Abstract
Perovskite solar cells (PSCs) demonstrate potential for sustainable energy transitions, however their commercialization is impeded by lead toxicity and environmental leakage risks. Herein, it is an effective way to implant a cellulose material with multiple adsorption modes and sites, hydroxypropyl methylcellulose phthalate (HPMCP), which plays a covering role in perovskite crystal and achieves environmental friendliness through effectually suppressing lead leakage. Meanwhile, we have devised a series of multidimensional experimental scheme to assess the impact of lead leakage on the ecosystem from the perspective of cell survival rates and plant growth. HPMCP-modified PSC has a relatively low impact on plant growth and germination, exhibiting a germination rate of 92.3% compared to the blank group (without lead contamination). Moreover, the HPMCP-modified PSCs acquire optimal power conversion efficiency of 26.27% and manifest remarkable environmental stability. This work establishes an environmental and health risk assessment benchmark for PSCs, enabling sustainable deployment.
Similar content being viewed by others
Introduction
Lead-based metal halide perovskite solar cells (PSCs) have emerged as an enormous potential next-generation photovoltaic technology, owing to its superior optoelectronic properties, long carrier diffusion length, and high defect tolerance1,2,3. Over the past few years, the certified power conversion efficiency (PCE) of PSCs has achieved 27.0%, rivaling silicon heterojunction solar cells4,5. However, lead leakage stands out as a pivotal challenge in the commercialization of lead-based PSCs. When perovskites decompose, the soluble lead salts are produced and lead ions (Pb2+) in the perovskite may dissolve out and enter the ecosystem through water bodies, soil, and other pathways6, thus posing a potential hazard to both the environment and human health due to lead’s high toxicity7,8.
To tackle this issue, several approaches have been reported to suppress infiltration of moisture and oxygen and alleviate the lead leakage issue via external encapsulation9,10,11. Whereas, the stability and lead leakage of perovskite can also be impacted by internal factors, such as defects and ion migration, which are not alleviated by external encapsulation. Thereby, addictive engineering emerges as a promising internal encapsulation technique for mitigating defects in perovskite films. Scientific studies indicate that the construction of polymer networks within perovskites can leverage the robust interaction between the plentiful functional groups in the polymer network and Pb2+ to effectively mitigate lead leakage12,13. The migration and transformation of traditional polymers used in internal encapsulation of PSCs in the environment can affect plant growth and development14,15. In comparison, natural cellulose and its derivatives not only possess multiple active sites but also exhibit superior environmental friendliness.
In addition, on the environmental impact concerning the lead leakage of PSCs, · especially the actual risks associated with important indicators of cell living and plant growth process remain unknown so far16. Most reports assess the inhibition of lead leakage from a monotonous impact perspective, such as measuring the content of lead leakage from PSCs in water or soil17 and the effects of the Pb-containing films on the growth of organisms like Escherichia coli18, lacking a systematic and multidimensional evaluation strategy. Hence, adopting a green polymer that can achieve internal encapsulation and effectively eliminate or minimize lead leakage19,20, as well as designing a multidimensional method to measure the ecological impact of lead leakage from perovskite, which are essential for bolstering the prolonged stability and the sustainable development of PSCs.
Herein, we apply an environmentally friendly hydroxypropyl methylcellulose phthalate (HPMCP) with multiple adsorption modes and sites into the perovskite layer to inhibit lead leakage. Meanwhile, to comprehensively assess the impact of perovskite lead leakage on the ecological environment, experimental protocols are designed to quantify both cellular survival rates and macroscopic plant development parameters. In standardized toxicity assays, the cell survival rate surpasses 95% after 7-day immersion in HPMCP-modified perovskite films, in sharp contrast to the control group which exhibits a meager survival rate of 2.89%. It can be concluded that HPMCP drastically suppresses the degradation of perovskite and Pb2+ release. Besides, it significantly bolsters the champion PCE, increasing from 23.43% (control) to 26.27% (HPMCP-modified). Notably, the modified PSCs demonstrate remarkable environmental stability, the initial efficiency of the device retains at 93% after 1200 h at 85% relative humidity (RH) without external encapsulation.
Results
Mechanism of lead capture
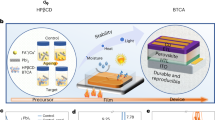
The hydroxypropyl methylcellulose phthalate (HPMCP) is incorporated into perovskite precursor as an internal encapsulation material to enhance the stability of PSCs and suppress lead leakage (Fig. 1a). To confirm the specific location of the HPMCP in perovskite films, scanning electron microscopy (SEM), energy-dispersive spectrometry (EDS) mapping and transmission electron microscopy (TEM) analysis have been carried out. SEM images demonstrate that the HPMCP fills grain boundary (GB) gaps, resulting in blurred boundary contours and an overall smoother, more uniform film morphology. The EDS mapping shows that the characteristic O element of HPMCP appears in the entire perovskite film (Fig. 1b; Supplementary Figs. 1 and 2). Due to the limited resolution of EDS mapping, we further analyze the TEM images and Young’s module of perovskite films. The TEM images show that HPMCP can physically encapsulate perovskite lattices and the substantial reduction on Young’s module of perovskite films confirms HPMCP’s comprehensive coverage, which is also applicable to large areas (Supplementary Fig. 3). Thus, the internal encapsulation of HPMCP efficiently protects GBs and perovskite surfaces, physically inhibiting the attack of external water and oxygen13,19,20.
Moreover, HPMCP possesses a rich array of functional groups and multiple binding sites, enabling to form various chemical interactions with perovskites (such as hydrogen bonds and coordination bonds), thereby maximizing its passivation efficacy and longevity on perovskite films. X-ray photoelectron spectroscopy (XPS) analysis reveals a systematic shift of Pb 4 f peaks, N 3 d peaks and N 1 s peak toward lower binding energies in the HPMCP-modified perovskite films (Fig. 1c; Supplementary Fig. 4). The chemical shifts suggest coordinative interactions between the HPMCP and undercoordinated ions in the perovskite lattice21,22,23. In addition, complementary Fourier transform infrared spectroscopy (FTIR) (Supplementary Figs. 5 and 6) provides further evidence of this coordination mechanism. Distinct peak shifts observed for both C = O and C-O groups in HPMCP upon interaction with PbI2 and FAI unequivocally corroborate the formation of coordination bonds and hydrogen bonds involving carbonyl and hydroxyl oxygen atoms with Pb2+ and FA cations. Compared with other cellulose derivatives of similar structures, HPMCP demonstrates superior lead leakage suppression capabilities (Supplementary Figs. 7 and 8). Therefore, under the dual effects of physics and chemistry, HPMCP coordinates with Pb2+ in the perovskite, efficiently coating, passivating and fastening non-coordinating Pb2+, thereby suppressing lead migration and leakage17.
Ecological impacts of lead leakage
Given the monotonous and limited availability of relevant characterization methods to assess the ecological impact of lead leakage from the degradation of perovskite (Supplementary Table 1), we have designed more macroscopic metrics, such as cellular survival rates and plant growth parameters, to provide more intuitive visualization of the environmental sustainability of HPMCP-modified perovskite solar cell (PSC) (Fig. 2a). It is well-established that lead exerts detrimental effects on human health even at low doses. Mitigating Pb toxicity and enhancing the biosafety of perovskite photovoltaics are critical to the commercialization and sustainable development of PSCs. To address this, we propose, for the first time, a systematic investigation into the cytotoxicity of perovskite films by evaluating the impact of Pb-containing films on growth and viability of adipose-derived stem cells (AMSCs) derived from animal tissue. In parallel, Polydimethylsiloxane (PDMS), a widely used coating agent24,25,26, is selected to investigate the environmental impact of perovskite modified with biocompatible encapsulants that meet biosafety requirements27,28,29,30,31, as a comparative counterpart to HPMCP. The control group (pristine perovskite film) exhibits a high concentration of Pb leakage, resulting in a high proportion of dead cells and a low cell viability, with a cell survival rate of only 2.89% after 7 days. In contrast, both the HPMCP group (HPMCP-modified perovskite film) and PDMS group (PDMS-modified perovskite film) demonstrate low cytotoxicity, with live cell counts comparable to those of the blank group (without Pb contamination), maintaining high and stable cell survival rates of 95.29% and 93.45% over the 7-day period, respectively (Fig. 2b, c). This is attributed to the internal encapsulation method, which inhibits Pb2+ release and thereby prevents cell apoptosis. To validate the relevance of cytotoxicity results, we employ the broadly representative L929 cell line for comparative assessment. The results still demonstrate consistently low cytotoxicity for PDMS and HPMCP coatings, indicating minimal influence of cell type on the relevance of the toxicity findings (Supplementary Figs. 9–11).
a The schematic illustrations of plant and cell testing methods. b The cell survival rate with or without the immersion of HPMCP/PDMS modified perovskite films. c MTT assay for quantitative determination of cell number. The impact of blank, control, PDMS and HPMCP groups on the cell growth performance. Green represents live cells, and red represents dead cells. All scale bars are 100 μm. d The lead release rate of the soil at different time with different PSCs buried. e The sprouting rate of plants at different time with/without different PSCs buried. f Indicators for measuring plant metabolism in blank plants and those grown with PDMS-modified and HPMCP-modified PSCs. g The Pb content in the plants with/without different PSCs buried. The error bars in (b), (d), (g) represent the standard deviation for three samples.
Additionally, we have conducted a multi-faceted assessment to evaluate the effects of PSCs on soil ecosystems and plant growth and germination. To explore the differences in Pb2+ release between the two encapsulants, the Pb release rate in the soil is tested by burying the PSC and analysing the soil over time. The lead release profiles over seven days exhibit distinct dynamics: the control sample (pristine PSC) displays rapid and sustained leakage, PDMS sample (PDMS-modified PSC) shows slow but progressive leakage, while HPMCP sample (HPMCP-modified PSC) undergoes an initial gradual release phase followed by stabilization.
What’s more, the germination rate is employed to measure the effect of Pb leakage on plant growth status. Firstly, we choose radishes for the plant growth and germination test. It is observed in Fig. 2e that the control sample, with its high lead leakage, completely inhibits plant growth and germination (0% germination rate). In contrast, the HPMCP sample exhibits a germination rate of 50% after 13 days, which is significantly closer to 55% of the blank sample (without Pb contamination) compared to the PDMS sample (45%). Secondly, we further evaluate the impact of lead leakage and lead toxicity on plant growth by analysing five key plant growth and metabolism indexes: photosynthesis rate32, malondialdehyde (MDA)33, H2O234, anthocyanins35, and soluble polysaccharides (SSC)36 (Fig. 2g, Supplementary Fig. 12 and Supplementary Table 2). The PDMS sample exhibits elevated MDA level and reduced photosynthetic rates, indicating more severe plant damage due to lead leakage37. Conversely, the HPMCP sample displays parameter values nearly aligned with those of the blank sample, demonstrating that HPMCP-modified PSC has minimal impact on normal plant growth and development, along with superior biocompatibility. Finally, to investigate the lead content of plant uptake from PSCs, the regular germinated plants are cultivated in the soil with PSCs to quantify lead absorption during their growth. The control sample reveals substantial lead accumulation in plants (362.45 mg/kg). In contrast, the HPMCP sample exhibits a lead content of only 11.54 mg/kg, much lower than that of the PDMS sample (17.87 mg/kg), demonstrating that the encapsulation of HPMCP effectively inhibits lead leakage and exhibits superior encapsulation performance (Fig. 2f). Above all, the incorporation of the cost-effective and environmentally friendly HPMCP effectively suppresses lead leakage and considerably mitigates the adverse effects of lead toxicity on plant growth and germination.
The difference in the effectiveness of the two encapsulants in inhibiting lead leakage may be attributed to their distinct interaction sites and the number of these sites. To explore the mechanism underlying this difference, XPS and FTIR analyses are conducted (Supplementary Figs. 4–6). XPS analysis reveals that the Pb and I peak shifts in HPMCP are fivefold larger than those in PDMS, demonstrating notably stronger interaction between HPMCP and PbI2 compared to PDMS. FTIR results further show that the characteristic peaks corresponding to C-N and N-H bonds in FAI exhibit more pronounced red shifts upon HPMCP incorporation compared to PDMS modification38. This indicates stronger hydrogen bonding interactions between HPMCP and FAI, which facilitates the formation of more stable perovskite structure with substantially reduced defects. Furthermore, in terms of binding sites and quantity, PDMS primarily interacts with perovskite through Si-O groups, whereas HPMCP employs multiple functional groups (C = O, C-O, and -OH) to establish both coordination bonds and hydrogen bonds with perovskite. This multidentate coordination capability enables HPMCP to achieve superior lead leakage suppression compared to PDMS39,40,41.
We further investigate the impacts of soil pH and mineral constituents on the degradation dynamics and environmental compatibility of PSCs. Mineral components exhibit negligible effects on the degradation of HPMCP-modified PSCs. However, the interaction between HPMCP and Pb2+ demonstrated slightly weakened efficacy under acidic conditions compared to neutral or alkaline environments (Supplementary Figs. 13–16). Crucially, HPMCP maintains exceptional environmental friendliness and stability, with secondary products arising from its strong Pb-binding capacity retaining favorable eco-compatibility profiles (Supplementary Fig. 17).
Our prior assessments primarily employ unencapsulated devices to evaluate the worst-case scenario of PSCs fully exposed to soil. On this basis, we further compare the environmental compatibility under intact external encapsulation and damaged external encapsulation. Compared to the control sample, the PSCs with internal encapsulation of HPMCP have a smaller impact on plant growth and higher environmental friendliness (Supplementary Figs. 18 and 19).
The Stability of HPMCP-modified perovskites
The moisture stability of the perovskite films is investigated. Target film retains black-phase after 60 days under high humidity conditions (65% RH at ambient temperature), while the control film degrades into yellow-phase (PbI2) (Fig. 3a and Supplementary Fig. 20). X-ray diffraction (XRD) analysis shows that the target film undergoes no changes, whereas the control perovskite film shows a pronounced increase in the PbI2 peak after 11 days. Even after 60 days, the intensity of the PbI2 peak in the control is still significantly higher than that in the target film, demonstrating that HPMCP effectively stabilize the films by inhibiting decomposition into harmful δ-phase42 (Fig. 3b and Supplementary Fig. 21). Since the high moisture sensitivity of FA-based perovskites leads to uncontrolled perovskite structural imperfections, the chemical interactions between the HPMCP and FAI are investigated by nuclear magnetic resonance (NMR) measurements. The 1H NMR chemical shift value of FAI exhibits a peak at approximately 8.8 ppm, corresponding to the -N-H group. Nevertheless, upon adding HPMCP to FAI, CsI and PbI2, the -NH2 peak of FAI is split into two peaks around 8.8 ppm, confirming the hydrogen bond interactions between -C = O in HPMCP and -N-H in FAI43,44. The blue shifts in CsI and PbI2 show that the encapsulation efficacy of HPMCP extends beyond FA-based PSCs to CsPbI3 systems (Fig. 3c and Supplementary Figs. 22 and 23). Furthermore, molecular dynamics calculations are implemented to elaborate the effect of water on perovskite, which demonstrate the lower loss of FA and MA in perovskite crystals due to the coating of HPMCP on perovskite (Fig. 3d, e). By establishing a scatter plot of the N element distribution on the X-Z plane, we observe that HPMCP-modified FA and MA exhibit negligible changes after 200 ps, by contrast, FA and MA in the control markedly deviate from their initial positions upon exposure to water and subsequent decomposition. The HPMCP modification effectively attenuates the detrimental impact of ambient moisture on the crystal structure of perovskite by improving the energetic barrier for the combination of H2O molecules with perovskites. We further delve into the chemical combination of FA+ with H2O or HPMCP to elucidate the functional mechanism leading to the decreased perovskite’s susceptibility to moisture43 (Supplementary Fig. 24). On the one hand, the interaction energy value of FA+ with HPMCP is lower than FA+ with H2O. Meanwhile, the encapsulation protection of perovskite by HPMCP reduces surface defects and vacancies in the perovskite, lowering the reactivity of water molecules with the perovskite. On the other hand, the hydrogen bonds between HPMCP and FA+ inhibit the binding of FA+ to water molecules and alleviate the erosion of the perovskite lattice by water molecules, thereby stabilizing the lattice and enhancing the stability of perovskite in humid environments.
a The images of fresh and aged perovskite films of control and target. b XRD patterns of control and HPMCP-modified perovskite films as a function of aging time in ambient air. c 1 H NMR spectra of FAI, and FAI + HPMCP mixed solution. d Snapshots of the system in 200 ps: perovskite-water and perovskite with HPMCP-water. e Scatter plot of N in X-Z plane of control and target perovskites. f, g GIXRD spectra with different ψ from 0° to 50° of control and target perovskite films. h Linear fit of GIXRD spectra of control and target perovskite films.
Furthermore, tensile stress lowers the energy required for defect formation and ion migration activation, rendering the perovskite surface extremely unstable and susceptible to irreversible degradation upon exposure to external environments45. The grazing-incidence X-ray diffraction (GIXRD) technique, employing the 2θ-sin2ψ method, can be utilized to monitor changes in residual stress on the surface of perovskite film. As shown in Fig. 3f, with increasing the ψ, the XRD peaks position of the control film shift to lower values of 2θ, whereas the target film demonstrates a considerable decrease in tensile strain, which is attributed to the enhanced crystallization of perovskite and decreased interfacial defects (Fig. 3g). The slope of the fitting curve of 2θ-sin2ψ reflects the magnitude of residual stress in perovskite films. As is displayed in Fig. 3h, the linear fit of 2θ-sin2ψ of control and target films presents the slope of −0.052 and −0.001, respectively. It confirms that the target perovskite film has released its tensile stress46. Hence, HPMCP modification regulates the residual stress on the surface and serves as a protective barrier against water vapor adsorption and permeation, thereby markedly enhancing the structural stability of PSCs47.
Photovoltaic performance
The top-view and cross-sectional SEM images (Fig. 4a) reveal uniform coverage of HPMCP over the perovskite film surface and GBs, accompanied by a more ordered and vertically aligned grain structure in the target group compared to the control group. The long molecular chains can infiltrate into the gaps of the GBs, acting as a “filler”46, which suppresses charge scattering and recombination at GBs, thereby enhancing the carrier mobility and electrical conductivity of the film48. Atomic force microscopy (AFM) and kelvin probe microscope (KPFM) analysis shows that the target group, with reduced surface roughness and a higher surface contact potential difference (CPD) compared to the control group, minimizes charge recombination losses45,49 and optimizes carrier extraction50 (Supplementary Figs. 25 and 26).
a The top view and cross-sectional SEM images of control and target perovskite films. b The in-situ UV-vis absorption of control and target perovskites during annealing. c TRPL spectra of control and target perovskite films. d The J-V curves of devices of control and target with the active area of 0.04 cm2. e Mott-Schottky plots of the control and target PSCs. f TPV of control and target PSCs. g Environmental stability of the unencapsulated PSCs at 25 °C and 85% relative humidity (RH) with or without HPMCP modified.
Ultraviolet-visible (UV-vis) spectroscopy and ultraviolet photoelectron spectroscopy (UPS) analyses indicate that HPMCP modification leads to negligible changes in the optical bandgaps of the perovskite films51,52, as well as better energy alignment and enhanced hole extraction at the interfaces of the perovskite film and hole transport layers (Supplementary Figs. 27 and 28). Furthermore, it can be observed from XRD that the intensity of the target group on the (100) crystal plane is similar to that of the control group, without much difference53 (Supplementary Fig. 29). We employ the in-situ UV–vis spectrum to analyse the crystallization kinetics of the perovskite films during the annealing process (Fig. 4b, Supplementary Fig. 30). Target film shows a prolonged intermediate region (0-13 s) compared with the control. The graphical representation of the relationship between absorption intensity and annealing time at a wavelength of 600 nm exhibits that the slope of the target group (0.01133) is lower than that of the control (0.01415). These results suggest that the incorporation of HPMCP slows down the crystallization process of the perovskite54, which is beneficial for the HPMCP-modified film to achieve larger crystal sizes and reduce the content of unreacted PbI2, thereby facilitating the formation of high-quality perovskite. Simultaneously, this result is also verified by in-situ photoluminescence (PL) (Supplementary Fig. 31) and further corroborated by SEM and AFM.
The recombination process of charges and the dynamics of charge excitation are characterized by steady-state PL and time-resolved photoluminescence (TRPL) decay measurements with the structure of ITO/MeO-2PACz/perovskite (Fig. 4c, Supplementary Fig. 32a). The target film exhibits lower fluorescence intensity and a shorter photovoltage lifetime of 445 ns compared to 462 ns in the control, which effectively decreases the trap density and inhibits charge recombination55. This result is further verified by the conductivity and PL mapping of perovskite films without hole transport layer (Supplementary Fig. 32b, c).
Afterward, to confirm the enhancement in photovoltaic performance of HPMCP-modified PSCs, we fabricate inverted PSCs with an ITO/MeO-2PACz/Al2O3/Perovskite/PEAI/PCBM/BCP/Ag configuration. Device modified with 0.05 wt% HPMCP not only exhibits exceptional repeatability but also achieves notably higher efficiency and better reproducibility than control (Fig. 4d, Supplementary Figs. 33–35). The champion power conversion efficiency (PCE) of target group is 26.27%, outperformed that of 23.43% of the control device. The champion device displays a short-circuit current density (JSC) of 25.92 mA/cm2, open-circuit voltage (VOC) of 1.20 V, and fill factor (FF) of 84.68%, which all surpasses the control one. According to the external quantum efficiency (EQE) spectra (Supplementary Fig. 36), the integrated current values of the control and target PSCs are 22.96 and 24.74 mA/cm2 respectively, which is consistent with the current values obtained from the J-V measurement56. The stabilized power output at the initial maximum power point (MPP) of target group is much beyond the control57 (Supplementary Fig. 37). Dark J-V measurements indicate that the HPMCP modification suppresses the charge recombination12 (Supplementary Fig. 38).
In addition, the dependence of VOC on light intensity and Nyquist plots demonstrate that HPMCP modification alleviates the defect-mediated non-radiative recombination43 (Supplementary Figs. 39 and 40). To investigate the defect passivation effect of HPMCP, we perform space-charge-limited current (SCLC) characterization to quantify the trap-filled limited voltage (VTFL) of PSCs. The target group exhibits lower VTFL values compared to the control, confirming effective defect passivation and facilitating carrier extraction and transport within the perovskite layer and across the relevant interfaces58 (Supplementary Fig. 41). Furthermore, capacitance-voltage (C-V) measurement is performed to assess the built-in potential (Vbi) of the devices (Fig. 4e). The target group exhibits a higher Vbi of 1.07 V compared to the control of 0.95 V, which suggests an enhanced impetus for carrier separation and transport42. Transient photovoltage (TPV) and transient photocurrent (TPC) techniques are commonly used to study carrier dynamics in PSCs. The target group signifies longer TPV decay and faster TPC decay, which represents slower charge carrier recombination and more efficient carrier transport and extraction59 (Fig. 4f, Supplementary Fig. 42). Finally, the HPMCP-modified PSCs demonstrate remarkable environmental stability, the initial efficiency of the device retains at 93% after 1200 h at 85% relative humidity (RH) without external encapsulation (Fig. 4g). The light stability of PSCs retains 83.7% of the initial PCE after 500 h of continuous light soaking, while that of the control device drops to 40.2%. Moreover, HPMCP-modified device maintains 86% of the original PCE at 85 °C under 85% relative humidity for 1000 h, significantly superior than that of the control one. Humidity stability tests conducted under ambient air with 85% RH without encapsulation reveal that the target group maintains 95% of its original efficiency after 1200 h, compared to 63% for the control (Supplementary Figs. 43 and 44).
Discussion
In summary, we utilize the internal encapsulation strategy to optimize the stability and suppress lead leakage of PSCs, as well as designing a systematic and multidimensional evaluation strategy to assess the ecological impact of lead leakage in perovskite. The multiple adsorption anchoring effects of HPMCP validly suppress lead leakage on PSCs and reduce lead absorption in plants by 96.8% compared to control. Moreover, HPMCP demonstrates exceptional ecological compatibility, exerting minimal impact on both plant and cellular growth. Eventually, the champion PCE of the inverted HPMCP-modified device reaches 26.27%. Moreover, this internal encapsulation exhibits exceptional humidity stability inhibiting the degradation of perovskite. The HPMCP-modified device without encapsulation retains 93% of its original PCE after 1200 h under 85% relative humidity in atmospheric ambiances. The internal encapsulation strategy and designated evaluation method for the influence of perovskite devices in ecology significantly accelerate the process of future commercial PSCs.
Methods
Materials
PbI2 (99.99%), PbBr2 (98%) and FAI (98%) were purchased from Polymer Light Technology Inc. CsI (98%), MACl (98%) and MABr (98%) was purchased from Aladdin. BCP and PCBM were purchased from Xian Polymer Light Technology Corp. MeO-2PACz was purchased from Tokyo Chemical Industry (TCI). HPMCP was purchased from Shanghai Adamas Reagent Co. Ltd. DMF, DMSO, chlorobenzene, ethanol, and isopropanol were purchased from Sigma-Aldrich. anhydrous alcohol (99.0%) and chlorobenzene (CB) were purchased from Sigma-Aldrich. Al2O3 and anhydrous isopropanol (IPA) were purchased from Aladdin. Silver (Ag) is purchased from commercial sources with high purity (≥99.999%). All reagents were used without any further purification.
Fabrication of Perovskite precursor solution
The perovskite composition is FA1.14MA0.06Cs0.07Pb1.3Br0.18I3.69. To prepare the precursor solution, 572 mg PbI2, 22 mg PbBr2, 196.1 mg FAI, 18.2 mg CsI, 8.8 mg MACl, and 6.7 mg MABr were dissolved in a 1 mL mixture of DMF and DMSO at a ratio of 4:1. For HPMCP-modified perovskite precursor solution, 5 mg HPMCP is dissolved in 1 mL of DMF, put it into original precursor solution at a ratio of 1:100. For HPMCP-modified perovskite precursor solution, 5 mg PDMS is dissolved in 1 mL of CB, then put it into antisolvent at a ratio of 1:100.
Device fabrication
The ITO substrates were cleaned by ultrasound using detergent, water, deionized water, ethanol, and isopropanol for 15 min by turn. Then, the ITO substrates were dried in a dry oven at 80 °C for 8 h, sequentially followed by plasma treatment for 5 min. For SAM deposition, 1 mg/mL MeO-2PACz in ethanol was spin-coated on the substrate at 4000 rpm for 30 s in a nitrogen glovebox, followed by annealing at 100 °C for 10 min. Subsequently, Al2O3 was spin-coated at 4000 rpm for 30 s, followed by annealing at 100 °C for 10 min. Then, the fully dissolved 1.4 M pristine and HPMCP modified perovskite precursor solution pre-stirred for 8 h were spin-coated onto the substrate via one-step method. The substrate was spin-coated at 1000 rpm for 5 s and 4000 rpm for 30 s. When the countdown was 35 s, 150 μL chlorobenzene serving as antisolvent was dropped onto the substrates. The substrate was then transferred to a thermostatic heater and annealed at 100 °C for 50 min. For PDMS modified film, the same as control device, However the only different step is putting 150 μL PDMS modified chlorobenzene serving as antisolvent was dropped onto the substrates. 1.5 mg/mL PEAI in isopropanol was dynamically spin-coated 4000 rpm for 30 s for the passivation of the perovskite surface, followed by annealing at 100 °C for 5 min. Then PCBM was spin-coated at 1500 rpm for 35 s, followed by annealing at 100 °C for 10 min. Subsequently, BCP was spin-coated at 4000 rpm for 30 s. Ag (100 nm) was thermally evaporated as the rear electrode. The aperture area of 0.04 cm2 was determined by a metal mask.
Film characterizations
FTIR spectra were recorded on Nicolet 8700 (Thermo Electron Corporation). XPS analysis was carried out on a Thermo ESCALAB 250Xi spectrometer with an Al. The AFM and KPFM images were obtained on an Oxford Instruments MFP-3D. The morphology of films was measured by AFM (nanoscope multimode Bruker, Jiangxi Science & Technology Normal University Analyses and Testing Center) and SEM (JEOL, JSM-7500F, 104 Japan) at an accelerating voltage of 5.0 KV. To measure the absorption or transmittance properties for the perovskite layers, the ultraviolet-visible spectra (UV-vis, SHIMADZU, UV-2600 spectrophotometer) was conducted. X-ray diffraction (XRD) was recorded by using a D8-Discover 25 diffractometer (Bruker, Jiangxi Science & Technology Normal University Analyses and Testing Center). The steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements at the peak emission of ~808 nm (on the excitation at 535 nm) were carried out by a steady state and lifetime spectrometer (FLS920, Edinburgh Instruments Ltd.). And the TRPL excitation fluence was ≈4 nJ/cm2 from a 405 pulsed laser with a wavelength of 405±8 nm and pulse width of 45 ps, at a repetition rate of 0.1 MHz. The PL decay data was recorded using time-correlated single photon counting technique.
Solar cells characterizations
The current density-voltage (J-V), current-voltage (I-V) and SCLC tests were characterized by using Keithley 2400 Sourcemeter. The ill uminated currents were measured under the solar simulated (Enli-Tech, 100 mW/cm2 and AM 1.5 G irradiation). The forward scan range was from 1.25 V to −0.2 V and the reverse scan range was from −0.2 V to 1.25 V, with 0.02 V for each step and the delay time is 50 ms. The incident photon-to-current conversion efficiency (IPCE) spectra was detected under monochromatic illumination (Oriel Cornerstone 260 1/4 m monochromator equipped with Oriel 70613NS QTH lamp), and the calibration of the incident light was performed with a monocrystalline silicon diode. Transient photocurrent and transient photovoltage decay curves of the devices were measured by Zolix DSR800; The EIS and Mott-Schottky were measured by an electrochemical workstation (Donghua DH7000D).
Release of lead ions in Soil analyses
Place the perovskite solar cell devices in 10 mL of phosphoric acid, then add 25 mL of encapsulated KI, let it stand for 10 min, add 10 mL of MIBK, shake for 5 min, and extract iodide. Determination of Pb2+ content in perovskite solar cell devices using atomic absorption spectroscopy. Store the perovskite solar cell devices in 50 g of soil. Clear the soil with deionized water on days 0.5, 1, 2, 3, 5, and 7, and then measure the Pb2+ in the soil using atomic absorption spectroscopy.
Phytotoxic analyses
Under conditions of 65% relative humidity, 25 °C temperature, and 255 μmol/mol CO2 concentration, various perovskite solar cell devices were buried in soil at a depth of 10 cm, with 20 radish seedlings planted above. The germination rate was calculated over a 13-day period. Subsequently, three randomly selected roots and stems from each group were dried, burned, and extracted using phosphoric acid, KI, and MIBK to determine the Pb2+ content in the plant species. The photosynthetic rate of plants was measured using an infrared gas analyzer (IR202, Yokogawa, Japan) in the upper leaf area of the plants for testing purposes. Plant leaves were crushed, mixed with activated carbon and 5 % TCA in a mortar, and ground to obtain a grinding solution. The soluble sugar content (SSC) was extracted from the grinding solution by boiling water. Then, 2 mL of the extract was taken, 0.5 mL of anthrone ethyl acetate and 5 mL of concentrated sulfuric acid were added. The absorbance was measured at 630 nm. The plant grinding solution was dissolved in PBS buffer. 1 mL of 1 mM KI was added and the absorbance was measured at 390 nm to determine the amount of H2O2. To the grinding solution, 67% TBA was added, and the mixture was kept at 100°C for 15 min, then cooled and centrifuged. The absorbance of the supernatant was measured at 450 nm, 532 nm, and 600 nm, respectively. 2 mL of distilled water was added to 2 mL of 67% TBA as a control. CMDA was calculated using the formula: CMDA = 6.45*(A532 - A600)-0.56*A450. Leaf samples were mixed with 1% HCl for 2.5 min. The slurry samples were then frozen at −20 °C. After filtration, the absorbance was measured at 530 nm to evaluate the anthocyanin content.
Cytotoxicity analyses
Adipose-derived stem cells (ADSCs) were incubated in DMEM medium containing 10% FBS and 1% PS at 37 °C under a humidified atmosphere with 5% CO2. ADSCs were seeded in 96 cell plates with a concentration of 104 cells/cell and incubated for 24 h. Cells were cultured using extraction solutions from different perovskite films (1 piece/50 mL). On the 1st, 4th, and 7th days, 100 μL of MTT (1 mg/mL) was added to each well and incubated for 4 h and further replaced by 100 mL of DMSO. The absorption values at 492 nm of cells in each well were measured by Multiskan MK3 (ThermoFisher, U.S.A.). And stain the cells on the first day using Calcein AM and PI, and observe the cells under an inverted fluorescence microscope (Leica, Germany).
Calculation details
The molecular sketches of all compounds were drawn using Material Studio 2020. Establish boxes for perovskite-water and perovskite-cellulose-water separately. Fixed crystal part of perovskite. Then perform a 200 ps NVT calculation using LAMMPS. Finally, post-processing is performed through Ovito.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information files and from the corresponding author upon request. Source data are provided with this paper.
References
Bati, A. S. et al. Next-generation applications for integrated perovskite solar cells. Commun. Mater. 4, 2 (2023).
Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 384, 189–193 (2024).
Zhu, H. et al. Long-term operating stability in perovskite photovoltaics. Nat. Rev. Mater. 8, 569–586 (2023).
Yang, Y. et al. Amidination of ligands for chemical and field-effect passivation stabilizes perovskite solar cells. Science 386, 898–902 (2024).
Zhou, J. et al. Highly efficient and stable perovskite solar cells via a multifunctional hole transporting material. Joule 8, 1691–1706 (2024).
Jang, J. H. et al. A novel approach for the development of moisture encapsulation poly (vinyl alcohol-co-ethylene) for perovskite solar cells. ACS omega 4, 9211–9218 (2019).
Tai, Q. et al. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 7, 11105 (2016).
Ding, B. et al. Dopant-additive synergism enhances perovskite solar modules. Nature 628, 299–305 (2024).
Liu, Z., Sun, B., Shi, T., Tang, Z. & Liao, G. Enhanced photovoltaic performance and stability of carbon counter electrode based perovskite solar cells encapsulated by PDMS. J. Mater. Chem. A 4, 10700–10709 (2016).
Wang, T. et al. Synergistic Defect Healing and Device Encapsulation via Structure Regulation by Silicone Polymer Enables Durable Inverted Perovskite Photovoltaics with High Efficiency. Adv. Energy Mater. 14, 2302552 (2024).
Chu, Q.-Q. et al. Encapsulation: The path to commercialization of stable perovskite solar cells. Matter 6, 3838–3863 (2023).
Li, Z. et al. Hyperbranched polymer functionalized flexible perovskite solar cells with mechanical robustness and reduced lead leakage. Nat. Commun. 14, 6451 (2023).
Cao, Q. et al. Environmental-friendly polymer for efficient and stable inverted perovskite solar cells with mitigating lead leakage. Adv. Funct. Mater. 32, 2201036 (2022).
Xu, L. et al. Methyl siloxanes in environmental matrices around a siloxane production facility, and their distribution and elimination in plasma of exposed population. Environ. Sci. Technol. 46, 11718–11726 (2012).
Wang, D.-G. et al. Concentrations of cyclic volatile methylsiloxanes in biosolid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere 93, 766–773 (2013).
Zhang, H. et al. Lead immobilization for environmentally sustainable perovskite solar cells. Nature 617, 687–695 (2023).
Dong, H. et al. Internal Encapsulation Strategy Using a Polymer Enables Efficient, Stable, and Lead-Safe Inverted Perovskite Solar Cells. Adv. Funct. Mater. 34, 2402394 (2024).
Yang, M. et al. Reducing lead toxicity of perovskite solar cells with a built-in supramolecular complex. Nat. Sustain. 6, 1455–1464 (2023).
Huang, Z. et al. Releasing nanocapsules for high-throughput printing of stable perovskite solar cells. Adv. Energy Mater. 11, 2101291 (2021).
Gong, C. et al. An Equalized Flow Velocity Strategy for Perovskite Colloidal Particles in Flexible Perovskite Solar Cells. Adv. Mater. 36, 2405572 (2024).
Yang, X. et al. Scalable flexible perovskite solar cells based on a crystalline and printable template with intelligent temperature sensitivity. Sol. RRL 6, 2100991 (2022).
Ahmad, T. et al. Encapsulation protocol for flexible perovskite solar cells enabling stability in accelerated aging tests. Energy Environ. Sci. 6, e12434 (2023).
Yang, X. et al. Low-temperature interfacial engineering for flexible CsPbI 2 Br perovskite solar cells with high performance beyond 15%. J. Mater. Chem. A 8, 5308–5314 (2020).
Deng, K., Liu, Z., Wang, M. & Li, L. Nanoimprinted grating-embedded perovskite solar cells with improved light management. Adv. Funct. Mater. 29, 1900830 (2019).
Li, Z. et al. Sulfonated Graphene Aerogels Enable Safe-to-Use Flexible Perovskite Solar Modules. Adv. Energy Mater. 12, 2103236 (2021).
Liu, H. et al. A low-cost and bendable “cage” for stable rigid and flexible perovskite solar cells with negligible lead leakage. Joule 9, 101816 (2025).
Nogami, S. et al. Evaluation of the rheological and rupture properties of gelatin-based hydrogels blended with polymers to determine their drug diffusion behavior. Polym. J. 54, 1477–1487 (2022).
Gazalian, D., Aliahmadi, A. & Rafati, H. Microencapsulation of Lactobacillus plantarum using cellulose-based polymers by spray-drying: A probiotic-delivery system for enhanced acid-resistance and storage stability. Int. J. Biol. Macromol. 311, 143634 (2025).
Cheng, W. et al. Sustainable cellulose and its derivatives for promising biomedical applications. Prog. Mater. Sci. 138, 101152 (2023).
Miranda, I. et al. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 13, 2 (2021).
Kuddannaya, S., Bao, J. & Zhang, Y. Enhanced in vitro biocompatibility of chemically modified poly (dimethylsiloxane) surfaces for stable adhesion and long-term investigation of brain cerebral cortex cells. ACS Appl. Mater. Interfaces 7, 25529–25538 (2015).
Bassi, D., Menossi, M. & Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 8, 2327 (2018).
Gamage, D. et al. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 41, 1233–1246 (2018).
Sofo, A., Dichio, B., Xiloyannis, C. & Masia, A. J. P. S. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci. 166, 293–302 (2004).
Abdel Latef, A. A. H., Kordrostami, M., Zakir, A., Zaki, H. & Saleh, O. M. J. P. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 8, 303 (2019).
Zhang, Y., Butelli, E. & Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 19, 81–90 (2014).
Kong, W., Liu, F., Zhang, C., Zhang, J. & Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 6, 1–8 (2016).
Luo, W. et al. Simultaneous Ultraviolet Conversion and Defect Passivation Stabilize Efficient and Operational Durable Perovskite Solar Cells. Adv. Funct. Mater. 34, 2400474 (2024).
Liu, C. et al. Flexible Indoor Perovskite Solar Cells by In Situ Bottom-Up Crystallization Modulation and Interfacial Passivation. Adv. Mater. 36, 2311562 (2024).
Li, Y. et al. Enhanced Corrosion Resistance of Ag Electrode Through Ionized 2-Mercaptobenzothiazole in Inverted Perovskite Solar Cells. Adv. Funct. Mater. 35, 2413245 (2024).
Kim, W. et al. Enhanced long-term stability of perovskite solar cells by passivating grain boundary with polydimethylsiloxane (PDMS). J. Mater. Chem. A 7, 20832–20839 (2019).
Yang, H. et al. Iodide Management and Oriented Crystallization Modulation for High-Performance All-Air Processed Perovskite Solar Cells. Adv. Mater. 36, 2411721 (2024).
Ning, L. et al. Intercepting the Chelation of Perovskites with Ambient Moisture through Active Addition Reaction for Full-Air-Processed Perovskite Solar Cells. Adv. Energy Mater. 14, 2401320 (2024).
Meng, H. et al. Inhibition of halide oxidation and deprotonation of organic cations with dimethylammonium formate for air-processed p-i-n perovskite solar cells. Nat. Energy 9, 536–547 (2024).
Ding, Y. et al. Stress regulation via surface micro-etching and reconstruction for enhancing triple-cation perovskite solar cells with an efficiency of 25.54%. Energy Environ. Sci. 17, 9268–9277 (2024).
Chen, Z. et al. Perovskite grain-boundary manipulation using room-temperature dynamic self-healing “ligaments” for developing highly stable flexible perovskite solar cells with 23.8% efficiency. Adv. Mater. 35, 2300513 (2023).
Wang, C. et al. Synergistic Toughening and Strain Releasing Strategy in Metal Halide Perovskite Photovoltaics. Adv. Funct. Mater. 34, 2410621 (2024).
Zhu, X. et al. A Surface-Reconstructed Bilayer Heterojunction Enables Efficient and Stable Inverted Perovskite Solar Cells. Adv. Mater. 36, 2409340 (2024).
Cao, R. et al. Methoxy-Based Passivator 4-Methoxyphenethylammonium Iodide as Multifunctional Passivator for High-Efficiency 3D/2D Flexible Perovskite Solar Cells over 23%. Sol. RRL 7, 2300680 (2023).
Zhan, C. et al. Indium Tin Oxide Induced Internal Positive Feedback and Indium Ion Transport in Perovskite Solar Cells. Angew. Chem. Int. Ed. 63, e202403824 (2024).
Li, Z. et al. Stabilized hole-selective layer for high-performance inverted pin perovskite solar cells. Science 382, 284–289 (2023).
Tang, X. et al. Macromers for Encapsulating Perovskite Photovoltaics and Achieving High Stability. Adv. Mater. 36, 2400218 (2024).
Ma, C. et al. Unveiling facet-dependent degradation and facet engineering for stable perovskite solar cells. Science 379, 173–178 (2023).
Li, K. et al. Self-Induced Bi-interfacial Modification via Fluoropyridinic Acid For High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 15, 2404335 (2024).
Zhang, Z. et al. Coordination engineering with crown ethers for perovskite precursor stabilization and defect passivation. Energy Environ. Sci. 17, 7182–7192 (2024).
Yang, Z. et al. Internal Capsulation Via Self-Cross-linking and π-Effects Achieves Highly Stable Perovskite Solar Cells. Adv. Mater. 36, 2410425 (2024).
Yang, Y. et al. A thermotropic liquid crystal enables efficient and stable perovskite solar modules. Nat. Energy 9, 316–323 (2024).
Cao, Q. et al. Co-Self-Assembled Monolayers Modified NiOx for Stable Inverted Perovskite Solar Cells. Adv. Mater. 36, 2311970 (2024).
Jiao, B. et al. Realizing stable perovskite solar cells with efficiency exceeding 25.6% through crystallization kinetics and spatial orientation regulation. Adv. Mater. 36, 2313673 (2024).
Acknowledgements
X.Y. acknowledges the financial support from the National Natural Science Foundation of China (52463027), the Science and Technology Project of the Education Department of Jiangxi Province (GJJ2201349), the Doctoral Start-up Funding of Jiangxi Science & Technology Normal University (2022BSQD03) and Jiangxi Provincial Key Laboratory of Advanced Electronic Materials and Devices (2024SSY03012). X.H. acknowledges the financial support from the National Natural Science Foundation of China (52173169, 52222312, 22461142139) and Natural Science Foundation of Jiangxi Province (20212BAB214055, 20224ACB204007). Z.H. acknowledges the financial support from the National Natural Science Foundation of China (52103244, 52473261) and Natural Science Foundation of Jiangxi Province (20232BAB214028). Z.X. acknowledges the financial support from the National Natural Science Foundation of China (12364013). S.D. acknowledges the financial support from the National Natural Science Foundation of China (52462041).
Author information
Authors and Affiliations
Contributions
Y. Y., J. Z. and H. Y. contributed equally to this work. X. Y., J. Z. and X. H. conceived and designed the experiments. Y. Y. fabricated the PSC devices. Y. Y., X. Y., H. Y. and X. H. completed the writing of the manuscript. Y. Y., J. Z. and X. Y. characterized and analyzed the crystallization kinetics and morphology of PSCs. Y. Y. and H. Y. performed PL, TRPL, in-stu UV, TPV, and TPC tests. Y. Y., J. Z., Y. L., Z. X., Z. H. and S. D. characterized the various photoelectric properties.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Fei Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Zhao, J., Yang, H. et al. Green encapsulants boost stability and sustainability in inverted perovskite solar cells. Nat Commun 16, 8993 (2025). https://doi.org/10.1038/s41467-025-64031-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64031-8