Abstract
Heterogeneous catalytic ozonation shows promise in destroying organic pollutants in water, yet developing catalysts with both high activity and stability remains challenging. In this study, we propose a catalyst design strategy involving the anchoring of electron-sharing sites near single-atom sites to construct bidirectional electron transfer interaction tunnels. The developed catalyst (MnN3–Fe1@FeN4) features Fe1@FeN4 atomic clusters as electron-sharing sites, coordinated Mn single-atom centers through shared nitrogen bridges, successfully establishing a synergistic system. The design not only enables rapid electron supply to ozone (i.e., the internal electron transport), reducing electron loss at single atoms through shared electron flow, but also facilitates electron transfer from pollutants to the catalyst via surface reactive species (i.e., the external electron transport), compensating for the electron depletion of active sites. By taking advantage of the dual bidirectional electronic transport interactions tunnels, MnN3–Fe1@FeN4 exhibits an extraordinary catalytic activity towards ozone, achieving over three times higher reactivity than Fe and Mn single atom catalyst and 2–4 orders of magnitude higher reactivity than conventional metal oxides. Moreover, the sustainability of the ozone catalytic activity surpasses almost all of the state-of-the-art catalysts. A catalytic ozone fixed-bed reactor with this catalyst has run continuously for over 260 h, treating 3125 times the bed volume of actual wastewater. Our work unveils a critical role of atomic clusters in modulating catalyst activity and long-term stability in heterogeneous catalytic ozonation, which could inspire innovative material design for more sustainable water purification applications.
Similar content being viewed by others
Introduction
The severe shortage of clean water around the world and the growing need for water reclamation are driving the demand for efficient and advanced water purification technoligies1,2. Advanced oxidation technologies (AOPs) mainly rely on highly reactive species to break down harmful contaminants in water3,4. Among the AOPs, the heterogeneous catalytic ozonation process is a promising solution5,6,7. It can effectively eliminate contaminants while enabling the repeated use of catalysts, providing an efficient and sustainable way to purify water. However, achieving and sustaining both high activity and high stability remains a formidable challenge. The catalysts generally contain active sites to effectively activate ozone to generate surface reactive species, such as hydroxyl radicals (•OH*), surface atomic oxygen (*O), and surface peroxide species (*O2)6. But the strong chemical activity can also make the catalyst itself more prone to chemical changes during the reaction, resulting in a decline in its stability. For example, metal ions may be overly oxidized or reduced during the reaction process, changing their oxidation states and thus losing the ability to activate ozone. Therefore, it is necessary to develop catalytic ozonation technologies that possess both high activity and high stability.
Compared to the conventional metal oxide catalysts, single-atom catalysts (SACs) are promising alternatives in increasing the catalytic ozonation activity because the high exposure sites of SACs facilitate the binding of O3 molecules to metal sites. Meanwhile, the strong interactions between the 3d orbital electrons of the metal sites and the oxygen 2p orbital electrons promote O3 decomposition into highly reactive surface species6. More critically, the geometry and electronic structure of the active sites in SACs can be directionally regulated at the atomic level, such as MnN4, FeN4, etc.8,9,10,11. This has great potential for achieving the goal of sustainable cycling of active electrons amongst the catalyst, ozone, and contaminants. Nevertheless, the high activity of SACs usually results in their instability during use, resulting in metal dissolution or loss of catalytic performance. The cause of instability is that isolated single-atom sites are in a state of electron loss during the catalytic ozone reaction12, weakening the bonding between the metal sites and ligand atoms (e.g., Me–N)13. Constructing single-atom sites with highly saturated coordination might enhance their stability, but such a strategy often works at the expense of catalytic activity. Moreover, enhancing catalytic activity by increasing the density of single-atom active sites is the most popular and effective strategy14. However, more active sites would simultaneously enhance the electron trapping of single-atom sites by O3, posing a more serious risk of demetalation. Therefore, it is crucial to enhance the catalytic activity of SACs while guaranteeing the sustainability of high activity. The dual metal sites in dual single-atom catalysts (DSACs) may collaborate with each other to provide electrons for O3 to enhance activity, and to a certain extent, the Me–N bond destruction is reduced. However, the amount of anchored dual metal sites is very limited (~1.0 wt%), thus DSACs still encounter the problem of insufficient electrons during long-term catalytic ozonation10.
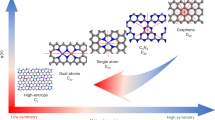
One possible solution to solve the above dilemma is to anchor an electron-sharing site (e.g., atomic clusters) close to SACs without reducing the site exposure of the SACs. By taking advantage of the strong electronic interactions and the short interaction distance between the SACs and electron sharing site, we may build a seamless electron transport interaction tunnel (Fig. 1). On the one hand, the construction of this tunnel can rapidly supply electrons to O3, while reducing the electron loss of single sites by shared electron flow within this tunnel, prolonging the site stability15. On the other hand, the construction of this tunnel can provide more empty orbitals for the catalyst to accept electrons16. This provides a base for the catalyst to rapidly extract electrons from pollutants via this tunnel to compensate for the electron loss of active sites17,18. Metal atomic clusters composed of two or more atoms exhibit a high density of delocalized electrons and accessible unoccupied orbitals, while their structural compatibility with SACs preserves the degree of active site exposure15,19,20. Therefore, the construction of electron transport interaction tunnel by atomic clusters near SACs are proposed to not only sustain high activity but also prolong the stability of the catalysts. Such potential has been largely unexploited so far due to the poor knowledge of their coordination structures, electronic configurations, and O3 catalytic mechanisms.
Catalytic ozonation paradigm of typical SACs (A). Conventional SACs lose electrons continuously during catalytic ozonation, resulting in low activity during long-term use. The concept of bidirectional electronic transfer interaction tunnel construction (B). The targeted anchoring of electron-sharing sites furnishes an ample supply of electrons and empty orbitals. This approach paves the way for realizing the concept of sustainable high activity. By establishing bidirectional electron-transport interaction tunnels, electrons can cycle within the catalyst, while simultaneously facilitating the extraction of electrons from contaminants present outside the catalyst.
In this study, we provide insights into the regulation of nanocatalysts with bi-directional electron transport interaction tunneling at the atomic level and reveal the underlying mechanism in enhancing catalyst activity and long-term stability in catalytic ozonation. First, we synthesize a Mn–Fe–NC catalyst consisting of three-coordinated Mn–N (MnN3 single atom) and four-coordinated Fe–N sites axially anchored by an Fe atom (Fe1@FeN4 atomic clusters) (named as MnN3–Fe1@FeN4), the structure of which is confirmed by performing the atomic/electronic structure analyses. Next, the activity and stability of the Mn–Fe–NC catalyst in eliminating pollutants are evaluated. The detailed mechanisms of the bidirectional electron transport interactions involving O3–catalyst–pollutants are revealed with a combination of multiple in situ analytical methods and theoretical calculations. Finally, the high activity, sustainability, and environmental robustness of the catalysts for the purification of real wastewater are demonstrated.
Results
Synthesis and characterization of catalysts
The preparation of Mn–Fe–NC by the proposed strategy that integrates nanocage encapsulation with a heterogeneous site deposition strategy is shown schematically in Fig. 2A. The obtained Mn–Fe–NC catalyst exhibited a typical Raman signal of carbon species, displaying two prominent peaks at ~1344 (D-band, crystal defects) and 1595 cm−1 (G-band, in-plane stretching of sp2 C), respectively. The significantly increased ID/IG values for Mn–Fe–NC (1.03) over Mn–NC (0.88) or Fe–NC (0.85) indicated that deposition of Fe sites altered the coordination environment of the original Mn–N, creating more defective sites (Supplementary Fig. 1), and the basal structure of Mn–NC template was retained (Supplementary Text 1). ICP-MS analysis showed that Mn and Fe quantities in Mn–Fe–NC catalyst were 0.95 wt% and 3.73 wt%, respectively (Supplementary Table 1).
Schematic of catalyst synthesis (A). High-resolution transmission electron microscope (HR-TEM) image (B) and energy dispersive X-ray spectroscopy (EDX) elemental mapping of Mn–Fe–NC catalyst (C). High-angle annular dark-field scanning transmission electron microscope (HAADF-STEM) image of Mn–Fe–NC catalyst (D). Site A (marked by red boxes) and Site B (marked by yellow circles) represent triatomic clusters and isolated single atoms, respectively. Insets in (D) depict the proportion of Site A and the Mn/Fe interatomic distances. Mn–Fe–NC catalyst structure analyzed by atomically resolved electron energy-loss spectroscopy (EELS) (E). Insets in (E) display atom-resolved EDX mapping. The intensity profiles obtained on Mn/Fe sites (F) and Fe–Fe sites (G). Mn K-edge X-ray absorption near-edge structure (XANES) spectra of Mn–NC, Mn–Mn–NC, Mn–Fe–NC catalyst and reference samples (H). Extended X-ray absorption fine structure (EXAFS) fitting curves of Mn–Fe–NC catalyst at the Mn K-edge (I) and Fe K-edge (J) in R space. Insets in (I, J) are the corresponding atom configurations. Purple balls for Mn, orange balls for Fe, blue balls for N, and cyan balls for C.
The HR-TEM image of Mn–Fe–NC catalyst showed the intact dodecahedral shape without visible metal agglomerates (Fig. 2B and Supplementary Fig. 5). Elemental mapping analyses manifested the homogeneous distribution of Mn, Fe, N, and C over Mn–Fe–NC, implying a uniform distribution of active sites (Fig. 2C). The atomic-scale homogeneity of Mn and Fe distribution was clearly presented through HAADF-STEM imaging coupled with atomic-resolution EELS and EDX elemental mapping (Fig. 2D, E and Supplementary Fig. 6)21. Notably, triatomic clusters (site A, marked by red boxes) accounted for 81% of the observed area, whereas 19% were characterized by isolated single atoms. The triatomic clusters display characteristic atomic distances centered at ~0.34 nm and ~0.25 nm, respectively. Based on Z-contrast analysis principles, the closer-spaced pairs (~0.25 nm) were conclusively assigned to Fe–Fe homonuclear configurations, while the wider-spaced pairs (~0.34 nm) corresponded to Mn/Fe heteronuclear arrangements (Fig. 2F, G). Therefore, in the Mn–Fe–NC catalyst, there exist binuclear sites composed of a Mn single atom and an Fe–Fe atomic cluster configuration. In Mn–NC template catalysts, we observed only a fragmentary distribution of Mn single-atom sites (Supplementary Fig. 7A), suggesting that the Mn/Fe binuclear sites observed in Mn–Fe–NC catalysts were generated through the deposition of Fe sites. Noted that more Mn single atom sites were observed in the control catalyst (Mn–Mn–NC) compared to Mn–NC (Supplementary Fig. 7B). The above results implied the success of our site deposition strategy.
XAFS analysis further determined the fine structure of binuclear sites in the Mn–Fe–NC catalyst. As shown in Fig. 2H, Mn K-edge XANES spectra showed that the adsorption threshold position of Mn–Fe–NC, Mn–Mn–NC, and Mn–NC were close to MnO and far from Mn2O3, demonstrating proximity to the divalent oxidation state of the Mn ion22. Notably, the absorption of Mn–Fe–NC shifted toward a lower energy relative to the Mn–Mn–NC or Mn–NC catalyst (Fig. 2H inset), implying that Fe deposition altered the coordination environments and electronic properties of the Mn site23. Furthermore, the Fourier-transformed (FT) Mn K-edge EXAFS oscillation and wavelet transform (WT) analysis of Mn–Fe–NC, Mn–Mn–NC, and Mn–NC catalyst confirmed the presence of coordination between Mn and N (Supplementary Figs. 11–13). EXAFS fitting result exhibited that the coordination numbers of Mn–N paths for Mn–Fe–NC catalyst were 3.2 (Fig. 2I), indicating that the single Mn atom mainly coordinates with 3N atoms to form Mn–N3 moieties. It should be noted that Mn atoms were observed to exist in the typical Mn–N4 moieties in Mn–NC and Mn–Mn–NC catalyst (Supplementary Fig. 15).
Additionally, the Fe K-edge XANES spectra showed that the position of the absorption threshold of the Mn–Fe–NC catalyst was close to the iron phthalocyanine (FePc) and Fe2O3 (Supplementary Fig. 16), suggesting that the valence state of the Fe species in Mn–Fe–NC is close to +322. The FT-EXAFS spectrum of the Fe K-edge for Mn–Fe–NC exhibited an intense peak at ∼1.46 Å in R-space, which could be attributed to the Fe–N bond, and a measurable Fe–Fe scattering peak was also present at ∼2.50 Å, signifying the presence of atomically dispersed N-coordinated Fe–Fe atomic clusters in Mn–Fe–NC catalyst (Supplementary Fig. 17). The second shell Fe–Fe bond at ∼4.5 Å was not obvious in the spectra of Mn–Fe–NC, implying that the size of the atom clusters was very small and has reduced structural periodicity24. Besides, the Fe 2p XPS spectrum showed no obvious zero-valent iron signal (~706.7 eV), indicating that Fe atoms in the atom clusters were coordinated by the substrate N/C atoms (Supplementary Text 2). The WT contour plot further revealed two characteristic peaks in the Mn–Fe–NC catalyst corresponding to Fe–N and Fe–Fe coordination at approximately 5.0 Å−1 and 7.5 Å−1, respectively (Supplementary Fig. 18). EXAFS fitting result exhibited that the coordination numbers of Fe–N and Fe–Fe paths for the Mn–Fe–NC catalyst were 3.8 and 1.0 (Fig. 2J). It was evidenced that the majority of Fe atoms in the Mn–Fe–NC catalyst form highly dispersed Fe1@FeN4 atomic clusters with nanoscale dimensions through quadruple N coordination on the plane and axial bonding with another Fe atom. Furthermore, distinct FT peaks corresponding to Mn/Fe atomic pairs were observed at the same position (R ~ 0.29 nm) in the EXAFS fitting curves for both Mn and Fe K-edges (Fig. 2I, J), confirming the successful formation of local Mn/Fe dual-site configurations in the Mn–Fe–NC catalyst, further supporting the HAADF-STEM observations.
Besides the above characterization analysis, we also used first-principles calculations to confirm the local coordination of Mn/Fe atoms in Mn–Fe–NC catalysts. Typically, Mn–NC and Mn–Mn–NC structures featured a single manganese atom bonded to four pyridine N atoms, whereas the Mn-atom and Fe-atom in the Mn–Fe–NC catalyst existed in a MnN3 single atom and Fe1@FeN4 atom cluster configurations, with a spacing of 0.34 nm between Mn and Fe sites, and all were stabilized by the support (Supplementary Fig. 20). The information obtained from the fine structure analysis was consistent with the theoretically validated results. Overall, we successfully constructed a catalyst MnN3–Fe1@FeN4, and its short site spacing allowed electrons to rapidly shuttle between various cores. The unique Fe1@FeN4 atomic clusters with abundant electrons and empty orbitals could serve as electron donor and acceptor sites for single-atom sites, forming an electron transport interaction tunnel.
Catalytic ozonation performance
The catalytic activity of the catalysts was evaluated by degrading OA as a representative compound. OA, as a frequent degradation product of many organic pollutants, is structurally stable and refractory to O3 oxidation (k < 0.04 M−1·s−1)25. Thus, its degradation is often employed to probe the catalytic ozonation activity of catalysts. As depicted in Fig. 3A, the removal of OA by O3 alone was negligible. In contrast, Mn–Fe–NC/O3 completely degraded OA in 15 min with the observed degradation rate constant (kobs) of 0.215 min−1, confirming the catalytic ozonation capability of Mn–Fe–NC catalyst. Notably, the kobs of Mn–Fe–NC catalyst were 3.5 times that of Mn–NC (0.062 min−1), 4.3 times that of Fe–NC (0.050 min−1), 4.2 times that of Fe–Fe–NC (0.051 min−1), and 2.2 times that of Mn–Mn–NC (0.097 min−1). The results suggested the strong interplay between the Mn and Fe constituents in Mn–Fe–NC. The XANES spectra shown in Fig. 2H also supported that Mn and Fe constituents were not isolated, and there was an electronic structure intermodulation effect23,26. In addition, Mn–Fe–NC outperforms numerous state-of-the-art ozonation catalysts in terms of mass activity for OA degradation, as evidenced in Fig. 3B and Supplementary Data 1. As an example, the OA degradation in the Mn–Fe–NC/O3 process was 1.2–14.8 times higher than that of the literature-reported Fe– or Mn–SACs, and 7294 and 174 times higher than that of the conventional FeOOH/O3 and TiO2/O3 process, respectively27,28.
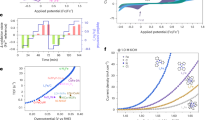
Performance evaluation of the prepared catalysts on oxalic acid (OA) degradation (A). Error bars represent the standard deviation, obtained by repeating the experiment twice. Conditions: [OA] = 100 mg·L−1, [O3]gas = 1.0 wt%, [catalyst] = 0.25 g·L−1, pH = 2.7. Comparison of mass activity for catalytic ozonation in terms of OA degradation (B)11,27,28,29,30,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69. Kinetics of benzotriazole (BTA), nitrobenzene (NB), 4-hydroxybenzoic acid (PHBA), 4-nitrophenol (4-NP), and phenol (PhOH) degradation in Mn–Fe–NC/O3 (C). Error bars represent the standard deviation, obtained by repeating the experiment twice. Conditions: [pollutants] = 69.4 μM·L−1, [O3]gas = 1.0 wt%, [catalyst] = 0.05 g·L−1, pHBTA = 4.70, pHNB = 5.00, pHPhOH = 5.75, pH4-NP = 4.00, pHPHBA = 4.60. Stability evaluation of Mn–NC/O3 and Mn–Fe–NC/O3 (D). Conditions: [pollutants] = 5.0 mgC·L−1, [O3]gas = 1.0 wt%, [catalyst] = 0.05 g·L−1.
Other organic pollutants commonly present in water were also examined, including compounds with electron-withdrawing groups (e.g., BTA, NB) and electron-donating groups (e.g., PhOH, 4-NP, and PHBA). These pollutants exhibit diverse properties, such as different pKa (3.98−9.95), logKow (1.23−1.91), and reaction rate constants with ozone (9.6 × 10−2 M−1·s−1–1.8 × 106 M−1·s−1) (Supplementary Table 7). As depicted in Fig. 3C and Supplementary Fig. 22, the kobs values of BTA and NB in the Mn–Fe–NC/O3 process were calculated to be 0.709 min−1 and 0.856 min−1, respectively, which were 15.7 and 5.1 times those in O3 alone. Similarly, the kobs of PHBA (1.451 min−1), 4-NP (1.666 min−1), and PhOH (2.095 min−1) in the Mn–Fe–NC/O3 process increased by 5.6 times, 6.4 times, and 6.2 times compared to O3 alone, respectively. Besides, TOC test results further demonstrated the effective mineralization of the pollutants (e.g., OA, NB and BTA) by the Mn–Fe–NC/O3 process (Supplementary Fig. 23). The performance of Mn–Fe–NC/O3 process in the presence of coexisting matrices, such as dissolved organic matter and inorganic ions (i.e., Cl−, SO42−, H2PO4−, and HCO3−) was also examined to evaluate its resistance to interference from the actual water background. As shown in Supplementary Figs. 24–26, NB degradation showed minor changes in kobs (<15%) in the presence of dissolved organic matter (2−5 mgC/L DOC), inorganic ions (Cl−, SO42−, H2PO4−, and HCO3−), and different pHs from 5.0 to 9.0. The exception was that NB degradation was inhibited by 30.8% at pH 3.0. Nevertheless, the results confirmed high efficiency and anti-interference activity of the Mn–Fe–NC/O3 process.
Furthermore, to evaluate the catalyst stability, repeated catalytic ozonation tests for NB degradation were conducted. NB represents the aromatic compound having low reactivity with O3 (9.6 × 10−2 M−1·s−1). The NB removal with Mn–Fe–NC catalyst could still be maintained after 50 repeated cycles (Fig. 3D), and the O3 decomposition efficiency also had minimal changes (Supplementary Fig. 27). In contrast, the catalytic activity of Mn–NC catalyst decreased sharply to 63.7% during continuous use for 12 cycles, implying the low stability of Mn–NC in O3 exposure. Besides, the stability of the Mn–Fe–NC catalyst was also significantly higher than that reported for single-atom ozone catalysts (Supplementary Data 2). Further analysis of PXRD and Raman spectra showed that the basic structure of the Mn–Fe–NC catalyst had no appreciable changes after 50 cycles (Supplementary Figs. 28 and 29). Moreover, based on a metal loss test, apparently low Mn (0.8 μg·mg−1) and Fe (0.004 μg·mg−1) ion leaching in Mn–Fe–NC/O3 was observed (Supplementary Fig. 30), whereas the leaching of Mn was 3.5 times and 5.0 times higher for Mn–NC/O3 (2.8 μg·mg−1) and Mn–Mn–NC/O3 (4.0 μg·mg−1), respectively. Overall, the remarkable activity of the Mn–Fe–NC/O3 process provides an opportunity for the advancement of water purification technologies in complex water matrices.
Reactive species generation pathway
The formation of interface species on the Mn–Fe–NC catalyst was identified by in situ DRIFTS and in situ Raman spectral characterization. Mn–Mn–NC catalyst was also tested for comparison. In Fig. 4A, Mn–Fe–NC catalyst exposed to O3 molecules displayed distinct peaks at 885 cm−1 and 1268 cm−1, indicating the concurrent generation of *O and *O229. It is due to the orbital hybridization and electron transfer of Mn 3d/Fe 3d-O 2p, resulting in strong interaction between O3 and metal sites7. O3 molecules with captured electrons were in a highly reactive state, followed by a rapid breakage of the O–O bond to form *O and *O25,30. Obviously, no signals of physically adsorbed *O3/*O3− at 1026–1054 cm−1 were detected, implying the advanced O3 interfacial decomposition capability of Mn–Fe–NC catalyst over even a long O3 exposure31. Conversely, a strong signal peak appeared at 1051 cm-1, implying that Mn–Mn–NC catalysts were weaker in decomposing O3 (Supplementary Fig. 31). Although more Mn active sites were designed to enhance the O3 adsorption of Mn–Mn–NC catalysts, the overloaded O3 decomposition capacity resulted in the accumulation of O3 molecules on its surface, threatening its stability (Supplementary Fig. 30). Meanwhile, the signal peaks of *O (952 cm−1) and *O2 (860 cm−1) in Mn–Fe–NC/O3 was clearly observed in the in situ Raman spectroscopy (Fig. 4B)10. It was noteworthy that the Me–O* stretching bands of *O adsorbed on Mn–Fe–NC catalysts blue shifted compared to those on Mn–Mn–NC catalysts (Supplementary Fig. 32). This implied that the electrons in 3d orbitals of the MnN3–Fe1@FeN4 in Mn–Fe–NC catalysts were more susceptible to transfer to the π* bonding orbitals in *O, modulating the d–π* transitions32. This was consistent with in situ DRIFTS insights.
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) spectra in Mn–Fe–NC/O3 (A). In situ Raman spectra in Mn–Fe–NC/O3 (B). Free energy diagrams of O3 evolution in MnN3–Fe1@FeN4 (C). Optimized structures for the O3 activation process on MnN3–Fe1@FeN4 with potential energy profiles (D). Computed Bader charge transfer of MnN3–Fe1@FeN4 to binding ozone (E).
The generation of other reactive species was also examined. As verified by in situ Raman, a distinct peak at 645 cm−1 (corresponding to the Mn/Fe–OH* intermediate) occurred upon O3 addition in Mn–Fe–NC33,34, whereas it was weaker in Mn–Mn–NC. Meanwhile, the result of quenching tests with 2 mM MeOH as a scavenger for •OH (k•OH = 9.7×108 M−1·s−1, kO3 = 0.02 M−1·s−1)35 indicated the generation of •OH in Mn–Fe–NC/O3 (Supplementary Figs. 33 and 34), but not in the Mn–Mn–NC/O3. Using coumarin as a probe, •OH* was observed to concentrate on the Mn–Fe–NC catalyst surface according to the fluorescence signal (Supplementary Fig. 35). Besides •OH, the typical ESR characteristic peak of TEMP-1O2 with three characteristic peaks was observed in Mn–Fe–NC/O3 (Supplementary Fig. 37). Above results support the rapid conversion of O3 to surface-active species (*O, *O2 and •OH*) and 1O2 on Mn–Fe–NC catalyst via a strong electronic interaction. In applications for abatement of electron-poor target molecules (e.g., OA, NB, and BTA, etc.), *O and •OH* can be the main ROS due to the high oxidation potential, whereas for electron-rich nucleophilic target molecules (e.g., PhOH, 4-NP, and PHBA, etc.), the role of *O2 and 1O2 may not be ignored.
In order to evaluate how these reactive species were formed from O3 evolution, DFT calculations were performed. As shown in Fig. 4C, the Eads value of the Mn atom in Mn–Fe–NC was −2.31 eV, where it was −1.11 eV for the Fe atom. It indicated that O3 can attach to both Mn and Fe atoms in Mn–Fe–NC, but preferentially attaches to the Mn atom. Besides, the Mn–Fe–NC showed much weaker binding affinity for pollutants than O3. For example, the adsorption energy of PhOH and OA on Mn sites was −0.51 eV and −0.41 eV, respectively (Supplementary Fig. 38), which was more positive than that of O3 (−2.31 eV). This ensured the effective adsorption of O3 on the metal atoms in Mn–Fe–NC. The free energy changes (ΔG) were further calculated for O3 decomposition. The ΔG values for O3 decomposition into *O and *O2 were −2.07 eV and −2.61 eV at the Mn and Fe atoms, respectively. The O3 evolution pathway on the Mn and Fe atoms in the Mn–Fe–NC catalyst is displayed separately in Fig. 4D and Supplementary Fig. 39. For the Mn atom (the red pathway), the generated *O was situated at the Mn atom, and *O2 was situated at the neighboring Fe atom. Subsequently, the attached *O2 on the Fe atom desorbs, and the re-exposed atom continues to adsorb and decompose O3 (as the blue pathway shows)10,36. Furthermore, the *O anchored on Mn/Fe binuclear sites spontaneously convert to •OH* rather than recombining into *O2 for subsequent O2 release (Supplementary Data 3). This design ensured that the Mn–Fe–NC catalyst could continuously decompose O3, which is superior to the Mn–NC catalysts. From the DFT calculation, the Eads value of the Mn site in Mn–NC was −1.71 eV, and the ΔG values for O3 decomposition into *O and *O2 were −0.83 eV (Supplementary Fig. 40), which were both more positive than the Mn site in Mn–Fe–NC.
Bidirectional electronic transfer interaction mechanisms
In order to reveal the electron transport interaction mechanisms that ensure the sustainable and high activity of Mn–Fe–NC catalysts, time-resolved XPS spectroscopy was employed to resolve the oxidation state changes of Mn and Fe during the catalytic ozone reaction. When Mn–Fe–NC was exposed to ozone, both Mn and Fe elements underwent the process of gaining and then losing electrons to different degrees, as the XPS spectra of Mn 2p and Fe 2p shifted left and then right to different degrees (Supplementary Fig. 41). In order to further determine the electron flow direction, Bader charge analysis was performed by DFT calculations. Result exhibited that electron transfer from MnN4 site in Mn–NC to O3 was 0.70 e, while Mn site lost 1.80 e (Supplementary Fig. 42). Continuous catalytic process would make continuous electron loss in Mn site, breaking the Mn–N bond and resulting in Mn leaching (Supplementary Fig. 30). In contrast, in MnN3–Fe1@FeN4 site of Mn–Fe–NC, the loss of electrons from interacting sites with O3 enabled neighboring sites to replenish electrons on time, preventing excessive loss of electrons in metal sites. As shown in Fig. 4E and Supplementary Fig. 43, the electron transfer from the Mn site to O3 was 0.75 e, while the Mn site lost 1.72 e. Meanwhile, the neighboring Fe site lost 1.09 e, and the axially coordinated Fe sites lost 0.53 e. The results indicated that the 0.23 e loss from the Mn site could be replenished from the neighboring Fe site by 0.18 e, and the axially coordinated Fe sites by 0.17 e. This electron interaction within the MnN3–Fe1@FeN4 was transported via the ligand N atoms shared between the Mn/Fe binuclear sites, that is, an internal electronic transport interaction.
More interestingly, we found that the construction of electron transport interaction tunnels was also favorable for extracting target contaminant electrons. As shown in Fig. 5A, the open-circuit potentials (OCP) of Mn–Fe–NC or Mn–NC catalyst-immobilized working electrode remarkably increased after the addition of O3 (ΔOCP+ozone) due to the rapid capture of electrons in active sites by adsorbed O3 molecules37. The electron-trapped O3 was converted to the transient intermediate O3•−, which rapidly decomposed to *O and *O2, as observed in in situ DRIFTS (Fig. 4A). The ΔOCP+ozone of Mn–Fe–NC/O3 was significantly higher than that of the Mn–NC/O3, confirming a more efficient electron transfer and interface species generation17. To further identify the roles of pollutants, NB and PhOH were added into the electrochemical systems, which showed a sudden potential drop (ΔOCP+pollounts). The result implied the electron-donating pathway from the pollutant to the catalyst. And it could be maintained after 50 repeated cycles of Mn–Fe–NC catalyst (Fig. 5B). The electron flow was further revealed by molecular orbital analysis17. The premise of electron-extraction in Mn–Fe–NC/O3 was based on the electron transfer from the highest occupied molecular orbital (HOMO) of pollutants (e.g., BTA, NB, PhOH, 4-NP, and PHBA) (Supplementary Fig. 45) to the lowest unoccupied molecular orbital (LUMO) of the reactive species in MnN3–Fe1@FeN438. As depicted in Fig. 5C, the LUMO of MnN4–O3* (−4.00 eV) was less negative as compared with those of MnN3–O3* (−5.49 eV) and Fe1@FeN4–O3* (−5.55 eV) of Mn–Fe–NC. Similarly, the LUMO of MnN4–O* (−3.57 eV) was much less negative as compared with those of MnN3–O* (−5.21 eV) and Fe1@FeN4–O* (−5.41 eV) of Mn–Fe–NC, and Mn–O*@Fe–O2* (−5.70 eV) or Mn–O2*@Fe–O* (−5.74 eV) formed by Mn–Fe synergistic decomposition of O3 (Fig. 5D). Notably, the strong inverse correlation between Δ(\({{{{\rm{LUMO}}}}}_{{{{\rm{catalyst}}}}/{{{{\rm{O}}}}}_{3}}-{{{{\rm{HOMO}}}}}_{{{{\rm{pollutants}}}}}\)) and kobs values of pollutants further confirmed effective electron extraction by the catalysts (Supplementary Fig. 46), as supported by in situ electrochemical results. The electrons of pollutants were transferred to the Mn–Fe–NC catalyst via surface reactive species, that is, an external electron transport interaction.
In situ electrochemical assay in Mn–Fe–NC (A) and Mn–Fe–NC used 50 times (B) in terms of open-circuit potentials. Conditions: [pollutants] = 5.0 mgC·L−1, [O3]gas = 1.0 wt%. Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) values of MnN4–O3* and MnN3–Fe1@FeN4–O3* (C). HOMO and LUMO values of MnN4–O* and MnN3–Fe1@FeN4–O*/O2* (D). Yellow and blue electron clouds indicate regions where the wave function is positive and negative. Schematic diagram of electronic transport interactions strategy in Mn–Fe–NC inducing sustainable high-activity in ozone catalysis (E).
Overall, the bidirectional electronic transport interactions in the Mn–Fe–NC catalyst are essential to maintain both high reactivity and stability. During the entire O3 catalytic process, the internal electron transfer interaction rapidly shuttles electrons between MnN3 single-atom sites and Fe1@FeN4 atomic clusters, thereby preventing the over-oxidation of these active sites. The reactive species generated on the catalyst surface can strongly extract pollutants' electrons by the external electron transport interaction, which not only oxidizes pollutants but also recharges electrons towards the Fe/Mn sites (Fig. 5E).
Practical decontamination application
For the real applications of nano catalysts relying upon catalyst slurries, the catalyst separation has been a key consideration39. So we developed the assembly of easily separable catalytic microspheres using an impregnation loading method to convert nanoparticles into coated silicate catalytic microspheres (named Mn–Fe–NC@SC, Fig. 6A). The SEM images demonstrated that Mn–Fe–NC catalysts are uniformly coated on the carrier microsphere surface without destroying the original dodecahedral shape of Mn–Fe–NC (Fig. 6B). Subsequently, a fixed-bed column reactor was used to test the efficacy of different types of target pollutants (BTA and PHBA, Supplementary Table 7) in the purification of various real water (Fig. 6C), including secondary effluent, aquafarm wastewater, and Pearl River water. Despite the complex matrix present in these water samples, PHBA was removed by >90% and BTA by >67% in 120 s, highlighting the great decontamination capability of Mn–Fe–NC@SC/O3 (Supplementary Fig. 47).
The picture (A) and scanning electron microscope (SEM) image (B) of Mn–Fe–NC@SC. A home-made fixed-bed column reactor (C). Continuous flow degradation tests of benzotriazole (BTA) removal from actual secondary effluent using Mn–Fe–NC@SC/O3 process (D). Schematic drawing of wastewater treatment system (E). Municipal sewage after biochemical treatment can undergo advanced treatment using the developed catalytic ozonation reaction system to achieve water recycling and reuse.
To further investigate the applicability and stability of Mn–Fe–NC@SC/O3, continuous flow degradation tests were performed, and the degradation of ozone-refractory BTA (kO3 = 2.4 × 102 M−1·s−1) in the secondary effluent was examined under a hydraulic retention time (HRT) of 5.0 min. As shown in Fig. 6D, the Mn–Fe–NC@SC/O3 system exhibited stable treatment capability, with <10.0% decrease in BTA removal efficiency after 260-h treatment. This corresponded to treating 125 L of wastewater or 3125 times the bed volume. Additionally, the Mn–Fe–NC@SC showed no significant detachment after 30 days soaking in ozone aqueous solution (no significant TOC signal detection, Supplementary Fig. 50), confirming its robustness. This sustainable high activity can significantly reduce process operating costs and energy inputs, implying a huge potential in reclaimed water reuse and industrial wastewater purification (Fig. 6E).
Discussion
In summary, our work provides fundamental insights into catalytic ozonation on binuclear centers (MnN3–Fe1@FeN4) catalysts and opens a strategy for effectively regulating the high activity and high stability of SACs. By anchoring atomic clusters close to single-atom sites, we can establish bidirectional electron transport interactions. The internal electron transport interaction enables rapid shuttling of electrons between MnN3 single-atom sites and Fe1@FeN4 atomic clusters. This pathway promotes the binding and decomposition of O3 and simultaneously prevents over-oxidation of single-atom sites. On the other hand, the external electron transport interaction allows the stabilized surface-active species to extract pollutants' electrons to transfer them to the catalyst. This pathway promotes oxidation of pollutants and also facilitates the recharging of electrons to atomic clusters. This bidirectional electron transport interaction strategy enables long-term stability and durability, superior to traditional SACs. This high activity and stability can also be achieved in complex environmental matrices, retaining decontamination capacity well after continuously treating actual wastewater. Overall, the strategy of constructing electron transport tunnels in catalysts provides ideas to develop advanced water treatment processes and can be extended to other SAC systems to handle the paradox of activity and stability.
Methods
Chemicals
The chemicals and reagents used in this study were provided in Supplementary Text 3.
Catalyst preparation
A facile nanocage encapsulated and integrated with a heterogeneous site deposition strategy was used to construct catalytic interfaces. First, 40 mM of Zn(NO3)2·6H2O and 4 mM of (CH3COO)3Mn·2H2O dissolved in dimethylformamide (DMF) were injected into a DMF solution containing 80 mM of 2-methylimidazole, well-mixed, and allowed to stand for 24 h at 120 °C. Mn displaced Zn in ZIF-8, followed by uniform growth of Mn–ZIF-8 nanocrystals. Then, the Mn–ZIF-8 was centrifuged and washed with methanol several times and dried at 60 °C under vacuum overnight. The as-obtained product was subsequently calcined at 1100 °C in a tube furnace under N2 flow for 1 h. The obtained material was acid-leached using a 0.5 M H2SO4 solution at 80 °C for 5 h to remove Mn clusters. The catalyst was obtained by calcining at 900 °C for an extra 3 h under N2 flow and was named as Mn–NC catalyst.
After that, 1.0 g of Mn–NC powder was dispersed into a 50% isopropanol solution containing 0.8 mM of Fe (II) chloride and 40 mM of cyanamide, sonicated for 2 h, and stirred for 5 h to ensure that the Fe and N sources were in full contact with the Mn sites. Then, the as-obtained mixture was collected and dried at 60 °C under vacuum overnight. Finally, the mixture was calcined at 1100 °C in a tube furnace under N2 flow for 1 h to obtain the Mn–Fe–NC catalyst.
The procedures for the synthesis of Fe–NC catalyst were like those of Mn–NC, except for the use of iron acetate instead of manganese acetate. The procedures for the synthesis of Mn–Mn–NC catalyst were like those of Mn–Fe–NC, except for the use of Mn (II) chloride instead of Fe (II) chloride. The procedures for the synthesis of Fe–Fe–NC catalyst were like those of Mn–Fe–NC, except for the use of iron acetate instead of manganese acetate.
Characterizations
Raman spectra were collected on a ZEISS LSM800 spectrometer with a 532 nm laser. Powder X-ray diffraction (PXRD) patterns were obtained on a Rigaku Ultima IV diffractometer with Cu Kα X-rays. The surface area and pore size distribution were obtained from nitrogen adsorption-desorption isotherms (Micromeritics ASAP 2460). Single-atom loading of the catalysts was determined by inductively coupled plasma-mass spectrometry (ICP-MS) after microwave ablation pre-treatment. A FEI TECNAI G2 F30 with electron acceleration energy of 300 kV was used to record the high-resolution transmission electron microscope (HR-TEM) and elemental mappings. A JEM ARM200F transmission electron microscope operated at 200 kV, equipped with a probe spherical aberration corrector, was used to obtain atomic resolution high-angle annular dark-field scanning TEM (HAADF-STEM) images of the samples and atomically resolved EELS spectra and elemental mappings. About 242 random sites in the HAADF-STEM images were analyzed by linear scanning using Digital Micrograph software10. Mn K-edge and Fe K-edge X-ray absorption fine-structure (XAFS) and extended XAFS (EXAFS) data were collected in fluorescence mode on the BL14W1 beamlines at the Shanghai Synchrotron Radiation Facility (Shanghai, China). The details of the XAFS experiment and data analysis were provided in Supplementary Text 4. X-ray photoelectron spectroscopy (XPS) measurements were conducted with a Thermo Scientific K-Alpha instrument.
Experimental procedures
Catalytic ozonation experiments were carried out in a homemade cylindrical reactor (diameter, 6.2 cm, height, 26.5 cm, and effective volume, 500 mL) at the bench scale (Supplementary Fig. 51). O3 was generated using an ozone generator (Anseros COM-AD-01, Germany) with a pure oxygen source, the concentration of which was monitored online by an ozone detector (Anseros GM-6000-OEM, Germany) to ensure stable gas O3 input (1.0 wt%). Degradation kinetics tests were conducted using model compounds, including oxalic acid (OA, 100 mg·L−1) and five typical micropollutants (i.e., benzotriazole (BTA), nitrobenzene (NB), phenol (PhOH), 4-nitrophenol (4-NP), and 4-hydroxybenzoic acid (PHBA)), each at 69.4 μM·L−1. The desired dosage of catalyst was added under magnetic stirring. The reaction was triggered once the gas mixture containing O3 and O2 was bubbled into the suspension at a flow rate of 0.30 L·min−1 through a rotameter. At certain time intervals, the samples were withdrawn from the reactor, and ozone was quenched with excessive Na2SO3 (0.50 M) solution immediately. In some tests, methanol (MeOH) and dimethyl sulfoxide (DMSO) were added as scavengers to evaluate the role of reactive species. All experiments were performed in duplicate.
Analytical methods
The concentrations of target compounds were measured by high-performance liquid chromatography (HPLC, Essential LC-16, Shimadzu, Japan) with detailed conditions listed in Supplementary Text 5. The TOC concentration of the sample was analyzed using a TOC analyzer. The dissolved O3 was measured by the indigo method5. The ion leaching was detected by inductively coupled plasma-optical emission spectrometry (ICP-OES). The surface-adsorbed oxygen species were identified by in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) in the range of 600–4000 cm−1 (Supplementary Text 6)29 and in situ Raman spectra (Renishaw inVia, UK) at a wavelength of 532 nm40. The distribution and content of •OH was semiquantitative analyzed by an inverted fluorescence microscope with 0.5 mM coumarin as a trapping agent41. Electrochemical tests were performed in a three-electrode cell connected to a CHI (660E) workstation, with a catalyst-modified glassy carbon electrode as the working electrode, a platinum wire as the counter electrode, and an Ag/AgCl electrode as the reference electrode (Supplementary Text 7).
Density functional theory (DFT) calculations
The optimized structure of the O3 activation process, the potential energy profile, and the Bader charge transfer associated DFT calculations are performed on Vienna Ab Initio Simulation Package (VASP) with the generalized gradient approximation (GGA)42 using the Perdew–Burke–Ernzerhof (PBE) functional43,44. We have chosen the projected augmented wave (PAW) potentials to describe the ionic cores and take valence electrons into account using a plane wave basis set with a kinetic energy cutoff of 450 eV. Geometry optimizations were performed with the force convergence smaller than 0.05 eV/Å. Spin-polarization effect was also considered. The DFT-D3 empirical correction method was employed to describe van der Waals interactions. The lattice parameter of the graphene and the size of the supercell of 6 × 6 × 1, 14.76 Å × 14.76 Å × 20 Å, were used for calculations of catalyst models. The k-points mesh of Monkhorst–Pack (2 × 2 × 1) was applied for all the calculation sections45. The DFT calculations associated with the front orbital theory are carried out with the Gaussian 16 program. All molecular geometries were optimized in the gas phase at the B3LYP-D3BJ/6–31 + G (d, p) level of theory with SMD as the solvation mode in water46,47,48. Optimized minima were verified by harmonic vibrational analysis to have no imaginary frequency at 298.15 K and 1 atm. To refine calculated energies, single-point calculations with a larger basis set 6–31 + G (d, p) were then performed based on these optimized structures with SMD as the solvation mode in water49,50,51.
Practical decontamination application experiment
The Mn–Fe–NC@SC composite catalyst system was successfully constructed by leveraging the hydration-induced solidification characteristics of silicate. Initially, silicate powder was mixed with water to form a slurry, which was subsequently processed into spherical carriers (∼1 cm diameter) through a granulation technique. Capitalizing on the gelation properties of silicate, the Mn–Fe–NC catalyst was uniformly loaded onto the surface of these spherical substrates. The composite system underwent a 12 h stationary curing process at ambient temperature, during which structural consolidation was achieved via a hydration-induced molding mechanism. This protocol ultimately yielded Mn–Fe–NC@SC composite materials with a catalyst loading capacity of 1 mg/g (10 mg catalyst per 10 g substrate). Practical decontamination application experiments using a fixed-bed column reactor filled with Mn–Fe–NC@SC microspheres. Experimental conditions: [O3]gas = 0.56 wt%, gas velocity: 100 mL/min, hydraulic retention time: 5 min. The efficiency of the fixed-bed column reactor was assessed using actual water samples artificially spiked with micropollutants, including aquaculture wastewater, Pearl River water, and secondary effluent from a municipal wastewater treatment plant in Guangdong Province. The basic water quality parameters were shown in Supplementary Table 9.
Data availability
All data generated in this study are provided in the Supplementary Information. Source data are provided with this paper. All the raw data relevant to the study are available from the corresponding author upon request. Source data are provided with this paper.
References
Naddaf, M. The world faces a water crisis-4 powerful charts show how. Nature 615, 774–775 (2023).
Hodges, B. C., Cates, E. L. & Kim, J. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Xu, J. et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 4, 233–241 (2021).
Zhang, G. et al. Redox-neutral electrochemical decontamination of hypersaline wastewater with high technology readiness level. Nat. Nanotechnol. 19, 1130–1140 (2024).
Song, Z. et al. Nanoscale sequential reactor design achieves effective removal of disinfection byproduct precursors in catalytic ozonation. Environ. Sci. Technol. 58, 12697–12707 (2024).
Ma, D. et al. Catalytic ozonation mechanism over M1-N3C1 active sites. Nat. Commun. 14, 7011 (2023).
Qu, W. et al. Refining asymmetric low-coordinated Fe-N3 motif to boost catalytic ozonation activity. Adv. Funct. Mater. 34, 2314187 (2024).
Zhou, X. et al. Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density. Proc. Natl. Acad. Sci. USA 119, e2119492119 (2022).
Liu, W. et al. Discriminating catalytically active FeNx species of atomically dispersed Fe–N–C catalyst for selective oxidation of the C–H bond. J. Am. Chem. Soc. 139, 10790–10798 (2017).
Qu, W. et al. Precisely constructing orbital coupling-modulated iron dinuclear site for enhanced catalytic ozonation performance. Proc. Natl. Acad. Sci. USA 121, e2319119121 (2024).
Wang, J. et al. Manipulating selectivity of hydroxyl radical generation by single-atom catalysts in catalytic ozonation: surface or solution. Environ. Sci. Technol. 56, 17753–17762 (2022).
Qu, W. et al. Accelerated catalytic ozonation in a mesoporous carbon-supported atomic Fe–N4 sites nanoreactor: confinement effect and resistance to poisoning. Environ. Sci. Technol. 57, 13205–13216 (2023).
Wan, X. et al. Iron atom–cluster interactions increase activity and improve durability in Fe–N–C fuel cells. Nat. Commun. 13, 2963 (2022).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Wang, Z. et al. Cooperation between dual metal atoms and nanoclusters enhances activity and stability for oxygen reduction and evolution. ACS Nano 17, 8622–8633 (2023).
Liu, Y. et al. Efficient proton-exchange membrane fuel cell performance of atomic Fe sites via p–d hybridization with Al dopants. J. Am. Chem. Soc. 146, 12636–12644 (2024).
Guo, J. et al. Fenton-like activity and pathway modulation via single-atom sites and pollutants comediates the electron transfer process. Proc. Natl. Acad. Sci. USA 121, e2313387121 (2024).
Pei, R., He, L., Zhao, Y. & Wang, X. The dynamic Lewis acid–carbene hybrid: pushing the electrophilicity of carbenes to the limit. J. Am. Chem. Soc. 145, 21733–21737 (2023).
Liu, H. et al. Decorating single-atomic Mn sites with FeMn clusters to boost oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 135, e202214988 (2023).
Wei, X. et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 9, 181–197 (2023).
Huang, H. et al. Clusters induced electron redistribution to tune oxygen reduction activity of transition metal single-atom for metal–air batteries. Angew. Chem. Int. Ed. Engl. 134, e202116068 (2022).
Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018).
Su, R. et al. Utilizing the oxygen-atom trapping effect of Co3O4 with oxygen vacancies to promote chlorite activation for water decontamination. Proc. Natl. Acad. Sci. USA 121, e2319427121 (2024).
Ao, X. et al. Markedly enhanced oxygen reduction activity of single-atom Fe catalysts via integration with Fe nanoclusters. ACS Nano 13, 11853–11862 (2019).
Xiao, J., Xie, Y., Rabeah, J., Brückner, A. & Cao, H. Visible-light photocatalytic ozonation using graphitic C3N4 catalysts: a hydroxyl radical manufacturer for wastewater treatment. Acc. Chem. Res. 53, 1024–1033 (2020).
Guo, Z. et al. Electron delocalization triggers nonradical Fenton-like catalysis over spinel oxides. Proc. Natl. Acad. Sci. USA 119, e2201607119 (2022).
Sui, M., Sheng, L., Lu, K. & Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 96, 94–100 (2010).
Beltrán, F., Rivas, F. & Montero-de-Espinosa, R. Catalytic ozonation of oxalic acid in an aqueous TiO2 slurry reactor. Appl. Catal. B Environ. 39, 221–231 (2002).
Xu, M. et al. Efficient catalytic ozonation over Co-ZFO@Mn–CN for oxalic acid degradation: synergistic effect of oxygen vacancies and HOO–Mn–NX bonds. Appl. Catal. B Environ. 322, 122085 (2023).
Zhang, T., Li, W. & Croué, J. P. Catalytic ozonation of oxalate with a cerium supported palladium oxide: an efficient degradation not relying on hydroxyl radical oxidation. Environ. Sci. Technol. 45, 9339–9346 (2011).
Wan, X. et al. Ozone decomposition below room temperature using Mn-based mullite YMn2O5. Environ. Sci. Technol. 56, 8746–8755 (2022).
Wei, D. et al. In situ Raman observation of oxygen activation and reaction at platinum–ceria interfaces during CO oxidation. J. Am. Chem. Soc. 143, 15635–15643 (2021).
Song, Z. et al. Molecular levels unveil the membrane fouling mitigation mechanism of a superpotent N-rGO catalytic ozonation membrane: interfacial catalytic reaction pathway and induced EfOM transformation reactions. Appl. Catal. B nviron. 319, 121943 (2022).
Sun, Z., Si, Y., Zhao, S., Wang, Q. & Zang, S. Ozone decomposition by a manganese-organic framework over the entire humidity range. J. Am. Chem. Soc. 143, 5150–5157 (2021).
Hoigné, J. & Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water—I: non-dissociating organic compounds. Water Res. 17, 173–183 (1983).
Wang, H. et al. An iron-containing metal–organic framework as a highly efficient catalyst for ozone decomposition. Angew. Chem. Int. Ed. Engl. 57, 16416–16420 (2018).
Wei, K. et al. 2D-Like catalyst with a micro-nanolinked functional surface for water purification. Environ. Sci. Technol. 58, 3007–3018 (2024).
Yang, M. et al. Unveiling the origins of selective oxidation in single-atom catalysis via Co–N4–C intensified radical and nonradical pathways. Environ. Sci. Technol. 56, 11635–11645 (2022).
Jiang, R. et al. Boosting the photocatalytic decontamination efficiency using a supramolecular photoenzyme ensemble. Sci. Adv. 10, eadp1796 (2024).
Li, W., Gibbs, G. & Oyama, S. Mechanism of ozone decomposition on a manganese oxide catalyst. 1. In situ Raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 120, 9041–9046 (1998).
Wang, J., Li, S., Qin, Q. & Peng, C. Sustainable and feasible reagent-free electro-Fenton via sequential dual-cathode electrocatalysis. Proc. Natl. Acad. Sci. USA 118, e2108573118 (2021).
Langreth, D. & Mehl, M. Beyond the local-density approximation in calculations of ground-state electronic properties. Phys. Rev. B 28, 1809–1834 (1983).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for Ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2010).
Zhang, X. et al. Identifying and tailoring C–N coupling site for efficient urea synthesis over diatomic Fe–Ni catalyst. Nat. Commun. 13, 5337 (2022).
Lee, C., Yang, W. & Parr, R. Results obtained with the correlation energy density functionals. Phys. Rev. B Condens. Matter Mater. Phys. 37, 785 (1988).
Hehre, W., Ditchfield, R. & Pople, J. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 56, 2257–2261 (1972).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Krishnan, R., Binkley, J. S., Seeger, R. & Pople, J. A. Self-consistent molecular orbital methods. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980).
Wei, S. et al. Self-carbon-thermal-reduction strategy for boosting the Fenton-like activity of single Fe–N4 sites by carbon-defect engineering. Nat. Commun. 14, 7549 (2023).
Zhou, Y. et al. Peripheral-nitrogen effects on the Ru1 centre for highly efficient propane dehydrogenation. Nat. Catal. 5, 1145–1156 (2022).
Ren, T. et al. Single-atom Fe–N4 sites for catalytic ozonation to selectively induce a nonradical pathway toward wastewater purification. Environ. Sci. Technol. 57, 3623–3633 (2023).
Yang, J. et al. Formation of both free hydroxyl radicals and surface oxygen during catalytic ozonation by single-atom iron: an overlooked pollutant-dependent oxidation mechanism. ACS EST Eng.5, 250–259 (2025).
Xie, Y., Peng, S., Feng, Y. & Wu, D. Enhanced mineralization of oxalate by highly active and Stable Ce(III)-Doped g-C3N4 catalyzed ozonation. Chemosphere 239, 124612 (2020).
Wang, Y. et al. Mechanistic investigations of the pyridinic N–Co structures in Co embedded N-doped carbon nanotubes for catalytic ozonation. ACS EST Eng.1, 32–45 (2020).
Zhang, T., Li, W. & Croué, J. P. A non-acid-assisted and non-hydroxyl-radical-related catalytic ozonation with ceria supported copper oxide in efficient oxalate degradation in water. Appl. Catal. B Environ. 121, 88–94 (2012).
Faria, P., Órfão, J. & Pereira, M. A novel ceria–activated carbon composite for the catalytic ozonation of carboxylic acids. Catal. Commun. 9, 2121–2126 (2008).
Beltrán, F. J., Rivas, F. J. & Montero-de-Espinosa, R. Iron type catalysts for the ozonation of oxalic acid in water. Water Res. 39, 3553–3564 (2005).
Orge, C. et al. Ozonation of model organic compounds catalysed by nanostructured cerium oxides. Appl. Catal. B Environ. 103, 190–199 (2011).
Andreozzi, R., Insola, A., Caprio, V., Marotta, R. & Tufano, V. The use of manganese dioxide as a heterogeneous catalyst for oxalic acid ozonation in aqueous solution. Appl. Catal. A Gen. 138, 75–81 (1996).
Beltrán, F., Rivas, F. & Montero-de-Espinosa, R. Ozone-enhanced oxidation of oxalic acid in water with cobalt catalysts. 2. Heterogeneous catalytic ozonation. Ind. Eng. Chem. Res. 42, 3218–3224 (2003).
Avramescu, S., Bradu, C., Udrea, I., Mihalache, N. & Ruta, F. Degradation of oxalic acid from aqueous solutions by ozonation in presence of Ni/Al2O3 catalysts. Catal. Commun. 9, 2386–2391 (2008).
Orge, C., Órfão, J., Pereira, M., Barbero, B. & Cadus, L. Lanthanum-based perovskites as catalysts for the ozonation of selected organic compounds. Appl. Catal. B Environ. 140, 426–432 (2013).
Yuan, Y., Garg, S., Ma, J. & Waite, T. Kinetic modeling-assisted mechanistic understanding of the catalytic ozonation process using Cu–Al layered double hydroxides and copper oxide catalysts. Environ. Sci. Technol. 55, 13274–13285 (2021).
Chen, S. et al. Efficient catalytic ozonation via Mn-loaded C-SiO2 Framework for advanced wastewater treatment: Reactive oxygen species evolution and catalytic mechanism. Sci. Total Environ. 858, 159447 (2023).
Restivo, J., Orge, C., Santos, A., Soares, O. & Pereira, M. Nano-and macro-structured cerium oxide–Carbon nanotubes composites for the catalytic ozonation of organic pollutants in water. Catal. Today 384, 187–196 (2022).
Shan, C. et al. Mesoporous Ce-Ti-Zr ternary oxide millispheres for efficient catalytic ozonation in bubble column. Chem. Eng. J. 338, 261–270 (2018).
Orge, C., Órfão, J., Pereira, M., Farias, A. & Fraga, M. Ceria and cerium-based mixed oxides as ozonation catalysts. Chem. Eng. J. 200, 499–505 (2012).
Kong, X., Garg, S., Mortazavi, M., Ma, J. & Waite, T. Heterogenous iron oxide assemblages for use in catalytic ozonation: reactivity, kinetics, and reaction mechanism. Environ. Sci. Technol. 57, 18636–18646 (2023).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant no. 22425607 and 22406206), the China Postdoctoral Science Foundation (grant no. 2023M743994), the Guangdong Basic and Applied Basic Research Foundation (2025A1515012018), and the Fundamental Research Funds for the Central Universities (grant no. 23qnpy88).
Author information
Authors and Affiliations
Contributions
Z.S. and X.Y. designed the study. Z.S., J.X., and L.Z. performed the experiments. Z.S. C.H., P.W., and X.Y. contributed to the data interpretation. Z.S. and X.Y. co-wrote the manuscript. All authors discussed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Seong-Ju Hwang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Z., Xu, J., Zhang, L. et al. Tailoring bidirectional electronic transfer interaction tunnels triggers sustainable and high activity of ozone catalysis for water purification. Nat Commun 16, 8121 (2025). https://doi.org/10.1038/s41467-025-63614-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63614-9