Abstract
Strain engineering is an effective approach to enhancing the activity of catalysts by tuning the electrical and geometric configurations. However, the impact of strain dimension on the sulfur redox kinetics and polysulfide adsorption configuration has yet been deciphered. Herein, we employ a biaxial-strained dichalcogenide catalyst with highly curved basal planes to activate the reserve metal atoms and realize efficient lithium-sulfur batteries. The high-dimensional strain enhances the exposure of Mo sites, thereby shifting the polysulfide adsorption mechanism from weak Li-S/Se bonding to strong S*-Mo bonding. Moreover, biaxial strain upshifts both d and p band centers, fostering the interfacial charge transfer and catalytic activity. Based on this mechanism, we obtain apparent correlations between biaxial strain and apparent activation energy for sulfur species conversion. This d-p hybridization dominated catalytic mechanism leads to obvious enhancement in capacity retention and rate performance. We showcase a 6 Ah-level multilayer pouch cell with a specific energy of 396 Wh kg–1 (based on masses of all components).
Similar content being viewed by others
Introduction
Lithium-sulfur battery (LSB) is notable due to the high theoretical specific capacity (1675 mAh g–1) and high specific energy (2600 Wh kg–1). However, the commercialization of LSBs is mainly plagued by the catastrophic lithium polysulfides (LiPSs, Li2Sx, 4 ≤ x ≤ 8) shuttling and sluggish reaction kinetics1,2,3,4,5. In the past decade, guided by the band theory, various electrocatalysts have been explored for sulfur cathode to enhance the reaction kinetics6,7,8. Strategies such as doping guest atoms, constructing heterostructures, alloying and amorphization, and introducing vacancies and strains have been reported to manipulate the band structure and modulate the active species adsorption and conversion kinetics9,10,11,12,13,14.
Fundamentally, the interaction between catalysts and active sulfur species can be mainly categorized as Li- or S- dominated orbital couplings. Recently, some descriptors have been proposed to direct the LSBs catalyst design, such as (1) the band (\(|\frac{{\varepsilon }_{{{\rm{p}}}}-{\varepsilon }_{{{\rm{d}}}}}{{\varepsilon }_{{{\rm{p}}}}}|+|\frac{{\varepsilon }_{{{\rm{s}}}}-{\varepsilon }_{{{\rm{p}}}}}{{\varepsilon }_{{{\rm{s}}}}}|\)) and lattice match (\(\frac{{r}_{{\mbox{Li}}-{\mbox{S}}}}{{r}_{{{\rm{catal}}}}}\)) indexes (for transition metal compounds), (2) the d–p coupling strength (εd/εp, for N4-coordinated metal single atoms), (3) the d orbital vacancies (for transition metal oxides), (4) p band center combined with subsurface metal cations Pauli electronegativity (\({\varepsilon }_{{{\rm{p}}}}\) + χm, for MXenes), (5) the p charge (for p-block metal sulfides), and (6) the number of valence electrons (for spinel sulfides)15,16,17,18,19,20. All of the these descriptors emphasize the impact of intrinsic band (electronic) factor on LiPSs adsorption and conversion. For a given catalyst, the band state is tuned by the crystal field, which splits the orbital into various configurations and synchronously influences the band center location, lattice parameters, as well as the electron spin states (for magnetic metals)13,21,22,23,24. It should be noted that the contribution degree of the band theory highly depends on the top surface atomic configuration; In other words, the d-band theory is expected to be inapplicable to the chalcogen atoms in metal chalcogenides.
Strain engineering is deemed a facile and effective route to tune the intrinsic activity of a catalyst material by modifying its electronic properties without changing the composition25,26,27,28,29. Till now, uniaxial strains such as in-plane tension, compression, and bending tension have been employed to alter the electron density around metal cations by adjusting the bond length or introducing vacancies30,31,32,33. Compared to these low-dimensional strains, biaxial strain provides a new dimension to tuning surface defects and optimizing the reactants adsorption configurations34,35. Particularly for nanostructured catalysts, biaxial strain is expected to endow the whole range of the basal plane with high activity by inducing vacancy formation (Fig. 1a).
2D metal dichalcogenides (such as MoS2, MoSe2, WS2, MoTe2, etc.), featuring atomically thin layers with high flexibility and strain sensitivity, are commonly employed as the strain loading matrices to catalyze various reactions36,37,38. In LSBs, catalytic reactions predominantly occur at the outer layer of the 2D catalysts. Hence, the interaction between LiPSs and 2D catalysts primarily arises from the Li s and chalcogen p orbital couplings, which is insufficient to anchoring LiPSs39,40,41. Although metal cations are considered to serve as Lewis acidic site to robustly bond with the S atoms in LiPSs (S*) via a stronger d–p hybridization in many compounds, metal cations in the 2D catalysts are mostly inactive on the basal planes, since the steric effect of the outer chalcogen sublayer shields metal catalytic activity (Fig. 1b)41,42,43. So far, there has been no systematic study dedicated to unlocking the contribution of the inner metal cations to sulfur redox on basal planes.
Here, to investigate the effects of the biaxial strain loading on the catalytic reaction in LSBs, molybdenum sulfide selenides (MSS) is selected as a case study37. As depicted by Fig. 1b, geometrically, biaxial strain enlarges the curvature of MSS surface and reduces the S and Se vacancies formation energies, triggering the homogeneous exposure of low-coordinated Mo sites on the basal plane. From the electronic aspect (Fig. 1c), biaxial strain elevates both the Mo d and S/Se p band centers (denoted as εd and εp, respectively) towards Ef, endowing the surface with sufficient sulphophilicity and lithiophilicity. Crucially, the vacancies induced by biaxial strain enhance the density of state (DOS) around Ef, ensuring the fast electrons transport. Different from the flat MSS, biaxially strained MSS unlocks the Lewis acidic Mo atoms; Hence, the polysulfides adsorption mechanism is transformed from weak Li-S/Se bonding to strong S*-Mo bonding. The experimental results indicate that such structural-electronic correlation becomes more pronounced when increasing the strain loading (herein, up to 1.8%), which endows the Li-S coin cells with improved capacity retention at 0.84 and 1.67 mA mgs–1 with the decay rates of 0.44‰ (200 cycles) and 0.12‰ (500 cycles) per cycle, respectively. The multilayer Li-S pouch cell with a low electrolyte-to-sulfur (E/S) ratio (3.5 μL mg–1) delivers high total capacity (~6 Ah), areal capacity (13.5 mAh cm–2), and specific energy (~396 Wh kg–1). This work may guide the rational design of high-dimensional strained catalysts for long-life conversion-type batteries.
Results
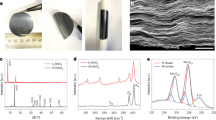
The biaxial strains with various degrees were introduced via the construction of spherical hollow MSS with different diameters via a hard template strategy (Fig. 2a and Fig. S1). Au nanospheres (NSs) with different sizes were first synthesized, followed by reacting with Mo(CO)6 to form a Mo outer layer (Fig. S2). Then, the Mo regions were treated by sulfurization and selenization to obtain Au@MSS shell (Fig. S3). After that, the inner Au core was etched by I/KI mixed solution. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images indicate the monodispersed hollow and spherical nanoshells with average diameters of 30.4 ± 3.6, 58.9 ± 2.9, and 119.8 ± 4.5 nm (Fig. 2b and Figs. S4–5). The uniform few-atomic-thick layers and the atomically curved MSS from the cross-sectional are clearly observed. Aberration-corrected high-angle annular dark-field scanning TEM confirms that the expanded lattice spacing of (002) planes (0.65–0.78 nm) compared with common MoS2. A closer inspection of the cross-sectional view of the nanoshell suggests the typical atomic arrangement of Mo, S, and Se in (002) plane with abundant Se and S vacancies (denoted as Sv, and Sev) evidenced by the dashed circles (Fig. S7). To investigate the biaxial strained atomic configuration, the geometric phase analysis is conducted (Fig. 2e, f and Fig. S6). The homogenous color in the strain mapping indicates that the atomic curvature generates a uniform strain distribution on different sizes of MSS spheres. The biaxial strain values were calculated around 1.8, 0.8 and 0.5%, respectively (denoted as MSS-1.8, MSS-0.8, and MSS-0.5). HAADF-STEM and the corresponding energy dispersive spectrometer (EDS) mapping images show the uniform distribution of Mo, S, and Se elements in circles (Fig. S4g). For comparison, the MSS plates without strain loading were also prepared via a facile hydrothermal method (Fig. S8, denoted as MSS-0). With the decreasing in diameter, the specific surface area evaluated by nitrogen adsorption-desorption isotherm increases from 227.8 (MSS-0.5), 254.4 (MSS-0.8), to 279.9 (MSS-1.8) m2 g–1, indicating more exposed active sites from larger biaxial strain (Fig. S9). Moreover, the Raman spectra show the A1g (226.9 cm–1) and E2g (449.5 cm–1) vibration peaks for 2H-MoSe2 and E12g (376.5 cm–1) and A1g (404.0 cm–1) for 2H-MoS2, verifying the structural nature of MSS (Fig. S10)35,44. Overall, the above results conclude that MSS with controlled biaxial strain has been successfully synthesized.
a Schematic of the morphologies of MSS with different biaxial strain loadings. b TEM, c, d AC-HAADF-STEM images of MSS-1.8. e, f HR-TEM and corresponding GPA images. g Comparison of the Sv and Sev formation energies on MSS with/without strain loading. h The FT-EXAFS k3χ(R) spectra of different samples and i the EXAFS wavelet transform analysis. j Calculated TDOS and PDOS of Mo-3d band in MSS-0 and MSS-1.8.
The influence of biaxial strain on geometric structure was firstly investigated by electron paramagnetic resonance measurements (Fig. S11). The intensity of the peak signal near the g value of 2.004 increases with the order of MSS-0, MSS-0.5, MSS-0.8, MSS-1.8, demonstrating that larger biaxial strain induces more anion vacancies. Correspondingly, the simulated formation energies of S and Se vacancies (denoted as Sv, and Sev) on biaxial strained MSS surface (<2 eV) are much lower than that on MSS-0 (>8 eV), confirming that strain could trigger the construction of Sv and Sev (Fig. 2g). The electronic structure and chemical environment of MSS were studied by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near-edge spectroscopy (XANES). The elemental stoichiometric ratios for MSS with different strain loadings are roughly around Mo:S:Se = 1:1.6:0.4 (Fig. S12a). With increased degree of biaxial strain, the signals of Moδ+ (δ < 4, ~228.1 eV, belongs to nonstoichiometric deficient MoS2 or MoSe2) in Mo 3d spectra becomes more apparent because of the rising content of Sv and Sev11,45,46. The appearance of Mo6+ in strained MSS can be attributed to the more delocalized local electric structure or surface oxidation (Fig. S12b)35. Such phenomena can also be reflected by the positive shift of both S 2p and Se 3d signals (Fig. S12c, d). In the Mo K-edge XANES spectra (Fig. S13a, b), the strained MSS exhibits the absorption edges between Mo foil and MoS2 references, implying that the average valence of Mo cations is between 0 and +4. In addition, there is a positive shift with the strain loading increase, which is attributed to the formation of Mo6+. Such a tendency is in accordance with the XPS results. From the extended X-ray adsorption fine structure and the corresponding wavelet transform (Fig. S2h, i, Fig. S13c–f, 14 and Table S1), the coordination numbers of the sum of Mo-S and Mo-Se in MSS-0.5, MSS-0.8, and MSS-1.8 are 5.2, 4.5, and 3.8, respectively, much lower than that (6) in pure MoS2 and MoSe2. This trend reveals that the increased strain results in weakened Mo-S and Mo-Se bonds and thus creates more anion vacancies. Notably, the peaks intensities of Mo-S (~2.40 Å), Mo-Se (~2.55 Å), and Mo-S/Se-Mo (~3.16 Å) bonds of MSS in R space decrease with increasing strain loadings, which is also indicative of the elevated concentrations of Sv and Sev.
Density functional theory (DFT) calculations were employed to unveil the impact of the biaxial strain on band structure. From the total DOS shown by Fig. 2j and Fig. S15, MSS-1.8 showcases typical conductor band structure with the Ef lying within a continuous band of states, implying a higher electron conductivity compared with that of MSS-0 (bandgap around 0.3 eV). For Mo atoms, the εd shifts from –1.898 eV (MSS-0) to –1.770 eV (MSS-1.8) with a more obvious orbital splitting, which is due to the re-arrangement of the electrons on vacancies-induced unsaturated Mo sites6,35. Similarly, the enhanced εp of S (from –3.027 to –2.972 eV) and Se (from –2.650 to –2.591 eV) can also be obtained in biaxially strained MSS (Fig. S16). The upshifts of both εd and εp are expected to render stronger LiPSs affinity via d–p hybridization with S*, and s–p hybridization with Li, respectively47.
We also calculated the interactions between MSS and LiPSs. Figure 3a, b presents the optimized molecular configurations of Li2S4 adsorption on the (002) facet of MSS-0 and MSS-1.8. Specifically, two Li atoms in Li2S4 tend to attach to the surficial S and Se atoms on MSS-0, while four S atoms remain a chain-like arrangement with S–S connection. On MSS-1.8, the S* are attracted to the exposed Mo sites. This changed geometric adsorption configuration on biaxially strained surface can also be found on other LiPSs molecules and Li2S2/Li2S (Figs. S17, 18). Compared to MSS-0, MSS-1.8 exhibits much lower adsorption energies toward various sulfur species (<–6 eV) (Fig. S19). Such elevated chemical interactions between the MSS and LiPSs is evidenced by the charge density difference analysis (Fig. S20). The charge transfer between MSS-1.8 and the Li2S4 cluster is much more pronounced compared to MSS-0. An obvious charge accumulation can be observed between Li and S in MSS-0, indicating the formation of Li-S bond. Similar charge density evolution also occurs between Mo in MSS-1.8 and S* atoms, in which the Lewis acidic Mo cations after adsorption are surrounded by a high electron density area along with the strong Mo-S* bonds43,48,49,50. A clearer illustration is provided by the Electron Localization Function 2D cross-sections (Fig. 3c, d), where the interface between MSS-1.8 and Li2S4 exhibits a higher degree of electron localization than that of MSS-0. As schematically shown in Fig. 3e, such transformation from Li-S/Se to Mo-S* dominated anchoring effect also occurs in the cases of Li2S6 and Li2S (Figs. S21, 22).
Optimized Li2S4 adsorption configurations on a MSS-0 and b MSS-1.8, with the corresponding c, d sliced 2D maps of the Electron Localization Function (ELF) (blue regions indicate electron depletion, and yellow regions indicate electron accumulation). e Schematic of the orbital hybridizations on MSS-0 and MSS-1.8. f XPS spectra of S 2p of Li2S6 and MSS before and after adsorption. In situ Raman spectra of the cells with g G/MSS-0 and h G/MSS-1.8. i PDOS of S 2p orbital in pristine Li2S4 and in the Li2S4 anchored on MSS-0 and MSS-1.8. j Comparison of the Li-S* bond length in Li2S4 before and after adsorption (Error bars represent standard deviation from three different bonds in the simulated models). k Simulated Li2S dissociation barriers and the corresponding Li+ pathways on MSS-0 and MSS-1.8.
In our experimental investigation, Li2S6 was selected to represent LiPSs since it has a higher solubility than Li2S4 and is prone to disproportionate in DOL/DME-based electrolyte (1,3-dioxolane/Ethylene glycol dimethyl ether, 1/1 v/v)51,52. From the comparison of the Mo 3d spectrum of MSS-1.8 after Li2S6 adsorption, the increased content of the Mo with low valence (Moδ+) in MSS-1.8 suggests the enhanced electrons densities. Meanwhile, the contribution of Mo in MSS-0 for adsorption is much lower with the negligible change of binding energy (Fig. S23a, b). Correspondingly, in the high-resolution S 2p spectrum (Fig. 3f), the upshift of terminal S atoms (ST2–, from ~161.4 to 162.6 eV) and bridge S atoms (SB2–, from ~162.9 to 164.2 eV) in Li2S6 indicate the loss of electrons49,50. This trend is also showcased by the S in MSS-1.8 (from ~163.9 to 164.1 eV) with a more pronounced shift than that in MSS-0 (from ~162.17 to 162.2 eV). With such intensified Li-S and Mo-S* dual interactions, MSS-1.8 renders a higher sulfur species fixation, as also supported by the adsorption test (Fig. S23c). Besides, based on the COMSOL simulation, the 120 nm-diameter vacancies-free MSS HS renders similar electric field gradient and LiPSs concentration distribution to those of the 30 nm-diameter vacancies-free MSS HS at 2.1 V (Fig. S24). By contrast, after introducing anion vacancies, the LiPSs concentration increases on the 30 nm-diameter MSS HS surface with intensified electric field. Thus, compared with the surface curvature, vacancies induced by the biaxial strain make dominating enhancement effect in the LiPSs capturing.
To investigate the shuttle effect regulation, in situ Raman spectroscopy with the signals collected from the separator/anode interface was conducted. In Fig. 3g, h and Fig. S25, the sulfur cathodes with bare graphene and G/MSS-0 demonstrate intense peaks located at ~215 (S82–), ~ 400 (S42–/ S62–), and 453 cm–1 (S42–/ S32–), which is due to the diffusion of LiPSs from cathode to the Li anode15,53. In sharp contrast, no obvious signals corresponding to LiPSs can be identified in the cell with G/MSS-1.8 during both the discharge and charge processes, verifying the effective shuttle effect alleviation.
To unveil the underlying LiPSs anchoring mechanism, the electronic structure was simulated with special emphasis on the Mo d, Li s, S* p, and S p-orbitals before and after Li2S4 and Li2S6 adsorption. From the projected density of state (PDOS) of Mo d-orbital, an obvious upshift of antibonding orbital can be identified in MSS-1.8, which signifies the strong orbital hybridization with enlarged energy splitting between antibonding and bonding orbitals (Fig. S26a)13,21. Correspondingly, the px and pz orbitals of S* largely overlap with the d-orbitals of Mo (especially for the regions marked by red rhombuses), which triggers the formation of twofold bonding (π and σ) and twofold antibonding (π* and σ*) states7,54. Meanwhile, there is no significant change of the Mo d band in MSS-0 after Li2S4 adsorption and the overlap level between Mo d and S* p, implying a much weaker bond (Fig. S26b). For the Li2S4 on MSS-1.8, the S* p-orbital shows more continuous distributions with the evident electronic concentration reduction around Ef when interacting with MSS-1.8, inferring that the electrons from the S* p-orbital were transferred to the unsaturated Mo d-orbital via a strong d–p orbital hybridization (Fig. 3i). A similar tendency can also be noticed in the cases of Li2S and Li2S6 adsorption (Figs. S27, 28). From the PDOS of Li s-band, the increased electronic concentrations below Ef validates the enriched electrons from S/Se via the s–p hybridization on both MSS-0 and MSS-1.8. A more continuous PDOS of Li on MSS-1.8 points out a tougher s–p hybridization than that on MSS-0 (Fig. S27a)55. Above results align with the XPS results. Moreover, during the LiPSs and Li2S adsorption, Ef lies across the total DOS of MSS-1.8, which guarantees the fast electrons conductivity during the redox process (Fig. S27c, d, and Fig. S28c). Apart from the bond strength, biaxial strain on MSS also influences the stability of the LiPSs molecules. Compared with Li-S bond lengths in pristine Li2S4 (~2.347 Å) and the Li2S4 on MSS-0 (~2.445 Å), the one in Li2S4 on MSS-1.8 is prolonged to ~2.727 Å. Similarly, the Li-S bond in Li2S was extended from ~2.086 to 2.557 Å after absorbed on MSS-1.8 (Fig. 3j). Such weakened Li-S bond is favorable for the dissociation of both soluble LiPSs and solid Li2S, promoting the phase conversion kinetics54. Moreover, the climbing image-nudged elastic band (CI-NEB) method analysis show that the Li-S bond decomposition barrier in solid Li2S is 0.18 eV on MSS-1.8, much lower than that on MSS-0 (0.93 eV), suggesting the improved decomposition ability of Li2S on MSS-1.8 (Fig. 3k and Fig. S29). In brief, curved MSS surface with abundant Sv and Sev simultaneously regulates the band structure and the exposure degree of Mo sites, where the strong d–p hybridization becomes the dominant interaction when trapping LiPSs. Such behavior is hardly realized in traditional flat 2D catalysts or those loaded low dimensional strains14,31. From the dynamic aspect, the emerged S*-Mo interactions gifted by biaxial strain further extends the Li-S bonds of LiPSs and reduces the dissociation barrier.
To better evaluate the mitigation of LiPSs shuttling in LSBs, the cells loaded with different catalysts were assembled with graphene as the conductive agent (denoted as G/MSS). During the discharge process at 0.167 mA mgs–1, all the cells yield two plateaus at approximately 2.3–2.4 V, and 2.1 V, which represent the generations of chains-like LiPSs, and solid Li2S2/Li2S, respectively (Fig. S30a). The sulfur specific capacity contributed by the high plateau (qH) of the cell with MSS-1.8 is 407.8 mAh g–1, illustrating that approximately 4 electrons are accepted by S8 molecule to form Li2S4 (Fig. S30b). Additionally, the cell with G/MSS-1.8 shows reduced overpotential and longer plateaus than other samples, implying a more complete conversion from sulfur to Li2S with lower residual LiPSs concentration in the electrolyte19,56. Based on the qH value and charge efficiency, the shuttle constant (ks) of 0.035 h–1 was obtained by G/MSS-1.8. Compared with the cells with G/MSS-0.8 (0.049 h–1), G/MSS-0.5 (0.056 h–1), and G/MSS-0 (0.069 h–1), a lower overcharge is required by G/MSS-1.8 to achieve complete oxidation of LiPSs to sulfur (Fig. 4a). The observed polarization in the voltage profiles exhibits a positive relationship with ks (Fig. S30c), indicating that higher biaxial strain degree results in enhanced charge efficiency and sulfur utilization.
a Comparison of ks for different catalysts. b Activation barriers at given discharge voltages for different catalysts. c Apparent activation energies of the LiPSs-Li2S conversion for different catalysts with corresponding activation entropy terms calculation. d Potentiostatic discharge curves and e corresponding SEM images of Li2S nucleation on different catalysts. Scale bar: 1 μm. f Typical DRT plot of LSB and g DRT plots (range from 10–6 to 100 s) of the cells at different charge-discharge states. h Contour maps of CV curves at various scan rates of the cells with G/MSS-1.8. i In situ XRD patterns of the cells with G/MSS-1.8 within one cycle. j Linear regression fitting between biaxial strain loading and Ea and ΔS0*. Error bars represent standard deviation from three independent cells.
Insights into the conversion reaction kinetics can be provided by linear sweep voltammetry (LSV) measurements. As shown in Fig. S31, the MSS-1.8 cell shows the highest onset potential and diffusion-limited current density in both sulfur reduction reaction (SRR) and sulfur oxidation reaction (SOR). Among all the tested samples, the Tafel slopes of MSS-1.8 give the lowest values of 48.4 and 58.8 mV dec–1 for SER and SOR, respectively, corresponding to accelerated reaction kinetics of MSS-1.8 for Li2S oxidation and elemental sulfur reduction57. Such enhanced SRR/SOR kinetics also aligns with the reduction in polarization overpotential on the MSS with higher strain loadings (Fig. S32). From the cyclic voltammetry (CV) curves of the Li2S6-based symmetric cells with different catalysts, the electrode with MSS-1.8 manifests higher peak current response and lower polarization compared with MSS-0.8, MSS-0.5 and MSS-0, corroborating the improved LiPSs redox kinetics (Fig. S33)15,47.
To quantify the sulfur redox kinetics on different catalysts, the activation energy barriers are obtained from the Arrhenius plot of charge-transfer resistance (Figs. S34, 35). The test was conducted at three voltage values of 2.4, 2.0, and 1.7 V to investigate the elemental S8 reduction, LiPSs redox, and Li2S oxidation, respectively9,19. The derived energy barrier at 2.4 V is 0.33 eV for the cell with G/MSS-1.8, lower than that of G/MSS-0.8 (0.43 eV), G/MSS-0.5 (0.49 eV) and G/MSS-0 (0.51 eV) (Fig. 4b). Similar trend is observed at 2.0 V and 1.7 V. Notably, at 2.0 V, all the samples have maximum barrier values, suggesting that the conversion from soluble LiPSs to solid Li2S2/Li2S is the rate determining step17,19. Since the sluggish nucleation rate of Li2S2/Li2S will lead to the LiPSs accumulation in the electrolyte and further results in the sulfur loss, the observed lower energy barrier fluctuation on MSS-1.8 from 2.4 to 1.7 V (0.33-0.42-0.24 eV) infers that biaxial strain could elevate the sulfur usage efficiency during the whole redox process. Similarly, from the reduction (cathodic) peak located at around 2.0–2.1 V (corresponding to the conversion from S42–/S62– to S2–) in the CV profiles, apparent activation energies (Ea) are obtained from the Arrhenius plot (Fig. S36). The order of Ea is G/MSS-1.8 < G/MSS-0.8 < G/MSS-0.5 < G/MSS-0 (Fig. 4c), which is in good agreement with the activation barrier obtained from the EIS tests47. According to the transition state theory, in a reaction involving significant adsorbate interaction, Ea will be augmented by the differential heat of adsorption, while the frequency factor is elevated by the activation entropy term (ΔS0*)58,59. A positive linear relationship between the biaxial strain degree, Ea, and ΔS0* is obtained (Fig. S37). Regarding the compensation effect, the higher biaxial strain creates more active sites for LiPSs adsorption, which results in a facilitated reaction by reducing the vibrational and rotational freedom of captured LiPSs17,19. Such propelled Li2S nucleation and oxidation rate on MSS-1.8 is evidenced by the smaller ΔRinternal size in galvanostatic intermittent titration profiles (Fig. S38). The trend is also manifested by the potentiostatic Li2S precipitation and dissolution test results (Fig. 4d, e and Figs. S39, 40). From the distribution of relaxation time (DRT) based on the in situ EIS test (Fig. 4f, g and Fig. S41), an inverse relationship between the diffusion impedance (region 1 and 2) and the state of charge (SOC) occurs in both cells, corresponding to the formation/dissolution of solid and insulating Li2S60. At the end of discharge (i.e., 0% SOC), the cell with MSS-1.8 exhibits a faster appearance and disappearance of the charge transfer resistance (Region 3) together with lower values, indicating the facilitated Li2S redox kinetics61,62.
From the contour maps of CV curves at various scanning rates (Fig. 4h), G/MSS-1.8 demonstrates higher current densities for two cathodic peaks and one oxidation peak compared to G/MSS-0 and G. For all these peaks from different samples, a diffusion limited process can be inferred (Fig. S42). According to the Randles–Sevcik equation (eq. (8) in Supplementary Information), MSS-1.8 delivers the highest Li+ diffusivity, which can be attributed to its enhanced ability to adsorb LiPSs and promoted redox kinetics, thereby avoiding the electrolyte viscosity increase and catalyst passivation. In addition to the LiPSs concentration, the desolvation process also contribute to Li+ transport. In bulk electrolytes, as shown in Raman spectra (Fig. S43), Li+ is predominantly coordinated by DME, as indicated by the apparent peak around 870 cm–1, which counts for 45.5% of the total DME molecules63. With the addition of MSS, the fraction of free DME (850 cm–1) increases with the weakening of Li+-DME coordination. Compared with MSS-0 (32.8%) and MSS-0.8 (27.5%), MSS-1.8 displays much lower Li+-DME interaction (22.6%). This trend is justified by the radial distribution functions (RDF) of Li+ (Fig. S44), where the g(r) values of Li-TFSI and Li-DME decrease after the participation of MSS-1.8 in the solvation sheath. The diminished interactions between Li+ and electrolyte species can be assigned to the improved lithiophilicity of MSS-1.8 with enhanced p-band center and the puncture effect formed by surface curvature. This would trigger the promoted Li+ desolvation via Li-S/Se bonds formation, benefiting the subsequent redox reaction64,65. Such tendency is reflected by the reduced contribution of double-layer relaxation impedance in the cell with MSS-1.8 during cycles (region 5 in DRT plots, Fig. 4g). The in-situ XRD patterns (Fig. 4i and Fig. S45) reveal the diffraction peak of Li2S (around 27.2°, JCPDF No. 23–0369) from the end of discharge to the beginning of charge process in the G/MSS-1.8. However, there are no obvious Li2S signals in the G/MSS-0 cathode within a cycle. Hence, we may infer that biaxial strain has effectively reduced the redox barrier and enhanced the sulfur utilization10,66,67.
Thereupon, under the hypothesis that catalytic activity is linked to the excitation of an electron to a localized state, we intend to correlate the biaxial strain loading to the thermal dynamic indexes of catalytic effect on MSS. As depicted in Fig. S46, linear correlations of strain value with Mo coordination number (R2 = 0.93) and with vacancy formation energy (R2 = 0.90 for Sv, R2 = 0.95 for Sev) can be achieved. Similarly, based on our previous studies, a higher strain dimension or a lower coordination number of cations in 2D catalysts will lead to an intensified upshift degree of metal d-band center, due to the strengthened electronic delocalization34,35. Hence, a relation chain can be constructed, namely, biaxial strain induces anion vacancies with a reduction in cation coordination number, exposing the cations with higher d-band center. Consequently, the biaxial strain loading induces a structure-electron relationship (Fig. 4j): Ea = –11.3∑(εx, εy) + 30.5; ΔS0* = –35.1∑(εx, εy) – 148.8.
The electrochemical performance of the LSBs was investigated by assembling coin cells with bare graphene, G/MSS-0, and G/MSS-1.8 in sulfur cathodes (see more details in “Method”). As shown in Fig. 5a, The cathode with G/MSS-1.8 delivers reversible discharge capacities of 1354, 975, 808, 705, and 623 mAh g–1 at 0.34, 0.84, 1.67, 3.3, and 6.7 mA mgs–1, respectively, higher than those with G/MSS-0 and G. When the current switches back to 0.84 mA mgs–1, a discharge capacity of 848 mAh g–1 is recovered. The corresponding voltage profiles of G/MSS-1.8 show more distinct and longer redox plateaus under the high rates compared with those of G/MSS-0 (Fig. S47), illustrating the boosted kinetics with higher sulfur utilization. Meanwhile, the increase in voltage polarization (ΔEp) with the current rate is slower in G/MSS-1.8 compared to that in G/MSS-0 (Fig. S48). From the galvanostatic charge-discharge process at 0.84 mA mgs–1 (Fig. 5b), the G/MSS-1.8 cell exhibits a high initial discharge capacity of 908 mAh g–1, which only slightly drops to around 829 mAh g–1 after 200 cycles, higher than that of bare G (482 mAh g–1) and G/MSS-0 (515 mAh g–1). In the long-term cycling test at 1.67 mA mgs–1, the cell with G/MSS-1.8 retains a reversible capacity of 682 mAh g–1 after 500 cycles, corresponding to a decay rate of 0.44‰ per cycle (Fig. 5c). The good reproducibility of the cell performance with MSS-1.8 catalyst was also showcased in Fig. S49. The advantage in capacity maintenance in coin cells with MSS-1.8 is illustrated by comparing with the previously reported catalysts (Table S4).
a Rate performance, and the cycling tests at b 0.5 C and c 1 C for the coin cells with the skip points of 3 and 5, respectively (Areal sulfur loading: 1.0 ~ 1.2 mg cm–1). d Cycling test of the coin cells with high areal sulfur loadings. The cycling stability of e single layer and f multilayer Li-S pouch cell with MSS-1.8.
Cell performance at increased sulfur loading is investigated. When the areal sulfur loading increases to 5 mg cm–2 under the E/S ratio of 7 μL mg–1 (Fig. 5d), the cathode with MSS-1.8 catalyst shows an initial capacity of 736 mAh g–1 (3.7 mAh cm–2) at 0.84 mA mgs–1 and a capacity retention of around 96% after 100 cycles (711 mAh g–1). When we continuously enhanced the areal sulfur loading to 6 mg cm–2 and reduced the E/S ratio to 5 μL mg–1, the areal capacity obtained at 0.084 mA mgs–1 is 8.8 mAh cm–2 (Fig. S50). After cycling at 0.34 mA mgs–1 for 60 cycles, an areal capacity of 6.3 mAh cm–2 with 94% capacity retention is achieved (Fig. 5e). Furthermore, a single layer Li-S soft packed pouch cell with ~0.7 g sulfur loading was assembled with an E/S ratio of 5 μL mg–1. The cell delivers a 0.9 Ah total capacity (~16 mAh cm–2) in the first cycle and around 8.3 mAh cm–2 after 45 cycles (Fig. 5f, g, Fig. S51 and Table S3). To further push the capacity, we assembled a multilayer pouch cell with a sulfur loading of 4.27 g (7.15 Ah capacity in theory) and a low electrolyte content (3.5 μL mg–1). The pouch cell reaches a total capacity of 6.11 Ah (85.5% sulfur utilization) at the initial discharge process with a practical specific energy of 396 Wh kg–1 (Figs. S52, 53 and Table S4). These coin cells and pouch cells with high sulfur loadings are well-placed among many reported cells (Fig. S54 and Tables S5 and S6), illustrating the potential of such high-dimension strained catalyst in grid-scale LSBs.
Discussion
We have demonstrated that biaxial-strained metal chalcogenides catalysts can efficiently boost the kinetic and stability performance of LSBs. Biaxial strain unlocks the inner Mo layer with upshifted d band center by inducing abundant anion vacancies on uniformly curved surface of the catalyst. As a result, the interaction between LiPSs and catalyst surface changes from the weak s–p hybridization to strong d–p hybridization. Owing to such a structural-electronic correlation, the biaxial strain can be a single geometric descriptor for predicting the sulfur species redox kinetics. The highest catalytic efficiency is realized by the metal chalcogenides catalyst with the maximum biaxial strain degree (1.8% in this work), which presents lower Ea with stronger LiPSs capture ability compared with other lower strained samples. The fabricated LSBs deliver improved cycling stability (decay rate is reduced by more than threefold) together with high rate performance (623 mAh g–1 at 6.7 mA mgs–1). The Li-S pouch cell with a sulfur loading >9 mg cm–2 and lean electrolyte (3.5 μL mg–1) showcases a specific energy of 396 Wh kg–1 (based on masses of all components) and maintains a total capacity around 3 Ah after 40 cycles. This work illustrates the positive effect of strained 2D nanocatalysts in boosting the reaction kinetics of conversion-type batteries.
Methods
Chemicals and materials
Chloroauric acid (HAuCl4, 99.9%), silver nitrate (AgNO3, ≥99.8%), ethylene glycol (EG, ACS reagent), poly-(diallyldimethylammonium) chloride (PDDA, Mw = 100 000 − 200 000, 20 wt% in water), sulfur powder (S, 99.9%), selenium powder (Se, 99.95%), molybdenum hexacarbonyl (Mo(CO)6, 98%), iodine (I, 99.8%), and potassium iodide (KI, 99%) were purchased from Sigma-Aldrich. Carbon nanotubes (CNT) were produced by XFNANO Tech Co., Ltd. Deionized (DI) water with a resistivity of 18.25 MΩ was generated by a Milli-Q water purification system. All chemicals were used without further purification.
Synthesis of Au nanospheres
A homogenous 60 mL of EG solution consisting of HAuCl4, PDDA, AgNO3, and HCl with concentrations of 0.5, 25, 0.01, and 1 mM, respectively, was maintained in an oil bath at 220 °C for 2 h to obtain Au nanospheres with a diameter of ~56 nm. Au nanospheres with a diameter of ~30 nm was synthesized with the above strategy and saturated oxygen (O2) in reaction solution. Moreover, larger Au nanospheres (~116 nm) were obtained at a lower reaction temperature of 200 °C with other parameters unchanged. Finally, the obtained products were washed 3 times with deionized water and collected by centrifugation at 14,100 ɡ.
Synthesis of biaxial strained MSS hollow spheres
5 mg of Au nanospheres were loaded on 20 mg of graphene in 10 mL of DI water, followed by sonication until a clear solution and dark red solid (graphene loaded with Au nanospheres) formed. After removing the DI water, the products were then heated in an oven at 60 °C for 5 h to obtain dried powder. 20 mg of powder underwent thermal treatment in a tube furnace at 550 °C for 0.5 h with 10 mg of Mo(CO)6 at the upstream of N2 atmosphere to synthesize AuMo alloy/graphene powder. Then the AuMo alloy/graphene was treated with Se and S mixed powder at 550 °C for another 0.5 h in N2 atmosphere, forming the Au@MSS/graphene structures. After removing Au core with I/KI mixture solution for 24 h, biaxial strained MSS hollow spheres with graphene (G/MSS) were obtained in large-scale.
Synthesis of the mixture of sulfur and G/MSS
100 mg as prepared G/MSS (with different strain loadings) mixture was grinded with sulfur powder at a mass ratio of 1/3, followed by heating at 155 °C in a sealed glass bottle (ampoule) for 30 h to guarantee the infusion of sulfur. The final sulfur content is around 75 wt%.
Electrodes fabrication and cells assembly
The cathode slurry was fabricated in a mortar with pestle based on mixing sulfur loaded G/MSS with poly(vinylidene fluoride) at mass ratio of 9:1, and N-Methyl-2-pyrrolidone was employed as the solvent. After that, the slurry was uniformly coated on carbon-coated Al foils by using a doctor blade on an automatic coating machine (MSK-AFA-II-VC, MTI). Then, the coated foil was dried at 55 °C in a vacuum oven for 12 h. By changing the coating thickness, the average areal sulfur loading was measured around 1.0–1.2, 5.0, and 6.0 mg cm–2 for the coin cell test. CR2032 coin cells were assembled based on the punched circled cathode (punching machine: MSK-T10, MTI), Celgard 2400 polypropylene (PP) separator, and a lithium metal foil. The electrolyte composition is 1 M LiTFSI in DOL/DME mixed solvent (1/1 v/v) with 2 wt% LiNO3 addition. For the cathode with sulfur loading around 1.0–1.2 mg cm–2, the E/S ratio was controlled at 15 μL mg–1. The E/S ratios were 7 and 5 μL mg–1 for the electrodes with areal sulfur loadings of 5.0 and 6.0 mg cm–2, respectively. Regarding the single layer pouch cell, the sulfur cathode includes rGO/S mixture (80 wt%, with mass ratio rGO/S = 1/7), MSS-1.8 (5 wt%), Super P (5 wt%), and CMC-SBR binder (5 wt%, arboxymethyl cellulose sodium, styrene-butadiene rubber, with mass ratio 1/1 in 45 wt% water solution) were mixed to obtain the slurry with the addition of water. For the multilayer pouch cell sulfur cathode, CNT/S composites (90 wt%, with mass ratio CNT/S = 1/9), conductive agent (1.67 wt%, rGO), MSS-1.8 (3.33 wt%), and LA133 binder (5 wt%) in a solid content of 1 g 5 mL−1 (H2O) were thoroughly ground and subsequently homogeneously coated on both sides of Al foils. In the pouch cell, Li foils (thickness 150 μm) served as the anodes, PP films (Celgard 2500) were utilized as separators, and aluminum-plastic film was employed as the packaging material. The electrolyte comprised DOL/DME (1:1 by volume) with 1.0 M LiTFSI and 3.0 wt% LiNO3 additives. The assembly process involved a multi-layer superposition technique, with both sides of the cell featuring lithium foil due to the double-coated cathode configuration. The detailed parameters of pouch cells are presented in Supplementary Information.
Electrochemical measurements
The cycling test for the cell was carried out within a charge–discharge potential range of 1.7–2.8 V (versus Li/Li+) on a LAND battery test system (CT3002AU, 5 V 2 mA, & 20 mA; CT3002K, 5 V 1 A & 6 A, Wuhan, China). The polarization of the cell was calculated based on the middle value voltage in the second cycle at 0.167 mA mgs–1, the specific capacity of the coin cells was calculated based on the mass of sulfur in the cathode. The ks was calculated from the charge and discharge capacities to analyze the LiPSs shuttling degree quantitatively. The Ea values for the liquid-solid conversion (LiPSs to Li2S) were obtained based on the fitting result of temperature-dependent CV peak current (j) by following the Arrhenius equation. Error bars were calculated based on measurements conducted on three independent cells, representing the standard deviations. CV, LSV, Li2S deposition/dissolution tests were performed on an electrochemical workstation (CHI760E, CH Instruments). Specifically, for the Li2S6 symmetric cell test, scan voltage was set within the range from –0.8 to 0.8 V, and the corresponding electrolyte was prepared based on dissolving 0.5 M Li2S6 in 1 M LiTFSI in DOL/DME mixed solvent (1/1 v/v). For the Li2S precipitation/dissolution test, electrolyte contains 0.5 M Li2S8 with 1 M LiTFSI in DOL/DME mixed solvent (1/1 v/v). In LSV test, SRR investigation was performed based on the electrolyte with 4 mM S8 in in DOL/DME mixed solvent (1/1 v/v), and SOR reaction was estimated in the 0.1 M Li2S/methanol electrolyte. The working electrode in above three tests was prepared by uniformly loading the different catalysts (MSS-0, MSS-0.5, MSS-0.8, and MSS-1.8 without graphene) on carbon paper (CP) with an areal loading around 1 mg cm–1. For the electrochemical Impedance Spectroscopy (EIS) tests at various temperatures for given voltages were conducted in the frequency range from 0.1 to 100 kHz based on the potentiostatic mode at an amplitude voltage of 5 mV. To maintain the voltage, each cell was discharged to the desired potential at 0.167 mA mgs–1, followed by a chronoamperometry process until the current became stable. Data are presented as mean ± standard deviation from three independent measurements. A quasi-stationary potential was applied for the in situ EIS test. The number of data points (per decade of frequency) is 12. All the specific capacities of the cells were normalized based on the weight of sulfur. Some specific detailed testing procedures were introduced in the corresponding Supplementary Figs. captions. Except for the temperature-dependent measurements, all the electrochemical tests were conducted at 25 ± 3 °C.
Characterization methods
The crystalline structures of the samples were determined using a Bruker D8 Advance diffractometer (Cu Kα radiation, λ = 0.15418 nm) with a scan rate of 5° min–1. Surface morphologies of the various samples were characterized by scanning electron microscopy (SEM, ZEISS SUPRA®55) equipped with an EDS. HRTEM images were acquired using a field-emission transmission electron microscope (FE-TEM, Talos F200X, FEI) with energy-dispersive X-ray spectroscopy (EDS) at 200 kV. Aberration-corrected high-angle annular dark field scanning TEM (HAADF-STEM) images were obtained using the Titan Themis G2 STEM at 300 kV. X-ray absorption fine structure (XAFS) data were collected at the BL14W1 XAFS beamline and analyzed using the Athena program. XPS measurements were conducted using a Thermo Fisher ESCALAB 250Xi. Electron spin resonance (ESR) spectra were acquired via a Bruker EMX plus 10/12 spectrometer, equipped with an Oxford ESR910 Liquid Helium cryostat, at the Steady High Magnetic Field Facilities. Raman spectra were recorded on a HORIBA LabRam HR800 Raman spectrometer with 532 nm laser excitation, covering the range of 200–2000 cm−1. Specific surface areas were measured by using a gas adsorption analyzer (Micromeritics ASAP 2020). The absorption spectra of Li2S6 in DOL/DME (1:1 v/v) solution were obtained using ultraviolet-visible (UV–vis) spectroscopy (UV-2550, Shimadzu), with a blank solution serving as the reference.
Calculations and simulations
The spin-polarized DFT computations for all the control catalysts were conducted by the Vienna Ab initio simulation package along with the commonly used projector-augmented wave (PAW) method68,69. The generalized gradient approximation generated by the Perdew-Burke-Ernzerhof was performed to calculate the exchange–correlation function. The van der Waals interactions in MSS were investigated with the DFT-D3 strategy in Grimme’s scheme70. For the interaction between the atomic core and related electrons in orbital, it was calculated by the PAW method. Moreover, the plane-wave basis set energy cutoff was 500 eV71. The geometry relaxation was conducted by the Brillouin zone with Gamma-centered 1 × 1 × 1 Monkhorst–Pack mesh grid. A vacuum spacing of approximately 15 Å was introduced in all slab models to minimize spurious interactions between periodic images of adsorbates72. The atomic positions of the bottom half of the structure were constrained73. All structures were fully optimized until reaching their total energies of <1 × 10–6 eV and average residual forces of <0.02 eV Å–1 74,75. The computational hydrogen electrode model was selected to evaluate the Gibbs free energy76. The CI-NEB method was employed to locate transition states77. The atomic coordinates of the optimized model for DFT calculations are provided as a separate Supplementary Data 1.
The molecular dynamics for RDF computation were conducted via the commonly used software package of GROMACS with a version of 2021.378,79. First of all, all the molecules were optimized in ORCA condition. The packmol was used to construct the system, along with the concomprised specific molecules confined in a 10 nm-edge sized cubic box80. The interactions between molecular and MSS were described using the OPLS-AA all-atom force field and the Universal Force Field. The atomic partial charges were assigned based on RESP charges, which was derived from Multiwfn computations81,82,83. Following energy minimization, the systems underwent a 2 ns pre-equilibration in the NPT ensemble using the Berendsen strategy. The production run was then performed in the NPT ensemble at 300 K with 1 fs of time step. The temperature and pressure in this system were tuned by V-rescale thermostat (τT = 1 ps) and Parrinello–Rahman methods (τP = 2 ps), respectively. After 20 ns of simulation, the RDF of the catalysts were computed using the built-in tools in GROMACS.
The COMSOL Multiphysics software was used to conduct the finite element analysis84. Without considering the possible side reactions. The following FEA model was defined by the law of mass conservation and electroneutrality assumption of related ions. The domain with corresponding size was presented in the figures. The size of computation domain for three models are set as 100 × 100, 100 × 100, 220 × 220 nm, with corresponding radii of the hollow spheres set as 15, 15, and 60 nm, respectively. The current and concentration distribution were tracked by using the tertiary current distribution and concentration diffusion equation, in which the flux of each ion in the electrolyte can be analyzed by the following Nernst–Planck equation:
where Ni, Di, ci, zi, F, T, R, and Φl present the the flux, diffusion coefficient, the concentration, the charge number, the Faradaic constant, temperature, gas constant, and electrolyte potential, respectively. l is the position along the diffusion region of thickness d (0 <l < d). Based on the steady-state continuity equations and the law of mass conservation, we have
The electroneutrality assumption was represented as
The boundary condition was set as follows:
where J stands for the diffusion flux. The simulation procedure commenced with an initialization of the current distribution, followed by a transient step. A 0 V electric potential was set on the outer surface of computation domain. The conductivity of electrolyte in bulk and the initial concentration in the electrolyte were set as 1 × 10–3 S cm–1and 1000 mol m–3, respectively.
Data availability
The data that support the findings of this study are available in the main text and Supplementary Information. The atomic coordinates of the optimized model for DFT calculations are provided with this paper (Supplementary Data 1). Source data are provided with this paper.
References
Song, Y.-W. et al. Phase equilibrium thermodynamics of lithium–sulfur batteries. Nat. Chem. Eng. 1, 588–596 (2024).
Zhou, J. et al. Healable and conductive sulfur iodide for solid-state Li-S batteries. Nature 627, 301–305 (2024).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Kim, S. C. et al. Solvation-property relationship of lithium-sulphur battery electrolytes. Nat. Commun. 15, 1268 (2024).
Han, Z. et al. Catalytic effect in Li-S batteries: from band theory to practical application. Mater. Today 57, 84–120 (2022).
Han, Z. et al. Engineering d-p orbital hybridization in single-atom metal-embedded three-dimensional electrodes for Li-S batteries. Adv. Mater. 33, e2105947 (2021).
Zhou, S. et al. Visualizing interfacial collective reaction behaviour of Li-S batteries. Nature 621, 75–81 (2023).
Peng, L. et al. A fundamental look at electrocatalytic sulfur reduction reaction. Nat. Catal. 3, 762–770 (2020).
Zhang, Y. et al. d-p Hybridization-induced “trapping-coupling-conversion” enables high-efficiency nb single-atom catalysis for Li-S batteries. J. Am. Chem. Soc. 145, 1728–1739 (2023).
Lin, H. et al. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ. Sci. 10, 1476–1486 (2017).
Zhou, T. et al. Twinborn TiO2–TiN heterostructures enabling smooth trapping–diffusion–conversion of polysulfides towards ultralong life lithium–sulfur batteries. Energy Environ. Sci. 10, 1694–1703 (2017).
Li, R. et al. Amorphization-induced surface electronic states modulation of cobaltous oxide nanosheets for lithium-sulfur batteries. Nat. Commun. 12, 3102 (2021).
Zhang, C. Y. et al. Surface strain-enhanced MoS2 as a high-performance cathode catalyst for lithium–sulfur batteries. eScience 2, 405–415 (2022).
Han, Z. et al. Machine-learning-assisted design of a binary descriptor to decipher electronic and structural effects on sulfur reduction kinetics. Nat. Catal. 6, 1073–1086 (2023).
Shen, J. et al. Revisiting the unified principle for single-atom electrocatalysts in the sulfur reduction reaction: from liquid to solid-state electrolytes. Energy Environ. Sci. 17, 6034–6045 (2024).
Sun, Y. et al. Counting d-orbital vacancies of transition-metal catalysts for the sulfur reduction reaction. Angew. Chem. Int. Ed. 62, e202306791 (2023).
Fang, M. et al. Effective screening descriptor for MXenes to enhance sulfur reduction in lithium-sulfur batteries. J. Am. Chem. Soc. 145, 12601–12608 (2023).
Hua, W. et al. Optimizing the p charge of S in p-block metal sulfides for sulfur reduction electrocatalysis. Nat. Catal. 6, 174–184 (2023).
Shen, Z. et al. Valence electron: a descriptor of spinel sulfides for sulfur reduction catalysis. Adv. Mater. 37, e2418090 (2025).
Li, H. et al. Regulating the spin state configuration in bimetallic phosphorus trisulfides for promoting sulfur redox kinetics. J. Am. Chem. Soc. 145, 22516–22526 (2023).
Ye, C. et al. The role of electrocatalytic materials for developing post-lithium metal||sulfur batteries. Nat. Commun. 15, 4797 (2024).
Dong, C. et al. Engineering d-p orbital hybridization with P, S Co-coordination asymmetric configuration of single atoms toward high-rate and long-cycling lithium-sulfur battery. Adv. Mater. 36, e2407070 (2024).
Li, B. et al. Origin of phase engineering CoTe2 alloy toward kinetics-reinforced and dendrite-free lithium-sulfur batteries. Adv. Mater. 36, e2309324 (2024).
Luo, M. & Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2, 17059 (2017).
Ding, F. et al. Tailoring planar strain for robust structural stability in high-entropy layered sodium oxide cathode materials. Nat. Energy 9, 1529–1539 (2024).
Su, J. et al. Strain enhances the activity of molecular electrocatalysts via carbon nanotube supports. Nat. Catal. 6, 818–828 (2023).
Wang, B. et al. Nanocurvature-induced field effects enable control over the activity of single-atom electrocatalysts. Nat. Commun. 15, 1719 (2024).
Zeng, R. et al. Origins of enhanced oxygen reduction activity of transition metal nitrides. Nat. Mater. 23, 1695–1703 (2024).
Jiang, M. et al. Strain-regulated Gibbs free energy enables reversible redox chemistry of chalcogenides for sodium ion batteries. Nat. Commun. 13, 5588 (2022).
Zhao, C. et al. The origin of strain effects on sulfur redox electrocatalyst for lithium sulfur batteries. Adv. Energy Mater. 14, 2302586 (2023).
Wang, B. et al. Zinc-assisted cobalt ditelluride polyhedra inducing lattice strain to endow efficient adsorption-catalysis for high-energy lithium-sulfur batteries. Adv. Mater. 34, e2204403 (2022).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Zhang, T. et al. Biaxially strained MoS2 Nanoshells with controllable layers boost alkaline hydrogen evolution. Adv. Mater. 34, e2202195 (2022).
Zhang, T. et al. Biaxial strain induced OH engineer for accelerating alkaline hydrogen evolution. Nat. Commun. 15, 6508 (2024).
Qi, Y. et al. Recent progress in strain engineering on van der Waals 2D materials: tunable electrical, electrochemical, magnetic, and optical properties. Adv. Mater. 35, e2205714 (2023).
Liu, Z. et al. Solution-processable microstructuring of 1T’-phase Janus MoSSe monolayers for boosted hydrogen production. J. Am. Chem. Soc. 146, 23252–23264 (2024).
Zhou, S., Kosari, M. & Zeng, H. C. Boosting CO2 hydrogenation to methanol over monolayer MoS2 nanotubes by creating more strained basal planes. J. Am. Chem. Soc. 146, 10032–10043 (2024).
Babu, G., Masurkar, N., Al Salem, H. & Arava, L. M. Transition metal dichalcogenide atomic layers for lithium polysulfides electrocatalysis. J. Am. Chem. Soc. 139, 171–178 (2017).
Wang, H. et al. High electrochemical selectivity of edge versus terrace sites in two-dimensional layered MoS2 materials. Nano Lett. 14, 7138–7144 (2014).
Lao, Z. et al. Band structure engineering and orbital orientation control constructing dual active sites for efficient sulfur redox reaction. Adv. Mater. 36, e2309024 (2024).
Wang, J. et al. Undercoordination chemistry of sulfur electrocatalyst in lithium-sulfur batteries. Adv. Mater. 36, e2311019 (2024).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. F. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Wang, M. et al. Identifying the evolution of selenium-vacancy-modulated MoSe2 precatalyst in lithium-sulfur chemistry. Angew. Chem. Int. Ed. 60, 24558–24565 (2021).
He, H. et al. Anion vacancies regulating Endows MoSSe with fast and stable potassium ion storage. ACS Nano 13, 11843–11852 (2019).
Li, Z. et al. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 8, 84–93 (2023).
Shen, Z. et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium–sulfur batteries. Nat. Catal. 5, 555–563 (2022).
Zhang, H. et al. Fe3O4-doped mesoporous carbon cathode with a plumber’s nightmare structure for high-performance Li-S batteries. Nat. Commun. 15, 5451 (2024).
Liang, X. et al. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun. 6, 5682 (2015).
Pang, Q., Kundu, D., Cuisinier, M. & Nazar, L. F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 5, 4759 (2014).
Pan, H. et al. On the way toward understanding solution chemistry of lithium polysulfides for high energy Li–S redox flow batteries. Adv. Energy Mater. 5, 1500113 (2015).
Gao, X. et al. Electrolytes with moderate lithium polysulfide solubility for high-performance long-calendar-life lithium-sulfur batteries. Proc. Natl. Acad. Sci. USA 120, e2301260120 (2023).
Liu, R. et al. Establishing reaction networks in the 16-electron sulfur reduction reaction. Nature 626, 98–104 (2024).
Yan, R. et al. Alveoli-inspired carbon cathodes with interconnected porous structure and asymmetric coordinated vanadium sites for superior Li-S batteries. Angew. Chem. Int. Ed. 63, e202404019 (2024).
Wang, J. et al. Coordinatively deficient single-atom Fe-N-C electrocatalyst with optimized electronic structure for high-performance lithium-sulfur batteries. Energy Storage Mater. 46, 269–277 (2022).
Mikhaylik, Y. V. & Akridge, J. R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 151, A1969 (2004).
Yang, Q. et al. Chlorine bridge bond-enabled binuclear copper complex for electrocatalyzing lithium-sulfur reactions. Nat. Commun. 15, 3231 (2024).
Zhao, X. et al. Unleash electron transfer in C-H functionalization by mesoporous carbon-supported palladium interstitial catalysts. Natl. Sci. Rev. 8, nwaa126 (2021).
Pan, A., Kar, T., Rakshit, A. K. & Moulik, S. P. Enthalpy-entropy compensation (EEC) effect: decisive role of free energy. J. Phys. Chem. B 120, 10531–10539 (2016).
Soni, R. et al. Lithium-sulfur battery diagnostics through distribution of relaxation times analysis. Energy Storage Mater. 51, 97–107 (2022).
Wu, G. et al. Optimizing s-p orbital overlap between sodium polysulfides and single-atom indium catalyst for efficient sulfur redox reaction. Angew. Chem. Int. Ed. 64, e202422208 (2024).
Lu, Y., Zhao, C.-Z., Huang, J.-Q. & Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 6, 1172–1198 (2022).
Miao, X. et al. Achieving high-performance lithium-sulfur batteries by modulating Li+ desolvation barrier with liquid crystal polymers. Adv. Mater. 36, e2401473 (2024).
Lin, X. et al. Alleviating OH blockage on the catalyst surface by the puncture effect of single-atom sites to boost alkaline water electrolysis. J. Am. Chem. Soc. 146, 4883–4891 (2024).
Geng, C. et al. Unveiling the role of electric double-layer in sulfur catalysis for batteries. Adv. Mater. 36, e2407741 (2024).
Li, H. et al. Developing high-power Li||S batteries via transition metal/carbon nanocomposite electrocatalyst engineering. Nat. Nanotechnol. 19, 792–799 (2024).
Conder, J. et al. Direct observation of lithium polysulfides in lithium–sulfur batteries using operando X-ray diffraction. Nat. Energy 2, 1–7 (2017).
Kresse, G. & Furthmüller JJCms. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B50, 17953–17979 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Xu, H. Atomic-scale insights into the electrochemical mechanisms of aluminum-sulfur batteries: a first-principles study of Al-S clusters on graphene. Chem. Phys. Lett. 851, 141492 (2024).
Xu, H., Guan, D. & Ma, L. The bio-inspired heterogeneous single-cluster catalyst Ni100-Fe4S4 for enhanced electrochemical CO2 reduction to CH4. Nanoscale 15, 2756–2766 (2023).
Xiao, W., Yoo, K., Kim, J. H. & Xu, H. Breaking barriers to high-practical Li-S batteries with isotropic binary sulfiphilic electrocatalyst: creating a virtuous cycle for favorable polysulfides redox environments. Adv. Sci. 10, e2303916 (2023).
Xu, H. et al. Theoretical design of core–shell 3d-metal nanoclusters for efficient hydrogen-evolving reaction. Energy Fuels 37, 16781–16789 (2023).
Xiao, W., Kiran, G. K., Yoo, K., Kim, J. H. & Xu, H. The dual-site adsorption and high redox activity enabled by hybrid organic-inorganic vanadyl ethylene glycolate for high-rate and long-durability lithium-sulfur batteries. Small 19, e2206750 (2023).
Peterson, A. A. et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Zheng, Q. et al. Cobalt single-atom reverse hydrogen spillover for efficient electrochemical water dissociation and dechlorination. Angew. Chem. Int. Ed. 63, e202401386 (2024).
Berendsen, H. J. C., van der Spoel, D. & van Drunen, R. GROMACS: a message-passing parallel molecular dynamics implementation. Comp. Phys. Commun. 91, 43–56 (1995).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Martinez, L., Andrade, R., Birgin, E. G. & Martinez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Jorgensen, W. L., Maxwell, D. S. & Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Rappé et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Wang, B. et al. Manipulating atomic-coupling in dual-cavity boride nanoreactor to achieve hierarchical catalytic engineering for sulfur cathode. Angew. Chem. Int. Ed. 63, e202406065 (2024).
Acknowledgements
This work is supported from the National Research Foundation, Singapore, under its Singapore-China Joint Flagship Project (Clean Energy), and Singapore Ministry of Education, under AcRF Tier 1 (RG80/22).
Author information
Authors and Affiliations
Contributions
J.-L.Y. conceived the research. T.Z. and H.J.F. supervised this project. J.-L.Y., W.Y., and J.L. carried out the experiments, and collected and analyzed the experimental data. T.Z. and T.X. conducted the material synthesis and characterization and analyzed the corresponding data. H.X. performed the simulations. J.-L.Y., T.Z., and H.J.F. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, JL., Xu, H., Xiao, T. et al. High-dimensional strain unlocks fast polysulfide redox kinetics for lithium-sulfur batteries. Nat Commun 16, 8910 (2025). https://doi.org/10.1038/s41467-025-63969-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63969-z