Abstract
Manganese ion (Mn²⁺) is crucial for various physiological processes, yet excessive levels disrupt cellular homeostasis and impair the function of multiple organelles. The transporter SLC30A10 plays a pivotal role in Mn²⁺ homeostasis by exporting Mn²⁺ from cells, preventing toxic effects. Mutations in the SLC30A10 gene result in Mn²⁺ accumulation and lead to disorders such as hypermanganesemia with dystonia 1 (HMNDYT1). Despite its physiological significance, the structural basis underlying Mn²⁺ binding and the detailed transport mechanisms of SLC30A10 remain unknown. Here, we present diverse conformations of high-resolution cryo-electron microscopy (cryo-EM) structures that reveal a Mn²⁺-binding site in SLC30A10, setting it apart from other SLC30 family transporters. Furthermore, we show that the HMNDYT1-associated D40A mutation interrupts Mn²⁺ binding and transport, identifying D40 as a potential therapeutic target. These findings provide structural insights into Mn²⁺ transport mechanisms mediated by SLC30A10, advancing our understanding of Mn²⁺ binding and potential targets for future therapeutic exploration.
Similar content being viewed by others
Introduction
Manganese (Mn) is an essential trace element crucial for numerous physiological processes, including metabolism, regulation of oxidative stress, immune responses, and brain development1,2. However, excessive accumulation of Mn²⁺ can lead to severe health issues, particularly affecting the nervous system, blood, and liver3,4. Mn toxicity affects multiple cellular processes, including Golgi, endoplasmic reticulum (ER), and endosomal function, thereby impairing protein folding, post-translational modification, intracellular transport and degradation5,6,7,8. In addition, elevated Mn²⁺ levels can disrupt mitochondrial function by impairing oxidative phosphorylation and increasing the production of reactive oxygen species (ROS), leading to cell death9,10,11,12. Given these detrimental effects, a comprehensive understanding of the cellular mechanisms regulating Mn²⁺ homoeostasis is critically important.
In mammals, SLC30A10 (also known as ZnT10) is a distinctive Mn²⁺ transporter, specifically exporting Mn²⁺ from cells to maintain Mn²⁺ homoeostasis, whereas other family members primarily transport zinc ions (Zn²⁺)13,14,15,16,17,18,19,20,21. Defects in the SLC30A10 gene lead to Mn²⁺ accumulation in various tissues, causing a condition known as hypermanganesemia with dystonia 1 (HMNDYT1), polycythemia, and cirrhosis22,23,24,25,26,27,28,29,30. Patients harbouring SLC30A10 mutation such as c.119 A > C (p.Asp40Ala) exhibit elevated Mn²⁺ levels, movement disorder, increased red blood cell counts, and chronic liver disease30. In mouse models, deletion of SLC30A10 in the central nervous system leads to significant Mn²⁺ accumulation in the basal ganglia and thalamus, underscoring its crucial role in Mn²⁺ efflux and homoeostasis31,32,33,34,35. Although previous studies, based on predicted models and mutagenesis experiments, have proposed several residues impacting SLC30A10’s Mn²⁺ transport and provided valuable insights into its functional properties14,36,37, the precise Mn²⁺ binding site and detailed transport mechanisms of SLC30A10 are yet to be fully elucidated.
In this study, we present cryo-electron microscopy (cryo-EM) structures of human SLC30A10, which reveal a Mn²⁺-specific binding site previously unidentified within the ZnT family. This finding, along with the delineation of two distinct conformations, advances our knowledge of Mn²⁺ transport. Site-directed mutagenesis, corroborated by functional assays in mammalian cells, has clarified the molecular mechanisms by which SLC30A10-mediated Mn²⁺ efflux, which reduces Mn²⁺ overload-induced oxidative stress and sustains cell viability, primarily through interactions at the Mn²⁺ coordination site (D40, N127, D248, S252). Our findings elucidate the pathogenesis of the spontaneous D40A mutation in SLC30A10, providing a theoretical basis for targeted therapy for HMNDYT1 treatment.
Results
SLC30A10 protects against Mn²⁺-induced cytotoxicity
Exposure to high levels of Mn²⁺ leads to toxic accumulation, particularly in the brain1. To investigate its impact, we treated nerve-derived SH-SY5Y cells with MnCl2 and measured intracellular Mn content using the cellular Fura-2 manganese extraction assay (CFMEA assay)38,39,40. The results showed a significant increase in Mn content, accompanied by cell death (Supplementary Fig. 1a, b). Normalizing Mn content to double-stranded DNA (dsDNA) using PicoGreen dsDNA assay further confirmed a marked elevation in Mn levels (Fig. 1a, Supplementary Fig. 1c). Cell survival assay showed that 100 μM MnCl2 exposure for 24 h caused ~20% cell death (Fig. 1b, c), highlighting the cytotoxic effects of excessive Mn²⁺.
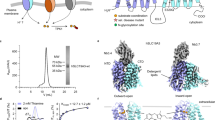
a Normalized Mn levels in SH-SY5Y cells (n = 5). b, c Flow cytometry detection (b) and statistical analysis (c; n = 3) of cell survival by Annexin V / PI. d Cell survival by Annexin V / PI in the absence or the presence of 100 μM MnCl2 for 24 h in Dox-inducible SLC30A10 overexpressed SH-SY5Y cells (n = 4). -Dox, without Dox treatment. +Dox, treated with 100 ng/ml Dox. e Normalized Mn levels in SLC30A10 transfected HEK293T cells by ICP-MS. Cells transfected with vector served as control. Data were normalized to control (n = 4). f In vitro Mn2+ transport assay of SLC30A10. Purified human SLC30A10 protein and calcein-salt were reconstituted into liposome. The fluorescence of calcein was quenched by Mn2+ that is transported into the liposome (n = 3). Transport was initiated by the addition of 100 μM MnCl₂. The fluorescence of MnCl2-free proteoliposome was measured to determine the background signal (F0). The fluorescence of 0 s was measured as maximum (max) fluorescence. The relative fluorescence was calculated using the equation (F/Fmax)-(1-F0/F0max). g, h Cryo-EM map of human SLC30A10 in the Mn2+-bound state in the side view. The two monomers are coloured in red and blue. i, j Cylinder representation of SLC30A10 in the Mn2+-bound state in the side view. Mn2+ is in purple spheres. The flexible region in the loops between TM4 and TM5 not observed in the structure are shown as dash line. k Cylinder representation of SLC30A10 in the Mn2+-bound state in the extracellular view. l Topology of SLC30A10 monomer. All bar graphs above represent mean ± SEM. Statistical analysis was performed using two-tailed unpaired t-test (a, c, e), one-way ANOVA with Dunnett’s multiple comparisons test (d), or two-way ANOVA with Sidak’s multiple comparisons test (f) (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant). The experiments were independently repeated 3 times.
Investigating the broader cellular effects, we observed that Mn²⁺ can induce oxidative stress, elevate ROS, and disrupt mitochondrial function, leading to cell death (Supplementary Fig. 1d–f). To pinpoint mechanisms that could counteract these effects, we screened several manganese transporters using quantitative real-time PCR (qRT-PCR). SLC30A10, localized on the plasma membrane, was the only efflux transporter that showed upregulated expression (Supplementary Fig. 1g), consistent with its established role in Mn²⁺ overload management41,42. Knockdown of SLC30A10 led to higher Mn²⁺ levels, elevated ROS content, decreased mitochondrial membrane potential (MMP), and reduced ATP levels, resulting in increased cell death (Supplementary Fig. 1h–m). Conversely, in SLC30A10-overexpressing cells, both the total Mn content in cells and the Mn levels within mitochondria were significantly reduced (Supplementary Fig. 1n–p), accompanied by decreased ROS levels, preserved mitochondrial function, and improved cell survival (Fig. 1d, Supplementary Fig. 1q–t). These knockdown and overexpression results align with previous findings that SLC30A10 plays a key role in regulating manganese homoeostasis13,31. Collectively, our findings underscore the critical function of SLC30A10 in maintaining mitochondrial integrity and protecting against Mn²⁺-induced cytotoxicity.
Cryo-EM structure determination and overall architecture of SLC30A10
To validate the specificity of SLC30A10 for Mn2+ transport, we overexpressed SLC30A10 in HEK293T cells and observed that it specifically reduced Mn content but had no effect on Zn levels (Fig. 1e, Supplementary Fig. 2a, 3a–d). We then purified full-length human SLC30A10 protein and reconstituted it into liposomes for ion transport assays (Supplementary Fig. 2b, c). These liposomes containing SLC30A10 exhibited significant Mn²⁺ transport activity, with the Km for Mn²⁺ determined to be 100.06 ± 24.53 μM (Fig. 1f and Supplementary Fig. 2d–f). Further tests confirmed no significant transport of other divalent ions such as Zn²⁺, Ca²⁺, or Mg²⁺, reinforcing SLC30A10’s specificity for Mn²⁺ (Supplementary Fig. 3e–g).
To gain deeper insight into the mechanism of Mn2+ transport by SLC30A10, we prepared full-length human SLC30A10 samples in the presence or absence of Mn2+ for cryo-EM and single-particle analysis (Supplementary Fig. 4, 5 and Supplementary Table 1). We obtained cryo-EM maps of SLC30A10 at 2.79 Å resolution in the presence of Mn2+ and 2.94 Å in its absence. The high-resolution maps enabled unambiguous assignment of most side chains. Under both conditions, SLC30A10 forms a homodimer consistent with other family members15,16,17,18,19,20,21 (Fig. 1g, h). Each monomer comprises a transmembrane domain (TMD) and a cytoplasmic C-terminal domain (CTD) (Fig. 1i–l). The CTD consists of three α-helices (α1, α2, α3), and four β-sheets (β1a, β1b, β2, β3). The TMD contains six transmembrane helices (TM1-TM6), connected by loops of varying lengths. Except for the excessively long and flexible loop between TM4 and TM5, all loops exhibit stable electron densities. The helix bundle near the extracellular region is tightly packed, while the cytoplasmic region is more loosely arranged, indicating that we captured the homodimers in the inward-facing (IF) conformation. We further explored structural heterogeneity in Mn2+ transport using 3D classification and refinement. This yielded an asymmetric dimer structure with a resolution of 3.34 Å in the presence of Mn2+, revealing heterogeneous conformations of the two subunits (Supplementary Fig. 4, 6). These conformations appear to represent a transition from the IF to outward-facing (OF) state.
Mn²⁺ binding site and coordination in SLC30A10
In the Mn²⁺-bound IF structure, we observed a Mn²⁺ ion nestled within the cytosolic cavity of SLC30A10 (Fig. 2a). The Mn²⁺ is coordinated by an atypical binding site formed by transmembrane helices TM2, TM4, and TM5, which has not been previously observed in this transporter family. Key residues, including D40 (TM2), N127 (TM4), D248 (TM5), and S252 (TM5), create an octahedral coordination around the Mn2+ (Fig. 2b and Supplementary Fig. 7a–c). Additional interactions involving E25 and M44 appear to further stabilize D40’s coordination with the Mn2+ through hydrogen bond, electrostatic forces, and hydrophobic effect (Supplementary Fig. 7d). In the Mn2+-free structure, the electron density at this binding site diminishes (Fig. 2c and Supplementary Fig. 7e). Molecular Dynamics (MD) simulations corroborate the coordination environment, confirming its critical role in Mn²⁺ binding and suggesting mechanisms for ion specificity (Fig. 2d, Supplementary Fig.8 and Supplementary Table 2).
a Electrostatic surface potential of the SLC30A10 inward-facing (IF). Mn2+ is in purple spheres. Negatively to positively charged regions are coloured from red to blue. The black box indicates the focused area shown in (b, c). b Zoomed-in view of the Mn2+-binding site in the side view. The density is contoured at 1.7σ. c The density map of the Mn2+-binding site. The density is contoured at 2σ. Blue, Mn2+ bound; orange, Mn2+ free. d Time-dependent variation of the distance between Mn2+ and the coordinating residue atoms in MD simulation. e Transport ability of SLC30A10 WT and mutations in HEK293T cells (n = 3). Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparisons test. f Structural comparison of Mn2+-bound SLC30A10 (IF) and Zn2+-bound ZnT1 (IF, PDB code:8XMF). The SLC30A10 is shown in blue, and ZnT1 in grey. The boxes indicate the focused areas shown in (g, h). g Zoomed-in view of the Mn2+-binding site from the extracellular view. Blue, Mn2+-bound SLC30A10 (IF); grey, Zn2+-bound ZnT1 (IF). h Comparison of the ZnT1 ion-binding site with the corresponding residues in SLC30A10. Blue, Mn2+-bound SLC30A10 (IF); grey, Zn2+-bound ZnT1 (IF); Mn2+ is in purple sphere; Zn2+ is in pink sphere. i Sequence alignment of human SLC30A10, ZnT1, ZnT3, ZnT4, ZnT7 and ZnT8 across a portion of the TM2 and TM5 region. j Transport ability of SLC30A10 WT and mutations in HEK293T cells (n = 3). Statistical analysis was performed using one-tailed one-sample t-test. All σ values are derived from locally extracted and resampled maps surrounding the Mn²⁺-binding site. All bar graphs above represent mean ± SEM from three independent experiments (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant).
To evaluate the functional importance of these Mn2+ binding residues, we performed site-directed mutagenesis and assessed Mn2+ transport in HEK293T cells. Cells expressing the D40A, N127A, D248A, S252A, E25A, and M44A mutants exhibited significantly higher intracellular Mn levels compared to those expressing wild-type (WT) SLC30A10. The D40A, N127A, D248A, and S252A mutants completely lost transport function, while the E25A and M44A mutants showed reduced Mn2+ transport capability (Fig. 2e, Supplementary Fig. 9). These results confirm the essential roles of these residues in Mn2+ transport by SLC30A10. Notably, a single-point mutation in SLC30A10 at position D40 (D40A) leads to Mn2+ accumulation in humans, causing HMNDYT1 disease (Supplementary Fig. 7f and Supplementary Table 3). The patient with HMNDYT1 is characterised by neurological impairment, including gait deviation, cognitive decline and language deficits30. This highlights how a loss-of-function mutation at a critical Mn2+ binding site directly contributes to the onset of HMNDYT1.
Structural comparison between ZnT1 and SLC30A10 reveals conserved transmembrane helices but a distinct Mn²⁺-binding site in SLC30A10 (Fig. 2f, g). While ZnT1’s canonical “HDHD” motif (two His, two Asp) forms a tight Zn²⁺ binding site, most of SLC30A10’s corresponding residues (N43, D47, H244, D248) do not directly coordinate Mn²⁺ (Fig. 2h, i). The only non-conserved residue N43 had no effect on Mn²⁺ transport when mutated to N43A or N43H, and neither did H244A. However, the H244N mutation moderately reduced activity, likely due to changes in electrostatic interactions. In contrast, D47A abolished transport and D47N/D47E reduced activity, suggesting a role in Mn²⁺ recruitment at the cytoplasmic face. D248N also abolished transport, consistent with its role in Mn²⁺ coordination. Multi-site mutations uniformly abolished Mn²⁺ transport, further underscoring the importance of these residues for transport function (Fig. 2j and Supplementary Fig. 11a, b). Although N43 mutations had little effect in SLC30A10, the H43N mutation in ZnT1 reduced Zn²⁺ transport and enabled Mn²⁺ transport (Supplementary Fig. 11c, d), confirming its importance in ZnT1. Previous studies investigating mutations at N43 have reported conflicting conclusions regarding its role in Mn²⁺ transport14,36,37. While some findings suggest N43 is critical for Mn²⁺ selectivity14, others report that N43A and N43H mutants retain transport activity comparable to WT36,37. Our results align with the latter and support the view that Mn²⁺ transport selectivity of SLC30A10 is not governed by a single residue, but rather involves more complex mechanisms. This contrast highlights a more complex selectivity mechanism in SLC30A10.
We further assessed non-conserved residues near the binding site with side chains projecting into the transport cavity S39, C93, G251, V254, and I291 (Supplementary Fig. 11e). Mutations in these residues, individually and in combination, impaired Mn²⁺ transport (Supplementary Fig. 11f), suggesting they may contribute to specificity by shaping the cavity or modulating ion accessibility.
Conformational transition of SLC30A10
We obtained both symmetric inward-inward facing (IF-IF) and asymmetric inward-outward facing (IF-OF) conformations of SLC30A10 in the presence of Mn2+. Significant conformational changes occur in the transmembrane domain (Fig. 3a–d and Supplementary Fig. 12a, b). In the OF protomer, TM5 rotates inward by 22.4°, closing the cavity that was previously open to the cytoplasm (Figs. 2a, 3c). Conversely, transmembrane helices TM1 and TM2 near the extracellular side rotate outward by 37.1° and 20.6°, respectively, creating an open cavity that extends into the extracellular space (Fig. 3d, e). This spatial separation prevents these residues from forming a stable coordination complex in the OF conformation. Structural comparisons between the OF protomers of SLC30A10 and related transporters, such as ZnT1, ZnT7, and ZnT8, showed similar backbone arrangements (Fig. 3f, g and Supplementary Fig. 12c–h). However, unlike in these counterparts, no ion-like density was observed near the ion-binding residues of SLC30A10, which were identified based on homology and structural alignment. This absence of density could suggest a lower ion-binding affinity at this site within the SLC30A10 compared to other members of ZnT family.
a Structural overlay of SLC30A10 inward-facing (IF) and outward-facing (OF). The IF conformation is coloured in blue, and OF conformation in green, respectively. The box indicates the focused area shown in panels (b−d). b−d Zoomed-in view of the overlay of IF and OF in sideview (b), intracellular view (c) and extracellular view (d). e Electrostatic surface potential of the transmembrane domain of SLC30A10 (OF). Negatively to positively charged regions are coloured from red to blue. f, g The OF conformation of SLC30A10 structure aligned with the ZnT1 (PDB code:8ZSZ), ZnT7 (PDB code:8J7W) and ZnT8 (PDB code:6XPE). SLC30A10, ZnT1, ZnT7 and ZnT8 are coloured in green, grey, yellow and purple, respectively. Zn2+ is in pink spheres.
a Proposed model for the Mn²⁺ transport mechanism of SLC30A10: The orientation and relative positions of helices and key residues are shown based on the structural data. Purple spheres represent Mn²⁺ ions. For clarity, only the transmembrane domain (TMD) of one protomer is shown. Mn²⁺ is attracted to a negatively charged cytoplasmic cavity (Red) and coordinated by D248, D40, N127, and S252. The ions are ultimately exported through an extracellular cavity, exiting the cell. b–e Statistical analysis of normalized Mn levels (b; n = 5), ROS levels (c; n = 3), mitochondrial membrane potential (d; n = 3) and ATP levels (e; n = 3) in the presence of 100 μM MnCl2 for 24 h in SH-SY5Y cells. f, g Flow cytometry detection (f) and statistical analysis (g; n = 3) of cell survival by Annexin V / PI staining in SH-SY5Y cells. All bar graphs above represent mean ± SEM from three independent experiments. Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparisons test (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05; ns not significant).
Mn2+ binding site is crucial for the protective function of SLC30A10
Our structural analysis proposes a working model for the Mn²⁺ transport mechanism of SLC30A10 (Fig. 4a). Mn²⁺ is initially attracted into a negatively charged cavity near the cytoplasmic side, then transferred to residue D248, where it forms a specific coordination binding site with D40, N127, and S252. Once Mn²⁺ is securely bound at this central site, the resulting coordinated interaction triggers conformational changes in TM5, TM1 and TM2. This transition shifts SLC30A10 from the IF to the OF conformation, allowing the release of Mn²⁺ into the extracellular space.
To validate this proposed model and investigate the role of SLC30A10 in protecting neuronal cells from Mn²⁺-induced cytotoxicity, we performed mutagenesis targeting key residues involved in Mn²⁺ transport. SH-SY5Y cells transfected with SLC30A10 mutants D40A, N127A, D248A, and S252A were exposed to MnCl₂ for 24 h. These mutants failed to reduce intracellular Mn levels (Fig. 4b and Supplementary Fig. 13a, b). Compared to cells expressing the WT SLC30A10, cells with mutant transporters showed decreased mitochondrial membrane potential, reduced ATP levels, and increased ROS accumulation, indicating impaired mitochondrial function (Fig. 4c–e and Supplementary Fig. 13c). Consequently, the mutants were unable to protect the cells from Mn2+-induced cytotoxicity (Fig. 4f, g). These results substantiate that the Mn²⁺ transport process, mediated by the specific Mn²⁺ binding site and adjacent negatively charged residues, is crucial for the protective function of SLC30A10 in neuronal cells. Our results elucidate SLC30A10’s critical role in reducing cellular Mn²⁺ levels, thereby preserving mitochondrial health and preventing the cytotoxic effects of Mn²⁺ overload.
Discussion
As a Mn²⁺ efflux transporter, SLC30A10 is upregulated in response to Mn²⁺ overload, playing a vital role in mitigating toxicity caused by excessive Mn²⁺ accumulation41,42. Our study confirms that elevated Mn²⁺ levels induce SLC30A10 expression, which in turn facilitates Mn²⁺ efflux, reduces oxidative stress, and maintains mitochondrial function. Conversely, a loss of SLC30A10 function correlates with increased cell death, while its overexpression not only decreases intracellular Mn²⁺ levels but also preserves mitochondrial integrity and enhances cell survival. These findings underscore the indispensable role of SLC30A10 in protecting cells from Mn²⁺ toxicity through its contributions to efficient Mn²⁺ efflux.
Although the role of SLC30A10 in Mn²⁺ transport is well recognised, the structural basis underlying its transport remains elusive. Previous studies based on homology modelling and sequence analysis proposed residues such as D40, N127, D248, and E25 as potential contributors to Mn²⁺ export14,36,37. These predictions are now experimentally validated by our structural analysis, which confirms that these residues form key components of the Mn²⁺-binding site. However, computational models alone often struggle to resolve the structural details of metal-binding environments, where precise side-chain positioning is essential for accurately identifying binding pockets and capturing conformational dynamics. By determining high-resolution cryo-EM structures of full-length human SLC30A10 in both Mn²⁺-bound and Mn²⁺-free states, we were able to overcome these challenges and gain mechanistic insights that were inaccessible through modelling approaches. In addition to validating the previously proposed residues, our structures identify S252 as a critical Mn²⁺-coordinating residue and implicate M44 in stabilising the binding environment. These findings refine the composition of the Mn²⁺-binding site and advance our understanding of how SLC30A10 mediates metal transport. Furthermore, we capture an outward-facing conformation of the transporter and report a cluster of structured water molecules adjacent to the ion-binding site, features that may play a role in Mn²⁺ coordination, and which have not been observed in other SLC30 family structures.
Functional validation supports the importance of these structural insights. Mutations in the coordinating residues, particularly D40A, abolish transport activity. Clinically, the D40A mutation corresponds to a spontaneous variant (c.119 A > C, p.Asp40Ala) found in patients with HMNDYT1, who exhibit neurological symptoms and liver damage due to the disrupted manganese homoeostasis30. Mouse models similarly show hepatic Mn²⁺ accumulation and metabolic alterations upon SLC30A10 deficiency34, though how SLC30A10 regulates liver metabolism requires further investigation.
By linking high-resolution structural insights with functional outcomes, our study provides a comprehensive mechanistic framework for understanding how SLC30A10 facilitates Mn²⁺ efflux and highlights the far-reaching consequences of mutations like D40A. These findings advance our understanding of the molecular mechanism of Mn²⁺ transport and offer potential therapeutic strategies to restore SLC30A10 function and address both neurological and hepatic manifestations of manganese toxicity.
Methods
Cell culture and transfection
HEK293T (SCSP-502) and SH-SY5Y (SCSP-5014) were purchased from the Cell Bank of the Chinese Academy of Sciences and were authenticated by the supplier using STR profiling. HEK293F (R790-07) was purchased from Invitrogen and have undergone supplier-performed quality control testing. HEK293T and SH-SY5Y cells were cultured in dulbecco’s modified eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (FBS, Moregate), 100 units/mL penicillin and 0.1 mg/mL streptomycin (MeilunBio) at 37 °C under 5% CO2. HEK293F cells were cultured in FreeStyle293 medium (Thermo Fisher Scientific) at 37 °C, under 8% CO2 on an orbital shaker platform rotating at 120 rpm. SLC30A10 over-expressed and knockdown SH-SY5Y cells were established by our lab and has not been authenticated. 1 μg/ml puromycin was added to the medium for SLC30A10-overexpressed and knockdown stable SH-SY5Y cells. The human SLC30A10 gene was cloned into the pLVX-Tet3G vector (Takara, 631847), and a doxycycline (Dox)-induced SLC30A10 overexpressing stable cell line was established by infecting SH-SY5Y cells with lentiviruses. The SLC30A10 was overexpressed when 100 ng/ml doxycycline (Dox) was added to the medium for 48 h. The SLC30A10 knockdown SH-SY5Y cells were generated using the pLKO.1 vector (Addgene, 8453), via infecting with viruses. The shRNA sequences for human SLC30A10 were GCCATCATATTCTATGTGCTT (shRNA1) and CCACGGACAAAGTCTTAACAA (shRNA2). PEI (Yeasen) was used for transient transfection of HEK293F cells, and Liposomal transfection reagent (MeilunBio) was used for HEK293T and SH-SY5Y transient transfection according to the manufacturer’s instructions.
Cellular Fura-2 Manganese Extraction Assay (CFMEA) assay
Site-directed mutagenesis of SLC30A10 was performed using homologous recombination PCR, and the sequences of all constructs were verified by DNA sequencing in BioSune. After cells were transfected with SLC30A10 wild-type or mutant plasmids, the CFMEA assay was performed to measure cellular Mn concentrations following a previously published protocol33. Briefly, cells were seeded in poly-D-lysine-coated 96-well plates with black edges and clear bottoms (Cellvis). After 24 h, cells were transfected with 200 ng of SLC30A10 plasmids per well, with vector-transfected cells serving as controls. For HEK293T cells, 300 μM MnCl2 was added. After 4 h of MnCl2 exposure, the medium was aspirated, and cells were washed twice with pre-warmed PBS. Then, 100 μL of assay buffer (PBS with 0.1% Triton X-100) containing 500 nM Fura-2 salt (Sigma) was added to each well. The plate was incubated at 37 °C for at least 1 h, protected from light. Fura-2 salt fluorescence was then measured using a BioTek Synergy2 Microplate Reader with excitation/emission wavelengths of 360 nm/535 nm. A standard curve for Mn concentration was generated using varying concentrations of MnCl2 and 500 nM Fura-2 salt. Mn concentrations and mass of Mn (MMn) in the cell extracts were calculated based on this standard curve. For SH-SY5Y cells, 100 μM MnCl2 was added, and Mn levels were measured after 24 h of treatment.
Manganese content and transport ability analysis
dsDNA from cell extracts post-CFMEA analysis was quantified using the PicoGreen dsDNA reagent (Yeasen). Briefly, 80 μL of 1:400 diluted PicoGreen reagent in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) was added to a 96-well plate. Then, 2 μL of cell extract from the CFMEA assay was added. After incubating for 5 min, protected from light, fluorescence was measured (excitation/emission wavelength: 480 nm/520 nm). A dsDNA standard curve was generated using varying concentrations of λDNA, and mass of dsDNA (MDNA) in each well was calculated using the linear equation derived from the standard curve.
Mn content relative to DNA (AMn) was calculated using the equation (MMn/MDNA), and data were represented as pg Mn/ng dsDNA. Finally, the data were normalised to the control group using the equation (AMn/AMncontrol). Mn2+ transport ability was calculated using the equation ((AMncontrol-AMn)/relative protein expression level). Relative protein expression levels were quantified by calculating the grayscale intensity from immunoblotting analysis using Fiji software. All statistical analyses, comparing wild-type and mutant cells, were performed using GraphPad Prism software.
Zinc transport assay based on HEK293T cell
The procedure of zinc transport assay is similar to the manganese transport ability analysis above. Briefly, 24 h after transfection, HEK293T cells were treated with ZnCl2 for 4 h. Then cells were washed twice with PBS and loaded with 100 μL of assay buffer (PBS with 0.1% Triton X-100) containing 1 μM FluoZin-3 salt (Sigma). After dark incubation at 37 °C for 2 h, the 96-well plates were measured using a BioTek Synergy2 Microplate Reader with excitation/emission wavelengths of 488 nm/525 nm. A standard curve based on the linear relationship of F-F0 was used to calculate the content of intracellular zinc. The measurement of dsDNA is the same as the method above. Zn2+ transport ability was calculated using the MZn/MDNA.
Relative Zn levels were also assessed using the membrane-permeable fluorescent dye FluoZin-3 AM, following a previously published method16. Cells were washed with PBS, treated with 60 µM ZnCl2 and 1 µM membrane-permeable FluoZin-3 AM (ThermoFisher Scientific) in DMEM for 50 min at 37 °C. After incubation, cells were washed three times with the buffer (20 mM HEPES-Na pH 7.4, 125 mM KCl, 5 mM NaCl, 10 mM glucose, and 10 µM phenanthroline) for microscopy imaging. Fluorescence intensities from cells without ZnCl₂ treatment were used to calculate the average background signal (F0). Relative Zn levels were then calculated as the ratio of F to F0 (F/F0).
Inductively coupled plasma mass spectrometry (ICP-MS)
Different cell models were analysed by ICP-MS. Transiently transfected HEK293T cells were used to assess changes in total cellular Mn levels, while stably transfected HEK293T cells were used to examine Zn levels. Stably transfected SH-SY5Y cells were utilized to quantify mitochondrial Mn content. To measure metal levels in HEK293T cells, cells were treated with 100 μM MnCl₂ for 1 h or 60 μM ZnCl₂ for 50 min. The cells were then harvested and washed with PBS containing 10 mM EDTA three times, followed by ICP-MS analysis15. For mitochondrial Mn quantification, SH-SY5Y WT and stable SLC30A10-overexpressing cells were treated with 100 μM MnCl₂ for 16 h. Mitochondria were then isolated using a mitochondrial isolation kit (Beyotime) according to the manufacturer’s instructions. Metal concentrations were measured by ICP-MS and normalised to total cell or mitochondrial weight.
Immunoblotting
Proteins were resolved in 10% SDS-PAGE, transferred to PVDF membranes (cytiva), and incubated with primary antibodies against DYKDDDDK tag (Flag tag, proteintech, cat#66008-4-Ig, lot#10027647, 1:5000, https://www.ptglab.com/products/Flag-tag-Antibody-66008-4-Ig.htm), GAPDH (proteintech, cat#60004-4-Ig, lot#10027647, 1:5000, https://www.ptglab.com/products/GAPDH-Antibody-60004-1-Ig.htm). The second antibody is Peroxidase AffiniPure Goat Anti-Mouse IgG (H + L) (YEASEN, cat#33201ES60, lot#P1203971, 1:10000). The relative protein expression levels were calculated using Fiji software.
Immunofluorescence and confocal imaging
HEK293T or SH-SY5Y cells were seeded on poly-D-lysine coated glass coverslips and grown for 24 h. Flag-tagged SLC30A10 and plasma-mCherry plasmids (Addgene, 55779) were co-transfected into cells for 16 h. After fixed in 4% PFA and permeabilised by Triton-X, the cells were blocked in blocking buffer (2% BSA in PBST). The cells were then immunolabeled at 4 °C overnight with the flag antibody (proteintech, cat#66008-4-Ig, lot#10027647, 1:5000, https://www.ptglab.com/products/Flag-tag-Antibody-66008-4-Ig.htm). The next day, the cells were washed three times with PBS, followed by incubation with a FITC fluorescein-conjugated secondary antibody (Beyotime, cat#A0568, lot#102522221208, 1:200, https://www.beyotime.com/product/A0568.htm) for 1 h. The nuclear was stained with DAPI (Yeasen) followed by three times washing with PBS. Finally, the coverslips were sealed with antifade mounting medium. Images were recorded by the Olympus FV3000 confocal system at 60× oil objective. All images shown are representative of at least three randomly selected fields.
qRT-PCR
Total RNA was extracted from SH-SY5Y cells using RNA isolation kit (Vazyme). cDNA was prepared with the RT SuperMix Kit (Vazyme). This kit could remove genomic DNA. qRT-PCR was performed with SYBR probes by StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The primer sequences used in this study are described in Supplementary Table 4.
Cell survival assay
After being treated with MnCl2, cells were digested, washed with PBS and immediately stained with propidium iodide (PI, Beyotime) and Annexin-V (AV, Beyotime). Then cells were analysed by flow cytometry (FACS Fortessa, BD) at once. Data were analysed by Flowjo (v10.8.1) software. Gating strategy was shown in Supplementary Fig.14a.
Mitochondrial membrane potential measurement
The JC-1 probe was used to detect the mitochondrial membrane potential. After being treated with 100 μM MnCl2 for 24 h, SH-SY5Y cells were collected and incubated with JC-1 probe at 37 °C for 30 min. Then the cells were washed twice with cold JC-1 buffer. Then, the fluorescence intensity of the probe was measured using a flow cytometer (FACS Fortessa, BD) in the FITC and PE channels. Data were analysed by FlowJo (v10.8.1) software. The gating strategy was shown in Supplementary Fig.14b.
ROS detection
The DCFH-DA probe was used to detect the ROS levels of cells. After being treated with 100 μM MnCl2 for 24 h, SH-SY5Y cells were collected and incubated with 10 μM DCFH-DA probe at 37 °C for 30 min. Then the cells were washed twice with PBS. The cells were detected by flow cytometry (FACS Fortessa, BD). Data were analysed by FlowJo (v10.8.1) software. The gating strategy was shown in Supplementary Fig.14c.
ATP detection
The CellTiter-Meiluncell luminescent reagent (MeilunBio) was used to detect the relative content of ATP in cells. SH-SY5Y cells were cultured in 96-well plates. After being treated with 100 μM MnCl2 for 24 h, cells were equilibrated at room temperature for 10 min. Then the cells were treated with CellTiter-Meiluncell luminescent reagent at room temperature for 10 min. The relative ATP levels were measured with luminescence using a microplate reader (Spark, TECAN). Data were analysed by GraphPad Prism software.
Expression and purification of SLC30A10
Gene encoding the full-length human SLC30A10 (Uniprot: Q6XR72) was cloned into a modified pEG vector, incorporating a 3 × Flag tag, a Twin-Strep tag and an HRV 3 C protease site at the N terminus. For protein expression, 1 L HEK293F cells were transiently transfected with 2 mg SLC30A10 plasmid and 4 mg PEI at a cell density of 2.5 × 106 cells/ml. Cells were cultured at 37 °C with 8% CO2 for 24 h and harvested by centrifugation at 1500 × g for 10 min. The cell pellets were resuspended in buffer A (20 mM HEPES, 150 mM NaCl, pH 7.4) containing 1% DDM, 0.1% CHS, 1 mM PMSF, 6 μg/mL protease inhibitor cocktail (Aprotinin:Pepstatin:Leupeptin (w/w) = 1:1:1). The resuspended cell pellets were dounced using a glass homogenizer, and agitated gently at 4 °C for 2 h. The lysate was centrifuged at 202,000 x g for 1 h to remove cell debris. The supernatant was incubated with flag resin (Genscript, L00432) at 4 °C for 1 h. Then the resin was collected in a gravity column and washed with 20 column volumes of buffer A containing 0.05% GDN. The protein was eluted using 0.4 mg/ml 3 × flag peptide (Everylab) with 3 column volumes. The eluted protein was concentrated with a 50 kDa cut-off Amicon Ultra spin (Millipore) and subjected to size-exclusion chromatography (SEC) using a Superose 6 Increase 10/300 GL column (Cytiva) equilibrated in buffer A containing 0.01% GDN. The peak fractions corresponding to purified SLC30A10 were collected and concentrated to 4 ~ 8 mg/mL for cryo-EM studies.
For the purification of SLC30A10 in the presence of manganese, the protein was purified following a similar protocol. Specifically, 1 mM MnCl2 was added, and the pH was adjusted to 6.0 using MES. In brief, Buffer B (20 mM MES, 150 mM NaCl, 1 mM MnCl2, pH 6.0) was used throughout the purification process to replace buffer A.
Proteoliposome preparation and transport assay
POPC, POPE, and POPG (Avanti Polar Lipids) were mixed with a ratio of 10:7:3 (wt:wt:wt) and dried in a vacuum centrifugal concentrator for 3 h to remove chloroform. The mixed lipid was resuspended in a reconstitution buffer (20 mM MES, 150 mM KCl, and 0.1 % GDN, pH 6.0) to a final concentration of 10 mg/ml. The purified SLC30A10 was added at 1:100 (wt / wt, protein: lipid), and dialysed at 4 °C in dialysis cassettes (thermo) against dialysis buffer (20 mM MES and 150 mM KCl, pH 6.0) for 6 days to remove the detergent. The reconstituted liposome was sonicated for 20 s and frozen-thawed for three cycles to incorporate Fura-2 salt (50 μM) or calcein-salt (250 μM). Then, the Fura-2/calcein-containing liposomes were separated from the bulk dye on a PD-10 column (cytiva). Liposomes were pelleted using ultracentrifugation at 100,000 g for 30 min and resuspended with reconstitution buffer (20 mM MES and 14.5 mM KCl, pH 6.0). The lower concentrations of KCl in the assay buffer established the desired membrane potential, and liposomes were added to 96-well black edge and clear-bottom plates (Cellvis).
To assess the transport specificity of SLC30A10 for different metals, 1 mM of MnCl₂, CaCl₂, ZnCl₂, or MgCl₂ was added to initiate transport, and Fura-2 was used to monitor metal uptake. Fluorescence of Fura-2 was monitored in a Spark multimode microplate reader (Tecan) with 360 nm / 535 nm (excitation/emission) for the Mn2+ transport assay. For groups of Ca2+, Zn2+, and Mg2+ transport assays, fluorescence of Fura-2 was measured at 340 nm/ 510 nm, 380 nm / 510 nm, respectively. The fluorescence ratio was normalised by the first value after the addition of ions.
Calcein was used to analyse the kinetics of SLC30A10. The quenching of calcein by Mn was measured with 492 nm/ 518 nm (excitation/emission) at 10 s intervals. The fluorescence of the MnCl2-free proteoliposome was measured to determine the background signal (F0). The fluorescence of 0 s was measured as maximum (max) fluorescence. Relative fluorescence was calculated using the equation (F/Fmax)-(1-F0/F0max). Initial transport rates were determined from the change in relative fluorescence within the first minute following the addition of MnCl₂ at the start of the assay. Kinetic analysis of SLC30A10 was performed by fitting the Michaelis-Menten equation to the initial transport rates43.
Cryo-EM sample preparation and data acquisition
Aliquots of 3.0 μl 4-8 mg/mL purified SLC30A10 samples in different conditions were applied to glow-discharged holey carbon grids (Quantifoil, R1.2/1.3, Au, 300 mesh). The grids were blotted for 3.5–4.0 s using Leica EM GP2 at 4 °C with 75% humidity, and plunge-frozen into the liquid ethane.
Cryo-EM datasets were collected on Titan Krios G4 cryo-electron microscope operated at 300 kV, equipped with a Falcon 4i Direct Electron Detector and a Selectris X energy filter (Thermo Fisher Scientific). Movie stacks were automatically collected using EPU at a magnification of 130,000× with a pixel size of 0.932 Å for a total dose per EER (electron event representation) movie of ∼50 e–/Å2. The defocus range was set between -0.8 to -1.8 µm.
Image processing
All datasets were processed in CyroSPARC (v4.5.3)44. For SLC30A10 in the presence of Mn2+, a total of 25,390 cryo-EM movies were collected. Motion correction was performed on all movies using MotionCor245 with a 5 × 5 patch alignment. The contrast transfer function (CTF) parameters for each micrograph were estimated using Gctf_v1.1846. Particle picking was conducted with Gautomatch_v0.56 (https://github.com/JackZhang-Lab/Gautmatch), and the motion-corrected micrographs with particle coordinates were imported into CryoSPARC for further analysis47. Particles were extracted and subjected to one round of two-dimensional (2D) classification to remove junk particles, resulting in 4,091,140 good particles for further 2D classification. Good classes were then used for ab initio reconstruction. Particles from classes displaying membrane protein characteristics and their corresponding cryo-EM maps were refined using non-uniform refinement under C2 symmetry, resulting in a consensus map. Multiple rounds of heterogeneous refinement and three-dimensional (3D) classification were performed on the original dataset to enrich particles in the symmetric IF-IF conformation. Selected particles were subjected to non-uniform (NU) refinement and local refinement with C2 symmetry imposed with a mask covering the transmembrane domain yielding an improved map at 2.79 Å resolution47. The remaining particles were subjected to additional rounds of heterogeneous refinement and focused 3D classification using a monomer mask. A small subset of classes was identified to adopt the asymmetric IF-OF conformation. A final map was produced by performing NU refinement and local refinement at 3.34 Å.
For SLC30A10 in the absence of Mn2+, data was processed similarly. In brief, a total of 6879 EER movie stacks were imported, and the selected particles were subjected to several rounds of 2D classification and a round of Ab initio reconstruction to generate the initial models. The 3D classification was performed with multi-round heterogeneous refinement in CryoSPARC. Particles belonging to a class with well-defined features were further refined using NU refinement and local refinement with C2 symmetry and yielded final maps at 2.94 Å.
Model building, refinement and validation
The predicted model of SLC30A10 was generated by AlphaFold248 and was fitted to the cryo-EM map density in UCSF ChimeraX49. The initial model was manually refined and adjusted using the phenix.real_space_refine in PHENIX50 and Coot51. Manganese ions and water molecules were manually added to the model to fit the corresponding densities observed in the map. The complete model was re-imported into PHENIX for further refinement and optimization. The statistics of cryo-EM data collection and model refinement are summarized in Supplementary Table 1. All figures were prepared with ChimeraX.
Molecular dynamics simulation
Molecular dynamics (MD) simulation was conducted for SLC30A10-Mn2+ system. The structure and force fields for the MD system were generated utilizing the CHARMM-GUI (v.3.7)52. The CHARMM36 force field for protein, POPC lipids and TIP3P water molecules model53,54. The force field of Mn was generated using the CHARMM General Force Field (CGenFF) programme55,56. Neutralization of the initial system was attained through the strategic addition of Na+ and Cl- ions, culminating in a final NaCl concentration of 0.15 M. The system comprised SLC30A10 (IF-IF homo) protein, Mn, POPC lipids, TIP3P water, and NaCl.
Subsequently, the energy minimization, equilibration and real MD production were performed by GROMACS (v.2023.3)57. The energy minimization process employed the steepest descent algorithms until the maximum force <1000 kJ mol-1 nm-1 to obviate any steric clashes or inappropriate geometry of the system. Throughout the ~2 ns equilibration process, the temperature of system was adeptly regulated at 303.15 K via berendsen thermostat for 500 ps (NVT ensemble) and pressure was maintained at 1 bar via berendsen thermostat for 1 ns (NPT ensemble)58. The hydrogen bonds were judiciously constrained using LINCS throughout the equilibration process59.
After pre-equilibration, MD simulation was carried out over 1000 ns, saving coordinates at 10 ps intervals. The temperature of the system was steadfastly held at 303.15 K through the Nose-Hoover thermostat and the pressure of the system was maintained at 1 bar using the Parrinello-Rahman barostat60. Long-range electrostatics interactions were computed using the particle mesh Ewald (PME)61 method with a cutoff of 12 Å. Short-range van der Waals interactions were smoothly switched to zero within the range of 10 Å to 12 Å. Periodic boundary conditions (PBC) were implemented in the MD systems. The SLC30A10-Mn system was run for three replicas with different initial velocities.
Statistical information
All data were analysed using GraphPad Prism 8 software (GraphPad Inc.). The unpaired Student’s t-test, one-sample t-test, and ANOVA were used to analysis the results as indicated in figure legends. Data in bar graphs are presented as mean ± SEM. Levels of statistical significance are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Other descriptive statistics and tests are provided in the Figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Source data are provided with this paper. The UniProt accession code of human SLC30A10 is Q6XR72. The 3D cryo-EM density maps of human SLC30A10IF-IF, SLC30A10IF-OF and SLC30A10Apo have been deposited to the Electron Microscopy Data Bank under the accession numbers EMD-62603 (Cryo-EM density map of SLC30A10 in Mn2+-bound state, determined in inward-facing conformation), EMD-62604 (Cryo-EM density map of SLC30A10, determined in asymmetric conformations-one subunit in an inward-facing Mn2+-bound and the other in an outward-facing Mn2+-unbound conformation) and EMD-62605 (Cryo-EM density map of SLC30A10 in the absence of Mn2+, determined in inward-facing conformation), respectively. The coordinates of human SLC30A10IF-IF, SLC30A10IF-OF and SLC30A10Apo have been deposited to the Protein Data Bank under the accession codes 9KVX (Cryo-EM structure of SLC30A10 in Mn2+-bound state, determined in inward-facing conformation), 9KVY (Cryo-EM structure of SLC30A10, determined in asymmetric conformations-one subunit in an inward-facing Mn2+-bound and the other in an outward-facing Mn2+-unbound conformation) and 9KVZ (Cryo-EM structure of SLC30A10 in the absence of Mn2+, determined in inward-facing conformation), respectively. The Protein Data Bank coordinates used for structural alignments are 8J7W (human ZnT7 of Zn2+-bound state in heterogeneous conformations one in IF and the other in OF conformation), 8ZSZ (human ZnT1 of Zn2+-bound state in OF conformation), 8ZSB (human ZnT1 of Zn2+-unbound state in OF conformation), 6XPE (human ZnT8 of Zn2+-bound state in OF conformation), and 8J7T (human ZnT7 of Zn2+-unbound state in OF conformation).
Code availability
The code and associated files for the MD simulations are available on GitHub at [https://github.com/Hanting-lab/MD-simulation/tree/main/SLC30A10].
References
Budinger, D., Barral, S., Soo, A. K. S. & Kurian, M. A. The role of manganese dysregulation in neurological disease: emerging evidence. Lancet Neurol. 20, 956–968 (2021).
Grandjean, P. & Landrigan, P. J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 13, 330–338 (2014).
Horning, K. J., Caito, S. W., Tipps, K. G., Bowman, A. B. & Aschner, M. Manganese is essential for neuronal health. Annu. Rev. Nutr. 35, 71–108 (2015).
Zhang, D. et al. Blood manganese and nonalcoholic fatty liver disease: a cohort-based case-control study. Chemosphere 287, 132316 (2022).
Liu, C. et al. Manganese activates autophagy to alleviate endoplasmic reticulum stress-induced apoptosis via PERK pathway. J. Cell. Mol. Med. 24, 328–341 (2020).
Mukhopadhyay, S., Bachert, C., Smith, D. R. & Linstedt, A. D. Manganese-induced trafficking and turnover of the cis-Golgi glycoprotein GPP130. Mol. Biol. cell 21, 1282–1292 (2010).
Mukhopadhyay, S. & Linstedt, A. D. Identification of a gain-of-function mutation in a Golgi P-type ATPase that enhances Mn2+ efflux and protects against toxicity. Proc. Natl. Acad. Sci. USA 108, 858–863 (2011).
Rokad, D. et al. Manganese exposure enhances the release of misfolded α-synuclein via exosomes by impairing endosomal trafficking and protein degradation mechanisms. Int. J. Mol. Sci. 25, 12207 (2024).
Diessl, J. et al. Manganese-driven CoQ deficiency. Nat. Commun. 13, 6061 (2022).
Kaur, G. et al. Affected energy metabolism under manganese stress governs cellular toxicity. Sci. Rep. 7, 11645 (2017).
Li, W. H., Xiang, Z. T., Lu, A. X., Wang, S. S. & Yan, C. H. Manganese-induced apoptosis through the ROS-activated JNK/FOXO3a signaling pathway in CTX cells, a model of rat astrocytes. Ecotoxicol. Environ. Saf. 262, 115326 (2023).
Morcillo, P. et al. Defective mitochondrial dynamics underlie manganese-induced neurotoxicity. Mol. Neurobiol. 58, 3270–3289 (2021).
Leyva-Illades, D. et al. SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity. J. Neurosci. 34, 14079–14095 (2014).
Nishito, Y. et al. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J. Biol. Chem. 291, 14773–14787 (2016).
Xue, J., Xie, T., Zeng, W., Jiang, Y. & Bai, X.C. Cryo-EM structures of human ZnT8 in both outward- and inward-facing conformations. Elife 9, e58823 (2020).
Bui, H.B. et al. Cryo-EM structures of human zinc transporter ZnT7 reveal the mechanism of Zn2+ uptake into the Golgi apparatus. Nat. Commun. 14, 4770 (2023).
Ishida, H., Yo, R., Zhang, Z., Shimizu, T. & Ohto, U. Cryo-EM structures of the zinc transporters ZnT3 and ZnT4 provide insights into their transport mechanisms. FEBS lett. 599, 41−52 (2024).
Li, Y. et al. Zinc transporter 1 functions in copper uptake and cuproptosis. Cell Metab. 36, 2118−2129.e6 (2024).
Long, Y. et al. Structural insights into human zinc transporter ZnT1 mediated Zn(2+) efflux. EMBO Rep. 25, 5006−5025 (2024).
Sun, C. et al. Structural insights into the calcium-coupled zinc export of human ZnT1. Sci. Adv. 10, eadk5128 (2024).
Sun, S. et al. The intestinal transporter SLC30A1 plays a critical role in regulating systemic zinc homeostasis. Adv. Sci. (Weinh) 11, e2406421 (2024).
Anagianni, S. & Tuschl, K. Genetic disorders of manganese metabolism11. Curr. Neurol. Neurosci. Rep. 19, 33 (2019).
Gulab, S., Kayyali, H. R. & Al-Said, Y. Atypical neurologic phenotype and novel SLC30A10 mutation in two brothers with hereditary hypermanganesemia. Neuropediatrics 49, 72–75 (2018).
Mukhtiar, K., Ibrahim, S., Tuschl, K. & Mills, P. Hypermanganesemia with dystonia, polycythemia and cirrhosis (HMDPC) due to mutation in the SLC30A10 gene. Brain Dev. 38, 862–865 (2016).
Quadri, M. et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477 (2012).
Quadri, M. et al. Manganese transport disorder: Novel SLC30A10 mutations and early phenotypes. Mov. Disord. 30, 996–1001 (2015).
Tavasoli, A. et al. A case of dystonia with polycythemia and hypermanganesemia caused by SLC30A10 mutation: a treatable inborn error of manganese metabolism. BMC Pediatrics 19, 229 (2019).
Tuschl, K. et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 90, 457–466 (2012).
Yapici, Z., Tuschl, K. & Eraksoy, M. Hypermanganesemia with dystonia 1: a novel mutation and response to iron supplementation. Mov. Disord. Clin. Pract. 7, 94–96 (2019).
Zaki, M. S. et al. Hypermanganesemia with dystonia, polycythemia and cirrhosis in 10 patients: six novel SLC30A10 mutations and further phenotype delineation. Clin. Genet. 93, 905–912 (2018).
Hutchens, S. et al. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J. Biol. Chem. 292, 9760–9773 (2017).
Liu, C. et al. Hypothyroidism induced by loss of the manganese efflux transporter SLC30A10 may be explained by reduced thyroxine production. J. Biol. Chem. 292, 16605–16615 (2017).
Mercadante, C. J. et al. Manganese transporter Slc30a10 controls physiological manganese excretion and toxicity. J. Clin. Investig. 129, 5442–5461 (2019).
Shi, J.-H. et al. Fructose overconsumption impairs hepatic manganese homeostasis and ammonia disposal. Nat. Commun. 14, 7934 (2023).
Taylor, C. A. et al. SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J. Biol. Chem. 294, 1860–1876 (2019).
Zogzas, C. E., Aschner, M. & Mukhopadhyay, S. Structural elements in the transmembrane and cytoplasmic domains of the metal transporter SLC30A10 are required for its manganese efflux activity. J. Biol. Chem. 291, 15940–15957 (2016).
Zogzas, C. E. & Mukhopadhyay, S. Putative metal binding site in the transmembrane domain of the manganese transporter SLC30A10 is different from that of related zinc transporters. Metallomics Integr. Biometal Sci. 10, 1053–1064 (2018).
Kwakye, G. F., Li, D., Kabobel, O. A. & Bowman, A. B. Cellular fura-2 manganese extraction assay (CFMEA). Curr. Protoc. Toxicol. Chapter 12, Unit12–Unit18 (2011).
Pradhan, S.H., Liu, J.Y. & Sayes, C.M. Evaluating manganese, zinc, and copper metal toxicity on SH-SY5Y cells in establishing an idiopathic parkinson’s disease model. Int. J. Mol. Sci. 24, 16129 (2023).
Zhang, H.-T. et al. PINK1/Parkin-mediated mitophagy play a protective role in manganese induced apoptosis in SH-SY5Y cells. Toxicol. Vitr. 34, 212–219 (2016).
Gurol, K. C. et al. PHD2 enzyme is an intracellular manganese sensor that initiates the homeostatic response against elevated manganese. Proc. Natl. Acad. Sci. USA 121, e2402538121 (2024).
Liu, C., Jursa, T., Aschner, M., Smith, D.R. & Mukhopadhyay, S. Up-regulation of the manganese transporter SLC30A10 by hypoxia-inducible factors defines a homeostatic response to manganese toxicity. Proc. Natl. Acad. Sci. USA 118, e2107673118 (2021).
Liziczai, M., Fuchs, A., Manatschal, C. & Dutzler, R. Structural basis for metal ion transport by the human SLC11 proteins DMT1 and NRAMP1. Nat. Commun. 16, 761 (2025).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. methods 17, 1214–1221 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. D., Struct. Biol. 74, 531–544 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. 114, 7830–7843 (2010).
Mark, P. & Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 105, 9954–9960 (2001).
Vanommeslaeghe, K. & MacKerell, A. D. Jr. Automation of the CHARMM general force field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. modeling 52, 3144–3154 (2012).
Vanommeslaeghe, K., Raman, E. P. & MacKerell, A. D. Jr. Automation of the CHARMM general force field (CGenFF) II: assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 52, 3155–3168 (2012).
Abraham, M. J. et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2, 19–25 (2015).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: linear Constraint Solver Mol. Simul. 18, 1463–1472 (1997).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Acknowledgements
We thank Z.C., A.D., and H.Z. at the Center of cryo-EM at Fudan University for their support with cryo-EM data collection; M.F. and Y.W. at CAS Center for Excellence in Molecular Plant Sciences for their assistance with ICP-MS analysis. We thank Q.Q. and Y.L. at Fudan University for providing the ZnT1 plasmid and for their suggestions on zinc levels detection. This work was supported by the grants from the National Science and Technology Innovation 2030 Major Projects of China (STI2030-Major Projects 2022ZD0212600 to H.Y.), the National Natural Science Foundation of China (32171216 to H.Y.), China Postdoctoral Science Foundation (2023M730689 to X.S.), and Fudan Undergraduate Research Opportunities Program (23216 to R.H.).
Author information
Authors and Affiliations
Contributions
H.Y. and X.S. conceived and designed the experiments. X.S. performed flow cytometry experiments, ICP-MS, proteoliposome assay, purified and prepared the samples for cryo-EM study, and collected cryo-EM data; J.K.Z. processed cryo-EM data, built models, and analysed the structures under supervision of H.Y.; P.S. performed MD simulations; X.S. and H.Z. performed cell-based ion export assay with help from R.H.; X.S. and S.W. performed the confocal imaging with assistance from X.G.; X.S., J.K.Z., P.S., and H.Y. analysed and interpreted the results. X.S., J.K.Z. and H.Y. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shen, X., Zhang, J.K., Sun, P. et al. Molecular mechanisms of SLC30A10-mediated manganese transport. Nat Commun 16, 8581 (2025). https://doi.org/10.1038/s41467-025-63616-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63616-7