Abstract
DNA deaminase toxins are involved in interbacterial antagonism and the generation of genetic diversity in surviving bacterial populations. These enzymes have also been adopted as genome engineering tools. The single-stranded (ss)DNA deaminase SsdA is representative of the bacterial deaminase toxin family-2 (BaDTF2), and it deaminates ssDNA cytosines without a strong sequence context dependence, which contrasts with the AID/APOBEC family of sequence-selective ssDNA cytosine deaminases. Here we report the crystal structure of SsdA in complex with a ssDNA substrate. The structure reveals a unique mode of substrate binding, in which a cluster of aromatic residues engages ssDNA in a V-shaped conformation sharply bent across the target cytosine. The bases 5’ or 3’ to the target cytosine are stacked linearly and make mostly sequence non-specific protein contacts, thus explaining the broad substrate selectivity of SsdA. Unexpectedly, SsdA contains a β-amino acid isoaspartate, which is important for enzymatic activity and contributes to the stability of SsdA as a toxin. Structure-function studies helped to design SsdA mutants active in human cells, which could lead to future applications in genome engineering.
Similar content being viewed by others
Introduction
A diverse family of toxins produced by Gram-negative bacteria is delivered to neighboring bacterial cells via the type VI secretion system (T6SS) to inhibit the growth of competing species and provide a growth advantage to the host strain. Such T6SS-delivered toxins include peptidoglycan hydrolases and phospholipases that promote lysis of target cells, membrane-disrupting/pore-forming proteins, metallopeptidases, NAD-glycohydrolases, (p)ppApp synthetases, and DNA endonucleases1. A recently discovered addition to this superfamily is the bacterial deaminase toxin family (BaDTF) proteins, which catalyze the deamination of cytosine to uracil and cause mutations in the genomic DNA of recipient cells2,3. In addition to causing lethal levels of DNA damage or DNA replication arrest, the activity of BaDTFs may facilitate the evolution of surviving target bacterial populations and contribute to the development of antibiotic resistance2.
BaDTF proteins are phylogenetically classified into at least three sub-families. The first class (BaDTF1) is represented by a double-stranded DNA (dsDNA) deaminase from Burkholderia cenocepacia, DddA. The deaminase toxin domain of DddA and its orthologs or their evolved variants have been adopted in the CRISPR-free, DddA-derived cytosine base editors (DdCBEs) that enable base editing of mitochondrial, chloroplast, and nuclear DNA targets3,4,5,6. Recent structural studies have revealed the mode of target engagement by DddA, featuring a unique tandem-displacement mechanism of base-flipping7. The second class (BaDTF2) is represented by a deaminase toxin from Pseudomonas syringae designated the single-stranded (ss)DNA deaminase toxin, SsdA2 (Supplementary Figs. 1 and 2). In contrast to DddA, which strongly prefers the 5’-TC motif in deaminating double-stranded (ds)DNA substrates, SsdA was reported to preferentially deaminate cytosines in ssDNA with little sequence-context preference2. Interestingly, BaDTF2 is evolutionarily more closely related to DYW motif-containing eukaryotic RNA deaminases than to other BaDTFs8,9, although SsdA and other BaDTF2 deaminases themselves lack the hallmark C-terminal DYW motif (Supplementary Figs. 1 and 3). The third class (BaDTF3) has been reported to target both ssDNA and dsDNA2,10.
The ssDNA-selective cytosine deaminase activity of SsdA is analogous to that of human APOBEC family deaminases, which provide innate antiviral immunity, promote the evolution of human cancers, and are used widely in base editing technologies11,12,13,14,15. However, in contrast to the human APOBEC deaminases with TC or CC-restricted editing preferences, SsdA was reported to have a much more relaxed target preference by deaminating cytosine preceded by any of the four bases (T, C, A, or G) and even exhibiting residual activity on dsDNA2. The structural basis of the broader substrate selectivity for SsdA is unknown. Here we report the crystal structure of SsdA in complex with ssDNA and corroborating biochemical data, which reveal a unique mechanism of substrate DNA recognition. High-resolution crystallographic data also show that SsdA contains a β-amino acid isoaspartate (isoAsp), which is important for enzymatic activity and may contribute to the stability of the toxin. Based on this structural information, we generated mutant derivatives of SsdA that can perform base editing in human cells and may be useful for future genome engineering applications.
Results
Overall structure of SsdA-ssDNA complex
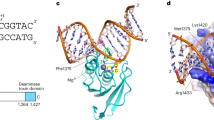
To understand how SsdA interacts with target DNA, we sought to crystallize P. syringae SsdA in complex with its preferred DNA substrate. As reported previously2, we observed that SsdA deaminates cytosines in ssDNA with any base at the 5’ (–1) position (Fig. 1a). By contrast, SsdA showed no deaminase activity on dsDNA, regardless of the length of the oligonucleotide substrate or the sequence around the target cytosine (Fig. 1a–c and Supplementary Figs. 4–6). Consistent with these results, we obtained crystals of the C-terminal toxin domain of SsdA (Lys259 to Glu409, with residue numbering according to PDB entry 7jtu2; Fig. 2a, b) with a 15-nucleotide (nt) ssDNA substrate containing a 5’-TCG target sequence in the middle. SsdA with substitution of the catalytically essential glutamic acid residue (E348A) was used to capture the enzyme-substrate complex (Fig. 2c). The structure of the SsdA-ssDNA complex was determined by molecular replacement phasing and refined to 1.94-Å resolution with excellent model quality and fit to experimental data (Rwork/Rfree = 16.3/19.0%, Table 1 and Supplementary Fig. 7). Two SsdA-ssDNA complexes are in the asymmetric unit with essentially the same conformations. The ssDNA bound to SsdA adopts a V-shaped conformation sharply bent away from the protein, with the target 2’-deoxycytidine nucleotide rotated out at the apex of ‘V’ and engaged in the Zn-bound active site (Fig. 2c). SsdA contains the core structural elements shared among the deaminase superfamily consisting of two α-helices and a layer of β-sheet8. The catalytically essential Zn ion is coordinated by a conserved triad motif comprising His346, Cys367, and Cys370 and positioned between the N-terminal ends of the two α-helices. His346 is stacked against the cytosine base in the active site pocket that is lined on the other side by Thr290 (Fig. 3a). Our SsdA structure in complex with ssDNA shows minimal structural changes from that in complex with the immunity factor/antitoxin (SsdAI) with the root mean square deviation (r.m.s.d.) of 1.3 Å and 0.65 Å for all and the main chain atoms, respectively, although the active site zinc ion was lost in SsdA bound to SsdAI (PDB 7jtu)2.
a DNA deamination activity of wildtype SsdA on 18-nt DNA substrates with different (underlined) –1 bases in the absence (ssDNA) or presence (dsDNA) of the complementary strand, examined in the EndoQ-mediated oligo cleavage assay43. A 10-fold molar excess of the bottom strand was used to ensure no residual ssDNA. Note that the lower band (smaller product) for the CC substrate results from deamination at –1 C, which is in an AC context. b DNA deamination assay for SsdA and human APOBEC3B (A3B) on a 28-nt DNA substrate containing four cytosines in different sequence contexts, in the absence or presence of varying amounts of the complementary strand. The largest product results from deamination only at the most downstream TC target, whereas the smaller products are generated by deamination of more upstream cytosines in the dinucleotide motifs indicated on the right. The results show that both SsdA and A3B are ssDNA-specific. However, whereas A3B is TC-selective, SsdA can deaminate at all four sites. c DNA deamination assay for SsdA and A3B on a 36-nt DNA containing a single cytosine, in the absence or presence of varying amounts of the complementary strand, highlighting ssDNA-specific deaminase activity of SsdA and A3B. The SsdA concentration was 50 nM in (a, c), and 25 nM in (b). The A3B concentration was 0.5 μM and 1.0 μM in (b) and (c), respectively. Gels are representative of three replicates. Duplicate experiments are shown in Supplementary Fig. 6. Annealing of the substrate oligonucleotides into dsDNA in the presence of different amounts of the complementary (bottom) strand is assessed in Supplementary Fig. 4.
a A schematic showing the domain organization of Pseudomonas syringae SsdA. PAAR: proline-alanine-alanine-arginine. The residue numbering is according to PDB entry 7jtu2. b Structure prediction by AlphaFold244 of full-length SsdA. The domains are color-coded as in (a). c The crystal structure of SsdAtox-ssDNA complex, viewed from three orthogonal orientations. The panel on the right shows the molecular surface of the protein colored according to electrostatic potential (blue: positive, red: negative).
a Close-up view of the SsdA-ssDNA interaction, with hydrogen bonds and salt bridges indicated by yellow dashed lines. An orange dashed line indicates an aromatic stacking interaction between Tyr334 and +1 G. b An orthogonal view looking down the narrow DNA-binding cleft, with the molecular surface of the protein colored according to electrostatic potential (blue: positive, red: negative).
Mechanism of ssDNA engagement
SsdA engages the sharply bent ssDNA in a deep positively charged groove between two protruding loops (Figs. 2c and 3). The loop on one side is centered on a short helical segment between Lys284 and Lys287, whereas that on the other side spans between Lys330 and Lys339. The narrow ssDNA-binding groove is most constricted between Val288 and Tyr334, where these residues project into the aperture over the deep active site pocket (Fig. 3b). The DNA bases on either the 5’ or 3’ side of the target C are each stacked along a linear axis, which are perpendicular to one another. A cluster of aromatic residues of SsdA: His332, Tyr334, Tyr342, and Phe343 serves as a scaffold to shape the ssDNA (Fig. 3a). Tyr334, in particular, plays a central role in stabilizing the V-shaped DNA conformation – It is π-stacked against the face of the +1 G base to set the direction of the strand downstream (3’) of the target C, while being hydrogen-bonded to –2 A and making a T-shaped aromatic stacking interaction with –3 A to position the upstream (5’) bases. His332 is hydrogen-bonded to the backbone phosphate group of –1 T, also stabilizing the DNA strand upstream of the target C. The thymine base immediately 5’ to the target C (–1 T) is exposed to the solvent and makes no protein contact, which explains the lack of strong sequence context preference of SsdA in deaminating ssDNA substrates.
In the deaminase active site, Thr290 plays a key role in poisoning the target (0) 2’-deoxycytidine nucleotide by stacking against the cytosine base and hydrogen-bonding to the deoxyribose moiety (Fig. 3a). The adjacent residues Lys289 and Ser300 interact with the phosphate backbone of +1 G and +2 A downstream of the target C. On the other side of the ssDNA-binding groove, Trp302 makes extensive van der Waals contact with the deoxyribose moieties of +1 G and +2 A, whereas Arg336 is hydrogen-bonded with the base and deoxyribose moieties of +2 A and +3 A, respectively, to stabilize the stacked nucleotides downstream of the target C.
A superposition of the antitoxin (SsdAI)-bound2 and the ssDNA-bound SsdA structures shows that SsdAI engages the tall loops on either side of the ssDNA-binding cleft and inserts Asp150 and Glu152 side chains into the ssDNA-binding cleft, which mimic the backbone phosphate groups of the nucleotides at +2 and +1 positions (Fig. 4 and Supplementary Fig. 8). Furthermore, Tyr334 of SsdA fits in a hydrophobic pocket of SsdAI and makes a T-shaped π-stacking with an antitoxin residue Phe154, which takes the position of the +1 G base of the ssDNA. Thus, as observed for DddI inhibition of DddA, SsdAI mimics both the shape and charge of the DNA substrate to achieve competitive inhibition with high affinity3,7.
a Crystal structure of the SsdAI-SsdAtox complex (PDB ID: 7jtu)2. The molecular surface is shown for SsdAI, colored according to electrostatic potential (blue: positive, red: negative). The side chains of acidic residues positioned similarly to DNA backbone phosphates are labeled. b SsdAtox-DNA complex in the same orientation as in (a). c Structure of the SsdAI-SsdAtox complex as in (a), after a 90° rotation. Some of the SsdAI residues are shown in sticks and visible through a semi-transparent surface. d SsdAtox-DNA complex in the same orientation as in (c).
Mutation analyses reveal key aromatic contacts
To complement the crystallographic observations above and to identify functionally important residues, we examined the ssDNA deaminase activity of various SsdA mutant derivatives in a biochemical assay (Fig. 5 and Supplementary Figs. 9, 10). Consistent with the key role played by Tyr334 in ssDNA engagement, SsdA Y334A showed no detectable activity. In contrast, SsdA Y334F retained a near wildtype-level activity, highlighting the importance of an aromatic interaction. On the other hand, SsdA Y342A had no activity, whereas SsdA Y342F was only weakly active. Similarly, a series of SsdA mutant derivatives H332A, H332Q, and H332T all showed no activity, whereas H332F showed only very weak, albeit detectable, activity. These results suggest that both the aromatic and polar interactions made by His332 and Tyr342 are important. P333A showed only a modest defect, indicating that Pro333 is less important than the neighboring residues His332 and Tyr334. A mutation of another residue Phe343 from the aromatic cluster, F343A, almost abrogated the deaminase activity. This suggests that Phe343, although it does not directly interact with ssDNA, plays a critical structural role. SsdA K289A, S300A, W302A, and R336A showed no or little activity, underscoring the importance of these mutated residues interacting with nucleotides downstream of the target C. Notably, Gly301 takes a backbone conformation only allowed for glycine (ϕ = ~ +120°, ψ = ~ +160°) to enable the unique positioning of Ser300 and Trp302 for ssDNA interaction, and this ‘SGW motif’ is among the most highly conserved residues in BaDTF2 members (Supplementary Figs. 1 and 2). For Val288 positioned near the mouth of the active site, substituting a positively charged (Lys) or a bulky aromatic (Trp) residue did not impair the SsdA activity, whereas an acidic (Glu) substitution at this position made SsdA slightly less active. Unexpectedly, SsdA K364A retained a near wildtype-level activity, suggesting that Lys364, despite its direct hydrogen bond with the 5’ phosphate of the target C, may be functionally less critical.
Deamination of ssDNA substrate (TC-18) by SsdA with various single amino acid substitutions for DNA-interacting residues around the active site (Fig. 3). Deaminated ssDNA oligonucleotide was cleaved by EndoQ43, yielding a band with faster mobility in gel electrophoresis. A chemically synthesized deamination product (TdU-18) was confirmed to be fully cleaved by EndoQ (2nd lane from the left). The SsdA concentration was 60 nM. Gels are representative of two replicates. A duplicate experiment is shown in Supplementary Fig. 9.
SsdA contains a β-amino acid
During the model building and refinement of the SsdA-ssDNA complex structure, it became apparent that the electron density for Asn294 could not be accounted for by the expected L-Asn and is more consistent with its β-linked isomer (Supplementary Fig. 7). To obtain stronger structural evidence, we crystallized SsdA without DNA and determined the structure at 1.33-Å resolution (Table 1 and Supplementary Fig. 11). The high-resolution electron density for this structure unambiguously showed that residue 294 of SsdA is a β-amino acid in both molecules in the asymmetric unit (Fig. 6a, b). The side chain of residue 294 forms bidentate hydrogen bonds in an idealized geometry with Arg272 from a neighboring α-helix. This suggests that the residue is L-β-Asp or isoaspartate (isoAsp), which is known to result from a spontaneous deamidation of L-Asn via a succinimide intermediate16. IsoAsp294 is in a tight turn between short β-strands, where its side chain also accepts a hydrogen bond from the preceding residue Ser293 and its main chain amide nitrogen donates a hydrogen bond to the carbonyl oxygen of Lys296 (Fig. 6a, b). Thus, isoAsp at this position plays an important structural role in stabilizing the protein fold. It is notable that isoAsp294 of SsdA is followed by Asp295, and this sequence context deviates from the more readily deamidated Asn-Gly and Asn-Ser motifs17,18,19, suggesting that the isomerization of SsdA Asn294 to isoAsp is conformation-specific.
a Crystal structure of the SsdA-ssDNA complex, with a β-turn containing isoAsp294 and the surrounding residues highlighted. Hydrogen bonds involving isoAsp294 are indicated by yellow dashed lines. b The composite omit 2Fo-Fc electron density contoured at 1.5σ for the higher-resolution SsdA structure without DNA. Hydrogen bonds involving isoAsp294 are indicated by yellow dashed lines, with distances in angstroms. c DNA cytosine deaminase activity of SsdA mutant derivatives with residue 294 changed to Ala, Asp, or Gly. WT denotes wildtype, which contains isoAsp294. The SsdA concentration in this experiment was 50 nM. Gel is representative of three replicates. A duplicate experiment is shown in Supplementary Fig. 9. d ssDNA-binding of SsdA mutant derivatives analyzed in a fluorescence polarization assay. Changes in fluorescence polarization (mean ± SD of three technical replicates) from the control sample containing no protein were plotted against the total protein concentration in the reaction. All proteins carried the E348A mutation to inactivate the DNA deaminase activity. ‘IsoAsp294’ denotes SsdA with only the E348A mutation. Y334A removes the key aromatic side chain of Tyr334, essential for the SsdA activity (Figs. 3 and 5). The determined KD was 28.0 (95% confidence interval: 22.6–34.8) μM for SsdA E348A, 33.6 (27.6–40.8) μM for N294A/E348A, and 42.3 (31.1–57.4) μM for N294G/E348A. The KD was not determined for N294D/E348A and Y334A/E348A due to weak binding. N.D., not determined. e Thermal melt curves and the Tm values for SsdA E348A (isoAsp294) and its variants that additionally carry an N294A, N294D, or N294G amino acid substitution, probed using CD spectroscopy.
To investigate the importance of isoAsp294 in the SsdA function, we tested the effect of N294A, N294D, and N294G mutations on SsdA activities. The alanine or glycine substitution should block the spontaneous post-translational isomerization to a β-amino acid. In addition, as Gly is achiral, Gly294 may better mimic the unique backbone conformation adopted by isoAsp294. The biochemical DNA deaminase assay showed that all 3 mutant derivatives are less active than wildtype SsdA, with N294A and N294G being more active among the mutant series, and N294D showing the most compromised activity (Fig. 6c and Supplementary Figs. 9, 10). Although L-Asp could form the succinimide intermediate and go through the isomerization, L-Asp294 of SsdA was likely not converted into isoAsp. The effects of the three mutations were further evaluated for ssDNA-binding in a fluorescence polarization assay (Fig. 6d). The crystallized protein SsdA E348A showed a KD of 28.0 μM for a 17-nt ssDNA containing a TCG motif. A double mutant Y334A/E348A, which has the key DNA-binding residue Tyr334 mutated, exhibited a much weaker binding with KD > 378 μM (95% CI). SsdA N294D/E348A showed even poorer binding (KD not determined due to the lack of saturation in binding). In contrast, SsdA N294A/E348A and N294G/E348A exhibited comparable affinities (KD of 33.6 μM and 42.3 μM, respectively) to SsdA E348A, consistent with what we observed in the DNA deamination assay above that substitution of Ala or Gly had milder effects than Asp at residue 294. Similar ssDNA-binding affinity and the effect of the amino acid substitutions were observed using microscale thermophoresis (MST), where N294D most severely compromised the ssDNA-binding of SsdA (Supplementary Fig. 12). These results suggest that, while isoAsp294 is not essential for the cytosine deaminase activity, it contributes to optimal ssDNA substrate-binding and deamination by SsdA.
To further investigate the structural roles of isoAsp294, we used circular dichroism (CD) spectroscopy. The CD spectra of SsdA E348A and its N294A, N294D, and N294G variants were indistinguishable at 20 °C, suggesting that these proteins share the same overall fold and secondary structures (Supplementary Fig. 13). However, heat-induced changes in the CD spectra during a temperature ramp show that SsdA E348A, which contains isoAsp294, is more thermostable with a higher melting temperature (Tm) than those of the N294A/D/G variants (Fig. 6e). Thus, isoAsp294 contributes to the thermal stability of SsdA, consistent with the network of hydrogen bonds observed in our structures.
SsdA mutant derivatives with activity in human cells
The sequence context-independent ssDNA deaminase activity of SsdA could be useful in base editing or other applications in human cells20. Thus, we tested the cytosine deaminase activity of SsdA in 293T cells using real-time, living cell-based ARSENEL assays representing all four different dinucleotide editing contexts (AC/CC/GC/TC)21,22. Wildtype SsdA fused to Cas9n (D10A) in the BE4max platform23 (Fig. 7a) showed activity on an episomal target with all four dinucleotide target motifs, although the editing efficiency on the GC target was very low (Fig. 7b and Supplementary Fig. 14). Curiously, a series of biochemically less active mutant derivatives of SsdA: N294A, N294D, and N294G, all showed significantly higher editing efficiencies than wildtype SsdA and were also able to edit an integrated chromosomal target in the ARSENEL assay, albeit with lower efficiencies compared to the APOBEC3A (A3A)-based base editor used as a positive control (Fig. 7c and Supplementary Fig. 14). The SsdA N294G-derived base editor was also able to edit the C5 position of the endogenous MUTYH locus (Fig. 7d–g), and it did not exhibit significant off-target activity at ~50 bp downstream of the target site (Supplementary Fig. 15). In all base editing experiments, immunoblot analyses confirmed that the SsdA-based editors were expressed, although at lower levels compared to the A3A-based base editor, possibly due to higher cytotoxicity (Supplementary Figs. 14 and 15). The results of these experiments demonstrate that SsdA is active in human cells and suggest that mutant derivatives may be promising candidates for use in base editing and other applications. Notably, recent work showed the utility of SsdA in base editing in mouse embryos24 and in plants25, consistent with our studies in human cells.
a Schematic diagram of the base editor constructs in the BE4max platform23 used in this study. Deaminase is either SsdA(259-409) or A3A. b, c Base editing efficiencies of wildtype (WT) and the indicated mutant variants of SsdA, or human A3A as positive control, quantified using flow cytometry. Editing efficiencies were assessed on four distinct ARSENEL reporters21 co-transfected along with the base editor constructs into 293T cells (b), or the ARSENEL reporters stably integrated into the chromosomal DNA of 293T cells (c). Column bars represent the mean ± SD from biologically independent triplicate experiments. Immunoblot analyses of the base editor expression are shown in Supplementary Fig. 14. d–g Chromosomal base editing efficiency of the indicated base editors on an AMBER reporter22 stably integrated into the genome and at the endogenous MUTYH locus, assessed in three biologically independent experiments. The schematic workflow is shown in (d). Editing efficiency on the AMBER reporter was measured as the percentage of GFP-positive (GFP⁺) cells by flow cytometry (e, mean ± SD). In sorted GFP⁺ cells, C-to-T conversion frequencies within the 20-bp sgRNA target window of MUTYH were determined by Sanger sequencing (representative chromatograms shown in (g), with cytosines numbered according to their position from 5’ to 3’ within the window, and quantified in bar graphs (f, mean ± SD). The base editor constructs contain an intron indicated by “i” in SsdAi and A3Ai.
Discussion
SsdA shapes ssDNA into a unique V-shape to engage the target cytosine in the active site (Figs. 2c and 3). The sharply bent ssDNA substrate, with the target deoxycytidine nucleotide flipped out for an engagement in the zinc-bound active site, is a shared feature with the APOBEC-family ssDNA deaminases. However, a superposition of the SsdA-ssDNA complex and A3A-ssDNA complex26 based on the conserved core structural elements of the deaminase fold shows distinct trajectories of the ssDNA backbones, with the DNA strands approaching the active site in orthogonal orientations (Fig. 8a). In the A3A/B-DNA complex, both the target (0) deoxycytidine and the 5′ neighboring (–1) deoxythymidine nucleotides are rotated out at the bottom of a “U”-shaped ssDNA and engaged by the enzyme through base-specific contacts, underlying the strong 5′-TC preference26. In contrast, in the SsdA-bound ssDNA, only the target deoxycytidine is rotated out toward the protein at the apex of the V-shape, whereas the bases up and downstream of the target cytosine are stacked linearly and do not make direct protein contacts, which explains the lack of a strong local sequence preference by SsdA (Fig. 1a, b and Supplementary Fig. 5). The –1 deoxythymidine nucleotide was observed to adopt the syn conformation in the SsdA-ssDNA structure (Fig. 3a). The modest preference for a pyrimidine at this position (Fig. 1a) could be due to favorable stacking with the –2 base or steric effect with surrounding atoms from the DNA backbone.
a Side-by-side comparisons between the SsdA-ssDNA complex structure (cyan, left) and the human A3A-ssDNA complex structure (magenta, right) (PDB ID: 5SWW)26. In each (upper and lower) pair, the two enzymes are shown in the same orientation based on a superposition of the conserved active site residues. The gray spheres represent a Zn ion in the active site. b A side-by-side comparison between the SsdA-ssDNA complex structure (cyan, left) and the DddA-dsDNA complex structure (yellow, right) (8E5E)7. Key hydrophobic residues of DddA Phe1375 and Met1379, which insert into the minor groove of dsDNA to promote base-flipping, are highlighted. The two enzymes are shown in the same orientation based on a superposition of the conserved active site residues. The gray spheres represent a Zn ion in the active site. The green sphere bound to DddA is a magnesium ion.
A structural comparison between the SsdA-ssDNA complex and the DddA-dsDNA complex7 highlights a remarkable diversity of DNA-binding mode among BaDTFs (Fig. 8b). The core of the deaminase fold comprising two α-helices packed against a β-sheet, is sandwiched between additional α-helices in SsdA, which support the extended loops on either side of the deep ssDNA-binding cleft. In contrast, the core of DddA is flanked on either side by loops making smaller protrusions, which together form a broader DNA-binding surface to accommodate the non-deaminated strand of dsDNA substrates. In the DddA-dsDNA complex structure, one of the core α-helices of the conserved deaminase fold is positioned in the minor groove and makes extensive contacts with both the backbone and bases of the dsDNA. DddA Phe1375, which is from the N-terminus of this helix and between the two zinc-coordinating Cys residues, and Met1379, one helical turn downstream, intercalate in dsDNA and promote flipping of the target cytosine (Fig. 8b). The corresponding residues of SsdA (Asp369 and Tyr373) are outside the DNA-binding groove and involved in stabilizing the protein fold.
SsdA undergoes minimal structural changes upon binding to ssDNA, with an all-atom and backbone r.m.s.d. of 0.91 and 0.55 Å, respectively, between our ssDNA-bound and free SsdA structures reported here (Supplementary Fig. 16). Including the antitoxin (SsdAI)-bound structure reported previously (PDB 7jtu)2, the largest structural deviation was observed when the two protein molecules in the asymmetric unit of the high-resolution DNA-free SsdA crystal structure were compared, which upon superposition yield an all-atom and backbone r.m.s.d. of 1.6 Å and 0.97 Å, respectively. The largest displacement was observed in a loop centered on Lys284, suggesting some flexibility of this region (Supplementary Fig. 17). This loop faces the base-pairing edges of the linearly stacked nucleobases downstream of the target cytosine in the SsdA-bound ssDNA (Fig. 2c), and thus, the flexibility might allow SsdA to accommodate ssDNA substrates with different sequences. However, this limited flexibility is not sufficient to enable SsdA with the deep and narrow ssDNA-binding groove (Fig. 3b) to accommodate dsDNA substrates, which explains the high specificity of SsdA for ssDNA (Fig. 1).
Deamidation of Asn into isoAsp in proteins is generally considered to be a spontaneous damage that negatively affects their stability and activity17,27. IsoAsp is recognized by protein L-isoaspartyl methyltransferase (PIMT), an enzyme conserved between prokaryotes and eukaryotes that methylates isomerized or racemized aspartyl residues to facilitate their conversion back to canonical L-Asp28. However, isoAsp in special cases has been reported to confer advantageous properties in bacterial, eukaryotic, and viral proteins29,30,31. For instance, an essential enzyme MurA from Enterobacter cloacae contains an isoAsp residue in a β-hairpin turn, which contributes to protein stability32. IsoAsp294 of SsdA is similarly in a hairpin turn that supports the positioning of DNA-interacting loops and thus contributes to the enzymatic activity as well as the overall stability. It is possible that isoAsp also serves to confer resistance against proteolytic degradation in target cells and thereby enhances the effectiveness of SsdA as an interbacterial toxin. Conversely, this post-translational modification could conceivably make SsdA susceptible to inactivation by the PIMT activity in receiving cells, which could explain the lower activity of wildtype SsdA than the N294 variants in human cells (Fig. 7). Regardless, the efficient base-editing activity of SsdA N294A/D/G in human cells and the structural information presented here provide a basis for the rational design of engineered DNA deaminases for novel applications.
Methods
Protein expression and purification
SsdA (residues 259–409) catalytically inactive mutant E348A, and double mutants N294A/E348A, N294G/E348A N294D/E348A, and Y334A/E348A were expressed in the E. coli strain BL21(DE3) using pET-24a expression vector with a C-terminal His6-tag. Transformed bacteria were grown in 4 liters of LB medium to an OD600 of 0.8, at which point the culture was supplemented with 100 μM ZnCl2 and induced by the addition of Isopropyl β-D-1-thiogalactopyranoside at a final concentration of 0.5 mM. After 20 h of shaking and incubation at 18 °C, the bacterial cells were collected by centrifugation at 4000 × g for 30 min. The cell pellets were resuspended in 20 mM Tris-HCl, pH 7.4, 0.5 M NaCl, 5 mM β-mercaptoethanol and 5 mM imidazole, lysed by the addition of lysozyme (0.4 mg/ml) and sonication. The lysate was centrifuged at 64,000 × g for 1 h at 4 °C to separate the supernatant from cell debris. The supernatant was collected and filtered through a 0.2 μm asymmetric polyethersulfone membrane and applied to a 5 mL Ni-NTA Superflow cartridge (QIAGEN). The His-tagged SsdA protein was eluted with a linear concentration gradient of imidazole and further purified by size-exclusion chromatography (SEC) on a HiLoad 26/600 Superdex 75 column operating with 20 mM Tris-HCl, pH 7.4, 0.2 M NaCl, 0.5 mM tris(2-carboxyethyl)phosphine (TCEP). Purified proteins were concentrated by ultrafiltration using Amicon centrifugal filters (3 kDa MWCO). The protein concentrations were measured on a Nanodrop 8000 spectrophotometer based on UV absorbance at 280 nm and the extinction coefficients calculated from the amino acid sequences. A3B C-terminal catalytic domain used as a control in ssDNA deamination assays was expressed in E. coli and purified as reported33.
X-ray crystallography
SsdA E348A was mixed with a 1.5-fold molar excess of 15 nt ssDNA (5’-AAAAAATCGAAAAAA-3’) in 10 mM Tris-HCl pH 7.4, 100 mM NaCl, and 0.5 mM TCEP at the final protein concentration of 27.5 mg mL−1. The SsdA-DNA complex was crystallized by the hanging drop vapor diffusion method using a reservoir solution containing 0.15 M NH4Cl and 22.5% PEG3350, pH 6.3, and the 1:1 volume ratio of mixing the protein-DNA complex and reservoir solutions in forming the drop. The DNA-free SsdA E348A crystals were obtained similarly, by mixing the protein at 55 mg mL−1 with a reservoir solution containing 1.8 M sodium phosphate monobasic monohydrate and potassium phosphate dibasic, pH 7.8. The crystals were cryo-protected by brief soaking in the respective reservoir solution supplemented with 20% ethylene glycol and flash-cooled by plunging in liquid nitrogen. X-ray diffraction data were collected at the NE-CAT beamline 24-ID-E of the Advanced Photon Source (Lemont, IL) for the SsdA-ssDNA crystal, and at the AMX beamline of NSLS-II (Upton, NY) for the DNA-free SsdA crystal. All datasets were processed with XDS34 for integration, followed by three other programs from the CCP4 Suite35: POINTLESS36, AIMLESS37 and TRUNCATE38 for reduction, scaling, and structure factor calculation, respectively. Anisotropic diffraction analysis and truncation were done with STARANISO. The structures were determined by molecular replacement with PHASER39 using the previously reported crystal structure of SddAtox bound to SddAI (PDBID 7JTU)2 as the search model. Iterative model building and refinement were conducted using COOT40 and PHENIX41. Modeling an L-Asn at residue 294 according to the genetic sequence renders the N294 side chain out of the density and results in a distorted geometry of the nearby backbone and strong positive and negative Fo-Fc densities. However, modeling the 294 position as isoAsp generates good geometry and clears the Fo-Fc map around the 294 position. A summary of crystallographic data statistics is shown in Table 1. Figures were generated using PyMOL (https://pymol.org/).
Cell-free protein expression and purification
Due to the toxicity of SsdA in bacterial cells, wildtype SsdA and various mutant derivatives with single amino acid substitutions used in the biochemical assay were expressed using NEBExpress® Cell-free E. coli Protein Synthesis System (New England Biolabs). Linear dsDNA templates used for expression were chemically synthesized as gBlock DNA fragments by Integrated DNA Technologies. The sequences of the gene fragments were confirmed by Sanger sequencing (ACGT). The cell-free expression mixture was incubated at 37 °C for 2.5 h and flash-frozen in liquid nitrogen to stop the reaction. His-tagged wildtype or mutant SsdA was affinity-purified with Dynabeads™ His-Tag Isolation and Pulldown kit (ThermoFisher Scientific). The purified proteins were confirmed by SDS-PAGE and their concentrations were quantitated based on band intensities in SDS-PAGE using Image J42 against previously quantitated SsdA E348A as a standard.
Biochemical DNA deaminase assay
The ssDNA oligonucleotide used in the deaminase assays in Figs. 1a, 5 and 6c is a 5′-fluorescein-labeled 18-nt DNA (FAM-5’-AAAAAAAATCGAAAAAAA-3’) and its variants with different –1 nucleobases (5’-AC, 5’-CC, 5’-GC). The reaction mixture contained 200 nM ssDNA substrate, wildtype or mutant SsdA at 25–60 nM (specific concentration used in each experiment mentioned in figure legends), 40 mM Tris–HCl, pH 7.4, 50 mM KCl, and 1.0 mM dithiothreitol. We also used 5’-fluorescein-labeled 24, 28, and 36-nt oligonucleotide substrates with different sequences (Supplementary Figs. 4, 5 and Fig. 1b, c). For testing the deaminase activity on dsDNA, an equimolar or 10-fold molar excess of unlabeled complementary strand was included in the reaction. After incubating at 37 °C for 50 min, pfuEndoQ was added to a final concentration of 1.0 μM, and the samples were further incubated at 60 °C for 30 min to cleave deaminated products. The reactions were stopped by the addition of formamide to 65% and heating to 95 °C for 10 min. The products were separated by 15% denaturing PAGE and scanned on a Typhoon FLA 7000 imager (GE Healthcare). For the quantitation in Supplementary Fig. 10, the band intensities were measured using Image J42. In each experiment, a control substrate representing the deaminated product (dU oligo) was treated with pfuEndoQ in parallel to confirm complete cleavage, and a non-deaminated substrate was incubated with EndoQ to confirm no cleavage.
Fluorescence polarization assay
The ssDNA-binding affinities of SsdA E348A, N294A/E348A, N294D/348A, N294G/348A, and Y334A/E348A were determined by fluorescence polarization/anisotropy measurements using a 3’-fluorescein-labeled 17-nt ssDNA probe (5’-GCGAAGTTCGGTTAACG-3’-FAM). A two-fold serial dilution series of protein concentrations ranging from 1.95 μM to 1.0 mM were mixed with the ssDNA probe at a final concentration of 18 nM in 20 mM Tris–HCl pH 7.4, 100 mM NaCl, 5 mM β-mercaptoethanol and the final volume of 100 μL in a black flat-bottom half-area 96-well plate. Changes in fluorescence polarization induced by protein binding were measured on a Tecan Spark 10 M microplate reader. Triplicated data were fit to the one-site specific binding model in GraphPad Prism 9 to determine the KD values and 95% confidence intervals.
Microscale thermophoresis (MST)
A 17-nt ssDNA probe with the same sequence as that used in the fluorescence polarization assay and a 5’ Cy5 modification was mixed with varying concentrations of SsdA E384A and its variants, N294A/E348A, N294D/348 A, N294G/348A, and Y334A/E348A, in 20 mM Tris–HCl pH 7.4, 100 mM NaCl, 5 mM β-mercaptoethanol. The MST measurement was carried out on Monolith X (NanoTemper) with the standard glass capillaries (MO-K022). Fluorescence emission at 670 nm was recorded 1.5 s after the IR laser was turned on, and the relative fluorescence intensity, normalized against the initial value, was plotted against protein concentration. Triplicated data were analyzed in MO.Control v2.6.6 to determine the KD values with 95% confidence intervals.
Circular dichroism (CD) spectroscopy
CD spectra were acquired for SsdA E384A and its variants, N294A/E348A, N294D/348 A, and N294G/348A, at 0.1 mg ml−1 on Jasco J-815 spectropolarimeter equipped with a temperature controller, using quartz cuvettes with a path length of 0.1 cm (Starna Ltd). The buffer condition was 50 mM sodium phosphate, pH 7.4, and 0.5 mM TCEP. The CD spectra at 20 °C and 60 °C were recorded for the wavelength range of 190–260 nm with a wavelength increment of 1 nm, with the number of scans of five. The data are presented as molar ellipticity (deg cm2 dmol−1) against wavelength. Heat-induced denaturation of these proteins was monitored by measuring CD spectra for the wavelength range of 208–222 nm with a step size of 1 nm and bandwidth of 1 nm, over the temperature range from 20 °C to 60 °C in 1 °C increments. Five scans at each temperature followed a 10-s equilibration period. Single-wavelength thermal melt curves were obtained by plotting the measured CD signals at 220 nm as a function of temperature. The curves were normalized by setting the minimum (most negative) CD value at 20 °C to 0 and the maximum (least negative) value at 60 °C to 1 for each protein. The melting temperature (Tm) was defined as the half-saturation point in a melt curve.
Episomal ARSENEL assay
Subconfluent 293T cells (ATCC) in 12-well plates were transfected with 300 ng of each ARSENEL reporter, 100 ng of each ARSENEL gRNA, and 300 ng of each base editor21,22. Transfections were performed using 1.8 μL TransIT-LT1 (Mirus) in 100 μL Opti-MEM (Thermo Fisher Scientific), incubated for 20 min at room temperature prior to addition to the cells. After 24 h, the transfected cells were PBS-washed, trypsinized, and analyzed by flow cytometry using a BD LSRFortessa instrument. For each condition, a minimum of 100,000 events were collected. Episomal editing efficiency was calculated as the proportion of eGFP⁺/mCherry⁺ double-positive cells relative to the total number of mCherry⁺ cells.
Chromosomal ARSENEL assay
293T cells were transduced with lentiviral vectors encoding each ARSENEL reporter (AC, CC, GC, TC)21,22 and selected with hygromycin to establish chromosomally integrated reporter pools. Following selection, >98% of cells in each pool were mCherry-positive by flow cytometry. For editing experiments, semiconfluent cells in 6-well plates were co-transfected with 200 ng ARSENEL gRNA, 100 ng pCMV-BFP (transfection marker), and 500 ng of each base editor. Transfections were performed using 2.4 μL TransIT-LT1 (Mirus) in 100 μL Opti-MEM (Thermo Fisher Scientific) and incubated for 20 min at room temperature before being added to cells. After 24 h, cells were washed with PBS, trypsinized, and analyzed on a BD LSRFortessa flow cytometer. At least 100,000 events were collected per condition. Chromosomal editing efficiency was calculated as the proportion of triple-positive (eGFP⁺/BFP⁺/mCherry⁺) cells relative to the BFP⁺/mCherry⁺ double-positive population.
Editing of the endogenous MUTYH locus
Ten million 293T cells harboring the chromosomally integrated TC dinucleotide ARSENEL reporter were seeded in 10 cm dishes to reach sub-confluency. Cells were co-transfected with 1 μg ARSENEL TC gRNA, 1 μg MUTYH gRNA, 1 μg pCMV-BFP (transfection marker), and 4 μg of each base editor using 21 μL TransIT-LT1 (Mirus) in 1 mL Opti-MEM (Thermo Fisher Scientific). The transfection mix was incubated for 20 min at room temperature before being added to the cells. After 24 h, cells were trypsinized, washed with PBS, and stained with Zombie Yellow viability dye (BioLegend). After 30 min, staining was quenched with FBS followed by an additional PBS wash. The entire sample was analyzed on a BD FACSAria Fusion flow cytometer, and GFP⁺ cells were sorted for downstream analysis. Cytotoxicity was assessed by calculating the percentage of Zombie Yellow–positive cells. Chromosomal editing efficiency was determined as the ratio of eGFP⁺/BFP⁺/mCherry⁺ triple-positive cells to the total BFP⁺/mCherry⁺ double-positive population. Genomic DNA was extracted from sorted cells, and the MUTYH locus was PCR-amplified, purified, and analyzed by Sanger sequencing. Frequencies of on-target and adjacent off-target C-to-T conversion events were quantified using EditR (http://baseeditr.com).
The guide RNA target sequence for MUTYH was 5’-TTCTCGTGGCCGGCGGCTGC-3’ and the sequencing primers were 5’-GTCTCAGAGGTCATGCTGC-3’ (forward) and 5’-GTGCTACGTTGCCATCCAC-3’ (reverse).
Immunoblotting
The remaining cells from flow cytometry experiments were collected for immunoblotting. The cells were PBS-washed and then lysed in RIPA buffer (ThermoFisher Scientific). A total of 24 samples were run on the same Criterion 4–20% gel (Bio-Rad), then transferred to a PVDF membrane. Primary antibodies were α-Tubulin Mouse mAb (#T5168, Sigma, 1:10,000), and SpCas9 Rabbit mAb (#Ab189380, Abcam, 1:10,000). Secondary antibodies used were goat anti-rabbit IRdye800 (LI-COR, #925-32211, 1:10,000) and goat anti-mouse IRdye680 (LI-COR, #926-69020, 1:10,000).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Atomic coordinates and structure factors for the SsdA-DNA complex and SsdA without DNA have been deposited in the Protein Data Bank (PDB) under the accession codes 9C64 and 9C63, respectively. Source data are provided with this paper.
References
Jurenas, D. & Journet, L. Activity, delivery, and diversity of Type VI secretion effectors. Mol. Microbiol. 115, 383–394 (2021).
de Moraes, M. H. et al. An interbacterial DNA deaminase toxin directly mutagenizes surviving target populations. Elife 10, e62967 (2021).
Mok, B. Y. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 (2020).
Guo, J. et al. A DddA ortholog-based and transactivator-assisted nuclear and mitochondrial cytosine base editors with expanded target compatibility. Mol. Cell 83, 1710–1724 e7 (2023).
Huang, J. et al. Discovery of deaminase functions by structure-based protein clustering. Cell 186, 3182–3195 e14 (2023).
Lee, S., Lee, H., Baek, G. & Kim, J. S. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat. Biotechnol. 41, 378–386 (2023).
Yin, L., Shi, K. & Aihara, H. Structural basis of sequence-specific cytosine deamination by double-stranded DNA deaminase toxin DddA. Nat. Struct. Mol. Biol. 30, 1153–1159 (2023).
Iyer, L. M., Zhang, D., Rogozin, I. B. & Aravind, L. Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 39, 9473–9497 (2011).
Takenaka, M. et al. DYW domain structures imply an unusual regulation principle in plant organellar RNA editing catalysis. Nat. Catal. 4, 510–522 (2021).
Vaisvila, R. et al. Discovery of cytosine deaminases enables base-resolution methylome mapping using a single enzyme. Mol. Cell 84, 854–866 e7 (2024).
Harris, R. S. & Dudley, J. P. APOBECs and virus restriction. Virology 479-480, 131–145 (2015).
Petljak, M. & Maciejowski, J. Molecular origins of APOBEC-associated mutations in cancer. DNA Repair 94, 102905 (2020).
Porto, E. M. & Komor, A. C. In the business of base editors: evolution from bench to bedside. PLoS Biol. 21, e3002071 (2023).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Venkatesan, S. et al. Perspective: APOBEC mutagenesis in drug resistance and immune escape in HIV and cancer evolution. Ann. Oncol. 29, 563–572 (2018).
Capasso, S., Mazzarella, L., Sica, F. & Zagari, A. Deamidation via cyclic imide in asparaginyl peptides. Pept. Res. 2, 195–200 (1989).
Aswad, D. W., Paranandi, M. V. & Schurter, B. T. Isoaspartate in peptides and proteins: formation, significance, and analysis. J. Pharm. Biomed. Anal. 21, 1129–1136 (2000).
Kato, K., Nakayoshi, T., Kurimoto, E. & Oda, A. Mechanisms of deamidation of asparagine residues and effects of main-chain conformation on activation energy. Int. J. Mol. Sci. 21, 7035 (2020).
Robinson, N. E. et al. Structure-dependent nonenzymatic deamidation of glutaminyl and asparaginyl pentapeptides. J. Pept. Res. 63, 426–436 (2004).
Xu, K. et al. Structure-guided discovery of highly efficient cytidine deaminases with sequence-context independence. Nat. Biomed. Eng. 9, 93–108 (2024).
Rieffer, A. E., Chen, Y., Salamango, D. J., Moraes, S. N. & Harris, R. S. APOBEC reporter systems for evaluating diNucleotide editing levels. CRISPR J. 6, 430–446 (2023).
St Martin, A. et al. A fluorescent reporter for quantification and enrichment of DNA editing by APOBEC-Cas9 or cleavage by Cas9 in living cells. Nucleic Acids Res. 46, e84 (2018).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846 (2018).
Kweon, J. et al. Efficient DNA base editing via an optimized DYW-like deaminase. Preprint at https://doi.org/10.1101/2024.05.15.594452 (2024).
Zhang, D. et al. Engineering a bacterial toxin deaminase from the DYW-family into a novel cytosine base editor for plants and mammalian cells. Genome Biol. 26, 18 (2025).
Shi, K. et al. Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat. Struct. Mol. Biol. 24, 131–139 (2017).
Shimizu, T., Matsuoka, Y. & Shirasawa, T. Biological significance of isoaspartate and its repair system. Biol. Pharm. Bull. 28, 1590–1596 (2005).
Ryttersgaard, C. et al. Crystal structure of human L-isoaspartyl methyltransferase. J. Biol. Chem. 277, 10642–10646 (2002).
Curnis, F. et al. Spontaneous formation of L-isoaspartate and gain of function in fibronectin. J. Biol. Chem. 281, 36466–36476 (2006).
David, C. L., Keener, J. & Aswad, D. W. Isoaspartate in ribosomal protein S11 of Escherichia coli. J. Bacteriol. 181, 2872–2877 (1999).
Mallagaray, A. et al. A post-translational modification of human Norovirus capsid protein attenuates glycan binding. Nat. Commun. 10, 1320 (2019).
Zhang, T., Hansen, K., Politis, A. & Muller, M. M. An unusually rapid protein backbone modification stabilizes the essential bacterial enzyme MurA. Biochemistry 59, 3683–3695 (2020).
Belica, C. A. et al. A real-time biochemical assay for quantitative analyses of APOBEC-catalyzed DNA deamination. J. Biol. Chem. 300, 107410 (2024).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Evans, P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. D Biol. Crystallogr. 67, 282–292 (2011).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
French, S. & Wilson, K. On the treatment of negative intensity observations. Acta Crystallogr. Sect. A 34, 517–525 (1978).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Shi, K. et al. Structural basis for recognition of distinct deaminated DNA lesions by endonuclease Q. Proc. Natl. Acad. Sci. USA 118, e2021120118 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Acknowledgements
X-ray diffraction data were collected at the Northeastern Collaborative Access Team beamlines of the Advanced Photon Source and the Center for Bio-Molecular Structure (CBMS) AMX beamline of NSLS-II, which are funded by the US National Institutes of Health grants NIGMS P30 GM124165 and P30 GM133893, respectively. This publication resulted from data collected during beamtimes obtained through NE-CAT BAG proposal #311950. This work was supported by NIH grants (NIGMS R35-GM118047 to H.A. and NCI P01-CA234228 to R.S.H. and H.A.) and a Recruitment of Established Investigators Award from the Cancer Prevention and Research Institute of Texas (CPRIT RR220053) to R.S.H. R.S.H. is the Ewing Halsell President’s Council Distinguished Chair at the University of Texas San Antonio and an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
L.Y. performed protein purification, crystallization, and biochemical analyses. K.S. performed crystallization, X-ray data collection, and structure analyses. Y.C., E.B.D., and R.S.H. performed base editing experiments. H.A. managed the project and wrote the first draft of the manuscript. All authors contributed to the editing of the manuscript and the generation of figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hiroshi Nishimasu, Yunbo Qiao and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yin, L., Chen, Y., Shi, K. et al. Structural basis for sequence context-independent single-stranded DNA cytosine deamination by the bacterial toxin SsdA. Nat Commun 16, 8841 (2025). https://doi.org/10.1038/s41467-025-63943-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63943-9