Abstract
Developing smart luminescent materials with off-on switchable responsiveness, which enables dark-to-bright state transitions in response to external stimuli, has garnered great interest across various domains. However, most of the reported systems with only monochromatic switching have limited their expansion toward advanced applications. Herein, a pressure-treated strategy is introduced to initially non-emissive isonicotinic acid (INA), achieving controllable switching from the dark state to high-quality multicolor emissions (i.e., blue, white, and yellow light). Experimental and theoretical analyses reveal that pressure-treated engineering can effectively modulate the intermolecular charge transfer strength within and between layers, enabling controlled emission color switching. Notably, the bright white light with CIE coordinates (0.31, 0.37) and the yellow light with CIE coordinates (0.42, 0.45) are retained under ambient conditions. The findings not only impart new vitality to nonemissive organic small molecules but also offer a new perspective for designing smart luminescent materials with multicolor and controllable properties.
Similar content being viewed by others
Introduction
Smart luminescent materials have recently attracted intense research interest due to their fundamental research significance and practical implications in diverse fields, such as information encryption, data security, and anti-counterfeiting technology1,2,3. Taking advantage of abundant structural diversity, flexible molecular design, and tunable optical properties, organic small molecules serve as ideal candidates for building smart luminescent materials, for which extensive studies have been reported4,5,6. The key factor for these smart responses is the response modes and resultant emission colors in response to external stimuli, including magnetic/electric fields, light, pressure, heat, etc7,8,9. Among them, the off-on switching mode can achieve a seamless transition from the dark state to the bright state, which is an ideal response mode for stimuli-responsive systems10,11. To improve the functionality and broaden the application range of materials, traditional smart luminescent materials with monochromatic switching characteristics are evolving toward the direction of achieving multiple yet controllable transitions12,13. However, the grafting of fluorophores via chemical tailoring requires tedious and time-consuming organic synthesis processes to endow the materials with switchability. Therefore, it remains a daunting challenge to develop an effective strategy for achieving free switching from dark to high-quality multicolor states within simple organic molecules.
Given that the molecular structure plays a pivotal role in determining their emission properties, manipulating structural configurations via external stimuli can endow materials with brand-new optical functional features14,15. Pressure, as a clean and effective tool, can modulate the crystal structure and optical properties of organic materials without altering their chemical composition16,17,18. In recent years, extensive research has been conducted on the precise regulation of intermolecular interactions, such as π–π stacking, Van der Waals forces, and hydrogen-bonding interactions, through pressure-treated engineering19,20,21. Meanwhile, a variety of novel phenomena have been observed, including piezochromism, pressure-induced blue-shifted emission, and pressure-induced enhanced emission22,23,24. Particularly, pressure-induced emission (PIE) affords a potential strategy to endow brand-new functional performance to initially non-emissive materials by triggering photoluminescence (PL)25,26,27. The present dilemma exists in high-performance PIE materials, typically reverting to their non-emissive state at ambient conditions due to high-pressure reversibility. More regrettably, research on multicolor PL interception within organic systems has not yet been conducted. Realizing efficient multicolor emissions in organic small molecules that initially lack luminescent properties remains one of the most challenging and high-value problems in this field.
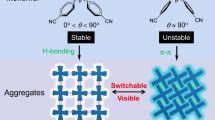
The ability to generate multiple emissive centers is a key task in obtaining multicolor emissive materials28,29,30. To realize the above protocols, many single-component strategies have been developed to construct dual emissions, such as dual fluorescence from two singlet states, fluorescence and phosphorescence, and dual phosphorescence with anti-Kasha’s behavior31,32. Among them, integrating two fluorescence into organic small molecule materials via dual charge transfer (CT) states is a highly promising strategy, but remains a bottleneck problem33,34,35. By introducing hydrogen bonding-assisted or π–π stacking-assisted weak interactions, the intermolecular CT affinity can be strategically enhanced, thereby effectively regulating the emission properties of materials (Fig. 1a, b)36,37,38,39,40. This further inspired us to explore the feasibility of achieving dual CT emissions from two singlet states by introducing hydrogen bonding-assisted intermolecular CT (H-CT) and π–π stacking-assisted intermolecular CT (π-CT).
a Schematic diagram of hydrogen bonding-assisted intermolecular charge transfer (H-CT). b Schematic diagram of π–π stacking-assisted intermolecular charge transfer (π-CT). c Proposed dual CT emissions from two singlet states in organic small molecules. Figure 1 is created using Microsoft PowerPoint.
In this work, we select the initially non-emissive isonicotinic acid (INA) as a paradigm to achieve pressure-controlled switching from a dark state to high-quality multicolor emissions (i.e., blue, white, and yellow light). The controllable switching of emission colors is greatly related to the regulation of intralayer hydrogen bonds and interlayer π–π stacking under different pressure treatments, thereby affecting the intermolecular dual CT intensity (Fig. 1c). More importantly, the bright white light with CIE coordinates of (0.31, 0.37) and the yellow light with CIE coordinates of (0.42, 0.45) are retained under ambient conditions. Furthermore, a prototype of white and yellow emitters based on pressure-treated samples is fabricated with excellent thermal, chromaticity, and fatigue stability, indicating its excellent potential for daylight illumination and display.
Results and discussion
Molecular structure and PL stimuli-responsive behaviors of INA
INA belongs to a triclinic crystal structure (space group P\(\bar{1}\)), with lattice parameters a = 6.410 Å, b = 7.234 Å, c = 7.503 Å, α = 103.415°, β = 114.664°, and γ = 105.715° at ambient conditions (Supplementary Fig. 1). Figure 2a presents the crystal structures with a unit cell dimension of 3 × 3 × 1, where one layer in the crystal layers is further extracted to investigate intermolecular interactions. Each INA molecule is confined by neighboring molecules with multiple intermolecular interactions, forming ordered multilayer molecule structures. The planar INA molecules are connected by hydrogen bonds to form a layered structure, while the main interaction between the different layers is π–π stacking. Intriguingly, the carboxyl group of the INA molecule is in the para-position of the nitrogen atom, resulting in an identical molecular orientation. Thus, the INA molecules within the plane are arranged linearly. We collected the UV-Vis absorption spectra of the INA at ambient conditions (Supplementary Fig. 2). The sample shows a strong absorption band at 285 nm, which is mainly attributed to the (π, π*) transition of the conjugated π-electrons in the pyridine ring41. However, the tiny oscillator strength of the small chromophore leads to non-emissive behavior in INA under 355 nm laser excitation (Supplementary Fig. 3)42. Considering the substantial presence of non-bonding interactions in the INA, PL turn-on behavior might be expected from structural modulation under pressure19,26.
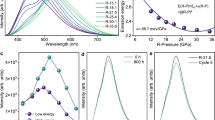
a Crystal structure of INA in the unit cell dimension of 3 × 3 × 1. The linear packing modes with tight dual hydrogen bonds of O − H ∙ ∙ ∙ N and C − H ∙ ∙ ∙ O are observed in the INA molecular array. The pink, blue, purple, and cyan spheres represent O, C, N, and H atoms, respectively. b A 3D colormap showing the pressure-dependent PL intensity and wavelength of INA. c Pressure-dependent PL intensity of INA. d PL spectra of INA before and after different pressure treatments. e Optical images of INA at 1 atm, 10.4 GPa, and after different pressure treatments. f The CIE chromaticity diagram of INA at 10.4 GPa and after different pressure treatments. g Normalized PL decay curves at R-20.0 GPa. Source data are provided as a Source Data file.
Experimental validation of the hypothesis was carried out through in situ high-pressure PL experiments using a symmetric diamond anvil cell (DAC), as shown in Supplementary Fig. 4. As expected, the emergence of a remarkable PIE was observed upon compression of the initial nonemission INA (Fig. 2b). With increasing pressure, PL intensity gradually enhanced and reached its maximum value at 10.4 GPa, followed by a progressive weakening. Compared to the initial state, the corresponding PL emission intensity exhibited a noticeable 246-fold enhancement at 10.4 GPa (Fig. 2c). The strong PL emission peak of INA at the released pressure state was centered around 525 nm, which exhibits a broader emission in the range of 340–820 nm (Fig. 2d and Supplementary Fig. 5). After conducting peak fitting analysis, two distinct emission peaks were identified at 468 and 557 nm, with the long-wavelength component being dominant (Supplementary Fig. 6). Notably, the wavelength exhibited a red shift from 525 nm to 590 nm upon complete release of pressure from 30.5 GPa. We named the samples released from 20.0 GPa and 30.5 GPa to ambient conditions as R-20.0 GPa and R-30.5 GPa, respectively, without needing to describe them individually in subsequent occurrences of the same situation. Optical micrographs revealed the trend of PL intensity in INA during compression, accompanied by a color change from non-emissive at 1 atm to brightening blue emission at 10.4 GPa (Fig. 2e and Supplementary Fig. 4c). As shown in Fig. 2f, the INA at R-20.0 GPa emitted bright a white emission with the CIE chromaticity coordinates of (0.31, 0.37) and the CCT of 6297 K. Meanwhile, the application of higher-pressure treatment allowed us to precisely modulate the emission from white light to bright yellow light, achieving chromaticity coordinates of (0.42, 0.45) and a CCT of 3597 K. To investigate the effect of sample morphology on the optical properties under high pressure, we further studied the in situ high-pressure optical properties of single-crystal samples (Supplementary Fig. 7). The PL experimental results showed that the single-crystal sample displayed an obvious PIE behavior under pressure. After different pressure treatments, the bright white and yellow emissions could also be observed at ambient conditions, consistent with the observations in powder samples. Compared to the initial PL quantum yield (PLQY) of merely 0.3%, the PLQYs for white and yellow light were measured to be approximately 25.0% and 16.8%, respectively (Supplementary Fig. 8). In addition, we also estimated the PLQY upon complete release of pressure at 20.0 GPa (Supplementary Table 1), which was close to the experimental measurement results. Notably, the PL intensities remained stable even up to five weeks after the complete release of different pressures to ambient conditions (Supplementary Fig. 9). To verify the origin of the two emission peaks after pressure treatment, we conducted time-resolved PL (TRPL) spectra (Fig. 2g). The INA lifetimes at different peaks all exhibited ns-level, implying that these emission peaks originate from singlet excited states with a fluorescent nature.
Structural evolution of INA under pressure
To gain a deeper insight into the relationship between the optical properties and structure of INA, we conducted in situ single-crystal X-ray diffraction (XRD) experiments under high pressure. With increasing pressure, all Bragg diffraction spots gradually shifted toward higher diffraction angles due to pressure-induced lattice contraction (Supplementary Fig. 10). When the applied pressure exceeded 10.4 GPa, the spots gradually broadened, and diffuse diffraction halos appeared. Meanwhile, no new peaks emerged during the entire compression process (Fig. 3a and Supplementary Fig. 11a). As shown in Fig. 3b and Supplementary Fig. 11b, the INA exhibited obvious anisotropic compression under pressure, with the b-axis demonstrating a higher compression rate compared to the a and c-axes. The continuous compression of the b-axis demonstrated that the interlayer molecular distance is gradually decreasing, which may lead to the gradual enhancement of π–π interactions43,44. The Rietveld analysis results for representative XRD data are shown in Supplementary Fig. 12 and Table 2. By fitting the third-order Birch−Murnaghan equation of state, the value of bulk modulus B0 in INA is determined to be 22.5(3) GPa with the derivative B′ = 7.6(4) (Fig. 3c). During the decompression process, the INA remained in a polycrystalline state (Supplementary Fig. 13). Upon complete release of the pressure from 20.0 GPa, most of the diffraction spots broadened and formed several discontinuous diffraction rings in the scattering pattern, indicating that the structural change was irreversible. Compared with the initial state, the main diffraction peaks were significantly broadened and weakened (Supplementary Fig. 14a). Notably, the relative intensities of the (011), (1-11), (002), and (21-1) diffraction peaks increased due to changes in the lattice constants and molecular geometry after pressure treatment45. Compared to 1 atm, we observed that the diffraction peak of the (020) lattice plane after pressure treatment was located at a higher angle (Supplementary Fig. 14b). Given that the orientation of the (020) lattice plane is nearly perpendicular to the direction of interlayer π–π stacking, variations in the diffraction angles are closely associated with the interlayer π–π stacking distance (Supplementary Fig. 14c). Consequently, the shift of the (020) plane confirms the enhancement of interlayer π–π stacking after pressure treatment. The pressure-volume (P-V) data were fitted by the Birch–Murnaghan equation of state, and the continuous volume collapse indicated that no phase transition occurred during the decompression process (Supplementary Fig. 14d).
a Changes in Bragg diffraction peaks as a function of pressure. b Compressibility indicatrix in the abc direction of INA. c Cell volume evolutions of INA upon compression. d Band gaps of INA during the compression process. e Comparison of the absorption spectra at 1 atm and after different pressure treatments. f Pressure dependence of IR shifts for selected peaks. g Representative Raman spectra of the INA between 50 and 250 cm−1 at 1 atm and R-20.0 GPa. h Comparison of PL decay curves at different pressures. i Pressure-dependent changes of radiative and non-radiative rates. Source data are provided as a Source Data file.
To elucidate the origin of the observed prominent PIE in the INA, we measured pressure-dependent UV-Vis absorption spectra using a symmetric DAC apparatus (Supplementary Fig. 15a, b). The emission spectra displayed significant changes upon compression, whereas the absorption spectra showed a continuous redshift with increasing pressure. As shown in Supplementary Fig. 15c, we used the direct bandgap Tauc plots to determine the bandgap values27,46. The band gap decreased at a rate of -39.28 meV/GPa during the compression process (Fig. 3d). However, the band gap recovered at a relatively slow rate during the decompression process (Supplementary Fig. 15d), indicating that this process is irreversible. When different pressures were fully released to ambient conditions, the CT absorption band tails were observed in the range of 310–450 nm (Fig. 3e). Compared to the initial state, the observed new CT absorption band tails after different pressure treatments confirmed a higher possibility of transition for the intermolecular CT components47,48.
To study the changes of intermolecular interactions in detail, especially the interactions of hydrogen bonds, we performed in situ high-pressure IR measurements on INA (Supplementary Figs. 16, 17). The IR absorption peaks at 702 cm−1 and 3215 cm−1 could be assigned to the carboxyl group out-of-plane bending vibration π(COOH) and the O − H stretching vibration ν(O − H), respectively49. These two vibration peaks remained essentially unchanged below 10.4 GPa but then exhibited a continuous blue shift (Fig. 3f). The stretching vibration frequency of ν(O − H) remained constant below 10.4 GPa, which was reflected in the unchanged O − H bond length. When the interatomic distances were shortened by compression, the length of hydrogen bonds decreased. It indicated that the O − H···N interactions were enhanced with increasing pressure. The strengthened hydrogen bond restricted the π(COOH), therefore suppressing the non-radiative vibrational process. As a result, the reduction of non-radiative transition losses has promoted emission enhancement within 10.4 GPa. Upon further compression, the O–H bond was shortened and the vibrational frequency increased, leading to an increase in the non-radiative process and fluorescence quenching. As shown in Supplementary Fig. 18, the peak shifts of the relevant chemical bonds affected by hydrogen bond interactions upon compression were irreversible during the decompression process. There were obvious redshifts in the IR vibrational peaks upon decompression compared to those in the compressed state at the same pressure (Supplementary Fig. 19e, f). Namely, the hydrogen bonds formed upon decompression were stronger than those formed during the compression process at the same pressure. After different pressure treatments, the ν(O − H) and ν(C − H) (C − H stretching vibration at 3054 cm−1) exhibited different redshifts compared to their initial states (Supplementary Fig. 20a)49. This further suggests that the pressure treatment enhances the intermolecular O − H···N strong hydrogen bonds and C − H···O weak hydrogen bonds. Notably, the π(C − H) (C − H out-of-plane bending vibration at 890 cm−1) exhibited a consistent redshift compared to the initial state, indicating that the enhanced interlayer π–π stacking after pressure treatment restricts its vibration (Supplementary Fig. 20b)50. We also found that ν(C − N) (C − N stretching vibration at 1028 cm−1) and τ(COO) (COO torsional vibration at 1308 cm−1) showed redshifts of 4 and 8 cm−1 compared to the initial state, respectively50. Furthermore, the ν(C − O) (C − O stretching vibration at 1338 cm−1) and ν(C = O) (C = O stretching vibration at 1617 cm−1) showed redshifts of 11 and 3 cm−1 relative to their initial states49,51. These observed redshifts demonstrated that the vibration freedom of corresponding bonds is constrained, attributed to the stronger intermolecular hydrogen bond interactions after pressure treatment.
Subsequently, we further conducted in situ high-pressure Raman scattering measurements to monitor responses of INA to high pressure (Supplementary Figs. 21, 22). As shown in Supplementary Fig. 21c, the Raman peak centers at 659 cm−1 can be ascribed to pyridine ring torsion vibrations τ(cccc)50. Notably, the τ(cccc) mode split into two peaks after the external pressure exceeded 10.4 GPa. It is generally considered that the further enhancement of interlayer ring-ring interactions can be interpreted as evidence for the subsequent PL quenching52. During the decompression process, the peaks located at 667 cm−1 and 680 cm−1 gradually merged into a single peak (Supplementary Fig. 22b). Specifically, the π–π interaction weakened upon decompression, leading to a gradual increase in PL intensity. Furthermore, some external modes disappeared, and the internal modes broadened upon compression. During the decompression process, the previously disappeared external modes did not recover, and the hysteresis effect could be observed (Supplementary Fig. 22c). When the pressure was completely released to ambient conditions, the Raman spectra did not return to their initial state, implying that the pressure-treated process is irreversible (Fig. 3g and Supplementary Fig. 23).
To get more insight into the emission features of INA, TRPL spectra at different pressures were recorded. All decay curves of the TRPL plots are fitted using a double exponential function, and the corresponding average lifetime values are summarized in Supplementary Table 4. At ambient conditions, the INA exhibits a short PL lifetime of 0.4 ns. As shown in Fig. 3h and Supplementary Fig. 24, the variations in lifetime under high pressure coincided with the observed changes in PL intensity. At 10.4 GPa, the average lifetimes reached 5.1 ns, which is ~12.8 times higher than that at ambient conditions. In addition, the recombination rates were calculated as the quantitative standard of the transition process by combining the PLQYs and average PL lifetimes under high pressure (refer to the Supporting Information for detailed methods regarding this)53,54. Notably, the radiative decay rate (kr) increased by a factor of 6 from 0.0075 ns−1 at 1 atm to 0.0478 ns−1 at 10.4 GPa, while the nonradiative decay rate (knr) was almost completely suppressed at 10.4 GPa (Fig. 3i). These observed changes directly demonstrated the promotion of radiative transitions and the suppression of nonradiative transitions, which are considered the direct factors contributing to the bright blue-light emission in initially nonemissive INA. Above 10.4 GPa, however, the kr experienced a notable decrease while the knr increased vigorously, resulting in a decline in PL with further compression. After pressure treatment, kr increased to 0.1914 ns−1 from an initial value of 0.0075 ns−1, while knr decreased to 0.5229 ns−1 from an initial value of 2.4925 ns−1 (Supplementary Fig. 25b). Based on the above discussion, the enhanced emission after pressure treatment can be explained by the significantly increased radiative recombination rate as well as the suppressed nonradiative loss.
Emission mechanism of INA after pressure treatment
We characterized the structural variations of INA based on the Rietveld refinements using the General Structure Analysis System (GSAS) software to explore the effect of intermolecular interaction on emission. As depicted in Fig. 4a, the interlayer molecules of the INA tetramer are arranged in a head-to-tail fashion to form slipped π-stacks, with the π–π interaction (dπ–π) about 3.999 Å at ambient conditions. Compared to the initial value, the interlayer π–π distance decreased to 3.612 Å after pressure treatment. These short contacts contribute to interlayer molecular coupling, thereby facilitating effective electron delocalization and overlap between molecules55. Furthermore, the O–H···N and C–H···O = C distances approached 1.428 and 2.501 Å after the pressure was completely released, respectively, which were significantly smaller than the distances of 1.571 and 2.645 Å at ambient conditions. The enhancement of hydrogen bonds between neighboring molecules in the same layer would promote the delocalization of the molecular orbitals within the layer, which reduces the excitation energy56. Accordingly, the irreversible lattice contraction after pressure treatment markedly modulated the intralayer hydrogen bonds and interlayer π–π stacking interactions, thereby exerting a significant influence on the PL properties of INA.
a The intralayer hydrogen bonds (dotted blue lines) and the distances of interlayer π–π interactions (dotted red lines) in the selected INA tetramer at 1 atm and R-20.0 GPa. b The UV–Vis absorption spectra and oscillator strengths of INA at 1 atm and R-20.0 GPa, calculated using the B3LYP/6-311 G(d,p) method with the Gaussian 09 program. c PL mechanism schematic illustration of INA before and after pressure treatment (Ex.: excitation). The calculated emission energies of the INA tetramer at the TD-DFT/B3LYP/6-311 G (d,p) level. d Hydrogen bond binding energy of INA. Source data are provided as a Source Data file.
To further elucidate the PL mechanism, we employed density functional theory (DFT) and time-dependent DFT (TD-DFT) methods at the B3LYP/6-311 G(d,p) level to study the electronic structure of INA and qualitatively analyze the PL origin. The optimized molecular geometry and spatial distributions of the molecular orbitals are presented in Supplementary Fig. 26 and Table 5. At ambient conditions, the electron density distribution of the highest occupied molecular orbital (HOMO) was primarily distributed on the nitrogen atom of the pyridine ring and the oxygen atom of the carbonyl. Whereas, the lowest unoccupied molecular orbital electron density (LUMO) was dominated by the entire INA. At this point, the intermolecular n–π* transition in a layer was the predominant charge transfer mode (accounting for 81.4%). The calculation results, however, indicate that the S1 state belonged to a forbidden electron transition with an oscillation intensity (f) value of 0.0000. This is why no absorption peak was detected in the UV–vis absorption spectra, and there was no emission at ambient conditions when excited with 355 nm. Compared with the initial value, the oscillator strength at 10.4 GPa was observed to be 0.0079, significantly increasing the radiative decay rate (Supplementary Fig. 27). It is suggested that the pressure-induced enhancement of hydrogen bonds can effectively break the forbidden intermolecular (n, π*) CT transition to light up the emission. After the complete release of the pressure, the S1 state was still dominated by the HOMO → LUMO transition. However, the S1 transition configuration of pressure-treated INA was different from that of the initial state, indicating that a new charge transfer channel has been formed after the phase transition. Electronic transition at this time was mainly assigned to the interlayer intermolecular n–π* transition, with the proportions of the transition process of 99.8%. As shown in Fig. 4b, the calculated pressure-treated absorption spectrum exhibited a subtle absorption band tail at 3.63 eV, which perfectly aligned with the experimental absorption spectrum. In addition, the molecular transition of S2 (H-2 → L, 99.6%) after the pressure treatment was much closer to that of S1 (H → L, 81.4%) at 1 atm, demonstrating that the intermolecular charge transfer in a layer still existed. For the INA tetramer, the S1 (3.63 eV) and S2 (3.88 eV) energies after pressure treatment were both higher than S1 (4.11 eV) at 1 atm. It is implied that the enhanced π–π interaction and hydrogen bond would promote the efficient intermolecular charge transfer between and within layers57.
Our speculation regarding the unique dual fluorescence emissions in the INA after pressure treatment was further verified by theoretical calculations (Supplementary Fig. 28 and Table 6). The emission wavelength depends on the inter/intralayer CT intensity. Notably, the H-CT emission mainly took place on the intermolecular (n, π*) transition within a monolayer of the INA tetramer, almost the same as that of the single-layer dimer from S1 → S0 (Supplementary Fig. 29)58,59. Meanwhile, the π-CT emission predominantly occurred on the intermolecular (n, π*) transitions between layers. As shown in Fig. 4c, the calculated H-CT and π-CT energies of INA after pressure treatment were 3.10 eV (400 nm) and 2.28 eV (544 nm), respectively, which correspond well with the dual fluorescence peaks at 468 nm and 557 nm at ambient conditions. To quantify the hydrogen bond strength, the hydrogen bond binding energies of INA were calculated before and after pressure treatment (Fig. 4d). The total energy of the structure increased by 0.35 eV after pressure treatment compared to the initial state, indicating that the pressure-treated structure is in a metastable state. When the pressure was completely released to ambient conditions, the hydrogen bond binding energy was 0.39 eV. Theoretical calculations revealed that the R-20.0 GPa sample needs to overcome an energy barrier of 0.39 eV to recover to its initial state, which is attributed to the enhanced hydrogen bonds. With the above considerations, the intermolecular CT within and between layers can be effectively optimized through pressure treatment, thereby achieving a strong white-light emission in initially non-emissive INA.
Optical applications of pressure-treated INA
We fabricated a series of phosphor-converted light-emitting diodes (pc-LEDs) to explore the applicability and stability of pressure-treated samples (Supplementary Fig. 30). Within the considered current range, white and yellow pc-LEDs were fabricated using individual R-20.0 GPa and R-30.5 GPa phosphors with CIE chromaticity coordinates of (0.31, 0.41) and (0.41, 0.47), respectively (Fig. 5a, b). Stability is an important performance of pc-LED devices. After nine cycles of heating/cooling between 298 and 393 K, the emission intensity of the white and yellow pc-LEDs remained at 93.1% and 90.9% of their original values, respectively (Fig. 5c and Supplementary Fig. 31a). This indicates that the pressure-treated samples exhibit higher thermal stability and reversibility. As shown in Fig. 5d and Supplementary Fig. 31b, we further tested the chromaticity stability of pc-LEDs using a low voltage (3 V) and different operating currents. The emission spectra of the white and yellow pc-LEDs presented increasing PL intensity under various input currents, demonstrating exceptional color stability (Supplementary Fig. 31c, d). Importantly, the white and yellow pc-LEDs exhibited excellent fatigue stability with slight changes in emission intensity over 100 h at ambient conditions (Fig. 5e and Supplementary Fig. 31e). As a demonstration, an operating white pc-LED explicitly illuminated three multicolor dolls, as shown in Fig. 5f.
a The emission spectra of operating devices by covering the different pressure-treated samples on a 365 nm near-UV chip, inset: photograph of operating white and yellow pc-LEDs. b The CIE chromaticity coordinates of the fabricated white and yellow pc-LEDs. c Heating-cooling cyclic stability of the white pc-LED in the range of 298-393 K. d PL spectra of the white pc-LED at different operating currents. e Integrated PL intensity of the white pc-LED as a function of aging times. The inset shows visual time-quenching behavior. f Photograph of three multicolor dolls under an operating white pc-LED. The device is operated at a voltage of 3.0 V (50 mA). Source data are provided as a Source Data file.
In summary, we successfully demonstrated a pressure-treated strategy for achieving controllable switching from the dark state to high-quality multicolor emissions (i.e., blue, white, and yellow light) in nonemissive INA. Systematically photophysical characterization results revealed that the pressure-induced enhancement of hydrogen bonds broke the previously forbidden intermolecular CT transition, thus triggering the PL turn-on behavior. After different pressure treatments, we obtained the bright white light with CIE coordinates of (0.31, 0.37) and the yellow light with CIE coordinates of (0.42, 0.45) at ambient conditions. The white-light emission was attributed to the cooperative effect between the interlayer CT emission assisted by π–π interactions and the intralayer CT emission assisted by hydrogen bonds, as revealed by experimental and theoretical investigations. Note that the significant redshift in emission to yellow light can be attributed to the enhanced π–π stacking, which results from the reduced interlayer distance after further pressure treatment. Based on these pressure-treated samples, the white and yellow pc-LEDs were successfully fabricated to demonstrate their applicability in daylight illumination and display. This discovery may not only deepen the understanding of organic small molecules with non-emissive properties but also open up a new path for the design of smart luminescent materials.
Methods
Characterization and high-pressure generation
INA, also known as pyridine-4-carboxylic acid, has the chemical formula C6H5NO2. The initial structural model was obtained from the Cambridge Crystallographic Data Centre (CCDC#2068819). Refinements of powder XRD patterns were accomplished using the GSAS software. We purchased from Damas-beta with a purity of 99% and used it directly without purification. The symmetric DAC employed a pair of type IIa ultralow fluorescence diamonds with a culet size of 400 μm to generate high pressure. The sample was loaded into a 150 μm diameter hole of the pre-indented T301 steel gasket, with a thickness of approximately 45 μm. Silicone oil with a viscosity of 10 cst was utilized as the pressure-transmitting medium (PTM) for high-pressure single-crystal XRD, UV-Vis absorption, and PL experiments, while KBr was employed as the PTM for IR measurements. These PTMs did not have any detectable effect on the behavior of INA under pressure. The sample and a small ruby ball were placed in the DAC chamber, and the pressure calibration was performed using the R1 fluorescence spectrum of the ruby60. The measurement accuracy of the present pressure calibration is ±0.1 GPa. The pressure gradient is below 1 GPa through the whole pressure range.
In situ high-pressure measurements
All the high-pressure experiments were conducted using a symmetric DAC apparatus at room temperature. The parameters were carefully maintained at a constant level throughout each high-pressure PL experiment to minimize the potential impact of varying excitation laser intensities and luminous fluxes on the resulting PL intensity of the intrinsic sample.
The in situ high-pressure UV-Vis absorption spectra were measured using a deuterium-halogen light source, while the PL measurements were conducted with a semiconductor laser operating at an excitation wavelength of 355 nm. The optical fiber spectrometer employed was the Ocean Optics QE65000 spectrometer. In situ PL and absorption images of the samples were captured using a Canon EOS 5D Mark II camera mounted on a Nikon Eclipse TI-U microscope. The TRPL experiments in the diamond anvil cell were conducted using a custom-built setup equipped with a 375 nm pulsed diode laser (LDH-P-C-375B, 40 ps). In situ high-pressure IR spectroscopy was performed on a Nicolet iN10 microscope spectrometer (Thermo Fisher Scientific, USA), employing a nitrogen-cooled mercury-cadmium-telluride (MCT) detector. Raman spectroscopy measurements were carried out using a Raman spectrometer (iHR 550, Symphony II, Horiba Jobin Yvon) with an excitation laser of 785 nm and a power output of 0.5 mW.
The in situ single-crystal XRD experiments under high pressure were conducted at beamline 17UM of the Shanghai Synchrotron Radiation Facility. The wavelength of the monochromated X-ray beam was 0.48337 Å (25.65 keV). The sample-to-detector distance and other detector parameters were calibrated using the CeO2 standard. The diffraction images were integrated and analyzed using the Dioptas program.
Calculation of PLQYs under high pressure
For the PLQYs under high pressures, we refer to the previously reported method to estimate53,54. By using the PLQY at ambient pressure as a reference, the PLQY can be calculated using the following formula61,62:
where Φ is the PLQY, ∫F(λem) is the integrated intensity of emission, A(λex) is the percentage of light absorbed at excitation wavelength, n is the refractive index, and the subscript 0 denotes the reference data at ambient pressure. Φ0 is the PLQY at ambient conditions of 0.3%, which is measured using a Hamamatsu multichannel analyzer c10027. The correction factor (n2/n02) is reckoned using the Clausius-Mossotti equation and the Lorentz-Lorenz equation based on the cell volume under pressure63,64:
in which the density ρ can be calculated from the cell volume. And the cell volume is acquired from the powder XRD data by using the Materials Studio program. RLL is called the Lorentz-Lorenz constant. RLL is related to polarizability α. The refractive index n at ambient pressure is 1.5423, which is determined by thin film interference. Based on the above estimation formula, we give the related parameters and calculated results of the PLQYs under high pressures as shown in Supplementary Table 1 below.
Calculation of k r and k nr under high pressure
Based on the PLQY (Φ) and lifetime (τ) measured under high pressure, the radiative and non-radiative recombination rates at different pressure points can be calculated using the following equations46:
in which Φ stands for PLQY, kr is the radiative carrier recombination rate, and knr is the nonradiative carrier recombination rate, τ stands for an average lifetime.
The analysis of in situ high-pressure XRD data
Fitting the P-V data obtained in the experiment to the third-order Birch-Murnaghan equation of state65:
where V0 and V are the volume at ambient and high pressure, respectively; B0 is the bulk modulus at ambient pressure, and B′ is the derivative of the bulk modulus for pressure.
The analysis of the PL decay curve
All PL decay curves obtained at different pressures were fitted using the double exponential function:
Thus, the average lifetime τ was calculated by the following equation:
Experimental observation of the bandgap under high pressure
Theoretical calculations indicate that INA exhibits a direct band gap at ambient conditions. Accordingly, the band gaps were determined by extrapolating the linear portion of the (αhν)2 versus hν in Tauc plots, where α denotes the absorption coefficient and hν represents the photon energy.
The fabrication process of multicolor pc-LED devices
The pressure-treated samples were mixed with epoxy resin glue at a ratio of 10 wt.%, and manually stirred using a stainless-steel rod for 3 min until completely uniform. These powders didn’t dissolve during fabrication and were embedded into the glue. Subsequently, the mixture was coated onto the surface of the commercial 365 nm near-UV chip until the microcavity was filled. Finally, the prepared pc-LEDs were cured in an oven at a temperature of 35 °C. These chips, with a power of 1 W and an emission maximum of 365 nm, were purchased from Shenzhen Looking Long Technology Co., Ltd. (Shenzhen, China). The performance of the prepared multi-color pc-LED devices was recorded using an automatic-temperature LED optoelectronic analyzer equipped with a temperature controller (ATA-500, EVERFINE, China).
Collection of pressure-treated phosphors by a Paris-Edinburgh press
To ensure the quantity of samples, high-pressure experiments of INA were conducted on the Paris-Edinburgh (PE) press to collect large-volume samples with different pressure treatments. The R-20.0 and R-30.5 samples were synthesized under high pressure using a VX3 PE press with double toroidal sintered diamond anvils, and the sample volume was 16.8 mm3. The synthesis of all samples was carried out using non-encapsulated T304 stainless steel gaskets. The system was driven by an automatic hydraulic oil syringe pump, and the pressure was estimated based on the Edinburgh group’s calibration curve66. The target oil pressures for 20 GPa and 30 GPa were 960 bar and 1400 bar, respectively. The rates of the compression and decompression were as follows: 10 bar/min below 100 bar, 0.5 bar/min from 100 to 400 bar, 0.2 bar/min from 400 to 800 bar, 0.1 bar/min from 800 to 1000 bar, and 0.05 bar/min from 1000 to 1600 bar. After releasing the pressure, the pressure-treated pellets were obtained after removing the surrounding gasket.
Theoretical calculations
In this work, all calculations were performed with the Gaussian 09 software package. The ground state (S0) geometry was optimized with the functional of B3LYP and 6–311 G(d,p) basis sets. The excited state energies were calculated using the TD-DFT method based on the optimized geometry.
Data availability
The authors declare that the main data supporting our findings of this study are contained within the paper and Supplementary Information. All other relevant data are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
Gaussian 09 code is available for download on the developer page: https://gaussian.com/.
References
MacKenzie, L. E. & Pal, R. Circularly polarized lanthanide luminescence for advanced security inks. Nat. Rev. Chem. 5, 109–124 (2020).
Zhang, C. et al. Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption. Nat. Commun. 8, 1138–1147 (2017).
Kim, J. H. et al. Nanoscale physical unclonable function labels based on block copolymer self-assembly. Nat. Electron. 5, 433–442 (2022).
Zhao, W. et al. Achieving single-molecule-based multi-color emissions by the regulations of intramolecular proton transfer and interphase transformation. Dyes Pigm 210, 111026 (2023).
Wei, P. et al. Multiple yet controllable photoswitching in a single AIEgen system. J. Am. Chem. Soc. 140, 1966–1975 (2018).
Shao, Y. et al. Unlocking multicolor emissions in the crystalline state through dimerization and configurational transformation of a single fluorophore. Chem. Mater. 36, 3223–3232 (2024).
Hu, S. et al. Lighting up nonemissive azobenzene derivatives by pressure. J. Am. Chem. Soc. 146, 28961–28972 (2024).
Woodward, A. N. et al. Thiazolothiazole fluorophores exhibiting strong fluorescence and viologen-like reversible electrochromism. J. Am. Chem. Soc. 139, 8467–8473 (2017).
Liu, X., Liao, Q., Yang, J., Li, Z. & Li, Q. Unveiling one-to-one correspondence between excited triplet states and determinate interactions by temperature-controllable blue-green-yellow afterglow. Angew. Chem. Int. Ed. 62, e202302792 (2023).
Li, Z. et al. Loading photochromic molecules into a luminescent metal–organic framework for information anticounterfeiting. Angew. Chem. Int. Ed. 58, 18025–18031 (2019).
Du, J. et al. Printable off–on thermoswitchable fluorescent materials for programmable thermally controlled full-color displays and multiple encryption. Adv. Mater. 33, 2008055 (2021).
Tang, Y. et al. Dynamic multicolor emissions of multimodal phosphors by Mn2+ trace doping in self-activated CaGa4O7. Nat. Commun. 15, 3209–3224 (2024).
Shi, Y. et al. Multiple yet switchable hydrogen-bonded organic frameworks with white-light emission. Nat. Commun. 13, 1882–1891 (2022).
Wang, Y., Yang, Y., Yang, X. & Zou, B. Pressure effects on metal/covalent-organic frameworks: structural and optical properties. Sci. China Chem. 67, 2890–2903 (2024).
Zhang, J. et al. Stimuli-responsive AIEgens. Adv. Mater. 33, 2008071 (2021).
Yang, Q. et al. Brightening triplet excitons enable high-performance white-light emission in organic small molecules via integrating n–π*/π–π* transitions. Nat. Commun. 15, 7778–7787 (2024).
Zhang, L., Wang, Y., Lv, J. & Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2, 17005–17021 (2017).
Ge, Y. et al. A distinctive HPHT platform with different types of large-volume press subsystems at SECUF. Matter Radiat. Extremes 9, 063801 (2024).
Xiao, Z. et al. Harvesting multicolor photoluminescence in nonaromatic interpenetrated metal–organic framework nanocrystals via pressure-modulated carbonyls aggregation. Adv. Mater. 36, 2403281 (2024).
Wang, Y. et al. Maximized green photoluminescence in Tb-based metal–organic framework via pressure-treated engineering. Angew. Chem. Int. Ed. 61, e202210836 (2022).
Yang, Y. et al. Regulating planarized intramolecular charge transfer for efficient single-phase white-light emission in undoped metal–organic framework nanocrystals. Nano Lett. 24, 9898–9905 (2024).
Zhai, C. et al. Molecular insertion regulates the donor-acceptor interactions in cocrystals for the design of piezochromic luminescent materials. Nat. Commun. 12, 4084–4093 (2021).
Fang, J. et al. Piezochromism in dynamic three-dimensional covalent organic frameworks. Angew. Chem. Int. Ed. 62, e202304234 (2023).
Zhao, Y. et al. Tricolor Ho3+ photoluminescence enhancement from site symmetry breakdown in pyrochlore Ho2Sn2O7 after pressure treatment. Phys. Rev. Lett. 125, 245701 (2020).
Wang, W. et al. Brightening blue photoluminescence in non-emission phthalic anhydride by pressure-driven molecular reassembly. Laser Photonics Rev, 2401173 (2024).
Zhang, T. et al. Brightening blue photoluminescence in nonemission MOF-2 by pressure treatment engineering. Adv. Mater. 35, 2211729 (2023).
Zhao, D. et al. Harvesting cool daylight in hybrid organic–inorganic halides microtubules through the reservation of pressure-induced emission. Adv. Mater. 33, 2100323 (2021).
Shen, Y., An, Z., Liu, H., Yang, B. & Zhang, Y. Excitation-dependent multicolour luminescence of organic materials: internal mechanism and potential applications. Angew. Chem. Int. Ed. 62, e202214483 (2023).
Yang, H. et al. Multicolour fluorescence based on excitation-dependent electron transfer processes in o-Carborane dyads. Angew. Chem. Int. Ed. 61, e202115551 (2022).
Huang, R. et al. Single-fluorophore-based organic crystals with distinct conformers enabling wide-range excitation-dependent emissions. Angew. Chem. Int. Ed. 61, e202211106 (2022).
Chu, B. et al. Aliphatic polyesters with white-light clusteroluminescence. J. Am. Chem. Soc. 144, 15286–15294 (2022).
Sun, H. et al. Stimuli-responsive dual-emission property of single-luminophore-based materials. Adv. Funct. Mater. 35, 2415400 (2024).
Chen, Z., Ho, C. L., Wang, L. & Wong, W. Y. Single-molecular white-light emitters and their potential WOLED applications. Adv. Mater. 32, 1903269 (2020).
Huang, W. et al. Trapping highly reactive photoinduced charge-transfer complex between amine and imide by light. Chem 10, 2829–2843 (2024).
Zhang, J. et al. White-light emission from organic aggregates: a review. Adv. Photonics 4, 014001 (2021).
Sun, J. et al. Charge-transfer exciton manipulation based on hydrogen bond for efficient white thermally activated delayed fluorescence. Adv. Funct. Mater. 30, 1908568 (2019).
Hu, J. et al. Developing through-space charge transfer polymers as a general approach to realize full-color and white emission with thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 58, 8405–8409 (2019).
Xie, Z. et al. Hydrogen-bonding-assisted intermolecular charge transfer: a new strategy to design single-component white-light-emitting materials. Adv. Funct. Mater. 27, 1703918 (2017).
Wu, J. R., Wu, G., Li, D. & Yang, Y. W. Macrocycle-based crystalline supramolecular assemblies built with intermolecular charge-transfer interactions. Angew. Chem. Int. Ed. 62, e202218142 (2023).
Pan, J. Q. et al. Photomodulation of charge transfer through excited-state processes: directing donor-acceptor binding dynamics. Angew. Chem. Int. Ed. 64, e202412790 (2025).
Mo, J.-T. et al. Bright long persistent luminescence in cluster-based MOFs derived from intra-ligand charge transfer characteristic. Chem. Eng. J. 483, 149343 (2024).
Chen, Y., Xie, Y. & Li, Z. Room-temperature phosphorescence of nicotinic acid and isonicotinic acid: efficient intermolecular hydrogen-bond interaction in molecular array. J. Phys. Chem. Lett. 13, 1652–1659 (2022).
Wang, C. & Li, Z. Molecular conformation and packing: their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 1, 2174–2194 (2017).
Fu, Z. et al. Pressure-induced emission enhancement by restricting chemical bond vibration. J. Mater. Chem. C. 9, 14578–14582 (2021).
Wang, K. et al. Structural properties and halogen bonds of cyanuric chloride under high pressure. J. Phys. Chem. B 115, 4639–4644 (2011).
Wu, M. et al. Pressure-induced restricting intermolecular vibration of a herringbone dimer for significantly enhanced multicolor emission in rotor-free truxene crystals. J. Phys. Chem. Lett. 13, 2493–2499 (2022).
Ni, J. et al. Deep-blue electroluminescence from phosphine-stabilized Au3 triangles and Au3Ag pyramids. Angew. Chem. Int. Ed. 61, e202213826 (2022).
Lu, B. et al. Piezochromic luminescence of AIE-active molecular co-crystals: tunable multiple hydrogen bonding and molecular packing. J. Mater. Chem. C. 6, 9660–9666 (2018).
Bardak, F., Atac, A. & Kurt, M. Infrared and Raman study of some isonicotinic acid metal(II) halide and tetracyanonickelate complexes. Spectrochim. Acta, Part A 71, 1896–1900 (2009).
Ramesh, G. & Reddy, B. V. Spectroscopic investigation on structure (monomer and dimer), molecular characteristics and comparative study on vibrational analysis of picolinic and isonicotinic acids using experimental and theoretical (DFT & IVP) methods. J. Mol. Struct. 1160, 271–292 (2018).
Atac, A., Karabacak, M., Karaca, C. & Kose, E. N. M. R. UV, FT-IR, FT-Raman spectra and molecular structure (monomeric and dimeric structures) investigation of nicotinic acid N-oxide: a combined experimental and theoretical study. Spectrochim. Acta, Part A 85, 145–154 (2012).
Wang, Y. et al. Pressure engineering toward harvesting the bright deep-blue-light emission in Y-based metal-organic frameworks. Adv. Funct. Mater. 33, 2300109 (2023).
Wang, Y. et al. Reaching 90% photoluminescence quantum yield in one-dimensional metal halide C4N2H14PbBr4 by pressure-suppressed nonradiative loss. J. Am. Chem. Soc. 142, 16001–16006 (2020).
Luo, H. et al. Regulating exciton–phonon coupling to achieve a near-unity photoluminescence quantum yield in one-dimensional hybrid metal halides. Adv. Sci. 8, 2100786 (2021).
Liu, J. et al. Through-space interaction of tetraphenylethylene: what, where, and how. J. Am. Chem. Soc. 144, 7901–7910 (2022).
Lee, K.-M. et al. Excitation-dependent visible fluorescence in decameric nanoparticles with monoacylglycerol cluster chromophores. Nat. Commun. 4, 1544–1552 (2013).
Yang, T. et al. Time-dependent afterglow from a single component organic luminogen. Research 2021, 9757460 (2021).
Yang, L.-L. et al. Understanding the AIE phenomenon of nonconjugated rhodamine derivatives via aggregation-induced molecular conformation change. Nat. Commun. 15, 999–1009 (2024).
Li, D. et al. White-light emission from a single organic compound with unique self-folded conformation and multistimuli responsiveness. Chem. Sci. 9, 5709–5715 (2018).
Mao, H. K., Xu, J. & Bell, P. M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. JGR Solid Earth 91, 4673–4676 (1986).
Zhou, M. et al. Study of high-pressure Raman intensity behavior of aromatic hydrocarbons: benzene, biphenyl and naphthalene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 97, 526–531 (2012).
Colangeli, L. et al. Raman and infrared spectra of polycyclic aromatic hydrocarbon molecules of possible astrophysical interest. Astrophysical J. 396, 369 (1992).
Zhang, C. F. et al. Charge transfer effect on Raman shifts of aromatic hydrocarbons with three phenyl rings from ab initio study. J. Chem. Phys. 150, 074306 (2019).
Dai, Y. et al. High-pressure-induced phase transition in 2,5-Diketopiperazine: the anisotropic compression of N–H···O hydrogen-bonded tapes. J. Phys. Chem. C. 122, 11747–11753 (2018).
Birch, F. Finite elastic strain of cubic crystals. Phys. Rev. 71, 809 (1947).
Hattori, T. et al. Development of a technique for high pressure neutron diffraction at 40 GPa with a paris-edinburgh press. High. Press. Res. 39, 417–425 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 12274177 to X.Y.) and the National Key R&D Program of China (Grant No. 2023YFA1406200 to X.Y.). The authors thank the staff from the Shanghai Synchrotron Radiation Facility (SSRF) at BL17UM and the User Experiment Assist System. The Kawai-Type Large-Volume Press experiments were performed at the B1 stations, Synergetic Extreme Condition User Facility (SECUF).
Author information
Authors and Affiliations
Contributions
X.Y. conceived the idea and designed the research. Z.X. prepared the sample and performed in situ high-pressure experiments and analyzed data. W.W. performed in situ single-crystal XRD experiments and analyzed data. Y.Y. and H.Z. assisted in performing experiments. Z.X., J.Y. and Y.Y. carried out the theoretical calculation. X.Y., Z.X. and B.Z. intensively discussed the data and wrote the manuscript. All authors discussed and commented on the manuscript and contributed to its writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, Z., Wang, W., Yu, J. et al. Controllable multicolor emission from initially non-emissive organic molecules by pressure engineering. Nat Commun 16, 8780 (2025). https://doi.org/10.1038/s41467-025-63834-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63834-z