen source and an alkoxyl group at the ortho position of the amide as a directing group [Fig. 2c, panel (1)]. Subsequently, several groups reported similar para-amination strategies for sp2 C-H bonds, utilizing hypervalent iodine reagent or metal catalyst employing a variety of nitrogen source, such as NFSI63, dibenzenesulfonimide64, azodicarboxylates65,66 and azoles67 [Fig. 2c, panel (2)]. In 2015, Nicewicz and co-workers reported an elegant site-selective C-H amination protocol of electron-rich arenes promoted by an organic photoredox-based catalytic system using azoles or ammonium salts as nitrogen source albeit with moderate to good regioselectivity (para Vs ortho) [Fig. 2c, panel (3)]68. Very recently, Zhang, Lei and Cai developed a facile, metal-free, dehydrogenative para-selective amination of nitroarenes through an aminyl radical coupling mechanism employing cyclic and acyclic secondary amine as nitrogen source and environmentally friendly O2 as an oxidant [Fig. 2c, panel (4)]69. However, the involvement of aminyl radicals excluded the employment of primary amines and non-nitroarenes. While these transformations have established the feasibility of the para-selective C-H amination of arenes with a variety of directing groups, the development of an effective regio-selective cross coupling of arenes with a number of N-nucleophiles under mild conditions is still in high demand. Herein, we report a practical, oxidant- and metal-free umpolung para-selective C-H amination and azidation of N-arylhydroxylamines with a wide range of N-nucleophiles. This transformation is efficiently promoted by FluoroSulfuryl Imidazolium Triflate (FSIT) under mild conditions (Fig. 2d).
Over the past decade, Sulfur(VI) Fluoride Exchange (SuFEx) chemistry, pioneered by Sharpless, Dong and co-workers70,71,72,has been extensively applied to the synthesis of numerous target molecules or functional materials employing SO2F2 or its surrogates (such as FSIT and AISF < ([4-(Acetylamino)phenyl]-ImidodiSulfuryl diFluoride>)) as effective and powerful hub. Moreover, in our recent studies, FSIT has been found to be an unique activator to achieve the umpolung cascade cross-coupling of N-arylhydroxylamines with various O- and S-nucleophiles for the efficient construction of para-functionalized anilides under simple conditions73. Inspired by the aforementioned protocols, we began to evaluate the possibility of realizing the metal- and directing-group-free para-C-H amination of N-arylhydroxylamines with diverse N-nucleophiles.
Results
Condition screening
We started our investigation by choosing N-phenylhydroxylamine 1a and aniline 2a as model substrates (Table 1). Screening of a series of bases, such as Et3N, DBU, DMAP, K2CO3, NaHCO3 and Na2CO3, was firstly conducted using MeCN as solvent at 0 oC under air atmosphere, indicating Na2CO3 was relatively promising with the generation of the desired product 3 in 33% yield (Table 1, entries 1-6). Notably, only trace amount of phenylsulfamoyl fluoride 1a′′, which was formed by the reaction of aniline 2a with FSIT72, could be observed. The subsequent solvent screening revealed that the participation of 1,4-dioxane was helpful, affording to the para-aminated anilide 3 in 49% yield using the mixed solvent of acetonitrile and 1,4-dioxane in 3:1 ratio (Table 1, entries 7-11). To our delight, the yield of product 3 could be further increased to 66% when the reaction was carried out at -20 oC, although the generation of N-phenylbenzamide 1a′ from the decomposition of N-phenylhydroxylamine 1a could not be fully suppressed (Table 1, entries 12-13). The employment of N2 atmosphere did not affect the yield of the target product 3 (Table 1, entry 14). Decreasing the loading equivalents of FSIT resulted in lower yield of para-aminated anilide 3 (Table 1, entry 15). Thus, the combination of 1.0 equivalent of N-arylhydroxylamine, 2.0 equivalents of aniline, 1.5 equivalents of FSIT, 2.0 equivalents of Na2CO3 as base, the 3:1 mixture of acetonitrile and 1,4-dioxane as solvent at -20 °C under air for 3 h, was selected as the optimal conditions for this cascade transformation. (Table 1, entry 12).
Mechanistic studies
To better understand the good chemoselectivity observed in this tandem transformation, particularly in the presence of multiple nucleophilic species and its reaction mechanism, we conducted a DFT study using the B3LYP-D3 functional (see pages S52-53 of the Supplementary Information and source data for computational details.). First, experimental data show that N-phenylhydroxylamine (1a) reacts more readily with FSIT than aniline (2a). The initial step involves the reaction of either nucleophile, 1a or 2a, with Na2CO3 to form an anionic nucleophile (as a Na-salt). Accordingly, we calculated the free energy changes for these reactions. As shown in Fig. 3a, the reaction between 1a and the base is exergonic by 5.9 kcal/mol, leading to the formation of the 1a-O nucleophile. In contrast, the reaction between 2a and the base is endergonic by 15.6 kcal/mol, forming the 2a-N nucleophile, indicating this reaction is thermodynamically less favorable than that of 1a. Therefore, thermodynamic calculations suggest that the N-phenylhydroxylamine anion forms more easily under basic conditions, supporting the experimental observation that the primary reaction occurs between 1a and FSIT.
Following the reaction of 1a with FSIT, the N-O bond undergoes cleavage, generating an imine cation. This cation can react with either aniline (2a) or the NaOSO2F nucleophile in solution. We calculated the free energy for both reactions, and the results show that the reaction between 2a and the imine cation is exergonic by 25.9 kcal/mol, whereas the reaction between NaOSO2F and the imine cation is only exergonic by 3.2 kcal/mol (Fig. 3b). These calculations demonstrate that, at this stage, it is more favorable for the imine cation to react with aniline (2a) rather than with other nucleophiles, which is consistent with experimental observations. Notably, we also considered the anionic forms of both nucleophiles (i.e., PhNH⁻ and FSO3⁻) in the free energy calculations, and found that the reaction of the imine cation with deprotonated aniline (PhNH⁻) is still much more favorable than with FSO3⁻ (see pages S52-53 of the Supplementary Information and source data for details). Thus, these calculations clearly explain why 1a, but not 2a, reacts with FSIT, and why the imine cation intermediate preferentially reacts with aniline over fluorosulfonate-based nucleophiles.
Lastly, we computed the reaction mechanism starting from the imine cation species. As shown in the free energy profile (Fig. 3c), the reaction between the imine cation (IN1) and aniline (2a) is exergonic by 25.9 kcal/mol, yielding the amine cation (IN2). Subsequent deprotonation of IN2 by Na2CO3 leads to IN3, which is exergonic by 23.1 kcal/mol. The carbonate base then interacts with the para-C-H bond of IN3 via hydrogen bonding to form intermediate IN4. This is followed by the deprotonation transition state (TS1), resulting in the formation of IN5. This step is facile (∆G≠ = 1.2 kcal/mol) and highly exergonic (∆G = 39.2 kcal/mol) relative to IN3, likely driven by aromatization. Finally, proton transfer from the carbonate back to the anionic nitrogen, along with the release of Na2CO3, yields the final product, IN6, with a slight exergonicity of 0.6 kcal/mol. Overall, the imine cation intermediate undergoes nucleophilic attack, deprotonation, rearomatization, and proton transfer to give the final amine product. It is important to note that the carbonate base plays a key role in this proton-shuttling process: deprotonating the para-C-H bond and returning the proton to the anionic nitrogen.
Substrate scope
With the optimal reaction conditions in hand, we began investigating the substrate scope of this cascade protocol by verifying the different amine nucleophiles (Fig. 4). Pleasingly, a wide array of primary anilines bearing halogen atom, cyanide, aldehyde, ketone, ester, sulfone, nitro, hydroxyl, alkyl and alkoxyl groups at the para, ortho or meta positions readily reacted with N-phenylhydroxylamine 1a under standard conditions, delivering the desired para-aminated anilides products 4-23 in moderate to good yields. In addition, 2,4-, 3,4-di- and 2,4,6-trisubstituted anilines and naphthylamines were well tolerated to give the corresponding products 24-30 in 41−73% yields. Notably, the excellent chemoselectivity of this cascade protocol could be proven by the aniline substrates with OH group, which could exclusively generate the N-attack products 19 and 25 rather than the O-attack products. We were pleased to find that herteroaryl amines, such as quinolinyl-, isoquinolinyl-, pyridinylamines with diverse substituents, were amenable to our current reaction conditions to afford the expected aminated products 31-41 in moderate to good yields, showing the potential applications of this methodology in preparing the pharmaceutical-relevant compounds. For the secondary amine substrates, such as N-methyl, N-phenyl amines, 1,2,3,4-tetrahydroquinoline, 2,3,4,5-tetrahydro-1H-benzo[b]azepine and pyrazole, this FSIT-promoted umpolung para-amination process still occurred to give the corresponding products 42-47 albeit with relatively lower yields. In addition to the above-mentioned nitrogen nucleophiles, we also explored other representative amination reagents, especially aliphatic amines. Unfortunately, they are not well applicable to the reaction to obtain the corresponding products (For details, see page S47 of the Supplementary Information.).
Method. A: 1a (0.20 mmol, 1.0 equiv.), 2 (0.40 mmol, 2.0 equiv.), Na2CO3 (0.40 mmol, 2.0 equiv.), FSIT (0.30 mmol, 1.5 equiv.) in 4 ml of MeCN/1,4-Dioxane (3/1) at −20 oC for 3 h. For 45: 2.0 equivalents of K2CO3 rather than Na2CO3 was employed as base. Yields were determined by the isolated products. FSIT fluorosulfuryl imidazolium triflate.
Given the good results with diverse arylamine nucleophiles, we decided to explore the substrate scope of N-arylhydroxylamine for this metal-free amination reaction, as shown in Fig. 5. N-Phenylhydroxylamines with several frequently-used N-protective groups (such as Ac, Cbz and Fmoc) smoothly reacted with 4-chloroaniline 2c under the standard conditions, affording to the corresponding para-aminated products 48-50 in high yields (70-78%). A broad range of N-phenylhydroxylamine with ortho- and meta-substituents, including electron-withdrawing and electron-donating functionalities, were well compatible to form the desired para-aminated products 51-62 in moderate to good yields. The 2,3-, 2,5-, and 3,5-disubstituted N-phenylhydroxylamines with various substituents were demonstrated as the suitable candidates for this cascade transformation to generate the desired products 63-71 in 53–75% yields. More importantly, N-phenylhydroxyl-amines connected with complex rings or natural product-relevant structures, such as cycloheptanol, adamantanol, furan-2-ylmethanol, menthol and perillyl alcohol, could also be compatible with this amination reaction to furnish the corresponding products 72-76 in good yields (58-80%) under the optimal conditions.
Encouraged by the successful assembling of secondary and tertiary amine at the para-position of the anilides, we were interested in the direct primary amination of our N-arylhydroxylamine substrates. Due to the strong coordinating property and nucleophilicity of ammonia and its analogs, the direct primary amination was much more challenging than the secondary and tertiary aminations in both metal-catalyzed and metal-free amination reactions. As far as we know, there are very rare examples for the efficient para-primary amination of arenes except for limited reports on metal-catalyzed cross-coupling amination reactions. After systematic screening of various aminating reagents and reaction conditions, we delightedly found that readily available diphenylmethanimine 77 can be employed as effective nitrogen source to accomplish the C-N bond formation in our protocol, followed by the practical removal of benzophenone under acidic conditions to obtain the para-primary aminated product 90 (For detailed optimization study, see page S9 of the Supplementary Information.). Gratifyingly, this facile successive protocol has a wide range of substrate scope (Fig. 6). N-Arylhydroxylamines bearing variety of substituents were compatible with this cascade reaction system, providing the corresponding products in both steps in good to excellent yields. Notably, the yields of p-aminobenzenamines 90-101 on the right side of Fig. 6 refer to the yields after hydrolysis of compounds 78-89 on the left side.
Method B: Step 1: 1 (0.20 mmol, 1.0 equiv.), 77 (2.0 mmol, 10.0 equiv.), FSIT (0.30 mmol, 1.5 equiv.), Na2CO3 (0.40 mmol, 2.0 equiv.) in 4 ml of MeCN/1,4-Dioxane (3/1) at -20 oC for 3 h. Step 2: Compounds 78-89 in 5 ml of THF/HCl (2.5/2.5) at 25 oC for 4 h, followed by treatment of 1 mol/l of KOH solution after removal of solvent. All yields were determined by the isolated products. FSIT fluorosulfuryl imidazolium triflate.
Having developed an efficient metal-free protocol for the synthesis of structurally diverse para-aminated anilides, we assumed that this method might provide a good opportunity to realize the goal of expanding the chemical space to other nitrogen-containing molecules, such as aryl azides. Due to their versatile reactivities and unique biological properties, organic azides have become widely applied in organic synthesis as highly valuable building blocks for the preparation of various nitrogen-containing heterocycles74,75,76, peptide molecules, functional materials and had extensive applications in polymer chemistry, drug discovery, biology and interface chemistry77,78,79,80,81. Although great effort has been devoted into the installation of the azide group onto the aromatic rings82,83, such as nucleophilic aromatic substitution84,85, diazotization pretreatment of aromatic amines followed by diazo transfer86,87, sonication-mediated Friedel-Crafts C-H azidation88 and copper-catalyzed cross-coupling of preactivated arenes89,90 or direct C-H bond azidation of anilines91, these methods still suffer from the harsh reaction conditions or the limited functional group tolerance.
To our delight, our current FSIT-promoted umpolung para-C-H functionalization strategy can be successfully extended to the direct para-C-H azidation of N-arylhydroxylamines using TMSN3 as azide source under the similar conditons (For detailed optimization study on para-azidation, see page S10 of the Supplementary Information.). A broad array of N-arylhydroxylamines bearing electron-withdrawing and electron-donating groups on the phenyl ring were well tolerated in this cascade transformation, affording the corresponding para-azidized anilide products 103-114 in moderate to good yields under standard conditions (Fig. 7).
Gram-scale reactions and synthetic applications
With these para-aminated anilide products in hand, a preliminary survey of their optical properties was carried out by choosing compound 33 as a representative. Similar to the general D-π-A system, the absorption of compound 33 does not change significantly in different polar solvents, while the emission shows a significant solvation effect. The maximum emission wavelength of compound 33 has been redshifted by ~70 nm from the non-polar solvent cyclohexane to the highly polar tetrahydrofuran (Fig. 8). To demonstrate the synthetic practicability of this protocol, a gram-scale synthesis of 5 was carried out under standard conditions. It was found that the gram-scale reaction proceeded smoothly to afford 1.08 grams of 5 in 67% yield (Fig. 9, panel 1). To illustrate the synthetic potential of the newly formed 1,4-diaminoaryl compounds, various reactions were performed under different conditions by choosing compound 6 as a representative (Fig. 9, paths a-e). For instance, the classical palladium-catalyzed Suzuki coupling of compound 6 with phenylboronic acid was accomplished to produce the corresponding biaryl product 115 in 70% yield (Fig. 9, path a)92; both nitrogen atoms of 6 could be efficiently methylated in the presence of iodomethane and sodium tert-butoxide to furnish 116 in 73% yield (Fig. 9, path b)93; compound 6 could be efficiently oxidized into N-((1E,4E)-4-((4-bromophenyl)imino)-cyclohexa-2,5-dien-1-ylidene)benzamide 117 in 82% yield employing iodobenzene diacetate as oxidant (Fig. 9, path c)94; the benzoyl group of 6 could be readily removed by hydrazine hydrate to afford N-(4-bromophenyl)benzene-1,4-diamine 11895, which could be further converted into 1-(3,5-bis(trifluorom-ethyl)phenyl)-3-(4-((4-bromophenyl)-amino)phenyl)thiourea 119 in 90% yield by the treatment with isothiocyanate (Fig. 9, path d and e)96.
In summary, we have developed a one-pot, metal-free, direct aromatic para-C-H amination and azidation of N-arylhydroxylamines, promoted by fluorosulfuryl imidazolium triflate (FSIT) under mild conditions. This cascade protocol exhibits excellent functional group tolerance, accommodating N-arylhydroxylamines with diverse functionalities and motifs relevant to natural products. A wide range of readily available nitrogen nucleophiles such as primary anilines, secondary amines, diphenylmethanimine and TMSN3, could be successfully introduced at the para-position of the anilides with moderate to good yields and excellent chemo- and regioselectivity. DFT studies were also carried out to explain the excellent chemoselectivity in the presence of multiple nucleophilic and electrophilic species. Ongoing research in our laboratory aims to extend this method to other nucleophiles and explore its synthetic applications in the preparation of more complex bioactive molecules.
Methods
Method A
The system of a mixture of 1 (0.2 mmol, 1.0 equiv.), 2 (0.4 mmol, 2.0 equiv.), Na2CO3 (0.4 mmol, 2.0 equiv.) in 1,4-dioxane (1 ml) and MeCN (3 ml), FSIT (0.3 mmol, 1.5 equiv.) was added in one portion. The reaction mixture was stirred at -20 oC for 3 h until the complete consumption of 1, which was monitored by TLC analysis. The reaction mixture was evaporated under reduced pressure and purified by column chromatography to give the desired product 3-76.
Method B
The system of a mixture of 1 (0.2 mmol, 1.0 equiv.), 77 (2 mmol, 10.0 equiv.) and Na2CO3 (0.4 mmol, 2.0 equiv.) in 1,4-dioxane (1 ml) and MeCN (3 ml), FSIT (0.3 mmol, 1.5 equiv.) was added in one portion. The reaction mixture was stirred at -20 oC for 3 h until the complete consumption of 1, which was monitored by TLC analysis. Afterwards, the reaction mixture was concentrated in vacuo and the residue was purified by flash column chromatography to afford the target compound 78-89.
The ketimine 78-89 (0.2 mmol, 1.0 equiv.) was suspended in 1/1 (v/v) ratio of 1 N HCl:THF (5 ml) solution and stirred at 25 oC for 4 h. Upon consumption of the ketimine as determined by TLC, THF was removed and the aqueous residue was basified using 1 N KOH to a pH of 10–14. The aqueous layer was then extracted three times with ethyl acetate (30 ml). The organic layer was dried with anhydrous Na2SO4, filtered and concentrated in vacuo to give the crude amine. Upon purification via column chromatography, the pure product 90-101 was obtained after solvent removal.
Method C
To a solution of 1 (0.2 mmol, 1.0 equiv.), TMSN3 102 (0.6 mmol, 3.0 equiv.) and K2CO3 (0.4 mmol, 2.0 equiv.) in 1,4-dioxane (1 ml) and MeCN (3 ml), FSIT (0.2 mmol, 1.0 equiv.) was added every half an hour in one portion until FSIT was completely added. The reaction mixture was stirred violently at -20 oC under air atmosphere for 3 h until the complete consumption of 1, which was monitored by TLC analysis. Afterwards, the reaction mixture was concentrated in vacuo and the residue was purified by flash column chromatography to afford the title compound 103-114.
Data availability
The X-ray crystallographic coordinates for the products reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition nos. CCDC 2376503 (58) and CCDC 2392892 (81). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Experimental procedures and characterization of new compounds are available in the Supplementary Information. Source data are provided with this paper. All data are available from the corresponding author upon request. Source data are provided with this paper.
References
Hili, R. & Yudin, A. K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2, 284–287 (2006).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Goldberg, F. W., Kettle, J. G., Kogej, T., Perry, M. W. D. & Tomkinson, N. P. Designing novel building blocks is an overlooked strategy to improve compound quality. Drug. Discov. Today 20, 11–17 (2015).
Zhao, Y., Huang, B., Yang, C. & Xia, W. Visible-light-promoted direct amination of phenols via oxidative cross-dehydrogenative coupling reaction. Org. Lett. 18, 3326–3329 (2016).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Shirley, M. Encorafenib and binimetinib: first global approvals. Drugs 78, 1277–1284 (2018).
Knölker, H.-J., Börger, C. & Schmidt, A. First total synthesis of murrastifoline B and an improved route to murrastifoline F. Synlett 25, 1381–1384 (2014).
Markham, A. Erdafitinib: First global approval. Drugs 79, 1017–1021 (2019).
Baqi, Y. & Müller, C. E. Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat. Protoc. 5, 945–953 (2010).
Candeias, N. R., Branco, L. C., Gois, P. M. P., Afonso, C. A. M. & Trindade, A. F. More sustainable approaches for the synthesis of N-based heterocycles. Chem. Rev. 109, 2703–2802 (2009).
Kunz, K., Scholz, U. & Ganzer, D. Renaissance of Ullmann and Goldberg reactions—progress in copper catalyzed C-N-, C-O- and C-S-coupling. Synlett 15, 2428−2439 (2003).
Guram, A. S. & Buchwald, S. L. Palladium-catalyzed aromatic aminations with in situ generated aminostannanes. J. Am. Chem. Soc. 116, 7901–7902 (1994).
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534–1544 (2008).
Inoue, F., Kashihara, M., Yadav, M. R. & Nakao, Y. Buchwald–Hartwig amination of nitroarenes. Angew. Chem. Int. Ed. 56, 13307–13309 (2017).
Chan, D. M. T., Monaco, K. L., Wang, R.-P. & Winters, M. P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 39, 2933–2936 (1998).
Lam, P. Y. S. et al. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 39, 2941–2944 (1998).
Liu, J. et al. From alkylarenes to anilines via site-directed carbon–carbon amination. Nat. Chem. 11, 71–77 (2019).
Wang, T. et al. Hydroxylamine-mediated C–C amination via an aza-hock rearrangement. Nat. Commun. 12, 7029 (2021).
Foo, K., Sella, E., Thomé, I., Eastgate, M. D. & Baran, P. S. A Mild, ferrocene-catalyzed C–H imidation of (Hetero)arenes. J. Am. Chem. Soc. 136, 5279–5282 (2014).
Paudyal, M. P. et al. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Liu, J. et al. Fe-catalyzed amination of (Hetero)arenes with a redox-active aminating reagent under mild conditions. Chem. Eur. J. 23, 563–567 (2017).
D’Amato, E. M., Börgel, J. & Ritter, T. Aromatic C-H amination in hexafluoroisopropanol. Chem. Sci. 10, 2424–2428 (2019).
Qiu, X. et al. Selective carbon-carbon bond amination with redox-active aminating reagents: a direct approach to anilines†. Chin. J. Chem. 39, 3011–3016 (2021).
Wang, T. et al. A metal-free direct arene C-H amination. Adv. Synth. Catal. 363, 2783–2795 (2021).
Ng, K.-H., Chan, A. S. C. & Yu, W.-Y. Pd-catalyzed intermolecular ortho-C-H amidation of anilides by N-nosyloxycarbamate. J. Am. Chem. Soc. 132, 12862–12864 (2010).
Yoo, E. J., Ma, S., Mei, T.-S., Chan, K. S. L. & Yu, J.-Q. Pd-catalyzed intermolecular C-H amination with alkylamines. J. Am. Chem. Soc. 133, 7652–7655 (2011).
Anand, M., Sunoj, R. B. & Schaefer, H. F. Non-innocent additives in a palladium(II)-catalyzed C-H bond activation reaction: insights into multimetallic active catalysts. J. Am. Chem. Soc. 136, 5535–5538 (2014).
Zhu, D. et al. Ligand-promoted ortho-C-H amination with Pd catalysts. Angew. Chem., Int. Ed. 54, 2497–2500 (2015).
Wang, H.-W. et al. Ligand-promoted rhodium(III)-catalyzed ortho-C-H amination with free amines. Angew. Chem., Int. Ed. 56, 7449–7453 (2017).
Kim, J. Y. et al. Rhodium-catalyzed intermolecular amidation of arenes with sulfonyl azides via chelation-assisted C-H bond activation. J. Am. Chem. Soc. 134, 9110–9113 (2012).
Grohmann, C., Wang, H. & Glorius, F. Rh[III]-catalyzed direct C-H amination using N-chloroamines at room temperature. Org. Lett. 14, 656–659 (2012).
Ryu, J., Shin, K., Park, S. H., Kim, J. Y. & Chang, S. Rhodium-catalyzed direct C-H amination of benzamides with aryl azides: a synthetic route to diarylamines. Angew. Chem. Int. Ed. 51, 9904–9908 (2012).
Shin, K., Baek, Y. & Chang, S. Direct C-H Amination of arenes with alkyl azides under rhodium catalysis. Angew. Chem., Int. Ed. 52, 8031–8036 (2013).
Yu, S., Wan, B. & Li, X. Rhodium(III)-catalyzed C-H activation and amidation of arenes using n-arenesulfonated imides as amidating reagents. Org. Lett. 15, 3706–3709 (2013).
Kim, H. J. et al. Hydrogen-bond-assisted controlled C-H functionalization via adaptive recognition of a purine directing group. J. Am. Chem. Soc. 136, 1132–1140 (2014).
Xing, Y.-K., Wang, Z.-H., Fang, P., Ma, C. & Mei, T.-S. Divergent synthesis of aryl amines and dihydroquinazolinones via electrochemistry-enabled rhodium-catalyzed C–H functionalization. Sci. China Chem. 66, 2863–2870 (2023).
Ryu, J., Kwak, J., Shin, K., Lee, D. & Chang, S. Ir(III)-Catalyzed mild C-H amidation of arenes and alkenes: an efficient usage of acyl azides as the nitrogen source. J. Am. Chem. Soc. 135, 12861–12868 (2013).
Kim, J. & Chang, S. Iridium-catalyzed direct C-H amidation with weakly coordinating carbonyl directing groups under mild conditions. Angew. Chem. Int. Ed. 53, 2203–2207 (2014).
Becker, P., Pirwerdjan, R. & Bolm, C. Acylsilanes in iridium-catalyzed directed amidation reactions and formation of heterocycles via siloxycarbenes. Angew. Chem. Int. Ed. 54, 15493–15496 (2015).
Hermann, G. N., Becker, P. & Bolm, C. Mechanochemical iridium(III)-catalyzed C-H bond amidation of benzamides with sulfonyl azides under solvent-free conditions in a ball mill. Angew. Chem. Int. Ed. 55, 3781–3784 (2016).
Bhanuchandra, M., Ramu Yadav, M., Rit, R. K., Rao Kuram, M. & Sahoo, A. K. Ru(II)-catalyzed intermolecular ortho-C-H amidation of aromatic ketones with sulfonyl azides. Chem. Commun. 49, 5225–5227 (2013).
Zheng, Q.-Z., Liang, Y.-F., Qin, C. & Jiao, N. Ru(ii)-catalyzed intermolecular C-H amidation of weakly coordinating ketones. Chem. Commun. 49, 5654–5656 (2013).
Shang, M., Zeng, S.-H., Sun, S.-Z., Dai, H.-X. & Yu, J.-Q. Ru(II)-catalyzed ortho-C-H amination of arenes and heteroarenes at room temperature. Org. Lett. 15, 5286–5289 (2013).
Thirunavukkarasu, V. S., Raghuvanshi, K. & Ackermann, L. Expedient C-H amidations of heteroaryl arenes catalyzed by versatile ruthenium(II) catalysts. Org. Lett. 15, 3286–3289 (2013).
Shin, K., Ryu, J. & Chang, S. Orthogonal reactivity of acyl azides in C-H activation: dichotomy between C-C and C-N amidations based on catalyst systems. Org. Lett. 16, 2022–2025 (2014).
Yadav, M. R., Shankar, M., Ramesh, E., Ghosh, K. & Sahoo, A. K. Ruthenium-catalyzed ortho-C-H mono- and Di-imidation of arenes with N-tosyloxyphthalimide. Org. Lett. 17, 1886–1889 (2015).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019).
Rössler, S. L. et al. Pyridyl radical cation for C-H amination of arenes. Angew. Chem. Int. Ed. 58, 526–531 (2019).
Ham, W. S., Hillenbrand, J., Jacq, J., Genicot, C. & Ritter, T. Divergent late-stage (Hetero)aryl C-H amination by the pyridinium radical cation. Angew. Chem. Int. Ed. 58, 532–536 (2019).
Peng, J., Xie, Z., Chen, M., Wang, J. & Zhu, Q. Copper-catalyzed C(sp2)-H amidation with azides as amino sources. Org. Lett. 16, 4702–4705 (2014).
Peng, J., Chen, M., Xie, Z., Luo, S. & Zhu, Q. Copper-mediated C(sp2)-H amination using TMSN3 as a nitrogen source: redox-neutral access to primary anilines. Org. Chem. Front. 1, 777–781 (2014).
Patel, P. & Chang, S. Cobalt(III)-catalyzed C-H amidation of arenes using acetoxycarbamates as convenient amino sources under mild conditions. ACS Catal. 5, 853–858 (2015).
Park, J. & Chang, S. Comparative catalytic activity of group 9 [Cp*MIII] complexes: cobalt-catalyzed C-H amidation of arenes with dioxazolones as amidating reagents. Angew. Chem. Int. Ed. 54, 14103–14107 (2015).
Liang, Y., Liang, Y.-F., Tang, C., Yuan, Y. & Jiao, N. Cationic cobalt(III)-catalyzed aryl and alkenyl C-H amidation: a mild protocol for the modification of purine derivatives. Chem. Eur. J. 21, 16395–16399 (2015).
Mei, R., Loup, J. & Ackermann, L. Oxazolinyl-assisted C-H amidation by cobalt(III) catalysis. ACS Catal. 6, 793–797 (2016).
Matsubara, T., Asako, S., Ilies, L. & Nakamura, E. Synthesis of anthranilic acid derivatives through iron-catalyzed ortho amination of aromatic carboxamides with n-chloroamines. J. Am. Chem. Soc. 136, 646–649 (2014).
Wang, P. et al. Ligand-promoted meta-C-H amination and alkynylation. J. Am. Chem. Soc. 138, 14092–14099 (2016).
Anugu, R. R., Munnuri, S. & Falck, J. R. Picolinate directed arene meta-C-H amination via FeCl3 catalysis. J. Am. Chem. Soc. 142, 5266–5271 (2020).
Yadav, N., Taneja, N., Musib, D. & Hazra, C. K. Practical access to meta-substituted anilines by amination of quinone imine ketals derived from anisidines: efficient synthesis of anti-psychotic drugs. Angew. Chem. Int. Ed. 62, e202301166 (2023).
Lv, Q., Hu, Z., Zhang, Y., Zhang, Z. & Lei, H. Advancing meta-selective C–H amination through non-covalent interactions. J. Am. Chem. Soc. 146, 1735–1741 (2024).
Sun, K., Li, Y., Xiong, T., Zhang, J. & Zhang, Q. Palladium-catalyzed C−H aminations of anilides with N-fluorobenzenesulfonimide. J. Am. Chem. Soc. 133, 1694–1697 (2011).
Yin, Y., Xie, J., Huang, F. Q., Qi, L. W. & Zhang, B. Copper-catalyzed remote C−H amination of quinolines with N-fluorobenzenesulfonimide. Adv. Synth. Catal. 359, 1037–1042 (2016).
Ji, D. et al. Metal-free remote C–H bond amidation of 8-amidoquinolines on the C5 position under mild conditions. Org. Lett. 18, 4478–4481 (2016).
Sahoo, H., Reddy, M. K., Ramakrishna, I. & Baidya, M. Copper-catalyzed 8-amido chelation-induced remote C−H amination of quinolines. Chem. Eur. J. 22, 1592–1596 (2016).
Zhu, H. et al. Silver(I)-Catalyzed C4–H Amination of 1-Naphthylamine Derivatives with Azodicarboxylates. Org. Lett. 20, 620–623 (2018).
Yi, H. et al. Coordination strategy-induced selective C–H amination of 8-aminoquinolines. Chem. Commun. 53, 6736–6739 (2017).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C-H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Zhang, Z. et al. Para-selective nitrobenzene amination lead by C(sp2)-H/N-H oxidative cross-coupling through aminyl radical. Nat. Commun. 15, 4186 (2024).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014).
Gao, B. et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017).
Guo, T. et al. A new portal to sufex click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F−SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Xi, Z. et al. Regioselective umpolung para-C–H functionalization of arylhydroxylamines. Nat. Synth. 2, 778–788 (2023).
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Himo, F. et al. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 127, 210–216 (2005).
Binder, W. H. & Sachsenhofer, R. ‘Click’ chemistry in polymer and materials science. Macromol. Rapid Commun. 28, 15–54 (2007).
Voskresenska, V. et al. Photoaffinity labeling via nitrenium ion chemistry: protonation of the nitrene derived from 4-amino-3-nitrophenyl azide to afford reactive nitrenium ion Pairs. J. Am. Chem. Soc. 131, 11535–11547 (2009).
Sletten, E. M. & Bertozzi, C. R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 44, 666–676 (2011).
Geurink, P. P., Prely, L. M., van der Marel, G. A., Bischoff, R. & Overkleeft, H. S. Photoaffinity labeling in activity-based protein profiling. Top. Curr. Chem. 324, 85–114 (2012).
Szkółka, A. et al. THETA as an efficient Cu-binding ligand for manual and automated “Click” synthesis: the rufinamide case. Org. Process Res. Dev. 28, 3257–3266 (2024).
Scriven, E. F. V. & Turnbull, K. Azides: their preparation and synthetic uses. Chem. Rev. 88, 297–368 (1988).
Brase, S., Gil, C., Knepper, K. & Zimmermann, V. Organic azides. An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 44, 5188–5240 (2005).
Keana, J. F. W. & Cai, S. X. New reagents for photoaffinity labeling: synthesis and photolysis of functionalized perfluorophenyl azides. J. Org. Chem. 55, 3640–3647 (1990).
Chehade, K. A. H. & Spielmann, H. P. Facile and efficient synthesis of 4-azidotetrafluoroaniline: a new photoaffinity reagent. J. Org. Chem. 65, 4949–4953 (2000).
Liu, Q. & Tor, Y. Simple conversion of aromatic amines into azides. Org. Lett. 5, 2571–2572 (2003).
Liu, C.-Y. & Knochel, P. Preparation of polyfunctional aryl azides from aryl triazenes. A new synthesis of ellipticine, 9-methoxyellipticine, isoellipticine, and 7-carbethoxyisoellipticine. J. Org. Chem. 72, 7106–7115 (2007).
Telvekar, V. N. & Sasane, K. A. Simple and efficient method for the preparation of aryl azides using sonication. Synth. Commun. 42, 1085–1089 (2012).
Zhu, W. & Ma, D. Synthesis of aryl azides and vinyl azides via proline-promoted CuI-catalyzed coupling reactions. Chem. Commun. 2004, 888−889 (2004).
Li, Y., Gao, L.-X. & Han, F.-S. Reliable and diverse synthesis of aryl azides through copper-catalyzed coupling of boronic acids or esters with TMSN3. Chem. Eur. J. 16, 7969–7972 (2010).
Tang, C. & Jiao, N. Copper-catalyzed C-H azidation of anilines under mild conditions. J. Am. Chem. Soc. 134, 18924–18927 (2012).
Liu, Y., Bai, S., Du, Y., Qi, X. & Gao, H. Expeditious and efficient ortho-selective trifluoromethane-sulfonylation of arylhydroxylamines. Angew. Chem. Int. Ed. 61, e202115611 (2022).
Hernandez-Perez, A. C. & Collins, S. K. A visible-light-mediated synthesis of carbazoles. Angew. Chem. Int. Ed. 52, 12696–12700 (2013).
Wang, L. & Fan, R. Divergent construction of nitrogen-containing polycyclic compounds with a dearomatization strategy. Org. Lett. 14, 3596–3599 (2012).
Yang, X., Shan, G. & Rao, Y. Synthesis of 2-aminophenols and heterocycles by Ru-catalyzed C–H mono- and dihydroxylation. Org. Lett. 15, 2334–2337 (2013).
Yuan, H. et al. Tandem approach to NOBIN analogues from arylhydroxylamines and diaryliodonium salts via [3,3]-sigmatropic rearrangement. Chem. Commun. 56, 8226–8229 (2020).
Acknowledgements
H.G. thanks the financial supports from the National Natural Science Foundation of China (22271176, 22471144), the Taishan Scholar Project of Shandong Province (tsqn202306015), Shandong University and Ningxia University. L.X. thanks the financial supports from the Taishan Scholar Project of Shandong Province (tsqn202312066), Shandong Provincial Natural Science Foundation Outstanding Youth Fund (ZR2024YQ016), Youth Innovation Team Program in Colleges and Universities of Shandong Province (2022KJ228) and Qilu Young Scholar Program of Shandong University.
Author information
Authors and Affiliations
Contributions
H.G. conceived and directed the project. Y.B. designed and performed experiments. H.J. performed the density functional theory calculations and mechanistic analysis. W.X. helped with the collection of some new compounds and data analysis. H.G. and L.X. wrote the manuscript with input from all other authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhen Zhang and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, Y., Jiang, HM., Xu, W. et al. Metal-free para-selective C-H amination and azidation of N-arylhydroxylamines. Nat Commun 16, 8399 (2025). https://doi.org/10.1038/s41467-025-63534-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63534-8