Abstract
Pulsed electromagnetic fields (PEMFs) enhance bone formation to combat osteoporosis, yet the mechanisms by which they promote bone health during aging remain unclear. This study shows PEMFs enhance new bone formation and innervation, promoting osteogenesis and reducing adipogenesis in mesenchymal stem cells (MSCs) in aging male mice. PEMF-induced osteogenesis is impaired by sensory nerve dysfunction in this model. Mechanistically, PEMFs stimulate sensory nerves to secrete semaphorin 3A (Sema3A), and depleting these nerves or knocking out Sema3a eliminates PEMFs’ bone-forming effects. Sema3A interacts with neuropilin-1 (Nrp1) in MSCs that express the leptin receptor, aiding osteogenesis and inhibiting adipogenesis in aging male mice. The activation of the “Sema3A-Nrp1” pathway is central for the anti-senescence effects of PEMFs on MSCs, and knocking out Nrp1 in MSCs that express the leptin receptor negates PEMFs’ benefits. Overall, PEMFs stimulate sensory nerves to produce Sema3A, which promotes osteogenesis, inhibits adipogenesis, and counters MSC senescence. This underscores their therapeutic potential for treating osteoporosis in aging males.
Similar content being viewed by others
Introduction
Bone is a metabolically active tissue essential for various physiological processes related to locomotion. It provides structural support, facilitates movement, stores minerals and growth factors, regulates mineral and acid-base homeostasis, protects vital structures, acts as muscle levers, and serves as a site for hematopoiesis. The general structure of bone consists of a central marrow space surrounded by periosteum and bone tissue1. Bone remodeling is a dynamic process involving the periodic replacement of old bone with newly formed bone at the same site, playing a crucial role in osteoporosis2. The balance between bone resorption and formation is vital for maintaining bone homeostasis. During remodeling, osteoblasts recruit osteoclast precursor cells into the osteon area, facilitating their interaction2. The bone remodeling cycle begins with osteoclastic bone resorption, followed by osteoblastic bone formation, which depends on the differentiation of bone marrow-derived mesenchymal stromal/stem cells (MSCs). Bone marrow-derived mesenchymal stem cells (BMSCs) serve as precursors for various cell lineages, including osteoblasts, chondrocytes, myoblasts, adipocytes, and fibroblasts3. Research indicates that defects in early mesenchymal precursors, likely MSCs, can lead to a decrease in osteoprogenitor cell production and may contribute to various musculoskeletal issues, including osteoporotic syndromes4.
Peripheral tissues, including the skin, joints, and the respiratory and gastrointestinal systems, are equipped with sensory innervation. They respond to external stimuli such as temperature, sound, odor, and taste (exteroception), while also receiving signals from various internal physiological systems, including the cardiovascular, respiratory, gastrointestinal, genitourinary, and nociceptive systems (interoception). Bone, as the largest organ, is densely innervated by primary afferent sensory nerve fibers and sympathetic nerve fibers throughout the periosteum, bone marrow, and mineralized bone5,6. Sensory nerve denervation has been linked to a decrease in trabecular bone mass due to decreased bone formation7,8. These sensory nerves play a crucial role in bone homeostasis by modulating skeletal interoception and secreting neuropeptides such as Calcitonin Gene-related Peptide (CGRP), nerve growth factor (NGF), substance P (SP), and semaphorin 3 A (Sema3A)9,10. Capsaicin-sensitive sensory neurons play a role in maintaining skeletal homeostasis, and their denervation leads to decreased new bone formation, resulting in a loss of trabecular connectivity and thickness11,12. Specifically, NGF-TrkA signaling in sensory nerves is essential for skeletal adaptation to mechanical loading13. Research by Chen indicated that prostaglandin E2 (PGE2), secreted by osteoblastic cells, activates PGE2 receptor 4 (EP4) in sensory nerves, thereby regulating bone formation by inhibiting sympathetic activity through the central nervous system14. This interoceptive signal is critical in regulating the lineage commitment of MSCs between osteoblasts and adipocytes11. Qiao’s study found that divalent metal cations can enhance bone formation by stimulating skeletal interoception in mouse injury models15. Additionally, implant-derived magnesium has been shown to promote CGRP-mediated osteogenesis in bone fracture healing in rats16. Furthermore, Epimedium treatment has been reported to mitigate osteoporosis effects through a brain/spinal cord/bone axis by increasing bone SP levels17. Given these insights, it is logical to explore the neurogenic potential of biomaterials in promoting bone formation.
Pulsed Electromagnetic Fields (PEMFs) are low-frequency magnetic fields characterized by specific amplitudes and waveforms, which exhibit stable variations in magnetic field amplitude over time. When tissues are exposed to PEMFs, they can generate a secondary electric field, similar to the natural conversion of mechanical energy into electrical energy18. Evidence suggests that PEMFs hold potential as a therapy for various bone disorders, including fresh fractures, nonunions, delayed unions, osteoarthritis, and osteoporosis19,20,21,22,23,24. Over the decades, the positive effects of PEMFs on osteoblasts, osteoclasts, and endothelial cells have been increasingly recognized19,20,21,22,23,25. For instance, numerous studies dating back to the 1970s in animal models confirm the osteogenic effects of biophysical stimulation on bone repair. Both pre-clinical and clinical research indicates that low-frequency PEMFs not only promote bone repair but also appear to enhance the osteogenic phase of healing, as evidenced by increased mineral apposition rate (MAR) in vivo26,27. In a 2005 study, Midura et al.28 subjected rat osteotomies to PEMF stimulation at two distinct frequencies and intensities (15 Hz, 2 mT versus 1.5 Hz, 0.02 mT), yielding comparable outcomes that demonstrated the higher frequency (15 Hz) treatment resulted in a doubling of both the apposition rate and the volume of the bone callus. In line with findings on proliferative effects, in vitro studies have demonstrated that biophysical stimulation induces (i) increased osteoblast differentiation, which enhances the production of collagen and key matrix glycoproteins such as osteocalcin and osteopontin29,30; (ii) stimulates the mineralization process31,32; and (iii) inhibits osteoclast differentiation while protecting against osteolysis33. Furthermore, extensive in vitro data suggest that PEMF positively influences chondrocyte proliferation, matrix synthesis, and chondrogenic differentiation by upregulating TGF-β and BMPs, while reducing anti-inflammatory cytokines through A2A and A3 adenosine receptors. This results in clinical translational studies demonstrating beneficial effects on pain relief and improved function in osteoarthritis of the knees, as well as enhanced bone formation in cases of osteoporosis34,35.
However, it has only recently been revealed that the nervous system may also be influenced by PEMFs. Studies have shown that PEMFs can influence growth factor activity and levels, thereby promoting nerve regeneration36. Specifically, PEMF treatment has been associated with increased median nerve distal latency and improved nerve conduction velocity37,38. These findings suggest that PEMFs could be involved in the physiology of nerves, although the exact effects remain unresolved. To date, it is still unclear whether the sensory nervous system contributes to the mechanisms through which PEMFs counteract osteoporosis. Sema3A, a highly conserved family of axon guidance molecules originally identified for their roles in directing axonal growth, are now recognized for its critical involvement in bone homeostasis39,40. While existing studies offer circumstantial evidence regarding the role of sensory nerves in bone development and maintenance, the extent to which signals derived from sensory nerves influence physical therapy for bone formation remains to be fully elucidated.
In this study, we aimed to investigate the mechanisms underlying PEMF-induced bone formation. Our findings indicate that PEMFs promote bone formation by stimulating sensory nerves. During the early stages, PEMFs induce the secretion of Sema3A from sensory nerves. Notably, increased levels of Sema3A stimulate neuropilin-1 (Nrp1) in leptin receptor (LepR)+ MSCs, shifting MSC differentiation from adipogenesis to osteogenesis and inhibiting senescence in aging bones. This research reveals a previously unknown role of PEMFs in bone formation mediated by sensory nerves.
Results
PEMFs preserve bone mass in aged mice
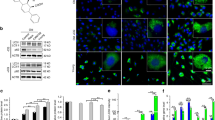
To investigate the effect of PEMFs on bone formation, young (4mon) and aged (20mon) male mice were treated with PEMFs (Fig. 1a). Micro-computed tomography (micro-CT) analysis of femoral cancellous bones are shown in Fig. 1b. PEMFs led to a significant increase in femoral cancellous bone volume fraction (BV/TV) (Fig. 1c), trabecular bone number (Tb.N) (Fig. 1d), and trabecular bone thickness (Tb.Th) (Fig. 1e), as well as a decrease in trabecular separation (Tb.Sp) (Fig. 1f), in aged mice compared to the sham group, while no such effects were observed in young mice. The serum level of procollagen peptide I (P1NP), a marker of osteoblastic bone formation, was significantly higher in PEMF-treated aging mice, while the level of the osteoclast bone resorption marker, carboxy-terminal collagen crosslinks (CTX), did not differ in either aging or young mice (Supplementary Fig. 2h, i). Calcein double labeling confirmed that aging mice treated with PEMFs exhibited increased bone formation and MAR compared to the non-PEMF-treated group. However, the number of tartrate-resistant acid phosphatase (TRAP)+ osteoclasts showed no significant difference following PEMF treatment in both aging and young mice (Supplementary Fig. 2a, b, j, k). In vitro, we investigated the potential effects of PEMFs on osteoclast activity by isolating bone marrow macrophages (BMMs) from 4-month and 20-month-old male mice. These BMMs were stimulated to differentiate into mature osteoclasts before being treated with PEMFs. The results indicated that PEMF treatment did not affect osteoclast activity, as evidenced by TRAP staining (Supplementary Fig. 2c, d). Additionally, analysis of osteoclast-related gene expression demonstrated that exposure to PEMFs did not influence osteoclast activity (Supplementary Fig. 2e–g).
4-month-old and 20-mon-old C57BL/6 male mice were treated with or without PEMFs for 4 weeks. a Schematic drawing of the PEMF device. Illustration created using Blender (version 4.3, Blender Foundation, Amsterdam). b Representative micro-CT images of distal femoral cancellous bones. c–f Quantification of the trabecular cancellous bone volume fraction (BV/TV), trabecular cancellous bone number (Tb.N), trabecular cancellous bone thickness (Tb.Th), and trabecular cancellous separation (Tb.Sp), n = 5 biological replicates for each group. g Immunofluorescence staining of femur sections using antibody against CGRP. DAPI stains nuclei blue. i Quantitative analysis of the CGRP+ sensory nerves (red). h Representative images of immunofluorescence staining of femur sections. Green: Perilipin+ cells; Red: Opn+ cells. DAPI stains nuclei blue. j, k Quantitative analysis of the number of Perilipin+ cells per mm² of primary trabecular tissue and the relative areas of Opn (N. Perilipin+ cells/Ar, Quantative relative area). Ar, tissue area, n = 5 biological replicates for each group. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA. P value: c P < 0.0001 (4mon Shamvs20mon Sham), P < 0.0001 (20mon PEMFsvs20mon Sham). d P < 0.0001(4mon Shamvs20mon Sham), P = 0.1401 (20mon PEMFsvs20mon Sham). e P < 0.0001(4mon Shamvs20mon Sham), P = 0.0018(20mon PEMFsvs20mon Sham). f P = 0.0007(4mon Shamvs20mon Sham), P = 0.0105(20mon PEMFsvs20mon Sham). i P < 0.0001 (4mon Shamvs20mon Sham), P = 0.0012 (20mon PEMFsvs20mon Sham). j P < 0.0001 (4mon Shamvs20mon Sham), P < 0.0001 (20mon PEMFsvs20mon Sham). k P < 0.0001 (4mon Shamvs20mon Sham), P < 0.0001 (20mon PEMFsvs20mon Sham). Source data are provided as a Source Data file.
These data indicate that PEMFs can effectively maintain bone formation in aged mice. In addition, PEMFs led to a marked increase in the number of CGRP+ sensory nerve fibers in aged group (Fig. 1g, i). We next examined the effect of PEMFs on osteogenesis and adipogenesis. Osteogenesis was indicated by the expression of osteoblast markers such as osteopontin (Opn), while adipogenesis was reflected by the number of Perilipin-expressing (Perilipin + ) cells. In the PEMF group, a decrease in the number of Perilipin+ adipocytes and an increase in Opn expression were observed in aging mice compared to the sham group, but no such changes were noted in young mice (Fig. 1h, j, k). Previous studies have reported that BMSCs can differentiate into osteoblasts and adipocytes41, we therefore speculated that BMSCs might be the target of PEMFs, and that PEMF could maintain the homeostasis of BMSC differentiation. To verify this, BMSCs isolated from 20-month-old mice treated with PEMF exhibited reduced expression of a subset of genes associated with adipogenic differentiation (Supplementary Fig. 2n–p) and increased expression of genes related to osteogenic differentiation, in contrast to BMSCs obtained from non-PEMF-treated 20-month-old mice (Supplementary Fig. 2l, m), with no such effects observed in young mice. Collectively, these data implied that PEMFs preserve bone mass and bone formation in aged mice, and a potential role of sensory nerves in the therapeutic effect of PEMFs. In the subsequent study, we applied an aging mouse model (20mon-old male mice) to further investigate this effect.
PEMFs promote bone formation via sensory innervation
To investigate whether sensory nerves participate in PEMF-induced promotion of bone formation, we created an inducible sensory denervation in iDTRAvil−/− mice by crossing Advillin-Cre mice with iDTR wide type (iDTR WT; iDTRwt) mice. It was discovered that administering 1 µg/kg of diphtheria toxin (DTX) three times a week for a period of four weeks can effectively induce sensory denervation in adult mice, specifically those that are 8 weeks old. This treatment leads to a decrease in CGRP expression, while the levels of PGP9.5 remain stable. This indicates that the denervation observed in iDTRAvil−/− mice primarily affects CGRP-positive fibers and does not impact other sensory neurons. Consistent with these findings, aging male iDTRAvil−/− mice, at 20 months of age, also exhibit a reduced number of CGRP-positive sensory nerve fibers when compared to their iDTRwt counterparts (Fig. 2b, h, Supplementary Fig. 1a and 3 a, b, g). Regarding the bone volume parameters, we found that 20mon male iDTRAvilfl/− mice showed significant decreases in BV/TV, Tb.N, and Tb.Th, and a significant increase in Tb.Sp as compared with iDTRwt mice (Fig. 2a, c–f). Because neural changes other than those in the sensory nervous system can affect bone metabolism indirectly, we performed pole tests and grip strength tests with iDTRAvilfl/- mice injected with DTX. No motor neural activity was influenced in this mouse model (Supplementary Fig. 3c–e). This implied the critical role of sensory innervation in maintaining bone volume. In aged mice (20 months), it has been shown that the number of CGRP+ sensory nerve fibers were increased after PEMF treatment in iDTRwt mice, whereas no significant change was observed in the iDTRAvilfl/− mice (Fig. 2b, h). Of note, while PEMFs could stimulate bone formation in iDTRwt mice, these effects were abolished in iDTRAvilfl/− mice (Fig. 2a). These results suggested that sensory innervation is required in the therapeutic effect of PEMFs. We then examined the impact of denervation on osteogenesis and adipogenesis in aged mice. Immunofluorescence staining demonstrated a higher level of adipogenesis and lower level of osteogenesis in iDTRAvilfl/− aged mice when compared to the iDTRwt aged mice (Fig. 2g, i, j). Additionally, PEMF therapy in aged iDTRwt mice significantly reduced adipogenesis, as indicated by the decreased number of Perilipin+ cells, while simultaneously enhancing osteogenesis, as evidenced by the increased expression of Opn (Fig. 2g, i, j). However, no significant changes were observed in that of iDTRAvilfl/− mice (Fig. 2g). In iDTRwt mice treated with PEMFs, we observed elevated serum P1NP levels and enhanced bone formation and MAR, as indicated by calcein double labeling; however, these parameters remained unchanged in iDTRAvilfl/− mice following PEMF stimulation (Supplementary Fig. 3f, h, i). To verify this in vitro, the BMSCs isolated from PEMF-treated 20mon-old male iDTRwt mice showed reduced expression of a group of genes associated with adipogenic differentiation (Supplementary Fig. 3l–n) and increased expression of genes related to osteogenic differentiation (Supplementary Fig. 3j, k), compared to BMSCs from non-PEMF-treated iDTRwt mice. No changes were observed in BMSCs from the PEMFs group compared to the non-PEMFs treated group in aged iDTRAvilfl/− mice (Supplementary Fig. 3j–n). Taken together, these data indicated that sensory innervation in aged bone tissues is essential for PEMF-induced new bone formation.
20-month-old male Adville-Cre::iDTR (iDTRAvifl/-) mice and iDTRwt mice were subjected to treatment with either PEMFs at a strength of 3.8 mT and frequency of 8 Hz for 1 hour, or were left untreated, over a period of 4 weeks. a Representative micro-CT images of distal femoral cancellous bones. b Immunofluorescence staining of femur sections using antibody against CGRP (red). DAPI stains nuclei blue. c–f Quantification of the trabecular cancellous bone volume fraction (BV/TV), trabecular cancellous bone number (Tb.N), trabecular cancellous bone thickness (Tb.Th), and trabecular cancellous separation (Tb.Sp), n = 5 biological replicates for each group. g Representative images of immunofluorescence staining of femur sections using antibodies against Perilipin (green) and Opn (red). DAPI stains nuclei blue. h Quantitative analysis of the CGRP+ sensory nerves (red), n = 5 biological replicates for each group. i, j Quantitative analysis of the number of Perilipin+ cells per mm² of primary trabecular tissue and the relative areas of Opn (N. Perilipin+ cells/Ar, Quantative relative area), n = 5 biological replicates for each group. Ar, tissue area. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA.P value:(c) P < 0.0001(sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (d) P = 0.0613 (sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (e) P = 0.0013 (sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (f) P = 0.0147 (sham iDTRwtvsPEMFs iDTRwt), P = 0.0062(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (h) P < 0.0001(sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (I) P < 0.0001(sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). (j) P < 0.0001(sham iDTRwtvsPEMFs iDTRwt), P < 0.0001(iDTRwt PEMFsvsiDTRAvifl/- PEMFs). Source data are provided as a Source Data file.
Sensory nerves mediate the effect of PEMFs on BMSC homeostasis through secretion of Sema3A
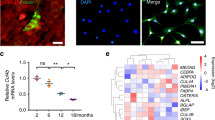
To further investigate the secretome of mouse dorsal root ganglion (DRG) sensory neurons, we performed Astral mass spectrometry coupled with data-independent acquisition (DIA) analysis on the supernatant of cultured DRG cells isolated from 20-month-old male mice in both the PEMF and non-PEMF groups (Supplementary Fig. 4a). An unsupervised hierarchical clustering analysis revealed differentially expressed proteins between the two groups. Gene ontology (GO) analysis (Supplementary Fig. 4b) indicated that these differentially expressed proteins were associated with synapse (GO:0045202), axon (GO:0030424), and neuron projection development (GO:0031175). Among the primary GO categories and the heatmap analysis, we observed that PEMF-treated DRG cells exhibited a significant increase in the secretion of the sensory neuropeptide Sema3A compared to the non-PEMF-treated group (Supplementary Fig. 4b,c). As described previously by Termini et al.42 at the bone level Sema3A, in addition to sensory neurons, is expressed by CD31+Sca-1− sinusoidal BM ECs (sBMECs) and CD31+Sca-1+ arteriolar BM ECs (aBMECs). And BMSCs are reported to secreted Sema3A43. We isolated these cells from aged mice in both the PEMF and non-PEMF groups. At baseline, we found that the expression and protein levels of the Sema3a gene were highest in sensory neurons. In contrast, very low levels were detected in both sBMECs and aBMECs, as well as in BMSCs (Supplementary Fig. 4d). Following PEMF treatment, Sema3a gene expression and protein level increased in sensory nerves, and remained stable in sBMECs, aBMECs and BMSCs (Supplementary Fig. 4d, e). These findings suggest that Sema3A, secreted by sensory neurons, may play a crucial role as a response molecule in regulating the exposure to PEMFs in aging mice. To quantify the kinetics of Sema3A secretion in response to PEMF, we utilized ELISA to measure Sema3A levels in the supernatants of cultured DRG cells treated with PEMFs. The results demonstrated a significant increase in Sema3A secretion following PEMF treatment, beginning at 1 hour, reaching peak levels at 3 hours post-treatment, then declining at 3 hours but remaining elevated between 12 to 24 hours (Supplementary Fig. 4f). This temporal pattern indicates that PEMF rapidly induces Sema3A secretion from sensory neurons, which may contribute to the observed effects on bone formation. In support, the co-immunostaining analysis revealed that Sema3A is predominantly expressed in sensory nerves within aging bone (Supplementary Fig. 4g, h). Notably, stimulation with PEMFs significantly enhanced the expression of Sema3A in CGRP+ sensory neurons in the bone, suggesting that PEMF stimulates Sema3A secretion from these neurons (Supplementary Fig. 4g–h).
To further investigate whether Sema3A secreted by sensory nerves exposed to PEMFs is essential for BMSC differentiation, in vitro experiments are conducted. We collected BMSCs from the sham group and the PEMF group aged male mice (20mon) and underwent flow cytometry (Fig. 3a). BMSCs from the PEMFs group exhibited an increased level of osteogenesis and reduced level of adipogenesis when compared to those in the sham group (Fig. 3b–d). This result indicated that BMSCs were the target of PEMFs in bone formation. To determine the direct effect of PEMFs on BMSCs in vitro, BMSCs were extracted from aged male mice and exposed to PEMFs. However, no significant difference was found between the sham and the PEMFs group in regards to the level of osteogenesis and adipogenesis. This result suggested that the effect of PEMFs on BMSCs needs a mediator (Fig. 3e–h). Notably, co-culturing BMSCs with the supernatant from DRGs treated with PEMFs resulted in an increased osteogenesis and a reduced adipogenesis (Fig. 3e–h). This finding implied that DRGs were essential for the therapeutic effect of PEMFs on BMSCs in vitro. Additionally, adding a specific anti-Sema3A antibody (1 μg/mL) to WT DRG co-culture with BMSCs negated the effects of PEMFs (Fig. 3e–h). Quantitative Real-time PCR (qPCR) analysis revealed that the impact of PEMF-stimulated DRGs on osteoblast differentiation and adipocyte formation was inhibited by the incubation with a Sema3A neutralizing antibody (Fig. 3i–m). Together, these data further proved that Sema3A secreted by sensory nerves mediated the therapeutic effect of PEMFs in vitro, and that PEMFs promote bone formation through controlling the differentiation of BMSCs.
a Bone marrow was collected from 20-month-old male mice that were treated with or without PEMF, and flow cytometry was performed to sort BMSCs. It was created in BioRender. Chen, S. (2025) https://BioRender.com/xi0onsj. Agreement number: FH28JKAJMF. b–d Representative images of Alizarin Red S (ARS) and Oil Red O (ORO) staining, along with quantification of ARS+ and ORO+ areas, were used to assess the levels of osteogenesis and adipogenesis. c, d n = 3 in each group from three independent experiments. e Schematic drawing of the experiment procedures. BMSCs extracted from 20-month-old mice were either cultured alone, exposed to PEMFs, co-cultured with null Sema3a or WT DRGs exposed to PEMFs, or treated with WT DRGs exposed to PEMFs in the presence of a Sema3A antagonist (1 μg/mL SM345431, United States). It was created in BioRender. Chen, S. (2025) https://BioRender.com/or7ob29. Agreement number: PX28JK9TNE. f–h Representative images of ARS and ORO staining, accompanied by quantification of ARS+ and ORO+ areas, were analyzed to assess the levels of osteogenesis and adipogenesis. g, h n = 3 in each group from three independent experiments. i–m qPCR analysis of adipogenesis- and osteogenesis-related gene expression in MSCs. n = 3 per group from three independent experiments. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparisons for c, d and g–m. P value: c P < 0.0001(ShamvsPEMFs). d P = 0.0117 (ShamvsPEMFs). g P < 0.0001 (ShamvsPEMFs+DRG). h P < 0.0001 (ShamvsPEMFs+DRG). i P < 0.0001 (ShamvsPEMFs+DRG). j P = 0.0002 (ShamvsPEMFs+DRG). k P = 0.0001 (ShamvsPEMFs+DRG). lP = 0.0223 (ShamvsPEMFs+DRG). m P = 0.0003 (ShamvsPEMFs+DRG). Source data are provided as a Source Data file.
Deletion of Sema3a in sensory nerves blocks the effect of PEMFs on bone formation
To further examine the role Sema3A in the regulation of bone formation in vivo, we generated conditional knockout Sema3a mice in sensory neurons by crossing the Sema3aflox/flox mice with the Advillin-Cre mice (Sema3aAvifl/-) (Supplementary Fig. 1a). Qpcr confirmed a reduction in Sema3a levels in the DRG (see Supplementary Fig. 5a). Additionally, pole and grip strength tests indicated no significant impact on motor activity, suggesting that the knockout of Sema3a did not influence overall neural function in 20-month-old male mice (Supplementary Fig. 5b–d). To investigate whether Sema3A secreted by sensory nerves is crucial for bone formation, we injected recombinant mouse Sema3A into 20mon-old male Sema3aAvifl/- (Sema3a knock out; Sema3a KO) mice and their wild-type Sema3a WT mice (Sema3awt) littermates. Our findings indicate that the beneficial effects of Sema3A on bone formation were diminished in the Sema3a KO mice (Supplementary Fig. 5e, f), suggesting that Sema3A derived from sensory nerves is essential for the bone remodeling microenvironment. In addition to the anti-Sema3A-specific antibody, isolating DRG from Sema3a knockout mice for co-culture with BMSCs negated the effect of PEMFs (Fig. 3e–m). Immunostaining for CGRP revealed a significant increase in the number of sensory nerve fibers in aged Sema3awt mice treated with PEMFs. In contrast, no such change was observed in Sema3a KO mice following PEMF treatment (Fig. 4a). Although PEMFs led to a marked increase in BV/TV, Tb.N, and Tb.Th in Sema3awt aged mice, no significant change was observed in Sema3a KO mice (Fig. 4b, e–g). Likewise, the increase in Tb.Sp could be reversed by PEMFs in Sema3awt mice but not in Sema3a KO mice (Fig. 4h). These data suggested the critical role of Sema3A in the therapeutic effect of PEMFs on bone homeostasis. In addition, this increased bone mass phenotype was accompanied by increased level of osteoblast differentiation and decreased adipocyte differentiation in Sema3awt mice after PEMF treatment, which was abolished in Sema3a KO mice (Fig. 4c, i and j). Consistent findings showed that the beneficial effects of PEMFs on MAR and P1NP levels were inhibited in the absence of Sema3a in sensory nerves (Supplementary Fig. 5g–i). Additionally, in vitro studies also showed BMSCs isolated from PEMFs+ Sema3awt mice showed increased osteogenic differentiation (Supplementary Fig. 5j, k), and decreased adipogenic differentiation (Supplementary Fig. 5l–n). These beneficial effects of PEMFs were not observed in Sema3a KO mice (Supplementary Fig. 5j–n). We next examined whether Sema3A could be secreted by BMSCs treated with PEMFs in vivo. We generated a conditional knockout Sema3a mice in the BMSCs by crossing Sema3a flox/flox mice with Prx-1-Cre (Sema3aPrx-1-Cre) mice (Supplementary Fig. 1a). At 20 months of age, deletion of Sema3a in BMSCs of Sema3aPrx-1-Cre mice had no significant influence on the bone formation parameters either in the sham group or the PEMF group when compared to the Sema3a WT (Sema3awt) mice (Supplementary Fig. 6a). PEMF treatment led to a marked increase in BV/TV, Tb.N, and Tb.Th and a significant decrease in Tb.Sp relative to the sham group of the Sema3aPrx-1-Cre aged mice, as well as Sema3awt mice (Supplementary Fig. 6b–e). This result suggested that Sema3A was primarily secreted by sensory neurons rather than BMSCs following PEMF treatment is responsible PEMF–induced bone formation.
20-month-old male Adville-Cre::Sema3a (Sema3aAvifl/-) mice and Sema3a flox/flox (Sema3awt) mice were treated with PEMFs at a strength of 3.8 mT and a frequency of 8 Hz for 1 hour, or remained untreated, over a duration of 4 weeks. a Representative images of immunofluorescence staining of femur sections using antibody against CGRP (red). DAPI stains nuclei blue. b Representative micro-CT images of distal femoral cancellous bones. c Double-immunofluorescence staining of femur sections using antibodies against Perilipin (green) and Opn (red). d Quantitative analysis of the CGRP+ sensory nerves (red), n = 5 biological replicates for each group. e–h Quantification of the trabecular cancellous bone volume fraction (BV/TV), trabecular cancellous bone number (Tb.N), trabecular cancellous bone thickness (Tb.Th), and trabecular cancellous separation (Tb.Sp). n = 5 biological replicates for each group. i, j Quantitative analysis of the number of Perilipin+ cells per mm² of primary trabecular tissue and the relative areas of Opn (N. Perilipin+ cells/Ar, Quantative relative area), n = 5 biological replicates for each group. Ar, tissue area. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA. P value: d P < 0.0001(Sema3awt PEMFsvsSema3awt Sham), P < 0.0001(Sema3awt PEMFsvsSema3aAvifl/- PEMFs). e P < 0.0001(Sema3awt PEMFsvsSema3awt Sham), P < 0.0001 (Sema3awt PEMFsvsSema3aAvifl/- PEMFs). f P = 0.4152(Sema3awt PEMFsvsSema3awt Sham), P = 0.0002 (Sema3awt PEMFsvsSema3aAvifl/- PEMFs). g P = 0.0133(Sema3awt PEMFsvsSema3awt Sham), P = 0.0029(Sema3awt PEMFsvsSema3aAvifl/- PEMFs). h P = 0.1060(Sema3awt PEMFsvsSema3awt Sham), P = 0.0172(Sema3awt PEMFsvsSema3aAvifl/- PEMFs). i P < 0.0001(Sema3awt PEMFsvsSema3awt Sham), P < 0.0001(Sema3awt PEMFsvsSema3aAvifl/- PEMFs). j P < 0.0001(Sema3awt PEMFsvsSema3awt Sham), P < 0.0001(Sema3awt PEMFsvsSema3aAvifl/- PEMFs). Source data are provided as a Source Data file.
PEMFs enhance the expression of Sema3A in the DRG by activating the cAMP/PKA signaling pathway
The signaling pathway involved in PEMFs upregulation of DRG Sema3A expression should be investigated. It has been described that PEMFs promote calcium-dependent activation of cAMP production, which, in turn, stimulates the cAMP response element binding protein (CREB)44. Since it was recently reported that Sema3a gene transcription is robustly induced by cAMP45, it is indicated that Sema3A in DRGs cells by PEMFs is mediated by cAMP. Our GO analysis suggests that cAMP plays a role in the response of DRG cells to PEMF treatment (Supplementary Fig. 4b). To validate this hypothesis, we isolated DRG from 20-month-old male mice and measured the intracellular concentrations of cAMP immediately following exposure to PEMFs at 1, 2, 3, 4, 6, 12, and 24 hours. We observed that cAMP levels in PEMF-treated DRG cells exhibited a trend similar to that of Sema3A (Supplementary Fig. 6f). Next, we analyzed the expression levels of downstream effectors of cAMP, including PKA. Using a detection kit, we measured PKA levels and found an increase at 1 hour, a peak at 2 hours, followed by a decline by 2 hours (Supplementary Fig. 6g). To determine whether activation of the cAMP–PKA pathway is necessary for the PEMF-induced secretion of Sema3A from DRG cells, we pretreated the DRG cells with the AC inhibitor 2’,5’-dideoxyadenosine (DDA) or the PKA inhibitor KT5720, both at a concentration of 10−6 M, added 12 hours prior to PEMF treatment. We then performed qPCR to analyze Sema3a mRNA levels in PEMF-treated DRG cells in the presence of cAMP and PKA inhibitors, which revealed that the beneficial effects of PEMFs were suppressed (Supplementary Fig. 6h, i). This confirms that PEMFs activate the cAMP/PKA pathway in DRG cells.
LepR+ MSCs are the main target of sensory neurons
Given that Sema3A secreted from sensory neurons regulates MSC differentiation, we aimed to identify which specific mesenchymal cell population is the target of Sema3A. Since leptin receptor (LepR)+ MSCs play critical roles in mediating adipogenesis and osteogenesis in adult mice46, we hypothesized that LepR+ MSCs could be the potential target of Sema3A. The LepR-Cre strain was crossed with the Rosa-LSL-tdtomato (tdtomato) strain, in which we could identify LepR-activated cells (tdtomato+) (Supplementary Fig. 1a). Capsaicin-induced sensory ablation was performed to confirm the important role of sensory nerves in bone formation13. Capsaicin or vehicle was injected into 20-month-old LepR-Cre; tdtomato mice for 1 week to generate the induced sensory denervation model, and mice were euthanized after another 2 weeks. The sensory ablation was confirmed by the immunofluorescence. After treated with capsaicin, the number of CGRP+ sensory nerves in the capsaicin group were significantly reduced when compared to the sham group (Fig. 5a, b). In addition, capsaicin group exhibited significantly reduced BV/TV, Tb.Th, Tb.N and an increase in Tb.Sp as compared with the sham group (Fig. 5c, d). Adipogenesis was increased and osteogenesis was decreased after capsaicin relative to control injection (Fig. 5e–g). In vivo fate mapping assays showed that tdtomato+adipocytes (Perilipin and tdtomato double-positive cells) were significantly increased after sensory denervation. PEMFs, lowered the number of tdtomato+ adipocytes that were induced by sensory denervation (Fig. 5h, i). These data suggested that LepR+ MSCs were the main target of Sema3A secreted by sensory neurons.
Twenty-month-old male LepR-Cre;tdTomato mice were injected with capsaicin (Cap; 30 mg/kg/day for 1 week) or vehicle for one week and subsequently euthanized two weeks later. a Representative images of immunofluorescence staining of femur sections using antibody against CGRP (red). DAPI stains nuclei blue. b Quantitative analysis of the CGRP+ sensory nerves (red), n = 5 biological replicates for each group. c Representative micro-CT images of distal femoral cancellous bones. d Quantification of the trabecular cancellous bone volume fraction (BV/TV), trabecular cancellous bone number (Tb.N), trabecular cancellous bone thickness (Tb.Th), and trabecular cancellous separation (Tb.Sp). n = 5 biological replicates for each group. e Double-immunofluorescence staining of femur sections using antibodies against Perilipin (green) and Opn (red). f, g Quantitative analysis of the number of Perilipin+ cells per mm² of primary trabecular tissue and the relative areas of Opn (N. Perilipin+ cells/Ar, Quantative relative area), n = 3 biological replicates for each group. h Representative images of immunofluorescence staining demonstrating the colocalization of perilipin (green) and tdTomato (indicating LepR+ cells) (red) in the femoral bone marrow of 20-month-old male LepR-Cre;tdtomato mice. The mice were treated with capsaicin (30 mg/kg/day for 1 week) or with PEMFs (3.8 mT, 8 Hz, 1 hour/day) for 4 weeks. i Quantitative analysis of tdtomato+adipocytes for each of the groups (marrow adipocytes labeled by white arrowheads), n = 5 biological replicates for each group. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student’s t test (b, d, f, g), two-way ANOVA for i. P value: b P < 0.0001 (ShamvsCap). d BV/TV: P < 0.0001(ShamvsCap); Tb.N: P = 0.0408(ShamvsCap); Tb.Th: P = 0.0302(ShamvsCap); Tb.Sp: P = 0.0013(ShamvsCap). f P = 0.0006(ShamvsCap). g P = 0.0020(ShamvsCap). i P < 0.0001(ConvsCap), P < 0.0001(CapvsCap+PEMFs). Source data are provided as a Source Data file.
Deletion of Nrp1 in LepR+ MSCs disturbs PEMF-induced bone formation
Previous studies have reported that Nrp1 is the receptor of Sema3A in MSCs42. We isolated LepR+ MSCs from 20-mon-old PEMF-treated male mice and found that Nrp1 expression was nearly 8-fold higher compared to the control group (Supplementary Fig. 7a). We speculated that PEMF-induced secretion of Sema3A from sensory neurons might combine with Nrp1 on LepR+ MSCs to preserve bone formation in aged mice. To test this hypothesis, we generated 20mon-old male LepR-Cre;Nrpfl/fl (Nrp1LepR-/-) mice to specifically delete Nrp1 in LepR+ cells (Supplementary Fig. 1a). The qPCR and flow cytometry data indicated a reduction in Nrp1 expression in LepR+ MSCs following the deletion of Nrp1 (Supplementary Fig. 7b–f, 8a, b). Pole tests and grip strength tests showed no changes in motor neural activity (Fig. 6a–c). Similar to bone phenotypes in AdvillinCre;iDTR mice, PEMF-induced bone formation was evidenced by bone formation parameters in PEMFs-treated Nrp1wt mice when compared to sham Nrp1 WT mice (Nrp1wt), while this effect was abolished in Npr1LepR-/- mice (Fig. 6d, e, Supplementary Fig. 7g–i). In addition, PEMFs increased the number of CGRP+ sensory nerves in Nrp1wt mice relative to the sham group, but this alteration was blocked in Nrp1LepR-/- mice (Fig. 6f, h). After treatment with PEMFs, double-immunofluorescence staining revealed a significant reduction in the number of Perilipin+ cells and a marked increase in Opn expression in Nrp1wt mice (Fig. 6g, i, j). However, this effect was absent in Nrp1LepR-/- mice, with no significant change in the number of those cells (Fig. 6g, i, j). To confirm this in vitro, the BMSCs isolated from 20-month-old PEMF-treated male Nrp1wt mice exhibited decreased expression of a cluster of adipogenic differentiation-related isoform genes (Supplementary Fig. 7l–n) and increased expression of osteogenic differentiation-related isoform genes (Supplementary Fig. 7j, k) compared to BMSCs isolated from non-PEMF-treated Nrp1wt mice. No changes were observed in BMSCs from Nrp1LepR−/− aged mice (Supplementary Fig. 7j–n). These findings suggest that Nrp1 signaling in MSCs is involved in PEMF-induced bone formation. As reported, Nrp1 is a quite promiscuous receptor, capable of interacting with several ligands besides Sema3A. In this regard, it is therefore important to define in more detail the pathway by which Sema3A induces the osteogenic differentiation of LepR+ MSCs through one or more of the four class A Plexins. The qPCR analysis of Plxna1 and Plxna4 expression in LepR+ MSCs from 20-month-old male mice revealed that PEMF treatment resulted in significantly higher levels of Plxna1 expression compared to the non-treated group, while Plxna4 levels were found to be nearly undetectable (Supplementary Fig. 7o), suggesting that Plexin A1 may play a key role in Sema3A signaling within LepR+ MSCs. To further investigate the role of Plxna1 in LepR+MSCs differentiation, we used adenovirus-mediated short hairpin RNA (shRNA) to knock down Plxna1 in LepR+MSCs from 20mon-old male mice. Notably, the knockdown of Plxna1 resulted in a reduction in osteogenic differentiation in LepR+ MSCs treated with Sema3A agonist, as evidenced by qPCR analysis and ARS staining (Supplementary Fig. 7p–s), highlighting its critical role in this process, and indicating that Nrp1/Plexin A1 serves as the primary mediator of Sema3A signaling in this context.
Behavioral testing was conducted on 20-month-old male LepR-Cre:Nrp1 (Nrp1LepR-/-) mice and Nrp1 flox/flox (Nrp1wt) mice. a, b The average turn time and total duration required to complete the pole test were recorded for both Nrp1LepR-/- and Nrp1wt mice, n = 5 biological replicates for each group. c Grip strength measurements of the forelimbs were taken for both Nrp1LepR-/- and Nrp1wt mice, n = 5 biological replicates for each group. d–j 20-month-old male Nrp1LepR-/- mice and Nrp1wt mice were treated with PEMFs at a strength of 3.8 mT and a frequency of 8 Hz for 1 hour, or remained untreated, over a duration of 4 weeks. d Representative micro-CT images of distal femoral cancellous bones. e Quantification of the trabecular cancellous bone volume fraction (BV/TV), trabecular cancellous bone number (Tb.N), trabecular cancellous bone thickness (Tb.Th), and trabecular cancellous separation (Tb.Sp). n = 5 biological replicates for each group. f Representative confocal images of immunofluorescence staining of CGRP (red). g Double-immunofluorescence staining of femur sections using antibodies against Perilipin (green) and Opn (red). h Quantitative analysis of the CGRP+ sensory nerves (red), n = 5 biological replicates for each group. i, j Quantitative analysis of the number of Perilipin+ cells per mm² of primary trabecular tissue and the relative areas of Opn (N. Perilipin+ cells/Ar, Quantative relative area), n = 5 biological replicates for each group. Ar, tissue area. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. * P < 0.05, **P < 0.01, ***P < 0.001 by two-tailed Student’s t-tests, for a–c; two-way ANOVA for e, h–j. P value: (a) P = 0.5811(Nrp1wtvsNrp1LepR-/-). (b) P = 0.8582(Nrp1wtvsNrp1LepR-/-). (c) P = 0.4937 (Nrp1wtvsNrp1LepR-/-). (e) BV/TV: P < 0.0001(Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs); Tb.N: P = 0.1112(Nrp1wt PEMFsvsNrp1wt Sham), P = 0.0656 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs); Tb.Th: P = 0.0411(Nrp1wt PEMFsvsNrp1wt Sham), P = 0.0209 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs); Tb.Sp: P = 0.0024(Nrp1wt PEMFsvsNrp1wt Sham), P = 0.0002 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). (h) P < 0.0001(Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). (I) P < 0.0001(Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). (j) P < 0.0001(Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). Source data are provided as a Source Data file.
Anti-senescence of MSCs is involved in the bone formation induced by PEMFs
To investigate the impact of PEMFs on MSCs, BMSCs isolated from aged mice and PEMFs treated mice to conduct RNA-sequence (seq). GO analysis indicated that the altered genes were associated with the cell cycle and cellular senescence (Fig. 7a). Meanwhile, BMSCs isolated from PEMF-treated 20-month-old mice exhibited decreased expression of a cluster of senescence and adipogenic differentiation-related isoform genes, along with increased expression of a cluster of osteogenic differentiation-related isoform genes compared to BMSCs isolated from non-PEMF-treated 20-month-old mice (Fig. 7b). SA-βGal staining showed that PEMFs led to a significant reduction in the number of SA-βGal+ cells and increase in the number of Brdu+ cells in Nrp1wt mice (Fig. 7c, d). However, this change was abolished in Nrp1LepR-/- mice, in which PEMF treatment resulted in no significant alteration (Fig. 7c, d). In consistent, qPCR results showed that the anti-senescence effects of PEMFs were blocked by Nrp1 deficiency (Fig. 7e–g). This result indicates that PEMFs have anti-senescence effects on MSCs, a finding supported by our previous study21, and suggests that Sema3A/Nrp1 signaling plays a role in this anti-senescence process.
20-month-old C57BL/6 male mice were treated with or without PEMFs for 4 weeks. BMSCs were isolated from femoral bone for RNA sequence. a The GO enrichment analysis of differentially expressed genes in mouse MSCs was performed. b Heat map of differentially expressed genes between MSCs from the non-PEMF and PEMF-treated groups. c Representative images of SA-βGal staining. d Representative images of Brdu staining. e–g qPCR analysis was conducted to assess the expression of senescence and anti-senescence-related genes in MSCs (n = 3). In vitro experiments were repeated 3 times. Data are represented as mean ± SEM, statistical analyses were conducted with a 95% confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA. P value: (e) P = 0.0023 (Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). (f) P = 0.0002 (Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). (g) P < 0.0001 (Nrp1wt PEMFsvsNrp1wt Sham), P < 0.0001 (Nrp1wt PEMFsvsNrp1LepR-/- PEMFs). Source data are provided as a Source Data file.
Discussion
In recent years, rapid progress has been made in the development of physical therapy for the treatment of musculoskeletal injuries. It has been shown that PEMFs have been proven to exert anti-inflammatory effects and are efficient in treating many bone disorders, including fresh fractures, nonunion and delayed fractures, osteoporosis, diabetic osteopenia, and osteonecrosis47,48. Particularly, it is a noninvasive and safe biophysical countermeasure, which appears to be a cost-effective way to achieve new bone formation. which should be the highest recommendation in clinical practice. However, it remains largely unclear how PEMFs induce new bone formation and the specific molecular and cellular mechanisms. We revealed a previously unknown role of PEMFs in bone formation through the sensory nerve. During the early stage, PEMFs induced the secretion of Sema3A from sensory nerves. Importantly, Sema3A from the increased sensory nerves stimulated Nrp1 in LepR+ MSCs, shifting their differentiation from adipogenesis to osteogenesis and inhibiting senescence in aging bone (Fig. 8).
PEMFs have the potential to alleviate osteoporosis in aged mice. Following PEMF treatment, sensory nerves release Sema3A, which then binds to Nrp1/Plexin-A1 on LepR+ MSCs. This interaction promotes osteogenesis while suppressing adipogenesis in aging mice. Furthermore, PEMFs contribute to the maintenance of BMSC homeostasis by enhancing self-renewal and reducing senescence, ultimately resulting in improved bone formation. The Scheme was created in BioRender. Chen, S. (2025) https://BioRender.com/70jouht. Agreement number: UE28JKAEK0.
The outcome of PEMF treatment in aging-related osteoporosis in vivo is encouraging, as indicated by the increases in BV/TV, Tb.Th as well as decreased Tb.Sp. Initially, we discovered that PEMFs did not significantly impact bone resorption; therefore, our primary focus shifted to the bone formation induced by PEMF treatment. Bone is a highly innervated organ that grows continually owing to the consistent presence of stem cells. The significant presence of CGRP+ sensory nerves in the PEMF group correlated strongly with MSC aggregation and enhanced osteogenesis, leading us to focus on sensory nerves. Amount of evidence shows that sensory nerves can regulate bone homeostasis, and loss of sensory nerves leads to decreased number of MSCs and osteogenic progenitor cells in bone and osteogenesis disruption11,13. In our study, we consistently observed sensory nerve dysfunction in deficient mice, which led to impaired PEMF-induced osteogenesis in an aging mouse model. This ultimately contributed to the development of osteoporosis. In addition, MSCs was activated after they were co-cultured with PEMFs-stimulated DRG. Such findings indicate that the osteogenic potential of PEMFs is strongly dependent on interaction with sensory nerves, suggesting that chemical cues derived from the PEMF synergistically activate sensory innervation and bone formation.
Sensory nerves can secrete various neurotransmitters that regulate bone homeostasis, as well as the behavior of BMSCs. In our study, we have explored the relationship between sensory nerve and the BMSCs. A previous study reported that Sema3A is a powerful protein that promotes osteogenic differentiation of bone marrow MSCs49. A drug-delivery system based on Sema3a gene therapy ameliorated bone loss in osteoporotic ovariectomized mice50. Therefore, Sema3A can be regarded as a key mediator in the neural-osteogenic interaction, acting as a paracrine factor essential for the osteogenic differentiation of BMSCs. However, in a previous study by Termini et al.42, at the bone level Sema3A, in addition to sensory neurons, is also expressed by sBMECs and aBMECs. To validate our hypothesis, we conducted additional experiments and discovered that Sema3A expression in sensory neurons is significantly greater during bone formation compared to sBMECs and aBMECs in aging bone. Besides, Sema3A expression in sensory neurons is upregulated in response to PEMF treatment, while levels in sBMECs and aBMECs remain relatively stable. In addition, Sema3A secreted by sensory neurons plays a vital role in promoting bone tissue formation for several reasons. First, sensory neurons are strategically positioned near osteogenic niches14, allowing for direct and efficient signaling to LepR+ MSCs. This anatomical proximity facilitates rapid transmission of signals that encourage osteogenic differentiation. Second, sensory neurons establish preferential contacts with LepR+ MSCs, creating a niche where Sema3A can directly influence these cells. Notably, Sema3A may be identical to CGRP, as both are encapsulated in synaptic vesicles and released from the axon terminals of DRG neurons16, this direct interaction is essential for the osteogenic differentiation of LepR+ MSCs, which could be the focus of our future research. Our findings indicate that sensory neuron-derived Sema3A plays a significant role in promoting bone tissue formation due to its strategic anatomical location, temporal dynamics, and direct signaling to LepR+ MSCs. While sBMECs and aBMECs also express Sema3A, the unique positioning and signaling capabilities of sensory neurons make their secretion of Sema3A particularly influential in bone formation. To further investigate whether Sema3A secreted by sensory nerves is crucial for bone formation, we injected Sema3A into Sema3a KO mice and their WT littermates. Our findings indicate that the beneficial effects of Sema3A on bone formation were diminished in the Sema3a KO mice, suggesting that Sema3A derived from sensory nerves is essential for the bone remodeling microenvironment. Since PEMFs enhance Sema3a expression in DRGs and increase the distribution of CGRP-positive sensory nerves, the elevated Sema3A levels in the femur may be due to the increased sensory innervation caused by PEMFs. Based on previous reports on the osteogenic effects of Sema3A and the present results, we conclude that PEMFs promote Sema3A secretion from DRGs to enhance the osteogenesis of MSCs. Our study provides evidence that Sema3A serves as a key mediator secreted by sensory nerves, influencing the effects of PEMFs on bone formation. However, we also acknowledge that other factors may contribute to this process25. To explore this further, we conducted experiments comparing the effects of PEMFs on wild-type and Sema3a knockout mice, as well as in vitro co-culture experiments with DRG and BMSCs. In our study, blocking of Sema3a expression in neurons in vivo attenuated new bone formation induced by PEMFs, whereas overexpression of Sema3A stimulated new bone formation. Besides, isolating DRG from Sema3a knockout mice for co-culture with BMSCs negated the effect of PEMFs. These experiments demonstrate that Sema3A significantly mediates the effects of PEMFs on bone formation. Other mechanisms, such as the modulation of osteoblasts or endothelial cells by PEMFs, may operate downstream of the Sema3A signaling induced by sensory nerves, with additional factors potentially playing a synergistic or complementary role. Therefore, we believe that a clear understanding of the mechanism by which PEMFs contribute to new bone formation through the nervous system will reveal the therapeutic potential of this physical therapy (Fig. 8).
Bone cells express receptors for Sema3A, and the activation of these receptors significantly promotes angiogenesis in bone51. LepR has been reported to mark MSCs in adult mouse bone marrow, and LepR-Cre is effective for in vivo fate mapping of MSCs46. It has been reported that LepR is a marker that is highly enriched in bone marrow MSCs, with approximately 0.3% of bone marrow cells expressing LepR+52. In adulthood, LepR+ MSCs play a major role in the remodeling process52 and LepR+ stromal cells formed a major cluster with the ability to partition into osteolineage and adipolineage cells11. Therefore, LepR-Cre could effectively trace cells in both osteoblast and adipocyte lineages. Indeed, our immunofluorescence staining results revealed that LepR+ MSCs were the main target of Sema3A secreted by sensory neurons exposed to PEMFs. Based on the analysis, Sema3A released from sensory nerve binds directly with the specific receptor Nrp1 on LepR+MSC, regulating BMSCs. Moreover, we found that knockout of Nrp1 in LepR+ MSC blocked decreased adipogenesis and increased osteogenesis and the ability of MSCs exposure to PEMFs. Understanding the Sema3A-Nrp1 crosstalk is the key revealing how Sema3A secreted from sensory nerves regulate the fate of MSCs. Further, Nrp1 is a quite promiscuous receptor, capable of interacting with several ligands besides Sema3A, e.g., VEGF-A, and modulating HGF signaling through the activity of the tyrosine kinase receptor (TKR) Met or EGF signaling via EGFR TKR. In this regard, our study demonstrated that Sema3A induces the osteogenic differentiation of LepR+ MSCs through Nrp1/PlexinA1. PEMFs could mitigate bone loss at the expense of decreased marrow adiposity, with the Sema3A/Nrp1 axis potentially playing a role in this process.
Cellular senescence is a cell state implicated in various physiological processes and, plays an important role in a wide range of age-related diseases, including OP53. Senescent MSCs have a reduced capacity to differentiate into osteogenic lineages and upregulate adipogenesis41,54,55. This phenotype highlights a pro-adipogenic microenvironment associated with aging. The reverse of aging MSCs can be influenced by targeting cellular senescence, which presents a potential therapeutic avenue for enhancing bone formation21. Our findings demonstrate that PEMFs can inhibit cellular senescence in MSCs from aging mice. Additionally, blocking the Sema3A/Nrp1 signaling pathway not only diminishes the effects of PEMFs but also increases the senescent population of MSCs in Nrp1LepR-/- mice. This suggests that PEMFs may help preserve senescent MSCs via the Sema3A/Nrp1 pathway.
In summary, we found that sensory nerve mediates PEMFs-induced bone formation. We acknowledge that in clinical settings, de novo bone formation is rarely observed in osteoporotic patients, with current therapies primarily aimed at restoring the balance between bone resorption and formation. In our study, PEMF exposure in 20-month-old mice led to a significant increase in osteogenesis, as evidenced by enhanced new bone formation and an increased number of trabeculae on Micro-CT, while osteoclast activity remained unchanged. This suggests that the benefit of PEMF treatment in aged bone may derive from its ability to rescue the impaired osteoblast function that is characteristic of senile osteoporosis rather than by initiating bone formation de novo. Importantly, the lack of effect in 4-month-old mice further underscores that the osteogenic response to PEMF is context-dependent and may be particularly relevant in the aging skeleton, where osteoblast activity is compromised. Although the extent of new bone formation observed in our murine model may differ from that seen in human patients, our findings indicate a potential therapeutic role for PEMF in improving the osteogenic capacity of aged bone. This mechanism could ultimately contribute to a net gain in bone mass and enhanced structural integrity, thereby indirectly restoring the balance between bone formation and resorption in osteoporotic conditions. The beneficial effects of PEMFs appear to be mediated by Sema3A, an axonal guidance molecule released from sensory neurons. Activation of Nrp1 receptor in LepR+MSCs by PEMFs inhibiting cellular senescence of MSC, inducing osteogenesis and suppressing adipogenesis. The discovery of PEMFs-induced bone formation through sensory nerve could revolutionize the current understanding of physical therapy for bone diseases.
Methods
Ethical statement
All mouse studies were conducted in accordance with protocol (2021215A), which was approved by the Experimental Animal Center of West China Hospital and the Institutional Animal Care and Use Committee (IACUC) of Sichuan University. These procedures adhered to the guidelines outlined in the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and complied with the principles of the Animal Welfare Act. All mice were euthanized, and all animal experiments were performed under isoflurane anesthesia. Every effort was made to minimize and prevent any suffering.
Animals and drug treatment
AdvillinCre, iDTR, Prx-1cre, Sema3afl/fl, LepRCre, Nrp1fl/fl, Rosa26-CAG-LSL-tdTomato mouse lines were purchased from Cyagen Biosciences (Guangzhou, China). C57BL/6 mice were purchased from BEIJING HFK BIOSCIENCE CO,LTD Experimental Animals (Beijing, China). All these mice were housed in specific pathogen-free conditions and analyzed with a mixed background. The mice were housed in a clean room with free access to water, in a humidity-controlled environment, and subjected to a 12-hour light/dark cycle (8 a.m. to 8 p.m.). They were maintained on a standard chow diet (25043213) at a constant temperature of 22-26 °C.
The genotypes of the mice were measured by PCR analyses of genomic DNA, which was extracted from mouse tails within the following primers: AdvillinCre: forward: AATGGCTCCCTGTTCACTGT, Reverse: AGGCAAATTTTGGTGTACGG, WT: TGACTAGGTAGAGGTGCAAATGTC; Prx1Cre:forward: GCTCTGATGTTGGCAAAGGGGT, Reverse: AACATCTTCAGGTTCTGCGGG; iDTR: forward: CCTATGACCATACAACTATCCTGGC, Reverse: GGGTGAGCATGTCTTTAATCTACC, WT: CACTTGCTCTCCCAAAGTCGCTC. Sema3afl/fl: forward: TCTTCCCCTGAGGTTCTGACATA; Reverse: CAACAGCCCTTTAAGTTAAGACCA. Nrp1fl/fl: forward: CAATAAATGGGGTGGCATTTGCAG; Reverse: ACTACAAAACCTCAAATGCTGGAG. LepRCre: forward: GTCATGAACTATATCCGTAACCTGG; Reverse: GACAGGCTCTACTGGAATGGAAC; Rosa26-CAG-LSL-tdTomato: forward: GGCATTAAAGCAGCGTATCC; Reverse: CTGTTCCTGTACGGCATGG;
Four-month-old and twenty-month-old male gene knockout and C57BL/6 mice were subjected to either PEMF treatment or a control condition. Serum was collected by centrifuging at 200 × g for 15 min and stored at −80 °C before analyses. Femurs, tibias, heart, liver, spleen, kidney tissues, and urine of the mice were also collected.
The drugs and compounds used in this study are as follows: diphtheria toxin (DTX, Sigma-Aldrich, D0564); Capsaicin (MilliporeSigma, M2028); Recombinant mouse Sema3A Fc chimera protein (R&D Systems, 5926-S3). Dosages and time courses are noted in the corresponding text and figure legends.
Behavioral analysis
Pole tests and grip strength assessments were conducted to evaluate changes in motor neural activity in iDTRAvifl/-, Sema3aAvifl/-, and Nrp1LepR-/- mice. All tests were carried out between 11:00 AM and 5:00 PM during the light cycle. For the pole test, a 9-mm diameter metal rod measuring 0.76 meters, wrapped in bandage gauze, was used as the pole. The time taken for each mouse to turn and the total duration to reach the base of the pole were recorded. Prior to testing, the mice underwent training for three consecutive days, with each training session consisting of three trials. In the grip strength test, neuromuscular strength was measured as the maximum force exerted by the mice (Biosed, USA). The mice were positioned to grasp a metal grid with their forelimbs. A gentle pull on the tail was applied, and the maximum holding force was recorded using a force transducer at the moment the mice released their grip on the grid. The peak holding strength was digitally recorded and displayed in kilograms.
PEMF treatment
Comprehensive details regarding the magnetic field device utilized in this study can be found in our earlier publication21. We employed a custom-designed PEMF exposure system, which consisted of a signal generator and a Helmholtz coil assembly featuring a three-coil array. Mice in the PEMF group were exposed to PEMF for 4 weeks, with parameters set at a frequency of 8 Hz, an intensity of 3.8 mT, and an exposure duration of 1 hour per day. The signal generator produced a pulsed burst waveform (burst width of 62.5 ms; pulse width of 62.5 ms) repeated at 8 Hz, maintaining an intensity of 3.8 mT and a duty cycle of 50%. The waveform was square-shaped. These intervention parameters were based on our previous research, which demonstrated their efficacy in enhancing osteogenesis21. An external infrared thermometer was used during PEMF exposure to confirm that the ambient temperature in the exposure area remained stable at 24 ± 2 °C throughout the session. Additionally, the exposure duration and PEMF parameters were consistent with prior studies that reported no significant thermal effects21.
For the placebo PEMF group, mice in the sham group remained in the exposure chamber for the same duration without any output. Following the PEMF intervention, the mice were returned to their housing. For in vitro PEMF treatment, a separate magnetic field device was set up within a cell incubator, with parameters of 8 Hz, 3.8 mT, and an exposure duration of 3 hours per day. A Gauss meter was used to monitor the magnetic field intensity within the coils during both in vitro and in vivo experiments, ensuring the stability of the equipment for each treatment.
Micro-computed tomography (micro-CT)
Left distal femurs were assessed by micro-CT (NMC-100, PINGSHENG, Shanghai, China) with the following parameters: 70 kV, 114 mA, and 700 ms integration time. Trabecular measurement scans were performed as follows: continuously obtaining 100 micro-CT images with a 10-μm layer spacing. Regions of interest were set from 0.6 mm to 1.5 mm of the distal growth plate in long bone tissue. Avatar software was used to recreate the complete femoral metaphyseal three-dimensional structure at the end of the reconstruction process and to perform data analysis. The region of interest of the trabecular bone was drawn beginning from 5% of the femur length proximal to the distal metaphyseal growth plate and extending proximally for another 5% of the total femur length. The following trabecular bone parameters were collected from the 3D analysis data: Tb.BV/TV, Tb.Th, Tb.N, and Tb.Sp.
Immunohistochemistry and immunofluorescence
At designated time points, mice femurs were collected and fixed in the 4% paraformaldehyde overnight, then transferred to 10% ethylenediaminetetraacetic acid for 21 days until the femurs were completely decalcified. Next, femurs were immersed in 20% sucrose solution with 2% polyvinylpyrrolidone (Sigma-Aldrich, PVP40) for 24 h and embedded in OCT (Sakura Finetek, Torrance, CA, USA). The 40-μm-thick coronal-oriented sections of the femurs were obtained using a cryostat microtome. The sections were then incubated with primary antibodies. The brain and DRG tissues from the mice were fixed in 4% paraformaldehyde, dehydrated with 30% sucrose, and then embedded in optimal cutting temperature compound OCT. Coronal sections of the brain and DRG tissues, each 10 μm thick, were subsequently obtained using a cryostat microtome. The following primary antibodies were used: Opn (R&D, AF808, 1:200), Perilipin (MilliporeSigma, P1873; 1:500; CST catalog 9349, 1:200), CGRP (Abcam, ab81887, 1:50; ab36001, 1:100), PGP9.5 (Abcam, ab108986, 1:300) and Sema3A (Santa Cruz Biotechnology, sc-74555; 1:200) overnight at 4 °C. Alexa 647-conjugated goat anti-rabbit antibody (Invitrogen, A32733, 1:500), Alexa 488-conjugated donkey anti-rabbit antibody (Invitrogen, A21206, 1:500), Alexa 555-conjugated donkey anti-goat antibody (Invitrogen, A21432, 1:500), Alexa 647-conjugated donkey anti-goat antibody (Invitrogen, A32849, 1:500). Alexa 488-conjugated goat anti-mouse antibody (Invitrogen, A32723, 1:500), Alexa 647-conjugated donkey anti-mouse antibody (Invitrogen, A31571, 1:500), while the nuclei were counterstained with Hoechst 33324 (Thermo Fisher Scientific). For Perilipin+ adipocytes, we quantified the number of cells per area using image analysis software. For Opn+ cells, we calculated the relative area of staining. Immunofluorescent images were captured with a Nikon (N-STORM & A1) confocal microscope and analyzed using ImageJ visualization software.
The samples were dehydrated in ethanol, embedded in paraffin, and sectioned into 5 μm-thick coronal-oriented slices using a rotary microtome. For hematoxylin and eosin (H&E) staining, specific slides were treated with hematoxylin (Thermo Fisher Scientific, 7231) for 3 minutes, followed by a 1-minute counterstaining with eosin (Epredia, 7111). TRAP staining (Sigma-Aldrich, 387A) was carried out on selected slides from each sample following the manufacturer’s guidelines. Briefly, the selected slides were pre-warmed to 37 °C in a water bath and then incubated in TRAP Staining Solution Mix at 37 °C for 15 minutes. The nuclei were counterstained with Methyl Green (Millipore Sigma, M8884). Images were taken using a polarized light microscope (Nikon Eclipse, Tokyo, Japan), and quantitative analysis was conducted using Image J software.
Calcein double-labeling assay
To evaluate dynamic bone formation ability, mice were administered intraperitoneally with calcein (20 mg/kg, SigmaAldrich, C0875) at 9 and 3 days before euthanasia. After fixation in 70% ethanol, the samples were dehydrated in gradient ethanol. Then, the calcein double-labeled bones were embedded in methyl methacrylate. 5-μm-thick longitudinal bone sections were made using a microtome and observed under a fluorescent microscope. MAR was measured using the OsteoMeasureXP Software (Osteo-Metrics, Inc.). MAR: the rate of formation of mineralized layer on the surface of trabecular bone, which is calculated by dividing the distance between two markers by the interval time between markers.
Primary mouse BMSC isolation and culture
Specifically, bone marrow cells were harvested from the tibiae and femora of male mice. These cells were then incubated with the following antibodies for 20 minutes at 4 °C: anti-Sca-1-PE (108108; BioLegend), anti-CD29-FITC (102206; BioLegend), anti-CD45-PerCP (103132; BioLegend), and anti-CD11b-APC/Cyanine7 (101226; BioLegend). Subsequently, fluorescence-activated cell sorting (FACS) was conducted using a BD Biosciences Aria model, and the data were analyzed with FACS DIVA software version (BD Biosciences). The sorted mouse Sca-1+CD29+CD45−CD11b− BMSCs were cultured for approximately 1 week until they reached 80–85% confluence. First-passage BMSCs were then trypsinized for about 1 minute and re-seeded in culture dishes to further enrich the cell population. The cells were maintained in α-MEM medium supplemented with 10% fetal bovine serum (Gibco), 100 μg/ml streptomycin (Gibco), and 100 units/ml penicillin (Gibco) under a humidified atmosphere of 5% CO₂ at 37 °C. For osteogenic differentiation, BMSCs were seeded into six-well plates at a density of 1.0 × 10⁶ cells per well and cultured in osteogenic induction medium containing 10 mM β-glycerol phosphate, 0.1 μM dexamethasone, and 50 μM ascorbate-2-phosphate for two weeks. After this period, cell matrix calcification was assessed by staining with 2% Alizarin Red (Hysigen Bioscience). In the adipogenic differentiation assay, BMSCs were plated in six-well plates at a density of 2.5 × 10⁶ cells per well and cultured in adipogenic induction medium composed of 1 μM dexamethasone, 5 μg/ml insulin, and 0.5 mM 3-isobutyl-1-methylxanthine for three weeks, with the culture medium being changed every 3 days. Lipid droplets in mature adipocytes were visualized using Oil Red O staining, following the manufacturer’s instructions (Hysigen Bioscience). For the cellular senescence assay, BMSCs were seeded in six-well plates at a density of 1.0 × 10⁶ cells per well and cultured for 24 hours. Senescent cells were then identified using a β-Gal staining kit (Cell Biolabs), according to the manufacturer’s instructions.
Cell sorting and flow cytometry analysis
To isolate and analyze CD45−LepR+ mesenchymal stem/progenitor cells (MSPCs) from the femora, we removed the soft tissues from femora of 20- and 4-month-old mice. The bones were then subjected to digestion with a protease solution (2 mg/ml collagenase A and 2.5 mg/ml trypsin in phosphate-buffered saline (PBS)) for 20 minutes to eliminate the periosteum and periosteal progenitors (step I). Subsequently, the bones were chopped into smaller fragments and digested in the protease solution for an additional hour (step II). The cells present in the supernatant were collected for flow cytometry analysis. After lysing red blood cells with a commercial ammonium-chloride-potassium lysis buffer (Quality Biological, Inc., Gaithersburg, MD, USA), the primary antibodies used were anti-CD45-APC (BD Biosciences, 559864, 1:200) and anti-LepR-biotin (BAF497; R&D Systems, 1:200). Flow cytometry was conducted using a 5-laser BD FACS instrument and FACS DIVA software (BD Biosciences), with cells gated as CD45- LepR + . Flow cytometric analyses were performed using FlowJo (Tree Star, Inc., Eugene, OR) and CellQuest software (BD Biosciences). Cell surface expression of Nrp1 was measured on LepR+MSCs via staining with goat anti-Nrp1, (R&D, AF566). To clarify that Sema3A promotes the osteogenic differentiation of LepR+MSCs through Plexin-A1, LepR+MSCs were subjected to Plxna1 knockdown using adenoviral particles of shPlxna1 and shControl, obtained from Sigma-Aldrich (TRCN0000079189). The bone marrow stromal cells were infected with the adenovirus for 8 hours prior to conducting further experiments, after which they were treated with a Sema3A agonist (Sino Biological, China).
Osteoclastogenesis in vitro
Primary BMMs were employed to simulate osteoclast differentiation in vivo. In brief, bone marrow cells were isolated from 4-month-old and 20-month-old male C57BL/6 mice and cultured at a density of 1 × 10^5 cells per well in a 96-well plate using α-modified minimal essential medium (α-MEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco). Following an initial incubation with 20 ng/ml macrophage colony-stimulating factor (M-CSF) (BioLegend, San Diego, CA) for 2 days, the adherent cells were designated as BMMs. These BMMs were then further stimulated with 50 ng/ml soluble receptor activator of nuclear factor kappa-B ligand (sRANKL) in the presence of 20 ng/ml M-CSF for varying durations to induce osteoclast differentiation.
DRG culture
For the isolation of DRG neurons, DRGs from the L2–L5 spinal levels were isolated in cold DMEM/F12 (Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12) medium (Invitrogen, Thermo Fisher Scientific, 11320033) and then digested with 1 mg/mL collagenase type A (Roche, Basel, Switzerland, 05349907103) at 37 °C. After trituration and centrifugation (200 × g), cells were resuspended and seeded on glass coverslips coated with ploy-D-lysine and laminin. The culture medium was replaced 6 h after seeding, and the adherent cells were further cultured at 37 °C with 5% CO2 for 3 days before use. The supernatant was collected following centrifugation at room temperature for 5 minutes at 200 × g.
The intracellular cAMP levels were measured using a cAMP enzyme immunoassay kit (Enzo Life Sciences, Farmingdale, NY) in accordance with the manufacturer’s instructions. The qualitative activity of PKA was assessed using PepTag non-radioactive protein kinase assays (Promega), following the manufacturer’s guidelines.
The compound treatment consisted of the Sema3A antagonist SM345431 (obtained from MCE), along with DDA and KT5720, also sourced from Sigma. The dosages and time courses are detailed in the accompanying text and figure legends.
Astral-DIA and bioinformatic analysis
The supernatant from DRG cell cultures was collected every 2 days using standard culture medium. The protein concentrations in the supernatants were concentrated using Amicon Ultra filters (10 kDa cut-off, UFC9010, Millipore, USA). Mobile phases A (100% water, 0.1% formic acid) and B (80% acetonitrile) were prepared, and the dried peptide samples were reconstituted in 0.1% formic acid before being centrifuged at 20,000× g for 10 minutes. Separation was performed using a Thermo Scientific Vanquish Neo UHPLC system. The samples were loaded onto an ES906 HPLC column (150 mm) and separated using an 8-minute gradient at a flow rate of 2.5 μL/min, with the following effective gradient: 0–4 minutes, mobile phase B increased linearly from 4% to 25%; 4–6.9 minutes, mobile phase B increased linearly from 25% to 35%; 6.9–7.3 minutes, mobile phase B increased linearly from 35% to 99%; and from 7.3 to 8.0 minutes, mobile phase B was maintained at 99%. The separated peptides were then transferred to a Thermo Astral mass spectrometer for acquisition in DIA mode. The main parameters were set as follows: normalized collision energy of 25%, default charge state of 2, resolution of 240,000, scanning frequency of every 0.6 seconds, scanning range of 380–980 m/z, and mass spectrometry AGC setting of 500%. Fragmentation ion scans were recorded with a maximum scan time of 3 ms, utilizing 300 2-Th scanning windows from 380 to 980 m/z. DIA-NN was employed to analyze the DIA data using a library-free approach. MS/MS data were searched against protein sequences downloaded from the Uniprot database with the following settings: enzyme: Trypsin/P; maximum missed cleavages: 2; fixed modification: carbamidomethyl (C); variable modifications: oxidation (M) and acetyl (N-terminus of protein); precursor mass tolerance: 20 ppm; fragment mass tolerance: 0.05 Da. The results were filtered at a 1% FDR, and only those protein groups that met this criterion were used for downstream analysis.
RNA sequencing
Total RNA was extracted from BMSCs isolated from 20-month-old male mice treated with PEMFs and from non-treated controls, using Trizol reagent (Thermofisher, 15596018) following the manufacturer’s procedure. The quantity and purity of the total RNA were assessed using the Bioanalyzer 2100 and the RNA 6000 Nano LabChip Kit (Agilent, CA, USA, 5067-1511). High-quality RNA samples with a RIN number greater than 7.0 were utilized for constructing the sequencing library. Complementary DNA (cDNA) libraries were prepared using the NEB Next® UltraTM RNA Library Prep Kit for Illumina® (NEB, USA) and subsequently sequenced on the Illumina NovaSeq 6000 platform. Raw reads were processed to remove low-quality reads and adapter sequences. The clean data were then mapped to the reference genome using Hisat2 v2.2.1. Differential expression analysis between the two groups was conducted using the DESeq2 R package (version 1.22.2). A gene was considered to be significantly differentially expressed if it exhibited a fold change of ≥ 2 and a qval of <0.05.
Quantitative real-time PCR
Total RNA for qPCR was extracted from cultured or sorted cells using the RNeasy Mini Kit (QIAGEN) following the manufacturer’s protocol. cDNA was synthesized from the purified RNA using random primers with the SuperScript First-Strand Synthesis System (Invitrogen). Subsequently, qRT-PCR analysis was performed using SYBR Green Master Mix (QIAGEN) in a thermal cycler, with two sets of primers designed for each target gene. Target gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (Gapdh) mRNA, and relative gene expression was determined using the 2 ΔΔCT method. The primers used for qRT-PCR were as follows: Mki67 (5′-ACCGTGGAGTAGTTTATCTGGG-3′ and 5′-TGTTTCCAGTCCGCTTACTTCT-3′); Cdkn2a (5′-GAAAGAGTTCGGGGCGTTG-3′ and 5′-GAGAGCCATCTGGAGCAGCAT-3′); Cdkn1a (5′- CCTGGTGATGTCCGACCTG-3′ and 5′-CCATGAGCGCATCGCAATC-3′); Pparg (5′-GTACTGTCGGTTTCAGAAGTGCC-3′ and 5′-ATCTCCGCCAACAGCTTCTCCT-3′); Alp (5′-CACGGCCATCCTATATGGTAA-3′ and 5′-GGGCCTGGTAGTTGTTGTGA-3′); Runx2 (5′-TCCACAAGGACAGAGTCAGATTACAG-3′ and 5′-CAGAAGTCAGAGGTGGCAGTGTCATC-−3′); Fabp4 (5′-AAGGTGAAGAGCATCATAACCCT-3′ and 5′-TCACGCCTTTCATAACACATTCC-−3′); Cebpa (5′-GCAAAGCCAAGAAGTCGGTGGA-3′ and 5′-CCTTCTGTTGCGTCTCCACGTT-−3′); Plxna1 (5′-GGGTGTGTGGATAGCCATCAG-3′ and 5′-GCCAACATATACCTCTCCTGTCT-−3′); Plxna4 (5′-ACAGGGCACATTTATTTGGGG-3′ and 5′-CACTTGGGGTTGTCCTCATCT-3′); Sema3a (5′-GGCTGGTTCACTGGGATTG-3′ and 5′-CCGTTTGCATAGTTTGCTCTGG-3′); Nrp1 (5′-GACAAATGTGGCGGGACCATA-3′ and 5′-TGGATTAGCCATTCACACTTCTC-3′); Nfatc1 (5′- GACTTCGATTTCCTCTTCGAGTTC −3′ and 5′- CTCGATTCTCGGACTCTCCAG −3′); Ctsk (5′- ATATGTGGGCCAGGATGAAAGTT −3′ and 5′- TCGTTCCCCACAGGAATCTCT −3′); Trap (5′- AAATCACTCTTTAAGACCAG −3′ and 5′- TTATTGAATAGCAGTGACAG −3′); Gapdh (5′-ACAACTTTGGCATTGTGGAA-3′ and 5′-GATGCAGGGATGATGTTCTG-3′).
ELISA and western blot testing
Sema3A concentrations in the serum and cell supernatant were determined by Sema3A ELISA kit ((Biomatik (EKU07258)) according to the manufacturer’s protocol. Mice serum and cell supernatants were collected. We also conducted a CTX ELISA on serum using a mouse kit (Immunodiagnostic Systems, AC06F1) and a P1NP enzyme immunoassay kit (Abbexa, abx258305). Western blot analysis was performed as previously reported21,56. In brief, cells were lysed in RIPA buffer and collected using a rubber policeman. Protein samples (20 µg) were loaded onto pre-cast electrophoresis gels (Bio-Rad) and separated by SDS-PAGE. The separated proteins were then transferred onto a PVDF membrane. The membrane was blocked with 5% nonfat milk and subsequently incubated with primary antibodies overnight at 4 °C, followed by incubation with secondary antibodies for 1 hour at room temperature. The blots were developed using the SuperSignal West Femto chemiluminescence kit (Thermo Scientific) and visualized by autoradiography. The primary antibodies used were as follows: anti-mouse Sema3A (Santa Cruz Biotechnology, sc-74555; 1:1000), and anti-GAPDH (Cell Signaling, 2118, 1:1000).
Statistical analysis
The data are expressed as mean ± SEM. These data are normally distributed; two-tailed Student’s t-test is used to compare between two groups. For analysis of the statistical significance of differences between more than two groups, we performed one-way or two-way analysis of variance (ANOVA) tests with Fisher’s LSD or with Tukey’s multiple comparison tests to assess statistical significance with a 95% confidence interval. The statistics is applied by GraphPad Prism10. Statistical differences were supposed to be significant when P < 0.05. All representative images of bones or cells were selected from a minimum of three independent experiments that yielded similar results, unless otherwise specified in the figure legend.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
RNA sequencing data have been deposited into the National Center for Biotechnology Information (NCBI) with accession codes PRJNA1293932 (https://www.ncbi.nlm.nih.gov/bioproject/1293932). Proteomics data have been deposited into Integrated Proteome Resources(iProX) with accession codes PXD066385 (https://www.iprox.cn/page/project.html?id=IPX0012689000). Source data generated in this study are provided in Figshare (https://doi.org/10.6084/m9.figshare.29623622). Source data are provided with this paper.
References
Buck, D. W. & Dumanian, G. A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 129, 1314–1320 (2012).
Siddiqui, J. A. & Partridge, N. C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 31, 233–245 (2016).
Kfoury, Y. & Scadden, D. T. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell 16, 239–253 (2015).
Bonyadi, M. et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl Acad. Sci. USA 100, 5840–5845 (2003).
Chen, W. G. et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 44, 3–16 (2021).
Lv, X., Gao, F. & Cao, X. Skeletal interoception in bone homeostasis and pain. Cell Metab. 34, 1914–1931 (2022).
Ding, Y., Arai, M., Kondo, H. & Togari, A. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone 46, 1591–1596 (2010).
Fukuda, T. et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 497, 490–493 (2013).
Wang, X. D. et al. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics 10, 4839–4850 (2020).
Wan, Q. Q. et al. Crosstalk between Bone and Nerves within Bone. Adv. Sci.8, 2003390 (2021).
Hu, B. et al. Sensory nerves regulate mesenchymal stromal cell lineage commitment by tuning sympathetic tones. J. Clin. Invest. 130, 3483–3498 (2020).
Offley, S. C. et al. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J. Bone Min. Res. 20, 257–267 (2005).
Tomlinson, R. E. et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl Acad. Sci. USA 114, E3632–e3641 (2017).
Chen, H. et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat. Commun. 10, 181 (2019).
Qiao, W. et al. Divalent metal cations stimulate skeleton interoception for new bone formation in mouse injury models. Nat. Commun. 13, 535 (2022).
Zhang, Y. et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 22, 1160–1169 (2016).
Liu, H. et al. Effects of water extract from epimedium on neuropeptide signaling in an ovariectomized osteoporosis rat model. J. Ethnopharmacol. 221, 126–136 (2018).
Zhu, S. et al. Effects of pulsed electromagnetic fields on postmenopausal osteoporosis. Bioelectromagnetics 38, 406–424 (2017).
Hao, X. et al. Bone deterioration in response to chronic high-altitude hypoxia is attenuated by a pulsed electromagnetic field via the primary cilium/HIF-1α axis. J. Bone Miner. Res. https://doi.org/10.1002/jbmr.4772 (2023).
Yan, Z. et al. High-specificity protection against radiation-induced bone loss by a pulsed electromagnetic field. Sci. Adv. 8, eabq0222 (2022).
Wang, T. et al. Pulsed electromagnetic fields attenuate glucocorticoid-induced bone loss by targeting senescent LepR(+) bone marrow mesenchymal stromal cells. Biomater. Adv. 133, 112635 (2022).
Wang, Q. et al. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone 154, 116211 (2022).
Wang, T. et al. Pulsed electromagnetic fields: promising treatment for osteoporosis. Osteoporos. Int. 30, 267–276 (2019).
Wang, T., Xie, W., Ye, W. & He, C. Effects of electromagnetic fields on osteoarthritis. Biomed. Pharmacother. 118, 109282 (2019).
Kar, N. S. et al. Pulsed-electromagnetic-field induced osteoblast differentiation requires activation of genes downstream of adenosine receptors A2A and A3. PLoS One 16, e0247659 (2021).
Canè, V., Botti, P. & Soana, S. Pulsed magnetic fields improve osteoblast activity during the repair of an experimental osseous defect. J. Orthop. Res. 11, 664–670 (1993).
Massari, L. et al. Biophysical stimulation of bone and cartilage: state of the art and future perspectives. Int. Orthop. 43, 539–551 (2019).
Midura, R. J. et al. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J. Orthop. Res. 23, 1035–1046 (2005).
Lohmann, C. H. et al. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J. Orthop. Res. 18, 637–646 (2000).
Zhou, J. et al. Effects of 50 Hz sinusoidal electromagnetic fields of different intensities on proliferation, differentiation and mineralization potentials of rat osteoblasts. Bone 49, 753–761 (2011).
Wang, Z., Clark, C. C. & Brighton, C. T. Up-regulation of bone morphogenetic proteins in cultured murine bone cells with use of specific electric fields. J. Bone Jt. Surg. Am. 88, 1053–1065 (2006).
Hartig, M., Joos, U. & Wiesmann, H. P. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur. Biophys. J. 29, 499–506 (2000).
Veronesi, F. et al. Pulsed electromagnetic fields and platelet rich plasma alone and combined for the treatment of wear-mediated periprosthetic osteolysis: An in vivo study. Acta Biomater. 77, 106–115 (2018).
Ongaro, A. et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. J. Cell Physiol. 227, 2461–2469 (2012).
Vincenzi, F. et al. Pulsed electromagnetic fields increased the anti-inflammatory effect of A₂A and A₃ adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 8, e65561 (2013).
Longo, F. M. et al. Electromagnetic fields influence NGF activity and levels following sciatic nerve transection. J. Neurosci. Res. 55, 230–237 (1999).
Kamel, D. M., Hamed, N. S., Abdel Raoof, N. A. & Tantawy, S. A. Pulsed magnetic field versus ultrasound in the treatment of postnatal carpal tunnel syndrome: A randomized controlled trial in the women of an Egyptian population. J. Adv. Res. 8, 45–53 (2017).
Tepper, O. M. et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 18, 1231–1233 (2004).
Xie, Y. et al. miR-196b-5p regulates osteoblast and osteoclast differentiation and bone homeostasis by targeting SEMA3A. J. Bone Min. Res. 38, 1175–1191 (2023).
Xu, K. et al. Selective promotion of sensory innervation-mediated immunoregulation for tissue repair. Sci. Adv. 11, eads9581 (2025).
Wang, T., Huang, S. & He, C. Senescent cells: A therapeutic target for osteoporosis. Cell Prolif. 55, e13323 (2022).
Termini, C. M. et al. Neuropilin 1 regulates bone marrow vascular regeneration and hematopoietic reconstitution. Nat. Commun. 12, 6990 (2021).
Liu, X. et al. Semaphorin 3A Shifts Adipose Mesenchymal Stem cells towards osteogenic phenotype and promotes bone regeneration in vivo. Stem Cells Int. 2016, 2545214 (2016).
Wang, Y. Y. et al. Pulsed electromagnetic fields promote bone formation by activating the sAC-cAMP-PKA-CREB signaling pathway. J. Cell Physiol. 234, 2807–2821 (2019).
Eberhard, D. et al. Semaphorin-3A regulates liver sinusoidal endothelial cell porosity and promotes hepatic steatosis. Nat. Cardiovasc. Res. 3, 734–753 (2024).
Tencerova, M. et al. Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell Rep. 27, 2050–2062.e2056 (2019).
Liu, H., Zhou, J., Gu, L. & Zuo, Y. The change of HCN1/HCN2 mRNA expression in peripheral nerve after chronic constriction injury induced neuropathy followed by pulsed electromagnetic field therapy. Oncotarget 8, 1110–1116 (2017).
Liu, H. F. et al. Pulsed electromagnetic fields on postmenopausal osteoporosis in Southwest China: a randomized, active-controlled clinical trial. Bioelectromagnetics 34, 323–332 (2013).
Qiao, Q. et al. Semaphorin 3A promotes osteogenic differentiation of BMSC from type 2 diabetes mellitus rats. J. Mol. Histol. 49, 369–376 (2018).
Yang, K., Miron, R. J., Bian, Z. & Zhang, Y. F. A bone-targeting drug-delivery system based on Semaphorin 3A gene therapy ameliorates bone loss in osteoporotic ovariectomized mice. Bone 114, 40–49 (2018).
Ma, Y. X. et al. Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration. Bioact. Mater. 9, 475–490 (2022).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Al-Azab, M., Safi, M., Idiiatullina, E., Al-Shaebi, F. & Zaky, M. Y. Aging of mesenchymal stem cell: machinery, markers, and strategies of fighting. Cell Mol. Biol. Lett. 27, 69 (2022).
Wong, P. F., Dharmani, M. & Ramasamy, T. S. Senotherapeutics for mesenchymal stem cell senescence and rejuvenation. Drug Discov. Today 28, 103424 (2023).
Wang, T. et al. Targeting cellular senescence prevents glucocorticoid-induced bone loss through modulation of the DPP4-GLP-1 axis. Signal Transduct. Target Ther. 6, 143 (2021).
Acknowledgements
Authors gratefully acknowledge the support of the National Natural Science Foundations of China (82102656 to T.W. and 82471388 to Z.H.); China Postdoctoral Science Foundation (2022T150452 to T.W., 2021M692299 to T.W.). Department of Science and Technology of Sichuan Province (2022NSFSC1392 to T.W.). The PostDoctor Research Project, West China Hospital, Sichuan University (2021HXBH021 to T.W.). Authors thank Xuanhe You (Orthopedic Research Institute of West China Hospital) for processing histological staining; thank Yaojia Zhou, Hongbin Wu (Experimental Animal Center of West China Hospital) for assistance in animal experiments; thank Yan Wang (Core Facilities of West China Hospital) for the help with flow cytometry measurement; thank Wenjuan Zeng (Institutes for Systems Genetics, West China Hospital) for the assistance with proteomic analysis. We thank AJE for editing this manuscript.
Author information
Authors and Affiliations
Contributions
T.W. and Z.H. designed the research, and T.W. wrote the manuscript. T.W., Z.L., C.W., J.C. and Y.M. performed the experiments and analyzed the data; S.C. helped with figure draw. T.W. and Z.H. proofread the manuscript; T.W., D.Z. and Z.H. supervised the experiments. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Junxuan Ma and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, T., Liang, Z., Wang, C. et al. Pulsed electromagnetic fields mediate sensory nerve regulation for bone formation in aging models. Nat Commun 16, 8223 (2025). https://doi.org/10.1038/s41467-025-63703-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63703-9