Abstract
Immune checkpoint blockade (ICB) has improved outcomes for patients with head and neck squamous cell carcinoma (HNSCC), but predictive biomarkers remain limited. Here, we use a time-resolved, multi-omic approach in a murine HNSCC model to characterize peripheral immune responses to ICB. Single-cell transcriptomics and T/B cell receptor analyses reveal early on-treatment expansion of effector memory T and B cell repertoires in responders, preceding tumor regression. These dynamic immune features inform a composite transcriptional signature that accurately predicts ICB response in independent human HNSCC cohorts. LiBIO outperforms existing biomarkers and generalizes to melanoma, non-small cell lung cancer, and breast cancer without retraining. These findings suggest that early treatment-induced changes in circulating immune repertoires reflect the host’s capacity to mount an effective antitumor response. This work provides a framework for leveraging transient peripheral immune dynamics to develop non-invasive, high-fidelity biomarkers for response to immunotherapy across cancer types.
Similar content being viewed by others
Introduction
Immune checkpoint blockade (ICB) has shown well-established efficacy in the treatment of numerous cancer types, including head and neck squamous cell carcinoma (HNSCC). However, only a subset of patients experiences a beneficial response, and these rates can vary widely among different cancer types1. This variability highlights the urgent need to identify reliable biomarkers that can predict which patients are likely to benefit from ICB treatment.
The FDA has approved several key biomarkers associated with response to ICB, including tumor mutation burden (TMB), PD-1 gene expression, and microsatellite instability (MSI)2. Higher TMB is associated with an increased likelihood of response, likely due to the greater availability of neoantigens that can be targeted by the immune system3,4. PD-1 protein expression is one of the commonly established biomarkers of ICB response across multiple cancer types, including non-small cell lung cancer (NSCLC) and melanoma5. MSI is another widely used biomarker of ICB response. Tumors classified as MSI-high tend to respond favorably to immunotherapy6. In HNSCC, two FDA-approved immunotherapy biomarkers are utilized: tumor mutational burden (TMB) and the programmed death-ligand 1 (PD-L1) combined positive score (CPS). The CPS measures the expression of PD-L1 on both tumor cells and immune cells, thereby providing a comprehensive assessment of the tumor’s interaction with the immune system7,8,9,10. Beyond these FDA-approved biomarkers, numerous other genomic and transcriptomic ICB biomarkers have been identified for different cancer types11,12. For instance, TIDE13 identified a T cell exhaustion signature that is predictive of ICB response in melanoma. IMPRES14 characterized gene pairs in the immune checkpoint pathway and identified pairs associated with ICB response. Greater numbers of tumor-infiltrating lymphocytes (TILs), inferring a pre-existing immune response within the tumor microenvironment, has been shown to correlate with better immunotherapy outcomes15,16,17. Additionally, gene expression profiles reflecting immune activation and cytokine levels can provide further insights into the likelihood of response18,19,20. Despite this progress, the predictive accuracy of existing biomarkers remains limited11. Additionally, these signatures are designed to characterize tumor-intrinsic and microenvironmental features, necessitating the collection of tumor tissue samples through biopsies or surgical procedures.
In contrast to invasive tumor sampling that introduce both cost and risk of morbidity, liquid biopsies offer a minimally-invasive alternative that can be performed with relative ease. Liquid biopsies are designed to capture two fundamental types of data: (1) information on the cancer cells themselves, including circulating tumor cells, circulating tumor DNA, exosomes, and more21,22,23; (2) information on immune cells circulating in the blood, whose abundance has been reported to be associated with ICB response, including monocytes, dendritic cell, myeloid cell, lymphoid cell21,23, and the neutrophil-to-lymphocyte ratio24. Most of these abundance signatures were developed as pre-treatment biomarkers and focused mainly on NSCLC and melanoma24,25. The antitumor immune response is inherently unidirectional and integrates the peripheral immune system through a sequential pathway: tumor → regional lymphatics → periphery → tumor. This framework underscores the need to refocus on a systematic investigation of peripheral immune events at more advanced time points during the treatment window with biomarker identification serving as the cornerstone of this effort.
Here, we conduct both bulk and single-cell transcriptomic sequencing and single-cell TCR sequencing at four time points across the ICB treatment span in a mouse HNSCC model. These include one pre-treatment time point and three on-treatment time points. We utilized these data to identify effective liquid biomarkers for anti-PD-1 treatment in human HNSCC and specifically learn which time point is the most informative and predictive with obvious preference to earlier time points. By analyzing the mouse data, we charted the clonal expansion of both T and B cells during ICB treatment, observing that responders and non-responders show a significant difference in the dynamics of their clonal repertoire expansion and pruning. Markedly, the strongest response to the ICB was detected in the earliest on-treatment time point, showing significant transcriptomic and TCR clonality differences between responders and non-responders. This aligns with the timing of tumor shrinkage initiation. Beyond our preclinical findings, we proceed to show that the ICB response related signatures derived using mouse data can successfully predict ICB response in HNSCC patients, demonstrating their potential clinical application. To our best knowledge, this is the first study that systemically characterizes the dynamic changes of circulating immune cells in the blood during ICB treatment across four sequential time points, associating these alterations with ICB response in patients.
Results
Study overview
We employed our previously characterized 4MOSC carcinogen-induced orthotopic HNSCC preclinical model26,27 to evaluate immune dynamics during ICB therapy. Two cohorts of mice were used, with one cohort comprising 45 mice for bulk RNA sequencing (RNA-seq) and the other comprising 16 mice for single-cell RNA sequencing (scRNA-seq) and single-cell TCR sequencing. Lesions developed consistently at expected time points26,27 (Fig. 1A, B). Blood samples were collected at four key intervals: pre-treatment (Day 4) and three on-treatment time points (Days 9, 17, and 24), aligned with the administration of anti-PD-1 therapy. Tumor growth and immune responses were monitored longitudinally to assess treatment efficacy (Fig. 1C, D). Bulk RNA-seq provided an overview of immune dynamics in the larger cohort, while scRNA-seq and scTCR sequencing in the smaller cohort delivered high-resolution insights into immune repertoire changes, supporting the study’s focus on peripheral immune biomarkers for predicting ICB outcomes.
A Schematic of the experimental design. Blood was collected at four time points: one pre-treatment time point (four days after cancer cell transplantation) and three on-treatment time points (days 9, 17, and 24). Anti-PD-1 treatment was initiated on day 4 following the first blood collection. The response to ICB treatment for each mouse was assessed based on RECIST criteria. This graphical abstract was created with BioRender.com. B Summary of in-house and publicly available HNSCC datasets used in this study. Three in-house datasets were generated, including an RNA-seq dataset from 45 mice across four time points, consisting of 15 ICB responders and 30 non-responders. Additionally, single-cell RNA-seq and TCR sequencing were performed on an independent cohort of 16 mice, comprising 7 responders and 9 non-responders. Another in-house dataset includes a single-cell RNA-seq dataset from ICB-treated HNSCC patients, containing 20 patients (9 responders and 11 non-responders). Furthermore, two publicly available single-cell ICB-treated datasets and four publicly available bulk RNA-seq ICB datasets were incorporated into the analysis. C Dynamic changes in tumor volume across time points in responders. The dashed line indicates the four time points at which whole blood were collected. D Dynamic changes in tumor volume across time points in non-responders. The dashed line indicates the four time points at which PBMCs were collected. Source data are provided as a Source Data file.
The 4MOSC model used in this study is a well-characterized, carcinogen-induced, orthotopic HNSCC model that exhibits a reproducible mixed-response to anti-PD-1 therapy (~30–40%), consistent with clinical response rates observed in human HNSCC. This model was originally described in Wang et al., Nat Comm 201927 and further refined in Saddawi-Konefka et al., Nat Comm 202226, where we demonstrated that response heterogeneity is driven by differences in immune activation and lymphatic function, rather than tumor-intrinsic clonal variation.
Single-cell analysis uncovers specific temporal changes in blood T and B cell abundances differentiating ICB responders from non-responders
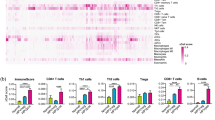
To profile the immune landscape and its dynamic changes during ICB treatment, we performed unsupervised clustering of cells from 63 samples collected from 16 mice across the 4 time points described above. This analysis identified 9 major cell types (SF1A, B). Among mononuclear cells, CD8+ T cells, CD4+ T cells, NK cells, and B cells are the dominant cell types in the blood samples. Comparisons of on-treatment to pre-treatment time points revealed significant increases in the abundance of CD8+ T cells, CD4+ T cells, and B cells following ICB treatment (one-tailed Wilcoxon rank-sum test, p < 0.05) (Fig. 2A, B). In contrast, the relative abundance of neutrophils decreased over time (Fig. 2B).
A Dynamic changes in the abundance of major cell types in blood. B Box plots showing the abundance changes of four cell types significantly influenced by ICB treatment (n = 16 biological replicates; each dot represents one mouse). C Abundance changes of effector memory CD8+ T cells in responders (n = 7) and non-responders (n = 9) across four time points. Each dot represents an individual mouse. D Abundance differences of effector memory CD8+ T cells between responders and non-responders. E Abundance changes of B cells in responders (n = 7) and non-responders (n = 9) across four time points. Each dot represents an individual mouse. F Abundance differences of B cells between responders (n = 7) and non-responders (n = 9). In box plots (B, C, and E), the center line indicates the median; the box spans the interquartile range (IQR, 25th to 75th percentile); whiskers extend to values within 1.5× IQR from the quartiles; and each dot represents one biological replicate (a single mouse). In (D and F), dots represent the mean, and error bars indicate the standard error of the mean (± SEM). The unit of study is the individual mouse. Statistical significance was assessed using a one-tailed Wilcoxon rank-sum test unless otherwise noted. The Mann–Kendall test was used to evaluate monotonic changes across time points. For all panels, the X-axis represents time points, and the Y-axis represents the fraction of cells out of the total measured. Source data are provided as a Source Data file.
To identify T cell subpopulations associated with ICB response, we further identified subsets of CD8+ T cells and CD4+ T cells by unsupervised clustering. To ensure accurate clustering and annotation of these subpopulations, we projected the CD8+ and CD4+ T cells onto a reference mouse T cell database (SF 1C, D). This analysis identified two dominant subtypes of CD8+ T cells including naïve CD8+ T cells and effector memory CD8+ T cells (Tem) (SF 1E, F), and three major subtypes of CD4+ T cells including naïve CD4+ T cells, Type 1T helper (Th1) cells and regulatory T cells (Tregs) (SF 1G, H). Among these T cell subpopulations, Tem (Fig. 2C) and Th1 CD4+ T cells increased following ICB treatment in responders (SF 2A). A monotonic increase in these two cell types was observed across all three on-treatment time points in responders, resulting in the highest accumulation levels at the late on-treatment time point (Day 24) (Mann–Kendall test, P-value for Tem 5.6 × 10−4, P-value for Th1: 9.0 × 10−5,) (Fig. 2C, D and SF 2A, B). This monotonic increase is not observed in non-responders following ICB treatment: While there was a modest increase in Tem at the early on-treatment time point (Day 9), this was followed by a sharp decline at the middle time point (Day 17) (Fig. 2C).
B cell abundance increased in both responders and non-responders following ICB treatment. There was no significant difference between responders and non-responders at the pre-treatment (Day 4) and late on-treatment time points (time points 4) (Fig. 2E). However, we observed a modest but statistically significant earlier increase in B cells among responders at the early on-treatment time point (Day 9) (one-tailed Wilcoxon rank-sum test, P = 0.027). In contrast, non-responders exhibited a delayed B cell accumulation that became apparent only at the middle on-treatment time point (Fig. 2F). These findings align with a previous study28 that demonstrated a predictive role for B cells in response to ICB in HNSCC.
Overall, distinct dynamic changes in immune cell composition were observed in blood samples following ICB treatment, highlighting key differences between responders and non-responders. Responders demonstrated an accumulation of T cell-mediated cytotoxic immune cells and a downregulation of suppressive immune cells contributing to enhanced immune activation. Conversely, non-responders initially accumulated immune response-related cells at the early on-treatment time point, followed by their decrease. Moreover, B cell accumulation occurred at different time points between responders and non-responders, further underscoring the distinct immune dynamics associated with ICB response. The accumulation of Tem and B cells began early in the on-treatment phase (Day 9) and negatively correlated with tumor shrinkage, which also started at Day 9 in responders (Fig. 1C, D). These results not only highlight the crucial role of Tem and B cells in ICB response but also illustrate that the early on-treatment phase serves as a watershed between responders and non-responders in HNSCC.
ICB treatment induces T cell and B cell clonal expansion that is predictive of ICB response
Single-cell TCR sequencing data enable the detection of expanding T cell clones. Analysis of TCR sequencing data uncovers a clonal expansion in CD8+ T cells within the blood Tem subpopulation during ICB treatment (Fisher’s exact test, odds ratio: 30.5, p < 2.2 × 10−16) (Fig. 3A, B). Moreover, these expanded clones were significantly enriched in responders compared to non-responders (Fisher’s exact test, odds ratio: 1.73, P-value: 3.75 × 10−5) (Fig. 3C) (SF 3A). The strongest expansion was observed at early on-treatment time points (one-tailed Wilcoxon rank-sum test, p = 0.027), followed by a dramatic decrease at the middle on-treatment time point (Fig. 3D). ICB treatment-induced clonal expansion is followed by subsequent pruning at the late on-treatment time point (Fig. 3D). In CD4+ T cells, the expanded clones are primarily enriched in the Th1 subpopulation (SF 3B, C), with the strongest expansion occurring at early on-treatment as well, without significant differences between responders and non-responders (SF 3D).
A UMAP of CD8+ T cells, with each dot representing a single-cell and colored by clone size. B Distribution of expanded clones (clone size ≥2, represented by red bars) and non-expanded clones (clone size = 1, represented by green bars) between effector memory CD8+ T cells and other CD8+ T cells. P-value and odds ratio were calculated using Fisher’s exact test. C Distribution of CD8+ T cell clone sizes between responders and non-responders across four time points. Statistical significance was determined using Fisher’s exact test. D Comparison of effector memory cell (Tem) clonal expansion estimated using single-cell TCR-seq data, between responders (n = 7) and non-responders (n = 9). The y-axis represents the average size of Tem clones, where higher values indicate greater clonal expansion. Each dot represents an individual sample. Statistical significance was determined using a one-tailed Wilcoxon rank-sum test. E Comparison of T cell clonal expansion estimated using bulk RNA-seq data, between responders (n = 15) and non-responders (n = 30). The y-axis represents the scaled Simpson index, where higher values indicate greater clonal expansion. Each dot represents an individual mouse. Statistical significance was determined using a one-tailed Wilcoxon rank-sum test. F Comparison of B cell clonal expansion estimated using bulk RNA-seq data, between responders (n = 15) and non-responders (n = 30). The y-axis represents the scaled Simpson index, where higher values indicate greater clonal expansion. Each dot represents an individual mouse. Statistical significance was determined using a one-tailed Wilcoxon rank-sum test. G AUC (Area Under the Curve) values for predicting ICB response based on the bulk B cell clonal expansion index, bulk T cell clonal expansion index, and single-cell Tem cell clonal expansion index at Day 9. In box plots (D–F), the center line indicates the median; the box spans the interquartile range (IQR, 25th to 75th percentile); whiskers extend to values within 1.5× IQR from the quartiles; and each dot represents one biological replicate (a single mouse). Source data are provided as a Source Data file.
To validate these findings, TCR analysis was performed using bulk RNA-seq data from an independent mouse cohort. The bulk TCR analysis results confirmed that T cell clonal expansion begins at early on-treatment time points and reaches completion by the late on-treatment time point, with responders exhibiting significantly stronger expansion than non-responders (one-tailed Wilcoxon rank-sum test, p = 0.026) (Fig. 3E). BCR analysis using bulk RNA-seq data further demonstrated significant B cell clonal expansion following ICB treatment (Fig. 3F). Responders exhibit increased B cell expansion at early (one-tailed Wilcoxon rank-sum test, p = 0.00024) and middle on-treatment time points (one-tailed Wilcoxon rank-sum test, p = 0.031), before returning to pre-treatment levels at the late time point (Fig. 3F). Interestingly, both T and B cell clonal expansion at early on-treatment time points exhibit strong predictive power for ICB response (Fig. 3G). Specifically, B cell clonal expansion demonstrates superior predictive ability compared to T cell clonal expansion at early on-treatment time points (Fig. 3G). This aligns with the timing of tumor shrinkage initiation in the responders (Fig. 1C, D) as well as the changes in cell abundance estimated from the single-cell data (Fig. 2D, F). Notably, B cell clonal expansion is predictive not only at the pre-treatment time point but also at two on-treatment time points, further underscoring the critical role of B cells in mediating the ICB response in HNSCC (Fig. 3G) (SF 4A–D).
Taken together, these findings underscore three key points: first, ICB-induced T and B cell clonal expansion occurs predominantly at early on-treatment time points and is followed by subsequent pruning by the late on-treatment time point despite continued PD-1 treatment; second, responders exhibit significantly stronger expansion in both T and B cells compared to non-responders; and third, this clonal expansion can be reliably detected in the blood using either single-cell TCR sequencing or bulk RNA-seq.
Serial Bulk RNA-seq analysis identifies the early on-treatment time point as most predictive of ICB response in the blood
To characterize the dynamic changes in biological functions following ICB treatment, we applied the fuzzy c-means algorithm to identify patterns of dynamic alterations in the expression of the different circulating immune cell types from bulk RNA-seq data. This analysis identified eight characteristic clusters (C1–C8) of gene expression patterns in both responders and non-responders (Fig. 4A, B). In responders, genes within five clusters (C1,3,4,5,8) were upregulated at the Day 24 compared to pre-treatment, whereas in non-responders, six clusters (C1,2,4,5,6,7) were downregulated comparing these time points. The upregulated clusters in responders were related to B cell immunity, immunoglobulin production, and associated pathways, including protein-RNA complex assembly, mRNA processing, and mitochondrial gene expression (Fig. 4A, B). In contrast, genes related to B cell activation and immunoglobulin production were downregulated in non-responders. These findings suggest that B cells play a crucial role in the response to ICB treatment and could serve as predictive biomarkers for ICB response in HNSCC28. In addition to the divergent dynamic changes between responders and non-responders, immune response pathways were activated early in both groups. However, in responders, additional pathways, such as myeloid leukocyte activation and chemokine production, were also activated to enhance and support the immune response. In non-responders, pathways related to innate immune response, leukocyte-mediated cytotoxicity, and cell killing were downregulated, thereby suppressing the immune response (Fig. 4A, B). Notably, the highest number of differentially expressed genes between responders and non-responders was observed at the early on-treatment time point (Day 9), highlighting its potential functional and predictive significance (SF 5A).
A Clusters of gene expression in responders. Each row of the heatmap represents one gene, and each column represents one time point. Colors indicate scaled gene expression levels. The line plot to the left of the heatmap shows the expression change pattern of each cluster. On the right side of the heatmap are the pathways enriched based on the genes within each corresponding cluster. B Clusters of gene expression in non-responders, with the same representation as in (A). C Machine learning models were trained for each time point using bulk RNA-seq data. The ICB response prediction performance of these time point-specific models was validated using five-fold cross validation. D Dynamic changes in gene expression between different time points were used to train a machine learning model. The ICB response prediction performance of this model was validated using five-fold cross validation. Source data are provided as a Source Data file.
To further assess the association between each time point and treatment outcome, we trained a lasso regression-based ICB predictor using bulk RNA-seq data from each time point. The five-fold cross validation reveals that the predictor trained at early on-treatment time point (Day 9) achieves the same performance as the late on-treatment time point (Day 24), indicating that the former can serve as an optimal time point for treatment efficacy (Fig. 4C). The early on-treatment time point (Day 9) emerges as the optimal window for assessing treatment efficacy, as it precedes observable tumor shrinkage and captures critical early immune dynamics associated with response. To investigate whether dynamic changes across time points could further enhance prediction accuracy, we built a predictor based on gene expression changes between time points. The changes between time points 4 and 2 outperformed any single-time point and dynamic change-based predictors in predicting ICB response, demonstrating that temporal dynamics may provide valuable insights for predicting ICB response but do not provide a marked increase in the prediction power over that of the early on-treatment time point (Fig. 4D).
Taken together, these results highlight distinct dynamic gene expression patterns between responders and non-responders, with differences in immune response and B cell activation. Furthermore, the observed temporal changes in gene expression serve as promising biomarkers for predicting ICB response, with the early on-treatment time point emerging as an optimal time for treatment assessment.
Analysis of mouse SC data identifies predictive blood Tem and B cell signatures that are predictive of ICB response in both bulk and SC expression cohorts of human patients
We demonstrated that ICB treatment induces clonal expansion and proliferation of Tem and B cells, particularly at early on-treatment time points, especially in responders. To translate these findings into a clinical context and improve outcome prediction for ICB treatment, we identified Tem and B cell gene response prediction signatures using single-cell expression data from the early on-treatment time point. An effector memory CD8+ T cell and B cell gene signature were identified, composed of the expression of 164 and 137 genes, respectively. Among the genes comprising the Tem signature, several well-established markers are present, including Cxcr3, Cxcr6, and Nkg7. Interestingly, we also identified genes previously characterized as markers of transitional T cells (e.g., Cx3cr1, Tbx21, Gzmb) (SF 6A, B). These transitional T cells differentiate from stem-like Tcf-1⁺ CD8⁺ T cells and do not progress to an exhausted state29,30. These cells exhibit enhanced functionality in T cell-mediated cytotoxicity and can be stimulated by anti-PD-1 inhibitors. This observation further supports the critical role of Tem cells in mediating the response to ICB. In the B cell gene signature, canonical B cell markers such as Cd19, Cd22, CD79a, CD79b, and Ms4a1 were identified. Additionally, novel genes not previously associated with these cell types were also identified (Supplementary Data 1).
To evaluate the predictive power of these mouse-derived signatures for predicting patients’ response, we tested our predictive biomarkers in a total of seven ICB-treated HNSCC cohorts. These included four bulk RNA-seq datasets31,32,33,34 and two single-cell datasets35,36 and one new in-house ICB-treated blood single-cell HNSCC dataset, previously unpublished. Both the Tem and B cell signatures demonstrated robust performance in predicting ICB response in blood samples. Specifically, the Tem signature achieved an average AUC of 0.69 ± 0.15, while the B cell signature achieved an average AUC of 0.74 ± 0.11 (Fig. 5A). To enhance accuracy and robustness, we combined the Tem signature and the B cell signature into a composite score, termed the LiBIO (Liquid Biomaker of Immunotherapy Outcomes) score, by simply calculating their mean value in an unsupervised fashion. Notably, this LiBIO score demonstrated greater robustness and predictive accuracy compared to either signature alone, achieving a mean AUC of 0.80 ± 0.10 (Fig. 5A). To assess whether dynamic changes in blood reflect corresponding immune activity within the tumor microenvironment, we first generated in-house bulk RNA-seq data from tumors of HNSCC mice treated with anti-PD-1. Tumor biopsies were collected 14 days after cell implantation from five responders and four non-responders (SF 7A). We also analyzed an independent murine dataset in which single-cell RNA-seq and TCR-seq were performed on tumors from ICB-sensitive and ICB-resistant HNSCC models. Across both datasets, effector memory T cell, B cell, and LiBIO scores were elevated in ICB-sensitive or responder tumors compared to resistant or non-responder tumors (two-tailed Wilcoxon rank-sum test; SF 7B, C). While not statistically significant due to limited sample size, the trends were consistent and biologically meaningful. Notably, in the ICB-sensitive tumors, T cell clonal expansion significantly increased following treatment (two-tailed Wilcoxon rank-sum test; SF7 D), mirroring the dynamics observed in the peripheral blood. These results suggest that the clonal expansion detected in the blood is reflective of tumor-intrinsic immune activation and support the notion that blood-derived immune signatures, such as the LiBIO score, capture biologically meaningful tumor immune dynamics during ICB therapy.
A, B AUC (Area Under the Curve) values for ICB response prediction based on Tem and B cell signature scores, as well as the combined score (calculated as the mean of Tem and B cell signature scores) in both blood (A) and tumor (B) HNSCC cohorts. C AUC values for the combined score compared to previously published transcriptomic signatures across 10 HNSCC cohorts. Each dot represents one HNSCC cohort, displayed using different colors and shapes. The box plot displays the median (center line), interquartile range (IQR; box limits: 25th to 75th percentile), and whiskers extending to 1.5× IQR from the quartiles. Two-tailed P-values were calculated using the Wilcoxon rank-sum test to compare the LiBIO score against other signatures. D Hazard ratios (HRs) for overall survival per 1-unit increase in the combined Tem and B cell score (LiBIO score), adjusted for age and sex, in three independent HNSCC cohorts: TCGA (n = 516), Foy et al. (n = 102), and INSPIRE (n = 12). Dots represent HR estimates; error bars indicate the 95% confidence interval (CI). Statistical significance was assessed using the Wald test. E, F Identification of fixed thresholds for the LIBIO score in single-cell (E) and bulk (F) cohorts. The X-axis represents the cohorts, while the Y-axis indicates the odds ratio (OR) of responders versus non-responders (see “Methods”). Orange bars correspond to training cohorts, and green bars represent independent validation cohorts. Source data are provided as a Source Data file.
We then turned to evaluate the Tem, B cell, and LiBIO scores in tumor datasets from HNSCC patients. Both the Tem and B cell signatures performed well, with comparable predictive accuracy in bulk and scRNA-seq datasets. Specifically, the Tem signature achieved an average AUC of 0.75 ± 0.087, and the B cell signature achieved an average AUC of 0.74 ± 0.12. Moreover, the LiBIO score outperformed both individual signatures, with an average AUC of 0.78 ± 0.046 (Fig. 5B). To benchmark the LiBIO score against FDA-approved biomarkers, we next compared it to the PD-L1 CPS. Due to the lack of publicly available ICB-treated HNSCC datasets with both transcriptomic profiles and clinical CPS annotations, we estimated CPS using a publicly available single-cell RNA-seq dataset (“Method”) previously incorporated in this study37. This dataset contains both malignant and immune cells, allowing for transcriptomic estimation of CPS based on CD274 expression. The LiBIO score showed superior predictive performance compared to the estimated CPS score in Receiver Operating Characteristic (ROC) analysis (SF 8A, B). Nonetheless, because CPS was inferred from transcriptomic data rather than immunohistochemistry, further validation in datasets with clinically measured CPS is warranted. Subsequently, we assessed the predictive efficacy of the LiBIO score across patient cohorts, comparing its performance to several contemporary transcriptomics-based biomarkers15,16,18,19,38,39,40,41,42,43. Overall, the LiBIO score exhibited superior predictive performance relative to alternative biomarkers (Fig. 5C). Interestingly, two functional CD8+ T cell related tumor signatures also performed strongly in bulk RNA-seq cohorts, highlighting the pivotal role of functional CD8+ T cells in mediating ICB responses. However, while these functional CD8+ T cell related signatures performed well in bulk datasets, their performance was less consistent in single-cell cohorts. In contrast, the LiBIO score demonstrated greater robustness and predictive accuracy across both bulk and scRNA-seq datasets (Fig. 5C). Additionally, survival analyses revealed that patients with higher LiBIO scores had significantly improved survival outcomes compared to those with lower scores. This predictive association was more pronounced in ICB-treated cohorts and remained significant in ICB-treatment-naïve datasets (Fig. 5D) (SF 9A–C). Importantly, although the signatures were derived from an HPV-negative HNSCC mouse model, they remained significantly correlated with patient survival after adjusting for HPV status (Fig. 5D).
A universal fixed threshold could further facilitate the clinical utility of the LiBIO score for evaluating ICB response in HNSCC patients. Learning from a few cohorts used to optimize this decision threshold, we identified a LiBIO threshold of 0.424 that best distinguished responders from non-responders in single-cell datasets (SF 10A) and 0.201 in bulk datasets (SF 10B) (“Methods”). To understand the differing thresholds between bulk and single-cell data, we analyzed the distribution of LiBIO scores across both data types. Single-cell cohorts exhibited higher LiBIO scores compared to bulk RNA-seq cohorts (SF 11A). This was accompanied by significantly higher estimated abundances of B and T cells in the single-cell cohorts (SF 11B) (“Methods”), likely due to technical differences. These observations help explain why the single-cell threshold (0.424) is higher than the bulk RNA-seq threshold (0.201). Using this optimized decision threshold, the odds ratio for response in the single-cell training dataset was 4.0 (Fig. 5E) and 5.0 in the bulk dataset (Fig. 5F). Reassuringly, when applied to independent HNSCC patient datasets, the mean odds ratio was even higher, at 5.6 ± 2.1 in single-cell datasets (Fig. 5E) and 4.70 ± 2.5 in bulk datasets (Fig. 5F). These findings highlight the utility of Tem and B cell signatures in predicting ICB response. Furthermore, a fixed LIBIO score threshold shows promise for clinical application, providing a robust and reliable method to distinguish responders from non-responders in HNSCC patients undergoing ICB treatment.
Cross-cancer validation of the LiBIO score as a predictive biomarker for ICB response
Tem and B cells play critical roles in mediating ICB responses, not only in HNSCC, but also across multiple cancer types17,44,45,46. To evaluate the broader applicability of the LiBIO score beyond HNSCC, we analyzed its predictive performance across 11 ICB-treated patient groups, including both pre-treatment and on-treatment samples, from four melanoma cohorts4,47,48,49, three NSCLC cohorts50,51,52, and two breast cancer cohorts53,54. The LiBIO score achieved an average AUC of 0.80 ± 0.09 in melanoma, 0.73 ± 0.23 in NSCLC, and 0.72 ± 0.10 in breast cancer cohorts (Fig. 6A). To further enhance clinical interpretability, we aimed to identify fixed decision thresholds for LiBIO score classification specific to each cancer type. For each data type, one cohort was designated as a training cohort to determine the optimal threshold that maximized the odds ratio for ICB response. The optimal thresholds identified were 0.196 for melanoma, 0.632 for NSCLC, and 0.383 for breast cancer (SF 12A–C). In the respective training cohorts, these thresholds achieved odds ratios of 4.7, 1.4, and 2.1 (Fig. 6B). We then applied the same thresholds to the remaining cohorts of each cancer type to evaluate generalizability. In these testing cohorts, LiBIO maintained strong performance, achieving average odds ratios of 3.4 ± 0.34 in melanoma (excluding the Riaz et al. cohort due to RNA-later biopsy preparation, which differs from FFPE used in the other cohorts), 2.3 ± 0.14 in NSCLC, and 1.3 in breast cancer (Fig. 6B). These results highlight the potential of the LiBIO score as a robust and clinically applicable biomarker for predicting ICB response across multiple tumor types.
A Area under the curve (AUC) values for LiBIO score-based prediction of ICB response across 11 patient groups. The x-axis labels indicate both the dataset source and the timing of sample collection relative to anti-PD-1 therapy, where “Pre” denotes samples collected before treatment initiation and “Post” denotes samples collected after treatment had begun. B Odds ratio (OR) for distinguishing responders from non-responders using fixed LiBIO thresholds specific to melanoma, NSCLC, and breast cancer (see “Methods”). Orange bars represent training cohorts used to determine the optimal threshold, and green bars correspond to independent validation cohorts. Source data are provided as a Source Data file.
Discussion
The host antitumor immune response is both dynamic and coordinated, characterized by the trafficking of immune cells from the tumor microenvironment to tumor-draining lymph nodes and subsequently into systemic circulation. This intricate yet synchronized immunobiology supports longitudinal peripheral sampling as a means to monitor the overall host immune response and serve as a surrogate for predicting tumor-specific immunotherapy outcomes. Here, we systematically characterized dynamic changes in the blood during ICB treatment across different time points in a mouse model of head and neck cancer. This approach establishes a platform for developing high-fidelity, non-invasive liquid biomarkers that enable real-time prediction and monitoring of immunotherapy outcomes. Consistent with this, our preclinical findings highlight the potential of longitudinal liquid biopsy approaches to track immune dynamics. Serial blood draws from oral cavity tumor-bearing mice treated with PD-1 blockade revealed an early, transient expansion of effector memory T (Tem) and B cell repertoires, preceding tumor regression. These findings underscore the pivotal role of Tem and B cells in mediating ICB responses in head and neck cancer7,28. Furthermore, temporal shifts in clonality and gene expression at early treatment time points emerged as strong predictors of ICB response, identifying these windows as optimal for assessment. Notably, the translation of a newly identified combined immune effector signature termed LiBIO to human cohorts demonstrated its broad applicability in predicting ICI outcomes, motivating its further prospective study. The LiBIO score not only demonstrates predictive utility for ICB response in both blood and tumor transcriptomic datasets but also exhibits prognostic value in both ICB-treated and ICB-naïve cohorts. While this suggests that LiBIO reflects core features of anti-tumor immune activation, future studies are needed to disentangle its predictive versus prognostic roles in distinct clinical contexts.
As with any study, our work has a few limitations. First and foremost, analogous time series data from tumors or blood of human HNSCC patients does not yet exist, which restricted our ability to directly compare the temporal dynamics observed in our mouse findings to human data. Additionally, we could only find one publicly available HNSCC patient cohort contains PBMC data, which could enable us to test our blood generated signature directly (at the pre-treatment time point). To partially overcome this challenge, we performed an additional blood-based analysis in a small in-house cohort we have generated. Due to the scarcity of PBMC datasets, we extended our validation to tumor-derived datasets. While several tumor datasets are available, most still have small sample sizes. Fortunately, two tumor cohorts with larger sample sizes were accessible, providing a more robust validation of the identified signatures. Another limitation to note is that single-cell TCR sequencing data can only capture a few hundred T cells for each sample, limiting the comprehensiveness of TCR tracing. This constraint hindered our ability to identify specific T cell clones associated with ICB response in our data.
We identified five days after ICB treatment as the early on-treatment time point in mouse that is optimal for assessing ICB response in mouse. This strategy may also be applicable for HNSCC patients, but obviously the best timing of such early on-treatment time point in humans requires further careful investigation. The resulting LiBIO score integrates the Tem and B cell signatures, and quite notably, its application using one fixed decision threshold yields a fairly high predictive odds ratio of response, which surpasses the predictive power of existing biomarkers and a transcriptomic based CPS score. Although this finding is encouraging, we emphasize that the CPS score was derived from RNA expression data and not standard clinical IHC measurements. Collectively, our preclinical insights support the premise that peripheral immune events can serve as a foundation for biomarker discovery, offering a non-invasive, biologically grounded approach to monitor and predict immunotherapy outcomes with high-fidelity. Finally, the approach presented lays a solid basis for developing similar biomarkers in other cancer indications.
Methods
All animal procedures were conducted in accordance with relevant ethical regulations and were approved by the Institutional Animal Care and Use Committee at the University of California, San Diego (UCSD) under protocol number S16200. The approved protocol, titled “Mouse model for cancer development and drug treatment of cancer”, was granted on August 23, 2022, and remains valid through July 21, 2025. It covers the use of Mus musculus (mouse) in full compliance with federal and institutional guidelines.
Mouse and anti-PD-1 treatment
C57BL/6J mice (6–8 weeks old) were housed under specific pathogen-free conditions and used in compliance with institutional animal care guidelines. Tumors were established by injecting 4MOSC1 cells (1 × 10⁵) into the tongue or buccal mucosa, as previously described. Mice were treated intraperitoneally with αPD-1 (clone RMP1-14, Bio × Cell, 200 µg) every three days starting on day 7 on-tumor implantation. Mice were evaluated at least three times per week to monitor tumor progression, weight, grooming, and general condition. Tumor size was recorded using calipers. Studies were concluded at predetermined endpoints or earlier if animals showed signs warranting humane euthanasia, such as >20% body weight loss, inability to groom or ambulate, distress, or tumor ulceration. According to institutional animal care guidelines, mice were euthanized if tongue tumors exceeded 8 mm or buccal tumors exceeded 10 mm, or in the case of ulceration. Both male and female mice were used in the study. Data were disaggregated by sex where relevant, and sex was considered during study design to evaluate any potential sex-based differences. No significant sex-based differences were observed; therefore, combined data are presented.
Sample collection
Mice were bled via retro-orbital puncture using heparinized EDTA-coated glass capillary tubes. Blood was immediately transferred into microcentrifuge tubes containing TRIzol reagent (Thermo Fisher Scientific) for RNA stabilization. Samples were processed according to the manufacturer’s instructions for RNA extraction. For scRNA-seq, blood was diluted in PBS + 0.04% BSA, and cell concentration and viability were determined using the Countess II Automated Cell Counter, targeting 700–1200 cells/µL for downstream analysis.
In-house HNSCC patient single-cell ICB cohort
Twenty pre-treatment PBMC samples were obtained from patients with newly diagnosed advanced-stage HPV-negative oral cavity cancers enrolled in a neoadjuvant immunotherapy clinical study. Deidentified PBMC were used for experimental purposes after patients provided full informed consent under NIH Biospecimen Protocol Number 18-DC-0051 (NCT03429036). The therapeutic agent used was bintrafusp alfa, a dual PD-L1 and TGF-β blocker. As part of this neoadjuvant study, clinical responses were assessed based on pathological responses, specifically by measuring tumor regression in the surgical specimens to determine the degree of tumor shrinkage following immunotherapy. Clinical response was treated as a continuous variable, represented by the percentage of tumor shrinkage. To classify patients into responder and non-responder groups, an arbitrary cutoff was applied. Samples with tumor shrinkage greater than 50% were categorized as responders, while those with 50% or less tumor shrinkage were categorized as non-responders.
Public HNSCC patient cohorts
Four publicly available bulk RNA-seq, Foy et al.31, INSPIRE34, Liu et al.33, Obradovic et al.32, and two single-cell RNA-seq, Bill et al.36, Luoma et al.35, datasets from ICB-treated HNSCC patients were collected. For the bulk RNA-seq data, Transcripts Per Million (TPM) values were obtained from the original publications. For the single-cell datasets, raw counts and cell annotation information were retrieved from the respective original studies. Data normalization and pseudobulk analysis for the single-cell data were performed using the NormalizeData and AverageExpression functions from the Seurat package55. Clinical information, including ICB response status and patient survival data, were also collected from the original publications. Response status of HNSCC patients was based on RECIST criteria56, with “CR/PR” patients classified as responders and “SD/PD” patients classified as non-responders.
Single-cell RNAseq data analysis
Raw scRNA-seq reads were barcode-deduplicated and aligned to the mm10 reference genome using Cell Ranger57 to generate count matrices. These count matrices were then used as input for Seurat to identify cell types and cellular states. Cells with more than 25% mitochondrial content or fewer than 500 expressed genes were removed from downstream analysis. Genes expressed in fewer than 3 cells were also excluded. Variable feature identification was performed using the FindVariableFeatures function with a parameter of 2000 features, followed by clustering with the FindClusters function. Marker genes for each cluster were identified using the FindMarkers function, and major cell types were annotated based on feature genes. Pseudobulk gene expression was calculated using the AverageExpression function.
CD8+ and CD4+ T cells were subset based on the expression of Cd8a, Cd8b1, and Cd4 using the scGate package58. Specifically, CD8+ T cells were identified using the signature Cd8a+, Cd8b1+, Cd4−, and CD4+ T cells using the signature Cd8a−, Cd8b1−, Cd4+. CD8+ and CD4+ T cells were then mapped to a mouse T cell database to annotate their subtypes using ProjecTILs59.
TCR and BCR repertoire analysis
Single-cell TCR sequencing data were aligned to the mouse mm10 V(D)J reference genome using Cell Ranger to obtain clonotype information for each cell. The filtered contig annotation file from the Cell Ranger output was used for downstream analysis. Bulk RNA-seq data were also used to identify T cell and B cell clones using TRUST460. Clone size was calculated by counting the number of identical clones in each sample. Clonal expansion was measured using the Simpson Index, calculated with the immunarch package61.
Bulk RNAseq data analysis
Mouse bulk RNA-seq data were first aligned to the reference genome mm10 to obtain raw counts. TPM values were then calculated based on raw counts and reference genome information. Genes were clustered using the fuzzy c-means algorithm62 from the e1071 package63. The optimal number of clusters was determined using the Elbow method. Gene functional annotation was performed using clusterProfiler64 and data visualization were performed using ComplexHeatmap65.
Identification of Tem and B cell signatures and signature score calculation
Cells from Day 9 samples were subset from the full dataset. The FindMarkers function from Seurat package was then applied to these subset cells to identify marker genes for each cell type. Marker genes for Tem and B cells were defined based on a false discovery rate (FDR ≤ 0.01) and fold changes (≥ 1.5). The signatures generated from the mouse single-cell data were then mapped to human gene symbols to ensure compatibility with human datasets. Genes not expressed in at least one of the six HNSCC cohorts were filtered out. The Tem and B cell signature scores for each sample were calculated using the ssGSEA algorithm66. The combined Tem and B cell score was computed as the mean value of the Tem and B cell scores.
Estimation of immune cell abundance
To estimate immune cell abundance across different data modalities, we applied complementary approaches tailored to single-cell and bulk RNA-seq datasets.
For single-cell RNA-seq cohorts, immune cell abundance was calculated directly based on cell type annotations. Specifically, we computed the relative abundance of B cells and T cells by dividing the number of annotated cells of each type by the total number of cells within each sample. Cell annotations were assigned using canonical markers and validated clustering from the original publications or our in-house pipeline.
For bulk RNA-seq cohorts, we estimated immune cell abundance using CODEFACS (COnfident DEconvolution For All Cell Subsets)67, a robust deconvolution framework that enables accurate inference of cell type-specific signals from bulk RNA-seq data. As the input reference signature, we used a curated single-cell RNA-seq dataset36 from HNSCC, which includes well-characterized malignant, immune, and stromal cell populations. This reference was used to deconvolve bulk expression profiles and extract relative abundance estimates for B cells and T cells across samples. All deconvolution steps were performed using default parameters unless otherwise specified.
Calculation of PD-L1 combined positive score from single-cell RNA-seq data
To approximate the clinically defined PD-L1 CPS using scRNA-seq data, we computed a transcriptomic analog based on the expression of CD274, which encodes PD-L1.
Cell annotations were obtained from the original publication and used to classify each cell as either a tumor cell or an immune cell. Non-relevant cell types (e.g., stromal or endothelial cells) were excluded from downstream analysis.
A cell was considered PD-L1 positive if more than two reads were mapped to the CD274 gene. This threshold was chosen to minimize potential noise from low-level or spurious expression often observed in single-cell RNA-seq data. For each sample, the CPS was calculated using the following formula:
ICB prediction performance evaluation and the determination of threshold of combined Tem and B cell score
The ICB response prediction performance was measured using the Area Under the Receiver Operating Characteristic (ROC) Curve (AUC) and the Odds Ratio (OR) of responders to non-responders. AUC is a standard metric in machine learning that evaluates the overall predictive performance of a classifier across all possible decision thresholds. The OR represents the odds of responding when the treatment is recommended, divided by the odds of responding when the treatment is not recommended. It quantifies performance at a specific decision threshold, making it a more clinically relevant measure. The detailed calculations for these two metrics are provided below:
The AUC is defined as the area under the ROC curve, which plots the true positive rate (sensitivity) against the false positive rate (1-specificity) at various threshold settings. The AUC is calculated using the following equation:
Where:
-
TPR is the True Positive Rate (sensitivity), calculated as \(\frac{{TP}}{{TP}+{FN}}\).
-
FPR is the False Positive Rate (1-specificity), calculated as \(\frac{{FP}}{{FP}+{TN}}\).
AUC values range from 0 to 1, where 1 indicates perfect model performance, and 0.5 indicates random chance.
The odds ratio (OR) is a measure of association between exposure (in this case, the treatment recommendation) and outcome (response to ICB treatment). It is defined as the odds of responding to treatment when it is recommended, divided by the odds of responding when it is not recommended. The OR is calculated using the following equation:
Where:
-
TP (True Positive) is the number of responders correctly identified as responders.
-
FP (False Positive) is the number of non-responders incorrectly identified as responders.
-
TN (True Negative) is the number of non-responders correctly identified as non-responders.
-
FN (False Negative) is the number of responders incorrectly identified as non-responders.
An OR greater than 1 indicates that the treatment increases the likelihood of response, while an OR less than 1 suggests that the treatment decreases the likelihood of response. An OR of 1 means the treatment has no effect on the odds of response.
Survival analysis
Cox proportional hazards regression68 was used to assess the association between gene signatures and interaction score and patient survival with age and sex as covariant. This model estimates the hazard ratio (HR) for each covariate, which represents the relative risk of an event (e.g., death or progression) occurring at any given time. Hazard ratios greater than 1 indicate increased risk, while values less than 1 suggest decreased risk. Kaplan–Meier survival curves were generated to estimate survival probabilities and visualize differences between high score group and low score group (samples were divided by the mean of score). Log-rank tests were used to compare survival distributions between the groups, with P-values indicating whether the differences in survival were statistically significant.
Statistics & reproducibility
Sample size was determined based on prior studies and statistical power calculations to ensure sufficient power to detect significant effects. No data exclusions were performed; all data points were included in the analysis. All experiments were replicated at least twice with consistent results, ensuring reproducibility. Mice were randomly assigned to experimental groups to control for potential confounding variables. Investigators were blinded to group allocation during data collection and analysis to minimize bias.
Unless otherwise stated, a one-tailed Wilcoxon rank-sum test69 was used to assess differences in distributions between two population groups. Odds ratios were calculated using Fisher’s exact test55. All statistical analyses were conducted using R version 4.4.170.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The bulk RNA sequencing data generated in this study are publicly available in the NCBI Gene Expression Omnibus (GEO) under accession code GSE299686. The single-cell RNA sequencing (scRNA-seq) and single-cell T cell receptor sequencing (scTCR-seq) data are publicly available under accession code GSE299683. Source data supporting the findings of this study are provided with this paper. Source data are provided with this paper.
Code availability
All original code used in this study has been deposited in GitHub at https://github.com/wbb1813/Time_series_mouse_ICB and is publicly available as of the date of publication. To ensure reproducibility and provide a permanent citation, the repository has also been archived in Zenodo with the https://doi.org/10.5281/zenodo.1585681571.
References
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Wang, Y. et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 11, 683419 (2021).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348, 124–128 (2015).
Riaz, N. et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 171, 934–949.e16 (2017).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Marcus, L., Lemery, S. J., Keegan, P. & Pazdur, R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 25, 3753–3758 (2019).
Gavrielatou, N., Doumas, S., Economopoulou, P., Foukas, P. G. & Psyrri, A. Biomarkers for immunotherapy response in head and neck cancer. Cancer Treat. Rev. 84, 101977 (2020).
Yilmaz, E. et al. Immunotherapy and biomarker testing in recurrent and metastatic head and neck cancers: ASCO guideline. J. Clin. Oncol. 41, 1132–1146 (2023).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Bauml, J. M., Aggarwal, C. & Cohen, R. B. Immunotherapy for head and neck cancer: where are we now and where are we going? Ann. Transl. Med. 7, S75–S75 (2019).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e14 (2021).
Liu, Y. et al. Predicting patient outcomes after treatment with immune checkpoint blockade: a review of biomarkers derived from diverse data modalities. Cell Genomics 4, 100444 (2024).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Auslander, N. et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat. Med. 24, 1545–1549 (2018).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Fehrenbacher, L. et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387, 1837–1846 (2016).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Chow, M. T. et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50, 1498–1512.e5 (2019).
Messina, J. L. et al. 12-chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep. 2, 765 (2012).
Lowery, F. J. et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 375, 877–884 (2022).
Möller, M., Turzer, S., Schütte, W., Seliger, B. & Riemann, D. Blood immune cell biomarkers in patient with lung cancer undergoing treatment with checkpoint blockade. J. Immunother. 43, 57–66 (2020).
Ao, H., Xin, Z. & Jian, Z. Liquid biopsy to identify biomarkers for immunotherapy in hepatocellular carcinoma. Biomark. Res. 9, 91 (2021).
Soyano, A. E. et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung Cancer patients treated with anti-PD-1 antibodies. J. Immunother. Cancer 6, 129 (2018).
Bagley, S. J. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106, 1–7 (2017).
Rizk, E. M. et al. Prognostic and predictive immunohistochemistry-based biomarkers in cancer and immunotherapy. Immunother. Cancer 33, 291–299 (2019).
Saddawi-Konefka, R. et al. Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC. Nat. Commun. 13, 4298 (2022).
Wang, Z. et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat. Commun. 10, 5546 (2019).
Chang, T.-G. et al. Tumor and blood B-cell abundance outperforms established immune checkpoint blockade response prediction signatures in head and neck cancer. Ann. Oncol. S0923753424049147 https://doi.org/10.1016/j.annonc.2024.11.008 (2024).
McManus, D. T. et al. An early precursor CD8 T cell that adapts to acute or chronic viral infection. Nature https://doi.org/10.1038/s41586-024-08562-y (2025).
Eberhardt, C. S. et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021).
Foy, J.-P. et al. Datasets for gene expression profiles of head and neck squamous cell carcinoma and lung cancer treated or not by PD1/PD-L1 inhibitors. Data Brief 44, 108556 (2022).
Obradovic, A. et al. Immunostimulatory cancer-associated fibroblast subpopulations can predict immunotherapy response in head and neck cancer. Clin. Cancer Res. 28, 2094–2109 (2022).
Liu, S. et al. Response and recurrence correlates in individuals treated with neoadjuvant anti-PD-1 therapy for resectable oral cavity squamous cell carcinoma. Cell Rep. Med. 2, 100411 (2021).
Cindy Yang, S. Y. et al. Pan-cancer analysis of longitudinal metastatic tumors reveals genomic alterations and immune landscape dynamics associated with pembrolizumab sensitivity. Nat. Commun. 12, 5137 (2021).
Luoma, A. M. et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 185, 2918–2935.e29 (2022).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Zhou, L. et al. Checkpoint blockade-induced CD8+ T cell differentiation in head and neck cancer responders. J. Immunother. Cancer 10, e004034 (2022).
Pabla, S. et al. Proliferative potential and resistance to immune checkpoint blockade in lung cancer patients. J. Immunother. Cancer 7, 27 (2019).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Wang, L. et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 9, 3503 (2018).
Chen, L. et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 8, 1156–1175 (2018).
Gibney, G. T., Weiner, L. M. & Atkins, M. B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 17, e542–e551 (2016).
Huang, L. et al. The RNA-binding protein MEX3B mediates resistance to cancer immunotherapy by downregulating HLA-A expression. Clin. Cancer Res. 24, 3366–3376 (2018).
Laumont, C. M. & Nelson, B. H. B cells in the tumor microenvironment: multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell 41, 466–489 (2023).
Principe, N. et al. Tumor infiltrating effector memory antigen-specific CD8+ T cells predict response to immune checkpoint therapy. Front. Immunol. 11, 584423 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e6 (2019).
Markovits, E. et al. MYC induces immunotherapy and IFNγ resistance through downregulation of JAK2. Cancer Immunol. Res. 11, 909–924 (2023).
Cui, C. et al. Ratio of the interferon-γ signature to the immunosuppression signature predicts anti-PD-1 therapy response in melanoma. Npj Genomic Med. 6, 7 (2021).
Ravi, A. et al. Genomic and transcriptomic analysis of checkpoint blockade response in advanced non-small cell lung cancer. Nat. Genet. 55, 807–819 (2023).
Cho, J.-W. et al. Genome-wide identification of differentially methylated promoters and enhancers associated with response to anti-PD-1 therapy in non-small cell lung cancer. Exp. Mol. Med. 52, 1550–1563 (2020).
Jung, H. et al. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 10, 4278 (2019).
Keenan, T. E. et al. Molecular correlates of response to eribulin and pembrolizumab in hormone receptor-positive metastatic breast cancer. Nat. Commun. 12, 5563 (2021).
Campbell, M. J. et al. Multi-platform biomarkers of response to an immune checkpoint inhibitor in the neoadjuvant I-SPY 2 trial for early-stage breast cancer. Cell Rep. Med. 5, 101799 (2024).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Andreatta, M., Berenstein, A. J. & Carmona, S. J. scGate: marker-based purification of cell types from heterogeneous single-cell RNA-seq datasets. Bioinformatics 38, 2642–2644 (2022).
Andreatta, M. et al. Interpretation of T cell states from single-cell transcriptomics data using reference atlases. Nat. Commun. 12, 2965 (2021).
Song, L. TRUST4: immune repertoire reconstruction from bulk and single-cell RNA-seq data. Nat. Methods 18, 627–630 (2021).
ImmunoMind Team. immunarch: an R package for painless bioinformatics analysis of T-cell and B-cell immune repertoires. https://doi.org/10.5281/zenodo.3367200 (2019).
Dunn, J. C. A fuzzy relative of the ISODATA process and its use in detecting compact well-separated clusters. J. Cybern. 3, 32–57 (1973).
Meyer, D., Dimitriadou, E., Hornik, K., Weingessel, A. & Leisch, F. E1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. CRAN (The Comprehensive R Archive Network, 2024).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS J. Integr. Biol. 16, 284–287 (2012).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Yi, M., Nissley, D. V., McCormick, F. & Stephens, R. M. ssGSEA score-based Ras dependency indexes derived from gene expression data reveal potential Ras addiction mechanisms with possible clinical implications. Sci. Rep. 10, 10258 (2020).
Wang, K. et al. Deconvolving clinically relevant cellular immune crosstalk from bulk gene expression using CODEFACS and LIRICS stratifies melanoma patients to anti-PD-1 therapy. Cancer Discov. candisc.0887.2021 https://doi.org/10.1158/2159-8290.CD-21-0887 (2022).
Cox, D. R. Regression models and life-tables. J. R. Stat. Soc. Ser. B Methodol. 34, 187–202 (1972).
Haynes, W. Wilcoxon rank sum test. In Encyclopedia of Systems Biology (eds. Dubitzky, W. et al.) 2354–2355. https://doi.org/10.1007/978-1-4419-9863-7_1185 (Springer New York, New York, NY, 2013).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2021).
Wang, B. et al. Longitudinal liquid biopsy identifies an early predictive biomarker of immune checkpoint blockade response in head and neck squamous cell carcinoma. https://doi.org/10.5281/zenodo.15856815 (2025).
Acknowledgements
This research is supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research. This work utilized the computational resources of the NIH HPC Biowulf cluster.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
B.W. and K.W. conducted computational analyses with assistance from S.S., S.M., S.P., S.R.D., D.W.; R.S.K., L.C., and S.T. performed the mouse experiments; X.Y. and C.A. generated the patient single-cell data. B.W. drafted the original manuscript, while P.A.D., K.W., S.R.D., J.S.G., R.S.K., and E.R. contributed to revisions. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
E.R. is a co-founder of Medaware Ltd. (https://www.medaware.com/), Metabomed (https://www.metabomed.com/), and Pangea Biomed (https://pangeamedicine.com/). He has divested and serves as an unpaid scientific consultant to the latter company. J.S.G. is a consultant/advisory board member for Pangea Biomed, Radionetics, and io9, and founder of Kadima Pharmaceuticals. The rest of the authors declare no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks N. Gopalakrishna Iyer, Muh-Hwa Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, B., Saddawi-Konefka, R., Clubb, L.M. et al. Longitudinal liquid biopsy identifies an early predictive biomarker of immune checkpoint blockade response in head and neck squamous cell carcinoma. Nat Commun 16, 8161 (2025). https://doi.org/10.1038/s41467-025-63538-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63538-4