Abstract
Recent advancements in implantable bioelectronic devices have increased the demand for biocompatible energy sources with long-term electrochemical and mechanical stability. Here, we present a tough hydrogel-based supercapacitor (THBS) fiber, fabricated via a thermal drawing process (TDP), that enables the integration of all components—electrodes, electrolyte, current collectors, and encapsulation—into a single, unified, and mechanically robust fiber-shaped architecture. Through thermal/mechanical optimization and the incorporation of self-healing properties, THBS fibers exhibit durable, high electrochemical performance under dynamic, high-curvature deformations mimicking in vivo physiological motions. Despite a thickness of only a few hundred microns, they maintain mechanical and electrochemical stability. Long-term functionality was confirmed over five weeks with minimal immune response. In vivo implantation demonstrated successful LED operation in a freely moving mouse, and successful optogenetic stimulation of both central and peripheral nervous systems. These results underscore the promise of THBS fibers as next-generation, fully biocompatible energy storage devices for advanced implantable bioelectronic systems.
Similar content being viewed by others
Introduction
Energy storage devices are vital for implantable biomedical electronics, including neurostimulators, pacemakers, and biosensors1,2,3,4,5,6, requiring reliable operation in physiological environments over prolonged periods. However, conventional energy sources present several critical challenges: toxicity in materials, limited flexibility and stability in dynamic biological environments, and the need for rigid encapsulation4,7,8. In addition, their reliance on redox reactions raises concerns about harmful side reactions and performance degradation, which compromise long-term safety in vivo. By contrast, biocompatible supercapacitors circumvent these issues and offer sufficient power9,10 for bioelectronics without the risks associated with redox reactions.
To meet evolving demands for implantable applications, biocompatible supercapacitors must feature non-toxicity, long-term stability, and mechanical adaptability to soft tissue environments, while maintaining high energy storage capabilities11. In particular, flexible, fiber-shaped designs are advantageous because they can reduce immune responses and adapt seamlessly to dynamic physiological movements12,13. Hydrogels have emerged as promising components for supercapacitors, offering rapid ion transport, low interfacial resistance, and excellent biocompatibility14,15,16,17,18,19,20,21,22. Despite these benefits, the development of biocompatible hydrogel electrodes and electrolytes remains constrained by limited material options that often compromise mechanical strength23,24,25,26. Achieving the necessary mechanical toughness and high electrochemical performance while minimizing interfacial resistance thus remains a major challenge27,28,29. In addition, hydrogels generally have limited durability under tensile and shear stresses, necessitating a mechanically optimized design that combines enhanced toughness and flexibility for reliable operation in biological systems30,31,32,33,34,35,36.
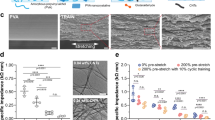
In this study, we present a thermal drawing process (TDP) to manufacture tough hydrogel-based biocompatible supercapacitor (THBS) fibers specially designed for safe, long-term in vivo implantation (Fig. 1a, b and Supplementary Figs. 1–3). This approach enables mass production by scaling down a preform from the centimeter to micrometer dimensions, achieving a highly adaptable form factor13,37,38,39,40,41,42,43 (bending stiffness of the THBS fibers ~10.3 N/m) (Supplementary Figs. 4 and 5). With a thickness of only a few hundred microns, these highly flexible THBS fibers allow minimally invasive insertion and adaptable placement throughout various tissues and organs (Fig. 1b). Fabricated entirely from biocompatible materials (polyvinyl alcohol (PVA), polycaprolactone (PCL), poly(ethylene-co-vinyl acetate) (EVA) and sodium chloride (NaCl))44,45,46, THBS fibers eliminate the risk of inflammatory responses caused by material toxicity (Fig. 1c). Biologically safe NaCl serves as the electrolyte ion source, enabling rapid migration to the electrode surface for efficient charge–discharge operations. Furthermore, to ensure mechanical durability and prevent electrical leakage, the internal structure of the fiber was fully encapsulated by an EVA layer during the TDP. All components were robustly integrated through the TDP, thereby preserving both tissue integrity and functional stability, and ultimately enabling exceptional operational longevity in vivo. The use of a conductive PCL (c-PCL) current collector facilitates effective longitudinal current flow, ensuring stable charge–discharge operations (Supplementary Fig. 6).
a Fabrication of THBS fibers via the thermal drawing process. The preform contains PVA-based hydrogel electrodes, PVA-based hydrogel electrolyte, c-PCL current collector, EVA encapsulation, and tungsten wires. b Illustration of the universally implantable THBS fibers. Their fiber-shaped form factor enables minimally invasive insertion and flexible positioning within tissue for biomedical applications. c Schematic illustration about THBS fiber’s biocompatibility. All components, including the electrode and electrolyte, are composed of biocompatible materials and encapsulated in EVA, which helps reduce inflammatory responses and ensures long-term in vivo usability. d Schematic illustration of the tough hydrogel network composed of PVA, PEG, SB, AC, and CB. The PVA-based dual-network forms hydrogen bonds with PEG and ionic coordination bonds with SB, providing self-healing capability and robust mechanical strength to enhance electrochemical performance and durability. e Microscopic and SEM images of THBS fibers showing uniform and continuous electrode-electrolyte alignment after TDP. The electrode layer includes AC and CB fillers (3 independent batches). f Optical images of a single THBS fiber under various deformations, including twisting, bending, knotting, and winding. g In vivo image of a freely moving mouse with an LED powered by implanted THBS fibers, demonstrating stable energy delivery for bioelectronic devices. b was created in BioRender. (Park, S. (2025) https://BioRender.com/kc2se3y).
The supercapacitor system incorporates a PVA-based dual-network hydrogel as both the electrolyte and electrode, specifically optimized for bio-implantable applications (Supplementary Figs. 7 and 8). This hydrogel leverages hydrogen bonds with polyethylene glycol (PEG)45,47,48,49,50,51 and ionic coordination bonds with sodium borate (SB)52,53,54,55 to ensure high toughness and self-healing ability (Fig. 1d). A borate-diol complex formed between PVA and SB facilitated a fully integrated network at the electrode-electrolyte interface, significantly enhancing capacitance. In the electrode, activated carbon (AC) with a high surface area was incorporated to enable charge storage, along with carbon black (CB) as a conductive additive to enhance electrical percolation. Microscopic side-view image, scanning electron microscopy (SEM), and confocal image confirmed the continuous interfacial alignment between the electrode and electrolyte, as well as the porous structures of the hydrogels after TDP, which enhance ion transport and electrochemical efficiency. (Fig. 1e and Supplementary Fig. 9). Because of their fiber-shaped form factor, THBS fibers effectively distribute mechanical stress, ensuring stability even under dynamic deformations like bending, twisting, and knotting that might occur during physiological movements in vivo. (Fig. 1f). As a practical demonstration, THBS fibers powered an LED in freely moving mice, highlighting their suitability for next-generation biomedical devices (Fig. 1g).
Results
Molecular design of biocompatible and tough PVA hydrogels
To fabricate fully biocompatible hydrogels suitable for the TDP, it is critical to understand their molecular fluidity under thermal conditions. Hydrogels can degrade or deform at high temperatures, making thermal processing especially challenging. The hydrogel within the THBS fiber remains tough at body temperature while exhibiting sufficient fluidity within the temperature range required for the TDP (Supplementary Figs. 10 and 11). Excessive fluidity during TDP can cause short circuits, so optimizing the molecular network to maintain supercapacitor alignment at higher temperatures is essential. To address this, a dual-network hydrogel composed of PVA, PEG, and SB48,56 was introduced for fabricating THBS fibers (Fig. 2a).
a Schematic illustration of molecular behavior in the hydrogels during the TDP. b XRD patterns of PVA/PEG, PVA/SB, and the electrolyte hydrogels, highlighting differences in crystallinity among the hydrogel formulations. c Stress-strain curves of the hydrogels, showing that the electrolyte hydrogel exhibits superior toughness and elongation. d Storage modulus (G’) and loss modulus (G”) of the electrolyte and electrode hydrogels, indicating the elastic and viscous properties critical for thermal drawability. e Loss tangent (tan δ) of electrolyte and electrode hydrogels, revealing optimal drawability at temperatures between 80 °C and 90 °C.
PVA, which contains abundant hydroxyl groups, forms two distinct bonding networks in the hydrogel: hydrogen bonds with PEG to increase crystallinity, primarily contributing to rigidity, and reversible ionic coordination bonds with SB to increase chain mobility and impart deformability. As shown in Fig. 2b, X-ray diffraction (XRD) analysis reveals a prominent peak at 20.0° for the PVA/PEG hydrogel, indicating high PVA crystallinity. In contrast, the PVA/SB hydrogel exhibits a broader and smaller peak, suggesting reduced crystallinity, likely due to the disruption of crystalline domains by ionic coordination. The PVA/PEG/SB electrolyte hydrogel exhibits an intermediate peak, reflecting a balance between rigidity and deformability. As detailed in the stress-strain analysis (Fig. 2c and Supplementary Table 1), this electrolyte hydrogel achieved a peak stress of 3.74 MPa and an elongation at break of 375%, significantly outperforming both the PVA/PEG (3.3 MPa, 321%) and the PVA/SB hydrogels (0.91 MPa, 373%). These superior mechanical properties result in an exceptional toughness of 7.01 MJ/m³ and a fracture toughness of 2172 J/m² (Supplementary Fig. 12). These findings highlight the synergistic effect of the dual bonding networks, where hydrogen bonds (PVA–PEG) enhance rigidity and ionic coordination bonds (PVA–SB) provide deformability. This design strategy, incorporating two distinct and complementary bonding networks, is analogous to the classical double-network hydrogel framework, where one network provides rigidity and the other imparts stretchability to achieve enhanced toughness31,48,57,58,59.
Rheological optimization for thermal drawability of PVA hydrogels
Rheological properties, such as storage modulus (G’), loss modulus (G”), and loss tangent (tan δ), play a pivotal role in designing thermally drawable hydrogels. The loss tangent, or G” to G’, indicates the hydrogel’s fluidity. When G’ and G” intersect and tan δ exceeds 1, viscous behavior prevails over elasticity, making the hydrogel drawable (Fig. 2d, e). Both the electrolyte and electrode hydrogels exhibit G’–G” intersections within the TDP temperature range of 80–90 °C, confirming their suitability for simultaneous drawing at these temperatures.
The dual-network structures of PVA hydrogels modulate these rheological properties. Notably, the PVA–SB bonds exhibit thermally reversible properties, altering the hydrogel’s viscoelastic behavior with temperature changes. The thermally cleaved PVA–SB bonds increase viscosity, causing tan δ to exceed 1 during TDP. Without SB, the PVA/PEG hydrogel maintains tan δ below 1 due to its high elasticity within the TDP temperature range, limiting its drawability. The PVA/SB hydrogel without PEG shows excessive viscosity compared to elasticity at temperatures over 40 °C, resulting in a tan δ value above 1, and causing the hydrogel to behave like a liquid, losing structural integrity after TDP (Fig. 2e). These findings highlight the thermal stability modulated by the dual-network of PVA–PEG bonds and PVA–SB bonds. By optimizing PEG and SB concentration in PVA hydrogels, tan δ can be maintained near 1 within the targeted temperature window, allowing precise control over drawability and mechanical properties. In addition to adjusting PEG and SB contents, we also optimized the PVA concentration in the electrolyte and the AC loading in the electrode to achieve favorable rheological properties for stable thermal drawing (Supplementary Figs. 13 and 14). This control is crucial not only for tailoring the mechanical robustness of supercapacitor fibers but also for preserving their electrochemical performance in dynamic physiological environments.
Electrochemical performance
Building on the successful design of tough hydrogels, we fabricated mechanically robust supercapacitor fibers via TDP. The key property of these fibers, their electrochemical performance, was verified using galvanostatic charge–discharge (GCD) and cyclic voltammetry (CV) measurements. GCD curves of THBS fibers containing saturated NaCl as the electrolyte ion source exhibit a symmetrical triangular profile, indicating minimal internal resistance and no apparent side reactions (Fig. 3a and Supplementary Fig 15). This observation is further supported by the CV curves across varying scan rates (Fig. 3b). The symmetric GCD curves, quasi-rectangular CV profiles, and absence of Faradaic peaks confirm that the charge storage in THBS fibers is dominantly electric double-layer capacitance, not pseudocapacitive. The fiber achieved a maximum areal capacitance of 268 mF/cm² and volumetric capacitance of 18.8 F/cm³ at a current density of 1.0 mA/cm², an areal energy density of 29.8 µWh/cm², and an areal power density of 8.48 mW/cm², surpassing most recently reported implantable supercapacitors5,60,61,62,63,64,65,66 (Fig. 3c, Supplementary Fig. 16 and Supplementary Table 2). The volumetric energy and power densities of the THBS fiber were 2.10 mWh/cm³ and 597 mW/cm³, and the length energy and power densities were 0.424 µWh/cm and 120 µW/cm, respectively.
(a) Galvanostatic charge–discharge (GCD) curves of THBS fibers at various current densities. (b) Cyclic voltammograms (CV) curves of THBS fibers at different scan rates, demonstrating stable electrochemical behavior. c Ragone plot combined with in vivo operational duration, comparing THBS fibers with previously reported biocompatible supercapacitors. Stable operation duration is defined as the period during which performance remains within 90–110% of the initial value. Light gray indicates devices with no data or stable operation lasting only 0 days. Black represents devices with stable operation for 1–8 days, while blue indicates devices with stable operation exceeding 9 days. d Capacitance retention of THBS fibers after bending at different angles (30°, 45°, 90°, 120°), as well as twisting (with a bending radius of 1.25 mm) (n = 9 independent samples per group). e Stability of THBS fibers immersed in PBS solution for 20 weeks, illustrating sustained electrochemical performance in physiological environments. f Capacitance retention of THBS fibers after 2000 charge–discharge cycles. g The GCD curves of THBS fibers connected in series and parallel. h Nyquist plots of coin cells before and after thermal treatment. i GCD curves of coin cells before and after thermal treatment. All electrochemical measurements were conducted at room temperature (~25 °C).
To evaluate durability under in vivo-like harsh conditions: continuous mechanical deformation, exposure to body fluids, and extended implantation periods, we conducted functional stability tests. Bending and knotting tests at various angles confirmed the fibers’ robustness, showing negligible changes in specific capacitance (Fig. 3d). Notably, the THBS fiber retained 96.48% of its initial capacitance after 1000 cycles of 90° bending (Supplementary Fig. 17), highlighting their exceptional mechanical and electrochemical stability during repeated deformation, simulating the dynamic conditions of in vivo implantation. In long-term immersion in phosphate-buffered saline (PBS), simulating body fluids, the encapsulated hydrogel maintained 98.8% and 92.1% of its original capacitance after 8 and 20 weeks, respectively (Fig. 3e). The slight degradation may result from factors such as gradual ion or moisture infiltration through the EVA encapsulation, subtle relaxation, and degradation of the hydrogel network. Nonetheless, further research is required to precisely identify the underlying cause of this degradation. Further cyclic tests highlighted the fibers’ durability, with 99.3% capacitance retention after 2000 charge–discharge cycles (Fig. 3f). Moreover, the system’s scalability was demonstrated by various configurations, including series and parallel connections, achieving higher voltage and increased capacitance. (Fig. 3g).
The excellent electrochemical performance of THBS fibers can be attributed to the thermal integration achieved during TDP. This process re-aligns and re-bonds the hydrogel network at the electrode-electrolyte interface, significantly enhancing its self-healing ability, reducing interfacial resistance, and increasing capacitance. To replicate the thermal environment during TDP, coin cell samples were subjected to heat treatment, and their electrochemical properties were evaluated (Supplementary Fig. 18). Optical microscopy images before and after thermal treatment confirmed successful integration at the electrode-electrolyte interface (Supplementary Fig. 19). Nyquist plots revealed a substantial decrease in internal resistance after treatment (Fig. 3h), leading to remarkable capacitance increase from 750.8 to 1067 mF/cm² (Fig. 3i). Thermal treatment also improved the mechanical stability of the interface by reinforcing the hydrogel’s self-healing properties18,28,67. By contrast, in control samples without SB, heat treatment did not induce effective interfacial healing, resulting in no significant decrease in internal resistance and minimal capacitance improvement (Supplementary Figs. 20 and 21). These findings underscore the critical importance of both the TDP and the material design in fabricating biocompatible, hydrogel-based supercapacitors with superior capacitance and interface stability.
Biocompatibility tests of the THBS fibers
To assess the potential biomedical applications of the THBS fibers, we conducted a comprehensive biocompatibility evaluation in vitro and in vivo. In the cell viability test, cytotoxicity of THBS fibers was investigated with 3T3 cells derived from mouse embryonic fibroblasts (Supplementary Fig. 22). Quantitative analysis showed no significant differences in cell viability after 24 h between the THBS fiber-embedded group (mean ± SD: 99.4 ± 0.364) and the sham control group (99.8 ± 0.111) (Fig. 4a–c and Supplementary Fig. 23).
Fluorescence images from cell viability tests with a THBH fibers and b control groups. c Quantitative analysis of cell viability after 24 h, showing a difference between the THBS fiber-embedded group and the control group (n = 5 independent samples; p = 0.0686). d–f H&E-stained tissue sections 1 month after implantation of THBS fiber and black silk. g Quantitative analysis of infiltrating cells based on H&E staining. The THBS fiber and sham control groups show no significant difference in immune response, while the black silk group exhibits significantly higher cell infiltration (n = 8 independent biological samples, THBS fibers vs Sham, p = 0.0590; Sham vs Black silk, p = 0.00074). h–j H&E-stained tissue sections after 5000 charge–discharge cycles, followed by a 1-week resting period to induce inflammatory responses. k Quantitative analysis of infiltrating cells from the H&E staining. The THBS fibers group shows lower immune response compared with uninsulated stainless-steel wires, reinforcing their biocompatibility for prolonged implantation (n = 20 independent biological samples, THBS fibers vs SS wires 0 cycle, p = 0.00091; THBS fibers vs SS wires 500 cycles, p = 4.23 × 10−10). Data are presented as mean values ± standard deviation (SD). All statistical comparisons were performed using unpaired two-sided t-tests (* indicates p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
To further evaluate long-term in vivo biocompatibility, we conducted 1-month implantation tests in mice and evaluated tissue immune responses via H&E staining (Fig. 4d–g and Supplementary Fig. 24). Statistical analysis revealed no significant difference in the number of infiltrating cells in the dermal layer between the THBS fiber group (34.3 ± 6.27) and the sham control group (27.3 ± 7.29), whereas the black silk group showed significantly higher infiltration (60.4 ± 17.6) (Fig. 4g). These findings confirm the excellent inherent biocompatibility of our THBS fibers, highlighting their suitability for long-term implantation into biological systems. To complement these long-term assessments, we also conducted a 1-week subcutaneous implantation study specifically designed to quantitatively evaluate local immune responses. This analysis incorporated blinded pathological scoring based on H&E staining and immunohistochemical (IHC) quantification of CD68 (macrophage marker) and TNF-α (pro-inflammatory cytokine) expression at the device–tissue interface. The THBS group exhibited no significant increase in fibrotic thickness, inflammatory cell infiltration, or immune marker expression compared to PDMS controls, reinforcing both histological and molecular evidence of biocompatibility (Supplementary Figs. 25 and 26).
We next evaluated immune response to repeated charge–discharge cycles of the implantable supercapacitor (Fig. 4h–k). Without proper encapsulation, leakage current during the charge–discharge process can cause significant tissue damage68,69. To assess the efficacy of EVA encapsulation, implantation tests were performed using THBS fibers and uninsulated stainless-steel (SS) wires as controls. After implantation, repeated charge–discharge cycles were applied, and tissue samples were harvested 1 week later to evaluate inflammatory responses. The THBS fiber subjected to 500 charge–discharge cycles showed significantly lower cell infiltration (19.1 ± 5.49), compared to the SS wires subjected to the same conditions (58.15 ± 16.15) (Fig. 4k). Moreover, THBS fibers subjected to 500 cycles exhibited lower cellular penetration than SS wires without charge–discharge (32.1 ± 14.34), likely attributable to the high flexibility of the THBS fibers. These results underscore the critical importance of encapsulation and flexibility in implantable supercapacitors, with the EVA layer in THBS fibers providing superior protection against charge–discharge-induced tissue damage.
In vivo application of the THBS fibers
To demonstrate the in vivo applicability of the THBS fibers, we employed them as an energy source for optogenetic stimulations of both the peripheral and central nervous system (CNS) (Fig. 5a–d). For the peripheral nervous system (PNS), the sciatic nerve was targeted, where stimulation elicits characteristic hindlimb movements. Serially connected THBS fibers coupled with an LED were implanted from a subcutaneous pocket around the hindlimb to the sciatic nerve, with the LED placed directly on the nerve (Fig. 5a and Supplementary Fig. 27). Each stimulation trial successfully induced optogenetic activation of the sciatic nerve, generating distinct hindlimb movements depending on which branch of the nerve stimulated (Fig. 5b). Upon discharge, the implanted THBS fibers emitted blue light from the LED, triggering upward movement via the sural nerve and downward movement via the peroneal nerve70,71 (Supplementary Fig. 28).
a Schematic illustrating optogenetic stimulation of the sciatic nerve in a mouse (PNS application). THBS fibers connected to an LED are implanted on the sciatic nerve and placed in a nearby subcutaneous pocket. Once charged, the fibers directly power the LED at the sciatic nerve, inducing hindlimb movement. b Hindlimb movement angles induced by optogenetic stimulation via THBS fibers targeting the sural or peroneal branch of the sciatic nerve. Error bars represent the mean with standard deviation (n = 5, independent stimulations). c Schematic illustrating optogenetic stimulation of the M1–M1 circuit in a mouse brain. THBS fibers connected to an LED are implanted on the M1 cortex and placed in a nearby subcutaneous pocket, while a conventional neural probe is implanted in the contralateral M1 cortex. Once charged, the fibers power the LED on the ipsilateral M1 cortex, triggering neuronal activation that extends to the contralateral M1 cortex. d Neuronal activity in contralateral M1 cortex before (i) and after (ii) THBS fibers induced optogenetic stimulation in M1 cortex. The top panels of (i, ii) show spike data from unit 1 (red) and unit 2 (blue). The middle panels display the neural stream data recorded by the implanted probe, and the bottom panels illustrate extracted spike unit data, classified using PCA and K-means clustering. e Photo of seamless long-term implantation of THBS fiber for in vivo cyclic testing. f In vivo cyclic voltammogram data from implanted THBS fiber over 5 weeks. g Normalized capacitance of the implanted THBS fiber over 5 weeks, demonstrating stable performance. a, c were created in BioRender. (https://BioRender.com/0idle7k, https://BioRender.com/l9s2ehd).
To further validate their applicability in the CNS, THBS fibers were also tested in the mouse brain (Fig. 5c). The M1–M1 circuit, known for its intra-hemispheric connectivity, was selected as the stimulation target69,72. THBS fibers were implanted from a subcutaneous pocket near the neck to the skull, and the coupled LED was positioned directly on the M1 cortex after removing a portion of the skull (Supplementary Fig. 29). The emitted blue light successfully activated the M1 cortex, which projects to the contralateral M1 cortex. Optogenetic stimulation at 100 mW/mm² for 10 s elicited increased spike activity in the contralateral M1 cortex, as evidenced by electrophysiological recordings before and after stimulation (Fig. 5d and Supplementary Fig. 30). Clustering of spike units, analyzed via principal component analysis (PCA), confirmed the recruitment of additional spike units post-stimulation, indicating the successful activation of the M1–M1 circuit.
To assess the long-term in vivo stability, the THBS fibers were implanted in a dorsal subcutaneous pocket of mice for 5 weeks. Their slim, one-dimensional form factor allows seamless implantation in various anatomical locations (Fig. 5e). Weekly CV tests performed over a 5-week period showed stable charge–discharge characteristics, with capacitance maintaining more than 99 % of its initial value (Fig. 5f, g). This longevity represents the longest in vivo operational lifetime reported for implantable energy storage devices, supporting the superior durability and effectiveness of the THBS fibers.
Discussion
The development of THBS fibers effectively addresses key challenges in implantable energy storage devices, including limited durability, biocompatibility, and long-term stability for extended bio-implantation. By employing the TDP, which enables robust integration of every component, including tough hydrogels, THBS fibers achieved both long-term bio-applicability and excellent electrochemical performance. The optimized PVA hydrogel matrix, incorporating PEG and SB, provides high mechanical toughness, self-healing properties, and stable performance under dynamic deformation. The integrated, mechanically robust fiber architecture ensures extended durability and prevents electrical leakage, thereby preserving both biological safety and functional stability, and ultimately enabling exceptional operational longevity in vivo.
Furthermore, in vivo experiments have confirmed the practical applicability of THBS fibers as a reliable energy source for bioelectronics, successfully demonstrating optogenetic stimulation of both peripheral and CNSs without adverse biological effects. Notably, THBS fibers are composed entirely of biocompatible materials, ensuring safe and seamless integration into complex biological systems. Overall, THBS fibers hold substantial potential for a wide range of bioelectronic applications, offering a reliable, flexible, and stable energy solution. Our one-dimensional design offers significant potential for integration into diverse medical devices, opening new possibilities for advancements in biomedical technology.
Overall, THBS fibers hold substantial potential for a wide range of bioelectronic applications, offering a reliable, flexible, and stable energy solution. Our one-dimensional design offers significant potential for integration into diverse medical devices, opening new possibilities for advancements in biomedical technology. Although THBS fibers are composed entirely of biocompatible materials and demonstrate stable in vivo performance, direct clinical translation demands additional validation beyond basic biocompatibility. This includes extended preclinical testing, comprehensive safety profiling, and alignment with formal regulatory approval processes. Furthermore, practical challenges remain, including the need for manufacturing scale-up, the development of sterilization protocols, and verification of device performance in large-animal models that more closely mimic human physiology.
The TDP used in this study offers a meaningful advantage in this regard, as it is inherently scalable and compatible with continuous, high-throughput production. This scalability supports a clear pathway toward the fabrication of implantable energy storage systems at clinically relevant quantities. With respect to sterilization, methods such as gamma irradiation or ethylene oxide treatment will need to be optimized to preserve hydrogel functionality; however, the mechanical and chemical stability of our encapsulated design provides a promising basis for withstanding such conditions. Additionally, the flexible, miniaturized geometry of the THBS fiber is expected to facilitate minimally invasive implantation and offer improved conformability in large-animal models, potentially surpassing the anatomical adaptability of rigid planar systems.
In parallel with these translational steps, technical integration with complementary systems will be essential to fully unlock the potential of this platform. A particularly important direction is the integration of THBS supercapacitors with wireless power transfer (WPT) systems. While WPT offers continuous and non-invasive energy delivery, it is susceptible to transient disruptions, misalignment, or sudden fluctuations in power demand. In these scenarios, THBS devices can serve as immediate, on-demand energy buffers, ensuring the uninterrupted operation of the implantable system. This co-development of biocompatible energy storage and WPT platforms presents a promising strategy toward realizing fully autonomous, closed-loop implantable bioelectronic technologies.
Methods
Materials
All chemicals were used without additional purification unless otherwise specified. Poly(vinyl alcohol) (PVA, Mw 89,000-98,000), poly(ethylene glycol) (PEG, Mw ~1000), sodium tetraborate decahydrate (SB, 99.5%), sodium chloride (NaCl, 99%), polycaprolactone (PCL, Mn 80,000), poly(ethylene-co-vinyl acetate) (EVA, vinyl acetate 18 wt. %), and multi-walled carbon nanotube (MWCNT, 50–90 nm diameter) were purchased from Sigma-Aldrich. Tetrahydrofuran (THF, 99%) was acquired from Daejung. CB was acquired from Alfa Aesar, and activated carbon (AC) from Nature’s Way. Tungsten wire (50 μm diameter) was obtained from Goodfellow.
Preparation of PVA-based electrolyte
A total of 12 g of PVA and 3 g of PEG were dissolved in 30 mL of deionized (DI) water by stirring at 90 °C for 1 h. The PVA/PEG solution was then mixed for 10 min using a Thinky mixer (model AR-100) to ensure uniform dissolution. The solution comprising 100 mg of SB dissolved in 6 mL of DI water was added, followed by an additional 10 min of mixing. The mixture underwent rotation within a vacuum mixer (HGT-400DIV, HANIL Global Technology) under vacuum conditions for 30 s. The prepared PVA gel was carefully cast into a mold to avoid trapping air bubbles. After 1 day of curing, it was immersed in a saturated NaCl solution for 10 min, and then stored at room temperature for 3 days.
Preparation of PVA-based electrode
A total of 2 g of PVA and 1 g of PEG were dissolved in 30 mL of DI water by stirring at 90 °C for 1 h. Then, 4 g of AC and 0.8 g of CB, corresponding to a polymeric matrix:AC:CB weight ratio of approximately 4:5:1, were added to the solution, along with 100 mg of SB dissolved in 6 mL of DI water. The remaining steps were identical to those used for electrolyte fabrication.
Preparation of c-PCL-based current collector
3.2 g of CB and 0.8 g of MWCNT were dispersed in 500 mL of THF via probe sonication (VC-505, Sonics & Materials, equipped with a 3 mm microtip) at 40% amplitude for 1 h. Next, 40 g of PCL was added to the CB/MWCNT solution and stirred overnight. The mixture was then poured into an aluminum tray and dried under vacuum for 24 h. The resulting c-PCL film was hot-pressed at 80 °C for five cycles, with the final press using a spacer to achieve a thickness of 2 mm.
Preform fabrication
THBS fibers were produced by TDP on a multi-layered preform with a carefully engineered internal structure. EVA was CNC-machined to form a structured block with seven void channels for two electrode gels, one electrolyte gel, two current collector composite bars, and two tungsten wires (50 µm in diameter). Two current collector composite bars were inserted into the machined EVA and consolidated via hot-pressing at 90 °C. The final preform measured approximately 24 mm in thickness, 28 mm in width, and 110 mm in length. Its seven void channels included two 5 × 5 mm channels (electrode gels), one 4 × 12 mm channel (electrolyte gel), two 2 × 12 mm channels (current collector composites), and two 2 × 3 mm channels (tungsten wires).
Thermal drawing process (TDP)
The two electrode gels and the electrolyte gel were loaded into the preform, and the open end was sealed with polyimide tape and a chemically resistant epoxy. The preform was drawn in a three-zone vertical tube furnace at top-zone 85 °C, a middle-zone 95 °C, and a bottom-zone 30 °C while continuously feeding tungsten wires.
Characterization
Morphological characterization
SEM images of the gels were obtained using a scanning electron microscope (Magellan 400, FEI company). Prior to imaging, each gel sample was promptly stored in a −40 °C refrigerator for 24 h immediately after preparation, followed by freeze-drying. Confocal imaging was performed using a high-resolution confocal laser scanning microscope (LSM 980 Airyscan 2, Zeiss). Hydrogels with a thickness of approximately 1 mm were imaged in bright-field mode using a 40 × objective. A z-stack of 101 consecutive 2D images was acquired at 0.2 μm intervals, covering a depth of approximately 20 μm from the surface. The exposure time was set to 10 ms, and the illumination was provided by a TL halogen lamp at a light source intensity of 3 V.
Chemical characterization
Temperature-dependent behavior of the gels was evaluated using Ultra Low Temp Differential Scanning Calorimetry (DSC 214 Polyma, NETZSCH) at a heating rate of 10 °C/min, from −80 °C to 200 °C. The degree of crystallinity of the gels was assessed via a High Resolution Powder X-Ray Diffractometer (SmartLab, RIGAKU). FTIR spectra were collected with a Nicolet iS50 FTIR Spectrometer (Thermo Fisher Scientific Instrument).
Rheological characterization
Rheological characteristics of PVA hydrogels were analyzed using a rheometer (MCR 302e, Anton Paar) equipped with an 8 mm diameter measuring plate. Prior to the measurements, the hydrogels were prepared as 8 mm diameter disks with a thickness of 1 mm. During the test, the hydrogels were first heated to 100 °C and held for ~30 s, then cooled to 40 °C at a rate of 2 °C/min while recording flow properties at a constant shear rate of 0.1 s⁻¹.
Mechanical characterization
Gel samples were cut to 2 mm by 40 mm with a 1 mm thickness, and strain-stress curves were obtained by stretching samples at 200 mm/min using a Materials Testing Machine (LS1, Ametek).
Electrochemical characterization
Long supercapacitor fibers were cut to the desired length and sealed with UV resin. Tests were performed on fibers with a width of >800 µm and a thickness of >650 µm. The fibers were galvanostatically cycled between 0 and 0.8 V at various current rates (0.5, 1, 1.5, 2, 2.5, 3 mA cm−2) using the WBCS3000L system (WonATech). CV measurements were conducted from 0 to 0.8 V at scan rates of 2, 5, 10, 20, and 30 mV s−1 using SP-300 Potentiostat (BioLogic).
Calculation method for electrochemical performance
The electrochemical performance of thermally drawn supercapacitor fibers was evaluated using GCD measurements. The capacitance was calculated from the discharge curves using the following equation:
where Q is the amount of charge, and ΔV is the operating voltage window.
The corresponding energy and power densities were calculated as:
where Δt is the discharge time.
The length-based, areal, and volumetric capacitances, as well as their corresponding energy and power densities, were calculated by dividing C by the length, projected area, and effective volume of the active gel electrode in both fiber and coin cell configurations.
Cell viability test
NIH/3T3 fibroblast cells (ATCC, Cat# CRL-1658) derived from mouse embryonic fibroblasts were used for cytotoxicity analysis. The cells were purchased from ATCC (US) and were authenticated by the provider. 3T3 cells were grown in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C in a 5% CO2 incubator. Supercapacitor fibers were placed in 24-well plates, and cells were seeded at 3 × 104/well. After 24 h incubation, the medium was removed and the wells were washed with Dulbecco’s phosphate-buffered saline (DPBS). The cells were stained with the DPBS solution containing 0.5 μM calcein-AM and 2 μM EthD-1 for live/dead analysis (green: live, red: dead). Cell viability was quantified using ImageJ software after fluorescence imaging (ECLIPSE Ts2-FL, Nikon).
Animal experiments
Male C57BL/6J mice and Thy1-ChR2-YFP transgenic mice (older than 8 weeks) were used in this study. Mice were housed under a 12-h light/dark cycle at 22–24 °C with ad libitum access to food and water. All animal experiments were carried out in accordance with protocols approved by the Korea Advanced Institute of Science and Technology Institutional Animal Care and Use Committee (KA2023-107).
In vivo biocompatibility tests
For long-term biocompatibility assessments, THBS fibers were surgically implanted into the dorsal subcutaneous pockets of wild-type male C57BL/6J mice for 1 month. For in vivo operational biocompatibility tests, THBS fibers were implanted in the same manner, then subjected to 500 cycles of charge–discharge cycles between 0 and 0.8 V at a scan rate of 100 mV s−1. Tissue samples were harvested 1 week later to evaluate the biocompatibility of the fibers under operational conditions.
H&E staining tests
Mice were anesthetized, perfused with saline, and followed by 4% paraformaldehyde. Skin tissues were collected and soaked in 30% sucrose in PBS for 1 week. Transverse sections (10 μm thick) were mounted on slides and stained with hematoxylin and eosin (H&E). Imaging was performed using a microscope (Leica DM5000, Leica Microsystems) with 20× lenses (Leica 506503, Leica Microsystems) and LAS X software (LAS version 4.0.0, Leica Microsystems). Epidermal regions (from the basal to the granular layer) were measured, and cell infiltrations were quantified in three randomly selected areas (100 μm²) per sample.
In vivo mouse optogenetic stimulation
For in vivo optogenetic stimulation, male Thy1-ChR2-YFP transgenic mice were used. Three THBS fibers were connected in series to a blue LED (473 nm, forward voltage of 1.7 V). Anesthesia was delivered with isoflurane (4% for induction, 1 % for maintenance in O2).
For the PNS study, the sciatic nerve was exposed by dissecting the vastus lateralis and biceps femoris muscles. The LED part was directly placed on the sciatic nerve, and the THBS fibers were implanted subcutaneously near the hindlimb.
For the CNS study, left and right M1 cortices (AP +0.1, ML ±1.2) were targeted using a stereotaxic apparatus. A microdrill was used to create a small opening in the skull, and the LED was placed on the M1 cortex. The THBS fiber was implanted in the dorsal subcutaneous pocket near the neck. Electrophysiological signals were recorded from the contralateral M1 cortex (AP +0.1, ML ±1.2, DV −0.4) using a neural probe (NeuroNexus) connected to a recording device (Labrat). Neural stream data acquired during in vivo optogenetic stimulation experiments were analyzed using Offline Sorter x64 (v4.0, Plexon Inc.) for spike sorting. All subsequent data processing and visualization were performed using MATLAB R2024a (MathWorks).
In vivo long-term performance stability
For long-term performance evaluation, THBS fiber was surgically implanted in the dorsal subcutaneous pocket of wild-type male C57BL/6J mice. Weekly CV measurements were performed in vivo for 5 weeks to assess cycling stability. To enable repeated electrochemical measurements, one end of the device was terminated with a gold pin, which was sutured externally to the skin such that the pin remained accessible outside the body (Supplementary Fig. 31).
Following implantation, the exposed gold pin area was disinfected with povidone-iodine and covered with a transparent, waterproof medical dressing (Tegaderm) to minimize infection and irritation. Electrochemical stability was assessed under minimally invasive and aseptic conditions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data supporting the findings of this study are available within the article, its Supplementary Information, and the Source data file. Any additional requests for information can be directed to and will be fulfilled by the corresponding authors. Source data are provided with this paper.
References
Yu, M., Peng, Y., Wang, X. & Ran, F. Emerging design strategies toward developing next-generation implantable batteries and supercapacitors. Adv. Funct. Mater. 33, 2301877 (2023).
Sheng, H. et al. Recent advances of energy solutions for implantable bioelectronics. Adv. Healthc. Mater. 10, 2100199 (2021).
Dissanayake, K. & Kularatna-Abeywardana, D. A review of supercapacitors: materials, technology, challenges, and renewable energy applications. J. Energy Storage 96, 112563 (2024).
Chodankar, N. R. et al. Revolutionizing implantable technology: biocompatible supercapacitors as the future of power sources. Adv. Funct. Mater. 34, 2406819 (2024).
Li, H. et al. Fully bioabsorbable capacitor as an energy storage unit for implantable medical electronics. Adv. Sci. 6, 1801625 (2019).
Krishnadoss, V. et al. In situ 3D printing of implantable energy storage devices. Chem. Eng. J. 409, 128213 (2021).
Krishnamoorthy, U., Lakshmipathy, P., Ramya, M. & Fayek, H. H. Navigating the future of healthcare with innovations and challenges in implantable battery technology for biomedical devices. Discov. Appl. Sci. 6, 584 (2024).
Bock, D. C., Marschilok, A. C., Takeuchi, K. J. & Takeuchi, E. S. Batteries used to power implantable biomedical devices. Electrochim. Acta 84, 155–164 (2012).
Deng, J., Sun, X. & Peng, H. Power supplies for cardiovascular implantable electronic devices. EcoMat 5, e12343 (2023).
Huang, X. et al. Materials strategies and device architectures of emerging power supply devices for implantable bioelectronics. Small 16, 1902827 (2020).
Xu, M. et al. Minimally invasive power sources for implantable electronics. Exploration 4, 20220106 (2024).
Lu, C. et al. Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits. Sci. Adv. 3, e1600955 (2017).
Kim, Y. et al. Multifunctional and flexible neural probe with thermally drawn fibers for bidirectional synaptic probing in the brain. ACS Nano 18, 13277–13285 (2024).
Yin, B.-S. et al. Elastic soft hydrogel supercapacitor for energy storage. J. Mater. Chem. A 5, 24942–24950 (2017).
Xu, Y. et al. Flexible solid-state supercapacitors based on three-dimensional graphene hydrogel films. ACS Nano 7, 4042–4049 (2013).
Sardana, S., Gupta, A., Singh, K., Maan, A. & Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. J. Energy Storage 45, 103510 (2022).
Cao, X. et al. Recent progress in multifunctional hydrogel-based supercapacitors. J. Sci. Adv. Mater. Devices 6, 338–350 (2021).
Zou, Y. et al. Flexible, all-hydrogel supercapacitor with self-healing ability. Chem. Eng. J. 418, 128616 (2021).
Parvini, E., Hajalilou, A. & Tavakoli, M. A bright future of hydrogels in flexible batteries and supercapacitors storage systems: a review. Int. J. Energy Res. 46, 13276–13307 (2022).
Liu, Y. et al. Biocompatible, high-performance, wet-adhesive, stretchable all-hydrogel supercapacitor implant based on PANI@ rGO/Mxenes electrode and hydrogel electrolyte. Adv. Energy Mater. 11, 2101329 (2021).
Guo, Y., Bae, J., Zhao, F. & Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 1, 335–348 (2019).
Cheng, T. et al. Conductive hydrogel-based electrodes and electrolytes for stretchable and self-healable supercapacitors. Adv. Funct. Mater. 31, 2101303 (2021).
Peng, J. et al. A mechanically robust all-solid-state supercapacitor based on a highly conductive double-network hydrogel electrolyte and Ti3C2Tx MXene electrode with anti-freezing property. J. Mater. Chem. A 9, 25073–25085 (2021).
Na, R. et al. Mechanically robust hydrophobic association hydrogel electrolyte with efficient ionic transport for flexible supercapacitors. Chem. Eng. J. 374, 738–747 (2019).
Lin, T. et al. One-pot synthesis of a double-network hydrogel electrolyte with extraordinarily excellent mechanical properties for a highly compressible and bendable flexible supercapacitor. ACS Appl. Mater. interfaces 10, 29684–29693 (2018).
Kuang, S. et al. A double cross-linked hydrogel electrolyte with high mechanical strength and excellent electrochemical performance for flexible supercapacitor and zinc ion capacitor. J. Alloy. Compd. 918, 165688 (2022).
Cheng, T. et al. Stretchable and self-healing interlocking all-in-one supercapacitors based on multiple cross-linked hydrogel electrolytes. Adv. Mater. Interfaces 9, 2201137 (2022).
Zhao, J. et al. A self-healing hydrogel electrolyte for flexible solid-state supercapacitors. Chem. Eng. J. 401, 125456 (2020).
Peng, H. et al. Physically cross-linked dual-network hydrogel electrolyte with high self-healing behavior and mechanical strength for wide-temperature tolerant flexible supercapacitor. Chem. Eng. J. 422, 130353 (2021).
Xu, Z., Chen, Y., Cao, Y. & Xue, B. Tough hydrogels with different toughening mechanisms and applications. Int. J. Mol. Sci. 25, 2675 (2024).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Nonoyama, T. & Gong, J. P. Tough double network hydrogel and its biomedical applications. Annu. Rev. Chem. Biomol. Eng. 12, 393–410 (2021).
Liu, Z. et al. A soft yet device-level dynamically super-tough supercapacitor enabled by an energy-dissipative dual-crosslinked hydrogel electrolyte. Nano Energy 58, 732–742 (2019).
Chen, J., Shi, D., Yang, Z., Dong, W. & Chen, M. A solvent-exchange strategy to develop stiff and tough hydrogel electrolytes for flexible and stable supercapacitor. J. Power Sources 532, 231326 (2022).
Chen, G. et al. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 8, 6776–6784 (2020).
Hua, M. et al. Tough-hydrogel reinforced low-tortuosity conductive networks for stretchable and high-performance supercapacitors. Adv. Mater. 33, 2100983 (2021).
Shen, Y. et al. Thermally drawn multifunctional fibers: toward the next generation of information technology. InfoMat 4, e12318 (2022).
Seo, H., Ryu, W. M., Jang, J. & Park, S. Thermally-drawn porous sutures for controlled drug release using thermally-induced phase separation. Mater. Horiz. 12, 779–787 (2025).
Ryu, W. M., Lee, Y., Son, Y., Park, G. & Park, S. Thermally drawn multi-material fibers based on polymer nanocomposite for continuous temperature sensing. Adv. Fiber Mater. 5, 1712–1724 (2023).
Loke, G., Yan, W., Khudiyev, T., Noel, G. & Fink, Y. Recent progress and perspectives of thermally drawn multimaterial fiber electronics. Adv. Mater. 32, 1904911 (2020).
Lee, Y., Won, J., Kim, D. Y. & Park, S. Microsensor-internalized fibers as autonomously controllable soft actuators. Small 21, e2409742 (2025).
Jeon, W. et al. Structurally Aligned Multifunctional Neural Probe (SAMP) using forest-drawn CNT sheet onto thermally drawn polymer fiber for long-term in vivo operation. Adv. Mater. 36, e2313625 (2024).
Yan, W. et al. Thermally drawn advanced functional fibers: new frontier of flexible electronics. Mater. Today 35, 168–194 (2020).
Osman, A. F., Alakrach, A. M., Kalo, H., Azmi, W. N. W. & Hashim, F. In vitro biostability and biocompatibility of ethyl vinyl acetate (EVA) nanocomposites for biomedical applications. RSC Adv. 5, 31485–31495 (2015).
Wang, Z., Ye, Q., Yu, S. & Akhavan, B. Poly ethylene glycol (PEG)-based hydrogels for drug delivery in cancer therapy: a comprehensive review. Adv. Healthc. Mater. 12, 2300105 (2023).
Li, R. et al. A simple, safe and easily accessible polyvinyl alcohol hydrogel for wound cleaning. J. Biomater. Appl. 36, 1737–1747 (2022).
Zhang, X. et al. Double-network hydrogel with high mechanical strength prepared from two biocompatible polymers. J. Appl. Polym. Sci. 112, 3063–3070 (2009).
Cui, L. et al. PVA-BA/PEG hydrogel with bilayer structure for biomimetic articular cartilage and investigation of its biotribological and mechanical properties. J. Mater. Sci. 56, 3935–3946 (2021).
Bodugoz-Senturk, H. et al. The effect of polyethylene glycol on the stability of pores in polyvinyl alcohol hydrogels during annealing. Biomaterials 29, 141–149 (2008).
Ahmed, A. S., Mandal, U. K., Taher, M., Susanti, D. & Jaffri, J. M. PVA-PEG physically cross-linked hydrogel film as a wound dressing: experimental design and optimization. Pharm. Dev. Technol. 23, 751–760 (2018).
Hu, O. et al. An antifreezing, tough, rehydratable, and thermoplastic poly (vinyl alcohol)/sodium alginate/poly (ethylene glycol) organohydrogel electrolyte for flexible supercapacitors. ACS Sustain. Chem. Eng. 9, 9833–9845 (2021).
Zhang, J. et al. A 3D printable, highly stretchable, self-healing hydrogel-based sensor based on polyvinyl alcohol/sodium tetraborate/sodium alginate for human motion monitoring. Int. J. Biol. Macromol. 219, 1216–1226 (2022).
Lu, B. et al. One-pot assembly of microfibrillated cellulose reinforced PVA–borax hydrogels with self-healing and pH-responsive properties. ACS Sustain. Chem. Eng. 5, 948–956 (2017).
Chen, L., Shao, J., Yu, Q. & Wang, S. High-strength, anti-fatigue, stretchable self-healing polyvinyl alcohol hydrogel based on borate bonds and hydrogen bonds. J. Dispers. Sci. Technol. 43, 690–703 (2022).
Ai, J., Li, K., Li, J., Yu, F. & Ma, J. Super flexible, fatigue resistant, self-healing PVA/xylan/borax hydrogel with dual-crosslinked network. Int. J. Biol. Macromol. 172, 66–73 (2021).
Riedo, C., Caldera, F., Poli, T. & Chiantore, O. Poly (vinylalcohol)-borate hydrogels with improved features for the cleaning of cultural heritage surfaces. Herit. Sci. 3, 1–11 (2015).
Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 15, 1155–1158 (2003).
Chen, Q., Chen, H., Zhu, L. & Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 3, 3654–3676 (2015).
Gong, J. P. Why are double network hydrogels so tough?. Soft Matter 6, 2583–2590 (2010).
Tian, W. et al. Implantable and biodegradable micro-supercapacitor based on a superassembled three-dimensional network Zn@ PPy hybrid electrode. ACS Appl. Mater. Interfaces 13, 8285–8293 (2021).
Sim, H. J. et al. Biomolecule based fiber supercapacitor for implantable device. Nano Energy 47, 385–392 (2018).
Sheng, H. et al. A thin, deformable, high-performance supercapacitor implant that can be biodegraded and bioabsorbed within an animal body. Sci. Adv. 7, eabe3097 (2021).
Lv, Q. et al. A degradable and biocompatible supercapacitor implant based on functional sericin hydrogel electrode. Adv. Energy Mater. 13, 2203814 (2023).
Lee, H. et al. Facile fabrication of a fully biodegradable and stretchable serpentine-shaped wire supercapacitor. Chem. Eng. J. 366, 62–71 (2019).
Lee, G. et al. Fully biodegradable microsupercapacitor for power storage in transient electronics. Adv. Energy Mater. 7, 1700157 (2017).
Wang, X. et al. An anticoagulant supercapacitor for implantable applications. Nat. Commun. 15, 1–17 (2024).
Peng, H. et al. A flexible and self-healing hydrogel electrolyte for smart supercapacitor. J. Power Sources 431, 210–219 (2019).
McCreery, D. B., Agnew, W. F. & Bullara, L. A. The effects of prolonged intracortical microstimulation on the excitability of pyramidal tract neurons in the cat. Ann. Biomed. Eng. 30, 107–119 (2002).
Chan, R. W. et al. Distinct local and brain-wide networks are activated by optogenetic stimulation of neurons specific to each layer of motor cortex. NeuroImage 263, 119640 (2022).
Kim, D. et al. Ultraflexible organic light-emitting diodes for optogenetic nerve stimulation. Proc. Natl. Acad. Sci. USA 117, 21138–21146 (2020).
Goto, K. & Ohashi, K. Skeletal Muscle Denervation: Sciatic and Tibial Nerve Transection Technique. In Skeletal Muscle Stem Cells (ed. Asakura, A.) 217–225 (Springer, 2023).
Skoven, C. S. et al. Dose-response relationship between the variables of unilateral optogenetic stimulation and transcallosal evoked responses in rat motor cortex. Front. Neurosci. 16, 968839 (2022).
Acknowledgements
This work was supported by the National Research Foundation of Korea (RS-2023-00207970, RS-2023-00225427, RS-2023-00303628, RS-2024-00403009), Regenerative Medicine grant (23B0103L1) and Boston-Korea Project (RS-2024-00467213) funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare), and New Faculty Startup Fund, SNUTI Research Startup for New Faculty of School of Transdisciplinary Innovations, and Creative-Pioneering Researchers Program through Seoul National University.
Author information
Authors and Affiliations
Contributions
S.J., H.S., Y.K., J.T.L., and S.P. conceived the concept and designed the study. S.J., H.S., Y.K., Y.L., and Y.J. fabricated the fibers and characterized the polymer materials for the thermal drawing process. S.J., H.S., and Y.K. designed the hydrogel matrix for the electrode and electrolyte. S.J. and H.S. performed the mechanical and rheological measurements of the hydrogels and analyzed the data. S.J. and Y.K. conducted the electrochemical measurements and data analysis. S.J. and S.L. carried out the cell viability tests and analyzed the data. Y.C. and Y.K. performed the in vivo biocompatibility tests and analyzed the data. Y.K. conducted the animal experiments and analyzed the data. S.J., H.S., Y.K., J.T.L., and S.P. participated in discussions throughout the study. S.J., H.S., and Y.K. wrote the draft. J.T.L. and S.P. supervised this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shofarul Wustoni, Fen Ran, and Hritwick Banerjee for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jeon, S., Seo, H., Kim, Y. et al. Fully biocompatible, thermally drawn fiber supercapacitors for long-term bio-implantation. Nat Commun 16, 8207 (2025). https://doi.org/10.1038/s41467-025-63649-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63649-y