Abstract
Viral strategies to antagonize the robust host innate immune response have a major function in the pathogenicity of viral infection and virus-associated cancers. Epstein-Barr virus (EBV) infection causes infectious mononucleosis (IM) and several human cancers. While latent EBV can reactivate in some nasopharyngeal carcinoma (NPC) cells, the impact of EBV reactivation on the anti-viral innate immune and immunotherapy response of NPC patients remains incompletely understood. Here, we reveal the function of the EBV-encoded BRRF2 protein as a pivotal regulator of the host immune system. We show that BRRF2, which is secreted via extracellular vesicles (EVs) from NPC cells undergoing EBV reactivation, specifically targets macrophages. It disrupts the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling pathway, which is crucial for innate immunity. BRRF2 inhibits the enzymatic activity of cGAS by interfering with the interaction of cGAS with dsDNA and reducing cGAS-DNA phase separation. Notably, our research shows a marked increase in the levels of BRRF2+ EVs in the bloodstream of NPC patients, which is closely associated with a diminished response to immunotherapy. By identifying BRRF2 as a potential biomarker for immunotherapy resistance, our findings provide deeper insight into the contribution of EBV to viral immunology and suggest further avenues for therapeutic intervention to increase the efficacy of immunotherapy.
Similar content being viewed by others
Introduction
Epstein-Barr virus (EBV), the first identified oncogenic human herpesvirus, is associated with approximately 1.5% of global cancer cases. These cancers include nasopharyngeal carcinoma (NPC), a head and neck epithelial tumor characterized by immune evasion mechanisms, and other malignancies, such as Burkitt lymphoma and gastric carcinoma1,2. Despite the approval of immune checkpoint inhibitors for the treatment of recurrence/metastatic NPC in China in 20213, current immunotherapies have limited success rates4,5, highlighting the need for new strategies against EBV-associated cancers.
Viruses have evolved strategies to counter robust host innate immunity and maintain infection and replication. For example, various viral proteins from herpesviruses, such as Kaposi’s sarcoma-associated herpesvirus (KSHV)6,7,8,9, human cytomegalovirus (HCMV)10,11,12, herpes simplex virus 1 (HSV-1)13,14,15, varicella-zoster virus (VZV)9, and EBV16 have been shown to target and inhibit key immune molecule, such as cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING). Open reading frame 52 (ORF52) from KSHV and the homologous BLRF2 protein from EBV have been reported to inhibit cGAS-deoxyribonucleic acid (DNA) phase separation and alter the innate immune response through the extraction of DNA from the cGAS-DNA droplet and its own phase separation and aggregation9. A direct interaction between ORF52 and cGAS/DNA has not been identified, but the ability of ORF52-DNA phase separation to form is significantly greater than that of cGAS-DNA phage separation9. The EBV deubiquitinase BPLF1 suppressed both cGAS-STING and RIG-I-mediated innate immune signaling by removing the ubiquitin modifications essential for downstream signaling activation, thereby facilitating viral immune evasion16. The ubiquitous presence of EBV in nonkeratinizing NPC and lymphoproliferative diseases signifies its pathogenic role17, with interactions between EBV variants and the tumor microenvironment increasing the risk of persistent infection and immunosuppression18,19,20. While previous studies focused on the role of latent EBV gene expression in immune escape in NPC21,22,23, some NPC cells exhibit EBV in its lytic cycle24,25,26, with unclear implications for the tumor environment and immunotherapy response.
The role of extracellular vesicles (EVs) in transferring viral proteins to uninfected or distant cells is increasingly recognized27,28. EVs, which are vital for cell communication and homeostasis29, can exacerbate viral pathogenesis and spread, as observed in viruses, such as human immunodeficiency virus (HIV)30,31 and human papillomavirus (HPV)32. While significant advances have been achieved in understanding EBV-containing EVs33,34,35, their roles in infection, spread, cancer development and immune modulation remain incompletely understood and warrant further investigation. EBV-encoded BRRF2, a conserved tegument protein in the gammaherpesvirus group, has been studied primarily for its role in virus production36. However, its potential influence on immune regulation remains unexplored.
Here, we show that BRRF2 in EBV-infected cells is transmissible via EVs, where it impacts recipient cells. BRRF2 binds to and inhibits cGAS, limiting cGAS-DNA phase separation. BRRF2 is a potential biomarker for the immunotherapy response and a therapeutic target in NPC therapy.
Results
Identification of EBV-encoded proteins in extracellular vesicles as regulators of innate immunity in NPC
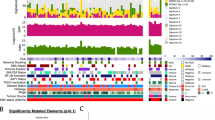
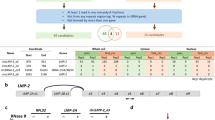
We explored the impact of the EBV lytic cycle on the microenvironment of NPC, with a particular focus on its potential regulatory effects on innate immune responses, by performing a systemic screen of EV-carried EBV proteins that might play a role in the regulation of innate immunity. EBV+ CNE2 cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) and sodium butyrate (NaB) to induce EBV reactivation. EVs were purified from those lytic cells according to the protocol shown in Fig. 1A. Sucrose density gradient ultracentrifugation separates components into distinct fractions based on their buoyant densities, resulting in different sucrose density ranges across the tubes. Each tube corresponds to a specific density with specific components. After sucrose density gradient ultracentrifugation, electron microscopy showed that the EVs and virus could be separated efficiently (Fig. 1B). EBV-encoded proteins and EV markers were detected by western blotting, and the concentration of EVs was determined by nanoparticle tracking analysis (NTA). Separated tube 7 contained mainly EVs without detectable EBV glycoprotein B (gB), while the separated tube 11 contained mainly EBV without detectable EV markers (Fig. 1C). EBV protein levels in EVs in separated tube 7 were also determined by mass spectrometry (MS). As expected, EBV reactivation promoted the secretion of EVs (Fig. 1C and D), and EVs from EBV lytic cells contained several EBV-encoded proteins, including the tegument proteins BLRF2 and BRRF2 (Supplementary Table 1). We constructed plasmids encoding all the EBV proteins to screen for EBV proteins that potentially inhibit cGAS-STING signaling. We successfully constructed plasmids encoding 78 EBV proteins, which represent the majority of the EBV proteome, as EBV encodes 84 proteins. We screened these plasmids for their ability to regulate cGAS-STING-mediated activation of the interferon β (IFNβ) promoter in HEK293T cells, and we identified several candidate EBV proteins that could markedly antagonize cGAS-STING-mediated signaling (Fig. 1E). Among these candidates, BZLF1, BRLF1, BLRF2 and BGLF4 have been reported to inhibit the EBV-induced expression of IFNβ37,38,39. This evidence suggested the reliability and validity of our screening experiment. A Venn diagram showed that BMRF1, BLRF2 and BRRF2 were the cargos of EBV-induced EVs and inhibitors of cGAS-STING signaling (Fig. 1F). Among them, BMRF140 and BLRF29,41 have been extensively studied previously. In addition, BGLF542,43,44, BPLF116, and LMP145,46,47 are well-characterized immune modulators. However, both the role of BRRF2 in suppressing cGAS-STING signaling and the underlying mechanism remain uncharacterized, as neither has been systematically investigated to date.
A Protocol for EV isolation and purification from the EBV+ CNE2 cell line. B EV isolation and purification from EBV+ CNE2 cells treated with or without NaB and TPA. Electron microscopy was used to identify EVs and viruses. The experiment was performed as three independent biological replicates. Representative images was shown. C EV isolation and purification from EBV+ CNE2 cells treated with or without NaB and TPA. Western blotting was used to detect EV markers (HSP70, CD63 and CD9) and an EBV-encoded protein marker (EBV-gB). Source data are provided as a Source Data file. D The concentration and size distribution of EVs from EBV+ CNE2 cells treated with or without NaB or TPA were measured using NTA. E EBV viral proteins were sorted by the suppression of cGAS-STING-mediated signaling. HEK293T cells were cotransfected with cGAS, STING, the IFNβ reporter (0.1 μg), the pRL-TK reporter (0.01 μg), and the indicated EBV viral protein plasmids (0.1 μg). The cells were harvested for dual-luciferase assays at 24 h after transfection. The experiment was performed as three independent biological replicates. The data are plotted as the means ± SEMs. F A Venn diagram was constructed to screen EV-derived EBV-encoded proteins involved in suppressing cGAS-STING-mediated signaling.
Circulating BRRF2+ EVs are potential biomarkers for predicting the effect of anti-programmed cell death protein 1 (PD-1) therapy in NPC
We first wanted to determine the clinical significance of circulating BRRF2+ EVs as biomarkers to predict the immunotherapy outcomes of NPC patients. We developed an immune-polymerase chain reaction (PCR) method to analyze BRRF2+ EVs in serum from NPC patients before anti-PD-1 therapy with a custom BRRF2-specific antibody. The custom BRRF2-specific antibodies were selected by immunizing rabbits with the purified BRRF2Δ322-573 protein. The affinity and specificity of the antibodies were characterized by enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR), western blotting analysis and immunofluorescence staining. The results indicated that the custom BRRF2-specific #12 and #14 antibodies are highly specific for BRRF2 and could be used in ELISAs (Fig. S1A–C and Fig. S2A, B). The #12 antibody could be also be used for western blotting and immunofluorescence staining (Fig. S1D, E). In competition assays, the #12 antibody did not compete with the #14 antibody, suggesting that they bind to distinct epitopes (Fig. S2C). We evaluated the linearity and dynamic range of the immuno-qPCR assay using the recombinant BRRF2 protein spiked into fetal bovine serum samples. Our results demonstrated that by incorporating PCR amplification technology, which provides enhanced sensitivity, the assay exhibited a linear dynamic range for BRRF2 protein detection between 0.24 and 15.63 ng/mL. After logarithmic transformation of the concentrations and linear fitting, we achieved an R² value of 0.9538, indicating excellent linearity across this range (Fig. S2D, E). These validation data confirm the robust analytical performance of our immuno-qPCR assay for quantifying BRRF2 protein levels in serum samples. Immuno-qPCR was performed, as shown in Fig. S2F (created with BioGDP.com48), and revealed higher BRRF2+ EVs levels in patients with a lower treatment response (Fig. 2A), suggesting a potential association between BRRF2+ EVs and immunosuppression, which warrants further investigation. The predictive value of BRRF2+ EVs for the anti-PD-1 therapeutic response was substantiated by the receiver operating characteristic curve analysis, which revealed high predictive accuracy, sensitivity, and specificity of 77.6, 71.7, and 82.7%, respectively (Fig. 2B, C and Supplementary Table 2). Multiplex immunohistochemistry (IHC) revealed the colocalization of the EBV lytic cycle proteins ZTA, and BRRF2 and the epithelial marker panCK in NPC tissues (Fig. 2D). These findings suggest that BRRF2 may be involved in immunomodulation during the EBV lytic cycle. These findings suggest that BRRF2+ EVs are potential biomarkers for predicting anti-PD-1 therapeutic outcomes in patients with EBV-associated NPC.
A Serum samples from a total of 50 HCs and 52 CR/PR and 47 SD/PD patients were collected. Circulating EVs carrying BRRF2 in serum samples from NPC patients pretreated with immunotherapy were determined by immuno-qPCR. The experiment was performed as three independent biological replicates. The data are shown as the means ± SDs. The cut-off value that maximized the Youden index was determined from the ROC analysis. Statistical differences were determined by nonparametric Kruskai-Wallis test with Dunn’s test for multiple comparisons. Specific p value are: CR/PR vs. SD/PD, p = 2.25e-05; CR/PR vs. HCs, p = 3.33e-07; and SD/PD vs. HCs, p = 2.81e-21. ***p < 0.001. B The area under the receiver operating characteristic curve (AUROC) was assessed for the discrimination of the CR/PR group from the SD/PD group according to the level of circulating EVs carrying BRRF2. C Confusion matrix for differentiating the CR/PR group from the SD/PD group based on the cutoff value (10.385). The x-axis represents the predicted immunotherapy response. The y-axis represents the actual immunotherapy response. The cutoff value that maximized the Youden index was determined from the ROC curve analysis. Significant differences were determined by the nonparametric Kruskal‒Wallis test. The data were obtained from at least three independent experiments. NPC nasopharyngeal carcinoma, CR complete response, PR partial response, SD stable disease, PD progressive disease, HCs healthy controls, CI confidence interval. D Representative images of BRRF2 (green), ZTA (yellow) and panCK (orange) staining in NPC patients’ tumor tissues. Scale bars, 5 μm.
BRRF2 promotes the secretion of BRRF2 from EVs
We then examined whether BRRF2 affects EV secretion and how BRRF2 accesses into EVs. First, HEK293T cells were transfected with Flag-BRRF2 or Flag-glutathione S-transferase (GST) as a control. Both EVs and cell lysates were collected for further comparison, which showed that Flag-BRRF2-expressing cells contained more EVs than control cells did (Fig. 3A, B). Sucrose density gradient ultracentrifugation revealed that BRRF2 was present in EVs from Flag-BRRF2-expressing cells (Fig. 3C). mCherry-BRRF2 was transferred into HEK293T cells, and the EVs were purified to visualize BRRF2 in EVs. Images of red fluorescence were captured using a confocal microscope (Fig. 3D). When the purified EVs were labeled with the CellMaskTM green plasma membrane dye, both green and red fluorescence were colocalized on the CD63 beads (Fig. 3E), indicating that BRRF2 is a component of the EVs. Furthermore, we examined the presence of BRRF2 in purified EVs from the EBV + CNE2 cell line with a custom BRRF2-specific antibody. As shown by western blotting, BRRF2 was highly enriched in the EVs derived from the EBV + CNE2 and Raji cell lines after treatment with TPA and NaB (Fig. 3F). More importantly, BRRF2 was detected in EVs from the serum of EBV-positive NPC patients (Supplementary Table 3 and Fig. 3G).
A HEK293T cells were transfected with Flag-GST or Flag-BRRF2 for 48 h. The cell supernatant was collected, and the EVs were isolated. Immunoblots were performed with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. B The concentration and size distribution of EVs from HEK293T cells transfected with Flag-GST or Flag-BRRF2 were measured using NTA. C EVs were purified using the sucrose density gradient ultracentrifugation. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. D Representative confocal image of purified EVs secreted from the HEK293T-mCherry-BRRF2 cell line. The experiment was performed as three independent biological replicates. E Representative confocal image of purified EVs carrying mCherry-BRRF2 (the cell mask was stained in green) captured by human CD63 isolation beads. The experiment was performed as three independent biological replicates. F Western blotting was performed to detect the expression of BRRF2 in EVs from EBV+ CNE2 and Raji cells treated with or without NaB and TPA. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. G EVs from 12 serum samples from NPC patients were collected by CD63 isolation beads, and the expression of BRRF2 was detected by immunoblotting. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file.
BRRF2 in EVs is captured by macrophages cells and suppresses innate immunity
EVs mediate cell-to-cell signaling by carrying a variety of cargos and transferring them between cells49. We propose that BRRF2 may be secreted in EVs from EBV-infected cells and function in recipient cells. A wide range of innate immune cells, including monocytes, dendritic cells (DCs), macrophages and neutrophils, are relevant to antiviral immunity50. Macrophages sense EBV and stimulate innate immune responses against EBV51. We analyzed RNA-seq data from the GSE102349 dataset using single-sample gene set enrichment analysis (ssGSEA) to investigate the relationship between BRRF2 expression and the tumor microenvironment composition. The tumor samples were stratified into BRRF2-high and BRRF2-low groups based on the median BRRF2 expression level. The composition of immune and stromal cells was then compared between these groups. The box plot (Fig. 4A) illustrates that, compared with the BRRF2-low samples, the BRRF2-high samples presented increased enrichment of macrophages, suggesting an immunestimulatory microenvironment in the presence of elevated BRRF2 expression. In a multiplex IHC study, BRRF2 expression was observed not only in tumor cells, which were characterized by positive signals from panCK antibodies but also in macrophages, which were characterized by cluster of differentiation 68 (CD68) staining within the tumor tissues of human NPC patients (Fig. 4B). An analysis of the spatial distribution within the tumor microenvironment revealed a significant difference in the average distance between tumor cells and CD68+ macrophages, which were stratified by BRRF2 expression status (Fig. 4C). Compared with BRRF2- tumor cells, BRRF2+ tumor cells presented a markedly reduced average distance to CD68+ macrophages. These results suggest that BRRF2 expression may enhance tumor-immune interactions through transfer from EBV-positive tumor cells to macrophages in the tumor microenvironment or release into the blood.
A The box plot shows the differences in the immune and stromal cell compositions between the two groups of tumor samples with different BRRF2 expression levels based on the RNA-seq data. p values were determined via an unpaired two-side ttest. Center line, median (50th percentile); box bounds, 25 and 75th percentiles (interquartile range, IQR); whiskers, extend from the bounds to the smallest or largest value within 1.5 × IQR of the bound; minima and maxima, the end points of the whiskers. B Representative images of panCK (green), BRRF2 (yellow) and CD68 (orange) staining in NPC patients’ tumor tissues. Scale bars, 5 μm. C The average distance from BRRF2+ or BRRF2- tumor cells to CD68+ cells was measured from the IHC images. Each dot represents a patient’s tumor tissue. The experiment was performed as three independent biological replicates. **p < 0.01 according to a two-sided paired t test. D HPMs and THP1 cells were incubated with HEK293T-induced EVs carrying mCherry-BRRF2 for 4 h, and mCherry-BRRF2 was internalized into the cells. E HPMs and THP1 cells were incubated with HEK293T-induced EVs carrying mCherry-BRRF2 for 4 h. mCherry signals were detected by flow cytometry. The median fluorescence intensity (MFI) is shown. (Means ± SEMs of n = 3 experiments. ****p < 0.0001 according to a two-sided unpaired ttest). F EVs from the HEK293T-Flag-BRRF2 cell line inhibited cGAS-STING activation induced by HT-DNA in THP1 and HPM cells. THP1 cells and HPMs were incubated with EVs from the HEK293T-Flag-BRRF2 cell line for 2 h and then transfected with 2 μg of HT-DNA for 6 h. Cell lysates were collected for immunoblotting with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. G After an incubation with EVs and transfection with HT-DNA, total mRNAs were extracted from THP1 cells for a qPCR analysis of the expression of the IFNβ, RANTES and IL6 mRNAs. The error bars are derived from triplicate experiments. The data are plotted as the means ± SEMs. ns indicates not significant. ****p < 0.0001 according to two-way ANOVA followed by Sidak’s multiple comparisons test.
We monitored whether EVs were captured by human primary macrophages (HPM) or THP1 cells by incubating purified EVs carrying mCherry-BRRF2 for 2 h. HPM and THP1 cells presented fluorescence signals, indicating the uptake of BRRF2 in these cells (Fig. 4D). Furthermore, mCherry fluorescence was also detected in HPM and THP1 cells via flow cytometry (Fig. 4E). Taken together, these data indicate that EVs containing BRRF2 are captured by macrophages.
We then investigated whether BRRF2, which is carried by EVs and internalized by macrophages, modulates the activation of the cGAS-STING pathway induced by herring testes (HT)-DNA. HT-DNA transfection induced the phosphorylation of STING, TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3) and increased the mRNA levels of IFNβ, processes regulated upon the activation of normal T-cell expressed and secreted factor (RANTES) and interleukin 6 (IL6), indicating the activation of the cGAS-STING signaling pathway. As expected, coculturing EVs carrying BRRF2 with macrophages prior to HT-DNA transfection abrogated both the increases in phosphorylated STING, TBK1 and IRF3 levels and the increases in the mRNA expression of IFNβ, RANTES and IL6 (Fig. 4F, G). These results suggested that BRRF2 transferred into cells via EVs and suppressed the cGAS-STING signaling pathway. Taken together, our results indicate that EBV-encoded BRRF2 can be transferred through EVs to macrophages, after which the cGAS-STING signaling pathway and proinflammatory cytokine gene expression are inhibited, contributing to further innate immune inhibition.
BRRF2 inhibits the dsDNA-triggered induction of downstream antiviral genes
HK1 cells were stably transduced with lentiviral constructs expressing BRRF2 or an empty control vector to examine whether BRRF2 is involved in the double-stranded DNA (dsDNA)-regulated antiviral gene expression. The transcription of IFNβ, RANTES and IL6 was detected by qPCR analysis. Notably, ectopic expression of BRRF2 inhibited the HT-DNA-induced transcription of the IFNβ, RANTES and IL6 genes compared with that in empty vector-transduced control cells (Fig. S3). Similarly, BRRF2 had similar effects on the suppression of the cGAS-STING pathway stimulated by HSV120 and poly(dA:dT) treatments (Fig. S3). These results suggest that BRRF2 inhibits the dsDNA-triggered induction of downstream antiviral genes.
BRRF2 interacts with cGAS
IFNβ promoter reporter assays were performed to explore how BRRF2 inhibits the cGAS-STING signaling pathway. The transfection of BRRF2 inhibited IFNβ promoter activation via the overexpression of cGAS and STING but not their downstream components TBK1 and IRF3-5D (Fig. 5A). We elucidated the molecular mechanisms underlying the negative regulatory role of BRRF2 in the innate antiviral response by examining whether BRRF2 could interact with cGAS by coimmunoprecipitation. The results showed that ectopic BRRF2 interacted with endogenous and overexpressed cGAS in HK1 and HEK293T cells, respectively (Fig. 5B, C). The BRRF2Δ322-573 and cGAS interaction could be fitted to a 1:1 simple binding model, and the calculated constant affinity was KD = 4.46 × 10−8 M (Fig. 5D). Domain mapping experiments revealed that N-terminus (1-96) of BRRF2 and the C-terminus of cGAS (213-522) were important for their interaction (Fig. 5E, F). Additionally, the removal of the N-terminus (1-96) of BRRF2 affected its ability to inhibit IFNβ reporter gene activation (Fig. 5G). These results suggest that BRRF2 inhibits the innate antiviral response by interacting with cGAS.
A BRRF2 targets the upstream cGAS–STING pathway. HEK293T cells were cotransfected with an empty vector, cGAS + STING (left panel), TBK1 (middle panel) or IRF3-5D (right panel) and the indicated vector or BRRF2. Twenty-four hours later, the cells were harvested for dual-luciferase assays. (Means ± SEMs of n = 3 repeated experiments. ns indicates not significant. ****p < 0.0001 according to one-way ANOVA followed by Tukey’s multiple comparisons test.) B BRRF2 directly interacts with cGAS. HK1 cells transfected with Flag-tagged BRRF2 or an empty vector were harvested for coimmunoprecipitations and immunoblot assays with BRRF2 antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. C Association of BRRF2 with cGAS. HEK293T cells were transfected with Myc-tagged cGAS and Flag-tagged BRRF2 or an empty vector for 24 h. Coimmunoprecipitations and immunoblots were performed with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. D Surface plasmon resonance (SPR) characterization of the interaction of the BRRF2 protein with cGAS. Kinetic analysis of the ability of purified BRRF2Δ322-573 (0.0625-1 μM) to immobilize cGAS. The binding responses at each concentration are shown as the results of the global fit analysis. The datasets were fit to a simple 1:1 interaction model for BRRF2Δ322-573 + cGAS. The experiment was repeated in triplicate. Representative result was shown. E HEK293T cells were transfected with cGAS-Myc and Flag-BRRF2 or the indicated BRRF2 mutants for 24 h. Coimmunoprecipitations and immunoblots were performed with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. F HEK293T cells were transfected with Flag-BRRF2 and cGAS-Myc with the indicated cGAS mutants for 24 h. Coimmunoprecipitations and immunoblots were performed with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. G HEK293T cells were cotransfected with cGAS, STING, or the IFNβ reporter (0.1 μg), the indicated BRRF2 WT or mutant plasmid (0.1 μg). The cells were harvested for dual-luciferase assays at 24 hrs after transfection. (Means ± SEMs of n = 3 experimental repeats. ns indicates not significant. *p < 0.05 and ****p < 0.0001 by one-way ANOVA followed by Tukey’s multiple comparisons test).
BRRF2 inhibits the binding of cGAS to DNA and cGAS enzymatic activity
As cGAS-DNA phase separation plays a key role in the activation of the cGAS enzyme activity, we investigated the influence of BRRF2 on the interaction of cGAS with DNA. The EBV BLRF2, which has been reported to inhibit the binding of cGAS to DNA, was used as a positive control in this experiment52. In the streptavidin pull-down assay, BRRF2 bound to HSV120 and dramatically inhibited the binding of cGAS to DNA (Fig. S4A). Strikingly, along with the increased expression of BRRF2, the amount of cGAS bound to HSV120 gradually decreased (Fig. S4B, C). These findings indicate that BRRF2 inhibits the interaction of cGAS with DNA in a dose-dependent manner.
Given the critical role of phase separation in cGAS enzymatic activation, we investigated whether BRRF2 inhibits the enzymatic activity of cGAS. An in vitro enzymatic reaction was performed by incubating cGAS with HSV120 in the presence of the substrates adenosine triphosphate (ATP) and guanosine triphosphate (GTP) and the reaction products were then analyzed by ion-exchange chromatography. Compared with the addition of the GST control, the addition of BRRF2 markedly reduced the production of cyclic guanosine monophosphate (GMP)-adenosine monophosphate (AMP) (cGAMP) in a dose-dependent manner (Fig. S4D). Along with the decrease in the level of the cGAMP product, the amounts of the residual substrates ATP and GTP subsequently increased, indicating that BRRF2 inhibited the enzymatic activity of cGAS (Fig. S4D). The cGAMP products from the GST control group could induce the transcription of antiviral genes, such as IFNβ, RANTES and IL6, in THP1 cells, according to the q-PCR analysis, whereas the induction of these genes was inhibited in the GST-BRRF2 groups, indicating that BRRF2 reduced the production of cGAMP and subsequently inhibited the transcription of the IFNβ, RANTES and IL6 genes (Fig. S4E–G). These results directly reveal that BRRF2 inhibits cGAS enzymatic activity.
BRRF2 inhibits cGAS phase separation in vivo and in vitro
dsDNA stimulation has been shown to induce cGAS phase separation to form cGAS‒DNA granules in the cytoplasm, which is critical for the production of cGAMP to induce signal transduction9,53. Ras GTPase-activating protein-binding protein 1 (G3BP1) colocalizes with cGAS–DNA foci and promotes the interferon response54. We generated stable HEK293T cell lines with continuous expression of green fluorescent protein (GFP)-cGAS to determine whether BRRF2 affects the ability of cGAS to form granules with dsDNA in cells. HT-DNA was transfected to induce the formation of cGAS–DNA foci in stable BRRF2-overexpressing cells. In GFP-cGAS-expressing HEK293T cells, HT-DNA transfection robustly induced GFP-cGAS granule formation. The proportion of cells exhibiting cGAS–DNA foci was nearly 60% following transfection with the vector control. However, this proportion decreased to approximately 20% when mCherry-BRRF2 was present (Fig. 6A), suggesting that BRRF2 inhibited GFP-cGAS granule formation. Similarly, HT-DNA also induced G3BP1 granule formation in HEK293T cells. The proportion of cells containing G3BP1 foci decreased from more than 50% to nearly 20% with the transfection of mCherry-BRRF2 (Fig. 6B). These results indicate that ectopic BRRF2 expression can prevent cGAS from forming granules with dsDNA.
A Left panel, Representative confocal images acquired from HEK293T/GFP-cGAS cells transiently expressing mCherry-BRRF2 or an empty vector, which were transfected with HT-DNA (2 μg/mL) for 12 h. The cells were fixed with 4% paraformaldehyde and stained with DAPI. Right panel, Histogram of the percentage of cells with GFP-cGAS foci. The data were collected from 100 cells. (The means ± SDs are shown. ****p < 0.0001 according to two-way ANOVA followed by Sidak’s multiple comparisons test). B Left panel, Representative confocal images acquired from HEK293T cells transiently expressing mCherry-BRRF2 or an empty vector, which were transfected with HT-DNA (2 μg/mL) for 12 h. The cells were fixed with 4% paraformaldehyde for immunofluorescence staining with the indicated anti-G3BP1 antibody. Right panel, Histogram of the percentage of cells with G3BP1 foci. The data were collected from 100 cells. (The means ± SDs are shown. ***p < 0.001, ****p < 0.0001 by two-way ANOVA followed by Sidak’s multiple comparisons test). C Diagram of the sedimentation assay used to separate the condensed liquid phase and the aqueous phase for immunoblot assays. D Separation of the condensed liquid phase and the aqueous phase of His-cGAS and HT-DNA. His-cGAS was mixed with or without HT-DNA for phase separation in the presence of GST or the indicated amount of GST-tagged BRRF2 for 1 h at 37 °C. The condensed liquid phase and the aqueous phase were prepared for immunoblot assays with the indicated antibodies. The experiment was performed as three independent biological replicates. Representative images was shown. Source data are provided as a Source Data file. E Representative DIC time-lapse imaging of Cy5-dsDNA and FITC-cGAS liquid droplets in the presence/absence of mCherry-BRRF2Δ322-573. The experiment was performed as three independent biological replicates.
We expressed and purified the His-tagged cGAS, GST-tagged BRRF2 and GST-only proteins to investigate whether BRRF2 inhibited cGAS phase separation in vitro. cGAS was incubated with HT-DNA supplemented with the indicated amount of GST-BRRF2, the GST tag or the same volume of control buffer. A sedimentation assay was performed to separate the condensed liquid phase and the aqueous phase (Fig. 6C). HT-DNA promoted cGAS phase separation and transformed cGAS from the aqueous phase into the condensed liquid phase. However, compared with the addition of the GST control, the addition of BRRF2 disrupted cGAS‒DNA phase separation and inhibited the conversion of cGAS into the condensed liquid phase in a dose-dependent manner (Fig. 6D). DIC time-lapse imaging revealed that dsDNA treatment promoted cGAS‒DNA liquid droplet formation (Fig. 6E). Strikingly, BRRF2 inhibited cGAS‒DNA phase separation and liquid droplet fusion (Fig. 6E). Taken together, these findings are consistent with the results in cells, as the BRRF2 protein inhibits cGAS phase separation.
BRRF2 facilitates EBV replication
We further investigated the function of BRRF2 during EBV replication. We first analyzed the correlation between the BRRF2 levels in NPC serum samples and the corresponding EBV DNA copy numbers. The R value was 0.455, indicating that BRRF2 expression was significantly positively correlated with EBV replication (Fig. 7A). Two different short hairpin RNAs (shRNAs) targeting BRRF2 were constructed and transfected into BRRF2-transduced EBV+ CNE2 cells, and the expression of BRRF2 was successfully knocked down (Fig. 7B). Similarly, in NaB- and TPA-activated EBV+ CNE2 cells, the BRRF2 mRNA was also knocked down following the transduction of these two specific shRNAs (Fig. 7C). Since a GFP gene was inserted into the genome of EBV, the GFP signal reflected the replication of EBV in NaB- and TPA-activated EBV+ CNE2 cells. BRRF2 knockdown dramatically decreased the number of GFP-positive cells, indicating that a reduction in BRRF2 expression restricted EBV replication (Fig. 7D). Similarly, the qPCR analysis revealed that the knockdown of BRRF2 expression markedly reduced the number of EBV copies in NaB- and TPA-activated EBV+ CNE2 cells (Fig. 7E, F). These results indicate that BRRF2 plays an important role in EBV replication.
A Positive correlation between EBV DNA and standardized EVs BRRF2 value. A scatter plot showing the significant positive relationship between EBV DNA levels (x-axis) and the standardized values of BRRF2 in extracellular vesicles (EVs) (y-axis). Each blue dot represents an individual sample. The solid blue line indicates the linear regression fit, and the surrounding gray shaded area represents the 95% confidence interval (CI) for the regression line. The plots on the top and right margins show the data distribution for each variable, respectively. R = 0.455 and p = 9.46e-06 in the Pearson correlation analysis. B Knockdown of Flag-BRRF2 in EBV+ CNE2 cells by shRNAs. EBV+ CNE2 cells transduced with Flag-BRRF2 were transfected with shRNAs for 24 h. Immunoblots were performed with the indicated antibodies. Source data are provided as a Source Data file. C EBV+ CNE2 cells transduced with BRRF2 shRNAs were treated with NaB + TPA. Twenty-four hours later, a qPCR assay was performed to analyze the expression of the BRRF2 mRNA. (The experiment was performed as four independent biological replicates. The data are plotted as the means ± SEMs. ****p < 0.0001 by two-sided Student’s t test). D–F EBV+ CNE2 cells transduced with BRRF2 shRNAs were treated with NaB + PMA. At the indicated time points, the cells were harvested to (D) count the number of GFP-positive cells through flow cytometry or (E, F) analyze the number of EBV genomic copies by qPCR. Means ± SDs of n = 3 technical replicates. *p < 0.05, **p < 0.01, and ****p < 0.0001 by two-way ANOVA followed by Tukey’s multiple comparisons test.
Discussion
In this study, we have identified and characterized an immunosuppressive mechanism in EBV infection and associated cancer progression, focusing on the role of EVs transporting the EBV tegument protein BRRF2. This protein interferes with cGAS-DNA phase separation in recipient cells, representing an innovative pathway for immune evasion.
EV research is often limited by the copurification of contaminants, and we adopted a multifaceted characterization approach in alignment with the guidelines established by the International Society for Extracellular Vesicles (ISEV)55. Our study employed sucrose density gradient ultracentrifugation, a widely used method for EV isolation. We complemented multi-techniques with additional quality control measures to mitigate the potential copurification of contaminants. Utilizing western blotting to evaluate a panel of EV-associated positive (e.g., CD63, CD81, and TSG101) and negative (e.g., calnexin) markers, we confirmed the enrichment of EVs while excluding cellular and nonvesicular contaminants. NTA provided quantitative data on EV size distribution and nonvesicular concentration, further confirming the physical homogeneity of our EV preparations. The MS results indicated a significant enrichment of canonical EV markers (e.g., CD63, CD9, and Flotillin-1) while excluding common contaminants. Electron microscopy enabled direct visualization of EVs, revealing their characteristic cup-shaped morphology and the absence of significant contamination by apoptotic bodies or other large vesicles. These combined efforts provide a high level of confidence in the purity and integrity of the EVs used in this study.
EBV, which is implicated in various severe cancers, including NPC, poses challenges for effective immunotherapy56,57. Our research contributes to the existing knowledge by exploring the mechanisms of immune response modulation during EBV infection, a key area with significant potential for improving health care outcomes. In contrast to prior studies that have focused primarily on the direct effects of viral infection on host cells58, our study emphasizes the role of EV-mediated intercellular communication in immune suppression. Previous studies have shown that EBV-infected cells release EVs containing viral and host-derived factors that influence recipient cells59,60,61. These EVs have been implicated in processes, such as immune modulation, intercellular communication and cancer development62,63. By investigating the role of EVs containing EBV-encoded BRRF2 in immune suppression, we expand upon these findings and provide further insights into the immunosuppression of EBV infection.
EBV has evolved multiple proteins to subvert the host immune response, such as BGLF5, BPLF1, and LMP1 serving as well-characterized examples16,21,42,43,44,45,46,47,64,65, and the involvement of BRRF2 in EVs may represent a distinct approach to immune modulation. While both BGLF542,43,44, BPLF116 and BRRF2 exhibit immune-suppressive functions, their molecular strategies differ fundamentally. BPLF1 employs a unique deubiquitinase activity to disrupt cGAS oligomerization, effectively blocking dsDNA recognition16. This contrasts with BGLF5’s mRNA destabilization mechanism42,44. BRRF2 hinders cGAS-DNA phase separation by directly interacting with cGAS. The orchestration of these processes by the aforementioned proteins may reflect a balance between pro- and anti-inflammatory signaling mediated by EBV during different stages of infection. This finding raises intriguing questions about how EBV orchestrates these interactions to dynamically modulate the immune response.
While we infer that BRRF2 might facilitate EBV replication, the correlation observed between serum BRRF2 and EBV DNA may reflect the tumor burden, with larger tumors releasing more BRRF2 and EBV DNA into the circulation. We did not specifically control for tumor size in our current analysis, which could indeed be an influencing factor in our study.
The identification of BRRF2 as a modulator of the cGAS-STING pathway adds to the growing understanding of the immune evasion strategies of EBV. While proteins, such as BGLF5, target upstream sensors44, BRRF2 appears to directly influence cGAS-STING signaling. Further investigations into the molecular mechanisms underlying the function of BRRF2, its interaction with other EBV proteins, and its role during lytic versus latent infection could reveal novel therapeutic targets for EBV-associated diseases.
Our findings show that EVs containing EBV-encoded BRRF2 from EBV-positive cells and high-copy NPC patient serum impair the cGAS-STING signaling pathway in recipient cells. This pathway is essential for the innate immune response. In addition to the NPC cells expressing BRRF2, uninfected macrophages expressed BRRF2. This mechanism of immune evasion via EVs provides a perspective that is distinct from the previously understood strategies employed by herpesviruses, which are known for their ability to counteract antiviral defenses.
An in-depth analysis revealed that BRRF2, along with other EBV proteins, counteracts cGAS-STING signaling, as evidenced by inhibited cGAS activation and decreased expression of proinflammatory cytokines. This finding complements previous studies that have identified various viral proteins involved in immune evasion but highlights a unique function of BRRF2 in this process.
We observed that BRRF2 hinders cGAS-DNA phase separation in cells and in vitro, necessitating a direct interaction between BRRF2 and cGAS. Although previous studies have reported that ORF52/VP22-type tegument proteins are able to restrict the formation of cGAS-DNA condensates9, these previous studies have not extensively explored the specific molecular interactions between viral proteins and cGAS. Our mutation analysis revealed that specific regions of BRRF2 and cGAS are critical for their interaction, with implications for a broader understanding of viral-host interactions.
Despite the development of immune therapies, their effectiveness in NPC patients is limited, with only an approximately 30% response rate4,5. Our research suggests that BRRF2 contributes to this limited response and may serve as a molecular marker for resistance to immunotherapy in NPC. This aspect of our study aligns with the growing body of research seeking to understand and overcome resistance to cancer immunotherapies.
Using an shRNA approach in CNE2-EBV cells, we found that BRRF2 aided in EBV viral infection. This model revealed that BRRF2 in EBV diminishes the effectiveness of viral infection, which aligns with findings from other studies focused on viral immunology.
While the study employs cell cultures to investigate the interaction between BRRF2 and the host immune system, these models might not fully replicate the complexity of human EBV infections and the human immune response. Translating these findings into effective clinical therapies presents significant challenges. The development of therapeutic agents targeting BRRF2 or the cGAS-STING pathway requires careful consideration of safety, specificity, and efficacy.
Our research elucidates how EBV employs BRRF2 to disrupt cGAS-DNA phase separation, a strategy distinct from previously known viral immune evasion tactics. The transfer of BRRF2 via EVs represents a sophisticated adaptation of EBV to host biology, significantly expanding our understanding of host-virus dynamics.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Sun Yat-sen University Cancer Center (protocol numbers: B2025-441-01 and B2025-469-01). Written informed consent was obtained from all participating patients for the use of their clinical parameters and biological samples for research purposes. For the healthy donor cohort, which consisted of retrospectively collected and fully anonymized residual serum from routine health check-ups, the requirement for informed consent was waived by the IRB.
Cell culture
The human acute monocytic leukemia cell line THP1 (ATCC, #TIB202), the murine breast cancer cell line EMT6 (ATCC, #CRL-2755), and the human NPC cell lines HONE1, CNE2, HK1 (provided from Dr. Sai-Wah Tsao, University of Hong Kong), and Raji (ATCC, #CCL-86), and latent EBV-infected CNE2 (EBV+ CNE2) cells were cultured with RPMI-1640 containing 10% fetal bovine serum at 37 °C with 5% CO2. The human embryonic kidney cell line HEK293T (ATCC, #CRL-3216) was cultured with DMEM containing 10% fetal bovine serum at 37 °C with 5% CO2.
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors using human lymphocyte separation medium (LTS1077, Dingguo). For the in vitro differentiation of monocytes into human macrophages (M0, primary macrophages), PBMCs were cultured in RPMI-1640 containing 10% fetal bovine serum and 50 ng/mL recombinant human macrophage colony-stimulating factor (M-CSF; Peprotech) for 7 days at 37 °C with 5% CO2.
Luciferase assays
HEK293T cells were transfected with the IFNβ reporter (0.1 μg), pRL-TK reporter (0.01 μg) or the indicated plasmids for 24 h. Control plasmids were added to ensure that each transfection received the same amount of total DNA. The firefly luciferase activity was assayed and normalized to that of Renilla luciferase.
Quantitative polymerase chain reaction (qPCR) assays
Total RNA was isolated using TRIzol reagent and precipitated with isopropanol. cDNAs were acquired for qPCR analysis to measure the mRNA levels of the indicated genes. The relative abundance of the target mRNAs was normalized to that of human GAPDH. The following primers were used:
human GAPDH forward, 5’-GAGTCAACGGATTTGGTCGT-3’;
human GAPDH reverse, 5’-GACAAGCTTCCCGTTCTCAG-3’;
human IFNβ1 forward, 5’-TTGTTGAGAACCTCCTGGCT-3’;
human IFNβ1 reverse, 5’-TGACTATGGTCCAGGCACAG-3’;
human RANTES forward, 5’-GGCAGCCCTCGCTGTCATCC-3’;
human RANTES reverse, 5’-GCAGCAGGGTGTGGTGTCCG-3’;
human IL6 forward, 5’-TTCTCCACAAGCGCCTTCGGTC-3’;
human IL6 reverse, 5’-TCTGTGTGGGGCGGCTACATCT-3’.
Western blot analysis
The cells were harvested and lysed with sample buffer for 10 min on ice. The supernatants were collected after centrifugation at 12,000 × g at 4 °C for 10 min. The protein concentration was determined using a BCA protein assay kit (Thermo Scientific #23225). The samples were boiled with bromophenol blue and 5% β-mercaptoethanol at 100 °C for 10 min and resolved via sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) on a 10% gel. Proteins were then transferred to polyvinylidene fluoride (PVDF) membranes. The membrane was blocked with 5% milk in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBST) for 1 h before being incubated with primary antibodies overnight and then incubated with secondary antibodies for another hour. Antibodies used in this study are listed in the Table S4.
Lentiviral transduction
HEK293T cells were cultured in 10 cm dishes and transfected with the indicated lentiviral plasmids, which contained exogenous genes or sh-RNA sequences, and the packaging plasmids psPAX2 and pMD2.G. At 48 h after transfection, supernatants containing lentiviruses were collected to infect the target cells.
Coimmunoprecipitation assays
The cells were harvested and lysed in NP40 lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP40 and 1% protease inhibitor cocktail) for 30 min on ice. The cell lysates were clarified by centrifugation at 12,000 × g at 4 °C for 15 min and then incubated with anti-Flag tag antibody conjugated agarose beads for 4 h. The protein bound beads were collected, washed five times with NP40 lysis buffer and then boiled in 2 × sample buffer for western blot analysis.
Virus preparation and infection
EBV, integrated with enhanced green fluorescent protein (EGFP), was prepared and produced in Akata cells by crosslinking with 0.8% goat anti-human IgG as previously described. The multiplicity of infection (MOI) of EBV was determined by qPCR to detect the BamH I-W fragment region of the EBV genome. Epithelial cells were exposed to EBV at 37 °C for 3 h and then washed twice to remove the unbound virus. Infected cells were cultured in fresh medium for the subsequent assays.
EBV reactivation
EBV latently infected CNE2 (EBV+ CNE2) cells were reactivated to enter the viral lytic cycle by the treatment with TPA (MCE #HY-18739) and NaB (MCE #HY-B0350A) for 12 h and then cultured in fresh medium. Flow cytometry was used to count the GFP-positive cells and a qPCR assay was performed to detect the BamH I-W and BALF5 fragment regions of the EBV genome to determinate the EBV titers. The following primers were used:
EBV BamH I-W forward, 5’-CCCAACACTCCACCACACC-3’;
EBV BamH I-W reverse, 5’-TCTTAGGAGCTGTCCGAGGG-3’;
EBV BALF5 forward, 5’-CGGAAGCCCTCTGGACTTC-3’;
EBV BALF5 reverse, 5’-CCCTGTTTATCCGATGGAATG-3’;
human ACTIN forward, 5’-TTTCCTGCCTGAGCTGACCT-3’;
human ACTIN reverse, 5’-ACACGGAGTACTTGCGCTC-3’.
Immunofluorescence staining and multiplex immunohistochemistry (IHC) analysis
The cells were cultured on coverslips in 24-well plates overnight. After receiving the indicated treatment, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 for 20 min. After being blocked with 3% bovine serum albumin (BSA) for 30 min, the cells were incubated with primary antibodies diluted in 1% BSA at 4 °C overnight and secondary antibodies diluted in 1% BSA at room temperature for another hour. The cells were mounted with Fluoroshield (Sigma) and examined using a Zeiss confocal microscope after being stained with 1 mg/mL 4’,6-diamidino-2-phenylindole (DAPI).
For multiplex IHC, formalin-fixed paraffin-embedded tissue sections (4 µm) were deparaffinized in xylene, rehydrated with graded ethanol solutions, and subjected to antigen retrieval using heated Tris-EDTA buffer (pH 8.0) for 2.5 min. Multiplex IHC was performed with an Opal 4-Color Fluorescent IHC Kit (PANOVUE #10001100050). The tissues were blocked with 10% fetal bovine serum in PBS for 10 min, incubated with primary antibodies at 4 °C for 8 h, and then treated with biotinylated secondary antibodies and streptavidin-HRP for 10 min. Signals were visualized using tyramide signal amplification (TSA) with Opal fluorophores (Opal 520, 570, 650), followed by DAPI nuclear staining. Negative controls without primary antibodies were used to adjust the background signals. Images were acquired using a Zeiss LSM880 microscope with Airyscan at 63× magnification. The tumor areas were analyzed using HALO V.3.0.1 (Indica Labs), with marker thresholds set based on the staining intensity. A spatial analysis was performed to determine the average distance of BRRF2+ and BRRF2- cells from CD68+ macrophages, and the phenotypic data were exported for further analysis.
Hematoxylin and eosin (H&E) staining
The collected paraffin-embedded tumor tissue sections were incubated with xylene three times for 2 min each. The sections were then hydrated by incubating them with 100% ethanol, 95% ethanol, 80% ethanol, 70% ethanol and running water for 2 min. Hematoxylin was used to stain the sections for 1 min, and the sections were placed under running tap water for 10 min. An eosin solution was used to stain the samples for 3 min, and the sections were placed under running tap water for 10 min. The slides were dried, a drop of per mount was placed over the tissue, and a coverslip was added. The tissues were observed using a Zeiss Axio Scan. Z1 microscope.
Protein expression and purification
His-tagged proteins were produced via the pSKB or pET22B vector, and the GST-tagged proteins were produced via the pGEX-6p-1 vector. Plasmids were sent to RecombX Gene (Guangzhou, Chian) to express and purify proteins. All recombinant proteins used in this study were expressed in E. coli Rosetta induced with 0.5 mM IPTG for 16 h at 18 °C and collected by centrifugation at 5000 rpm for 15 min. E. coli cells were resuspended in His lysis buffer (50 mM Tris-HCl [pH 7.4], 500 mM NaCl, 30 mM imidazole, 2 mM β-mercaptoethanol and 1% protease inhibitor cocktail) and lysed with a high-pressure homogenizer to purify 6His-cGAS, 6His-EGFP-cGAS and 6His-sumo-mCherry-BRRF2Δ322-573. The cell lysates were clarified by centrifugation at 20,000 × g for 2 h and purified on Ni-NTA agarose beads (Thermo Scientific). After extensive washing with lysis buffer three times, the proteins were eluted with His elution buffer (50 mM Tris-HCl [pH 7.4], 500 mM NaCl, 300 mM imidazole, 2 mM β-mercaptoethanol and 1% protease inhibitor cocktail), further purified on an AKTA purifier (GE Healthcare), and eluted with buffer containing 50 mM Tris-HCl [pH 7.4], 500 mM NaCl, 2 mM β-mercaptoethanol and 1% protease inhibitor cocktail. The 6His-sumo tag in 6His-sumo-mCherry-BRRF2Δ322-573 was cleaved by PreScission Protease at 4 °C overnight. Cleaved protein samples were further purified on Ni-NTA agarose beads. The flow-through samples were further purified on an AKTA purifier (GE Healthcare) and eluted with buffer containing 50 mM Tris-HCl [pH 7.4], 500 mM NaCl, 2 mM β-mercaptoethanol and a 1% protease inhibitor cocktail.
E. coli cells were resuspended in GST lysis buffer (50 mM Tris-HCl [pH 7.4], 600 mM NaCl, 2 mM β-mercaptoethanol and 1% protease inhibitor) and lysed with a high-pressure homogenizer to purify GST-BRRF2/GST. The cell lysates were clarified by centrifugation at 20,000 × g for 2 h and subsequently purified on glutathione agarose beads (Thermo Scientific). After extensive washing with lysis buffer three times, the proteins were eluted with GST elution buffer (50 mM Tris-HCl [pH 7.4], 600 mM NaCl, 10 mM GSH, 2 mM β-mercaptoethanol and 1% protease inhibitor), further purified on an AKTA purifier (GE Healthcare), and eluted with buffer containing 50 mM Tris-HCl [pH 7.4], 600 mM NaCl, 2 mM β-mercaptoethanol and a 1% protease inhibitor cocktail. The peak samples were collected, the concentrations were measured, and the samples were stored at −80 °C until use.
Development and characterization of the rabbit anti-BRRF2 monoclonal antibody
Purified BRRF2Δ322-573 was sent to ABclonal Technology to generate a specific BRRF2 monoclonal antibody. Briefly, the BRRF2Δ322-573 protein was injected subcutaneously at multiple sites. Five injections were performed over several months. The rabbits’ blood samples were collected periodically to monitor the immune response by ELISA. After a satisfactory immune response was achieved, B cells were isolated from the rabbits’ spleens. The isolated B cells were fused with myeloma cells to produce monoclonal antibodies indefinitely. After obtaining the purified monoclonal antibodies, we characterized the specificity of the antibodies for BRRF2 and their affinity using ELISA, SPR, western blotting, and immunofluorescence staining.
In vitro phase separation assays
For imaging, 50 μM purified cGAS (2% EGFP labeled) was mixed with 10 μM 45-bp dsDNA (2% Cy5 labeled) in 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 1 mg/mL BSA to a final volume of 45 μL. Liquid droplets were observed in glass-bottom cell culture dishes for differential interference contrast (DIC) imaging (Zeiss LSM880). Images were captured every 10 s for 10 min. Then, 5 μL of BRRF2Δ322-573 (2% mCherry labeled; 30 μM) was added and mixed. Images were captured immediately.
For the sedimentation assay, purified cGAS was mixed with an increasing gradient of GST-BRRF2 or the GST control in buffer supplemented with 20 mM Tris-HCl [pH 7.4], 150 mM NaCl and 10 μM HT-DNA and incubated at 37 °C for 1 h. The samples were subsequently centrifuged at 10,000 × g for 5 min. The supernatant and pellet were separated into two tubes immediately. The pellet fraction was washed once and resuspended in buffer with 20 mM Tris-HCl [pH 7.4] and 150 mM NaCl in the same volume as the supernatant fraction.
Kinetic analysis by surface plasmon resonance (SPR)
The SPR analyses were performed at 25 °C using BIAcore 8000 equipment (BIAcore, GE Healthcare). The equipment was preconditioned in water and running buffer (HBS-EP: 10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% P20). A carboxymethylated dextran CM5 research-grade sensor chip was docked and normalized with HBS-EP buffer. Purified BRRF2Δ322-573 was diluted in 10 mM sodium acetate, pH 4.5, to a final concentration of 10 μg/mL and immobilized on the flow cell 2 of a carboxymethylated dextran CM5 research-grade sensor chip using the amine coupling method according to the manufacturer’s instructions. The final surface contained 7500 RUs of immobilized BRRF2Δ322-573. Kinetic analyses were performed at a 30 μL/min flow in HBS-EP by injecting a range of duplicate concentrations of the anti-BRRF2 antibody. A series of running buffer injections was used as blanks for correcting instrument noise. Regeneration was completely achieved with a 120 s pulse of 10 mM glycine (pH 1.5) at a 30 μL/min flow, with no noticeable alteration in the binding properties. The data were processed using BIA evaluation software version 4.1 (BIAcore, GE Healthcare). Kinetic parameters were calculated using a global analysis by fitting the data to a simple 1:1 binding model for the anti-BRRF2 antibody–BRRF2Δ322-573 interaction.
Antibodies were injected sequentially without regeneration in between (the contact time was set to 60 s) to perform a competitive analysis with SPR. The reaction units (RUs) of a specific antibody pair were subtracted from the RUs of a specific control pair (running buffer followed by antibodies) to evaluate the strength of the competition. We assessed the competitive binding of the #12 antibody and #14 antibody to BRRF2 by determining the corrected RUs of the BRRF2Δ322-573 protein after contact with antibody #12 (mAb_RU). The corrected RUs (mock_RU) were obtained by subtracting the RUs of PBS.
DNA pull-down assays
HEK293T cells were lysed in NP40 lysis buffer for 30 min on ice. The cell lysates were collected by centrifugation at 12,000 × g at 4 °C for 15 min. The DNA oligonucleotide HSV120 was conjugated to biotin (EZ-link psoralen–PEG3–biotin) via UV (365 nm wavelength) irradiation for 1 h on ice and then precipitated with ethanol and sodium acetate. The cell lysates were incubated with biotinylated HSV120 for 1 h, followed by an incubation with streptavidin agarose for 2 h at room temperature. The agarose beads were collected and washed three times with NP40 lysis buffer and then boiled in 2 × sample buffer for western blot analysis.
In vitro cGAS activity assays
Purified cGAS and BRRF2 or GST were mixed with reaction buffer (20 mM Tris-HCl [pH 7.4], 2 mM ATP, 2 mM GTP, and 5 mM MgCl2) in the presence of 0.1 μg/mL HSV120 and incubated at 37 °C for 1 h. The samples were then heat inactivated at 100 °C for 5 min and centrifuged at 16000 × g for 10 min. The supernatants were collected and filtered with a 10 kDa ultrafiltration filter (Millipore) for MonoQ analysis. The column was first equilibrated in buffer A (20 mM Tris-HCl [pH 8.5]). The samples were then loaded onto the column and eluted with a gradient of buffer B (20 mM Tris-HCl [pH 8.5], 1 M NaCl) in buffer A (20 mM Tris-HCl [pH 8.5]) from 1% to 50%.
EV isolation, purification and characterization
EBV-positive CNE2 cell lines and BRRF2-expressing HEK293T cell lines were cultured for certain durations. The supernatants were collected and centrifuged at 10,000 × g for 45 min, and the cell debris was discarded. EVs were pelleted from filtered supernatants at 100,000 × g for 75 min, washed, and then sucrose density gradient ultracentrifugation was used for further purification. Finally, the EVs were dissolved in 200 μL of PBS and analyzed by western blotting to determine their presence and purity.
After purification, the hydrodynamic size and concentration of EVs were measured using nanoparticle tracking analysis (NTA) with a NanoSight NS300 (Malvern). A 5 μL sample of EVs or EBV was applied to a glow-discharged grid with a continuous carbon layer (Electron Microscopy, China) and incubated for 1.5 min. Next, the grid was stained with 8 μL of uranyl acetate for 40 s. Finally, the grid was transferred to a Talos 120 C transmission electron microscope (Thermo Fisher Scientific) and imaged with a Ceta 16 M CMOS detector (Thermo Fisher Scientific).
For the analysis of human serum samples, a commercial Exosome-Human CD63 Isolation/Detection Kit (Thermo Fisher, #10606D) was used according to the manufacturer’s instructions. A western blot analysis was conducted to verify the presence of BRRF2 and the EV marker HSP70.
Mass spectrometry
Isolated exosomes were sent to Oebiotech or Wininnovate Bio to run LC-MS/MS. Biological replicates (n = 3 per sample) and technical replicates (n = 3 per extraction) were processed to account for variability. Briefly, The lyophilized peptide fractions were re-suspended in ddH2O containing 0.1% formic acid, and 2 ul aliquots of which were loaded into a nanoViper C18 (Acclaim PepMap 100, 75 μm × 2 cm) trap column. The online Chromatography seperation was performed on the Easy nLC 1200 system (ThermoFisher). The trapping, desalting procedure were carried out with a volumn of 20 μL 100% solvent A (0.1% formic acid). Then, an elution gradient of 5–38% solvent B (80% acetonitrile, 0.1% formic acid) in 60 min was used on an analytical column (Acclaim PepMap RSLC, 75 μm × 25 cm C18-2 μm 100 Å). DDA (data-dependent acquisition) mass spectrum techniques were used to acquire tandem MS data on a ThermoFisher Q Exactive mass spectrometer (ThermoFisher, USA) fitted with a Nano Flex ion source. Data was acquired using an ion spray voltage of 1.9 kV, and an interface heater temperature of 275 °C. For a full MS survey scan, the target value was 3 × 106 and the scan ranged from 350 to 2000 m/z at a resolution of 70,000 and a maximum injection time of 100 ms. For the MS2 scan, only spectra with a charge state of 2–5 were selected for fragmentation by higher-energy collision dissociation with a normalized collision energy of 28. The MS2 spectra were acquired in the ion trap in rapid mode with an AGC target of 8000 and a maximum injection time of 50 ms. Dynamic exclusion was set for 25 s.
The MS/MS data were analyzed for protein identification and quantification using PEAKS Studio 8.5. The local false discovery rate at PSM was 1.0% after searching against Homo sapiens database with a maximum of two missed cleavages. he following settings were selected: Oxidation (M), Acetylation (Protein N-term), Deamidation (NQ), Pyro-glu from E, Pyro-glu from Q for variable modifications as well as fixed Carbamidomethylation of cysteine. Precursor and fragment mass tolerance were set to 10 ppm and 0.05 Da, respectively.
EV transfer studies
THP1 cells and human primary macrophages (HPM) were seeded into 6-well plates and cocultured with purified EVs for 2 h. Then, the cells were centrifuged at 200 × g for 5 min to remove EVs and transfected with 2 μg of HT-DNA for 6 h. Total THP-1 RNA was isolated via the TRIzol method, and total THP-1 protein was extracted with RIPA buffer for the detection of cGAS‒STING signaling pathway activation and proinflammatory cytokine gene expression.
Clinical samples
For multiplex immunofluorescence staining, NPC patients with high EBV copy numbers (>2000 copies) were recruited between January 2016 and December 2021 at Sun Yat-sen University Cancer Center in Guangzhou, China. Patients’ tumor samples were collected by puncture or surgery, fixed with formalin and embedded in paraffin.
For immuno-qPCR, a total of 98 pathologically confirmed RM-NPC patients with high EBV copy numbers (>2000 copies) who were receiving anti-PD-1 immunotherapy and 50 healthy individuals were recruited between January 2016 and December 2021 at Sun Yat-sen University Cancer Center in Guangzhou, China. The TNM stage was established based on the eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer staging system for NPC. Serum samples were collected from patients before anti-PD-1 treatment. According to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), a total of 52 NPC patients responded to anti-PD-1 immunotherapy (defined as the PR/CR group), and 47 patients did not respond (defined as the SD/PD group). The serum was separated by centrifugation at 3500 rpm for 10 min at room temperature and then frozen at −80 °C until use.
Immuno-qPCR
The procedure for immuno-qPCR refers to the literature66. 96-well microplates were coated with anti-CD63 antibody (800 ng/mL) and anti-BRRF2 capture antibody (#12, 1000 ng/mL) in PBS overnight at 4 °C. Plates were washed three times with washing buffer (0.05% Tween-20 in PBS, pH 7.2-7.4) and blocked with blocking buffer (0.5% BSA + 0.2% NaN3 + 5 mM EDTA in PBS) at 37 °C for 2 h. After washing, serum samples were added and incubated overnight at 4 °C to capture EVs via plate-bound anti-CD63 antibodies. Following washing, IP Buffer was added and incubated at 37 °C for 2 h to lyse the captured EVs, releasing intravesicular contents including BRRF2 protein. The released BRRF2 was captured by the immobilized anti-BRRF2 antibodies. After washing, biotinylated anti-BRRF2 detection antibody (#14, 100 ng/mL) was added and incubated at 37 °C for 1 h. After washing, streptavidin (10 nM) was added and incubated for 20 min at 37 °C, followed by washing and addition of biotinylated DNA probes (5 pM). Quantification was conducted using EZ Color SYBR Green qPCR Master mix with the following program: 98 °C for 30 s; followed by 35 cycles of 98 °C for 10 s, 55 °C for 5 s, and 72 °C for 5 s (with fluorescence recording). Primers and probes used in the assay are listed below:
Biotinylated DNA probe, 5’-Biotin-GAATTCGAGCTCGGTACCCGGGGATCCTCTAGAGTCGACCTGCAGGCATGCAAGCTTGGCACTGGCCGTCGTTTTACAACGTCGTGACTGGGAAAACCCTGGCGTTACCCAACTTAATCGCCTT-3’;
forward primer, 5‘-GAATTCGAGCTCGGTACCCGG-3’;
reverse primer, 5’-AAGGCGATTAAGTTGGG-3’.
Analysis of single-cell transcriptome data
The raw data of NPC RNA-seq data (GSE102349) were aligned to the EBV strain M81 genome using STAR. Reads uniquely mapped to BRRF2 were selected and counted by Feature Counts as the expression level of BRRF2 in the samples. The samples were divided into high- and low-expression groups based on the median expression of BRRF2. The ssgsea function from the GSVA package was then used to perform gene set enrichment analysis (GSEA) for microenvironmental cell gene sets using the RNA-seq data.
Statistical analysis
GraphPad Prism v8.0.1 software was used to perform the statistical analysis. All of in vitro experiments were performed in at least triplicate using biologically independent samples, unless noted otherwise. Technical replicates (repeated measurements of the same sample) were averaged before statistical analysis. The information on the statistics is available in the figure legends. A p value less than 0.05 was regarded as statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data from this article has been uploaded onto the Research Data Deposit public platform (https://www.researchdata.org.cn/) (RDD No. RDDB2025283825). The data from GSE102349 [PMID: 28851814 and 39744943] were reused for investigating the relationship between BRRF2 expression and the tumor microenvironment composition. This data can be found at NCBI GEO link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE102349. All other data are available in the article and its Supplementary files or from the corresponding author upon request. Source data are provided with this paper.
References
Wong, K. C. W. et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 18, 679–695 (2021).
Farrell, P. J. Epstein-barr virus and cancer. Annu. Rev. Pathol. 14, 29–53 (2019).
Huang H. et al. Immunotherapy for nasopharyngeal carcinoma: current status and prospects (Review). Int. J. Oncol. 63, 97 (2023).
Wang, F. et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 30, 1479–1486 (2019).
Bossi, P., Gurizzan, C. & Chan, A. Immunotherapy for nasopharyngeal carcinoma: the earlier the better. JAMA 330, 1954–1955 (2023).
Bhowmik, D. et al. Cooperative DNA binding mediated by KicGAS/ORF52 oligomerization allows inhibition of DNA-induced phase separation and activation of cGAS. Nucleic Acids Res. 49, 9389–9403 (2021).
Zhang, G. et al. Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc. Natl. Acad. Sci. USA 113, E1034–E1043 (2016).
Ma, Z. et al. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc. Natl. Acad. Sci. USA 112, E4306–E4315 (2015).
Xu, G. et al. Viral tegument proteins restrict cGAS-DNA phase separation to mediate immune evasion. Mol. Cell 81, 2823–2837 (2021).
Zou H. M. et al. Human cytomegalovirus protein UL94 targets MITA to evade the antiviral immune response. J. Virol. 94, e00022 (2020).
Su C., Zheng C. Herpes simplex virus 1 abrogates the cGAS/STING-mediated cytosolic DNA-sensing pathway via its virion host shutoff protein, UL41. J. Virol. 91, e02414-16 (2017).
Fu Y. Z. et al. Human cytomegalovirus DNA polymerase subunit UL44 antagonizes antiviral immune responses by suppressing IRF3- and NF-kappaB-mediated transcription. J. Virol. 93, e00181 (2019).
Zhang, J. et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe 24, 234–248 (2018).
Huang J. et al. Herpes simplex virus 1 tegument protein VP22 abrogates cGAS/STING-mediated antiviral innate immunity. J. Virol. 92, e00481-18 (2018).
Deschamps T., Kalamvoki M. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus 1. J. Virol. 91, e00535-17 (2017).
Lui, W. Y. et al. Suppression of cGAS- and RIG-I-mediated innate immune signaling by Epstein-Barr virus deubiquitinase BPLF1. PLoS Pathog. 19, e1011186 (2023).
Xu, M. et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 51, 1131–1136 (2019).
Lo, K. W., Chung, G. T. Y. & To, K. F. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 22, 79–86 (2012).
Tsang, C. M., Lui, V. W. Y., Bruce, J. P., Pugh, T. J. & Lo, K. W. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol. 61, 84–100 (2020).
Gourzones, C., Barjon, C. & Busson, P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 22, 127–136 (2012).
Kase, K. et al. Epstein-Barr virus LMP1 induces soluble PD-L1 in nasopharyngeal carcinoma. Microorganisms 9, 603 (2021).
Liao, C. et al. Anoikis resistance and immune escape mediated by Epstein-Barr virus-encoded latent membrane protein 1-induced stabilization of PGC-1alpha promotes invasion and metastasis of nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 42, 261 (2023).
Huo, S. et al. EBV-EBNA1 constructs an immunosuppressive microenvironment for nasopharyngeal carcinoma by promoting the chemoattraction of Treg cells. J. Immunother. Cancer 8, e001588 (2020).
Cochet, C. et al. Expression of the Epstein-Barr virus immediate early gene, BZLF1, in nasopharyngeal carcinoma tumor cells. Virology 197, 358–365 (1993).
Raab-Traub, N. & Flynn, K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47, 883–889 (1986).
Jin, S. et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 30, 950–965 (2020).
Crescitelli, R., Lässer, C. & Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 16, 1548–1580 (2021).
Han, Q. F. et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 21, 207 (2022).
Chen, C. et al. Tumor-suppressive circRHOBTB3 is excreted out of cells via exosome to sustain colorectal cancer cell fitness. Mol. Cancer 21, 46 (2022).
Madison, M. & Okeoma, C. Exosomes: implications in HIV-1 Pathogenesis. Viruses 7, 4093–4118 (2015).
Teow, S. Y., Nordin, A. C., Ali, S. A. & Khoo, A. S. B. Exosomes in human immunodeficiency virus type I pathogenesis: threat or opportunity? Adv. Virol. 2016, 9852494 (2016).
Liu, J. et al. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J. Mol. Sci. 15, 758–773 (2014).
Nanbo, A., Kawanishi, E., Yoshida, R. & Yoshiyama, H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J. Virol. 87, 10334–10347 (2013).
Meckes, D. G. Jr et al. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 107, 20370–20375 (2010).
Sun, L. & Meckes, D. G. Jr Methodological Approaches to study extracellular vesicle miRNAs in Epstein(-)Barr virus-associated cancers. Int J. Mol. Sci. 19, 2810 (2018).
Watanabe, T. et al. The C-terminus of Epstein-Barr virus BRRF2 Is required for its proper localization and efficient virus production. Front. Microbiol. 8, 125 (2017).
Morrison, T. E., Mauser, A., Wong, A., Ting, J. P. Y. & Kenney, S. C. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15, 787–799 (2001).
Wang, J. T. et al. Glycogen synthase kinase 3 negatively regulates IFN regulatory factor 3 transactivation through phosphorylation at its linker region. Innate Immun. 20, 78–87 (2014).
Rafiee, N., Ravanshad, M., Asadi, B., Kianfar, R. & Maleki, A. Investigation of IL-2 and IFN-gamma to EBV Peptides in stimulated whole blood among multiple sclerosis patients and healthy individuals. Intervirology 64, 203–208 (2021).
Zhang, Q., Holley-Guthrie, E., Ge, J. Q., Dorsky, D. & Kenney, S. The Epstein-Barr virus (EBV) DNA polymerase accessory protein, BMRF1, activates the essential downstream component of the EBV oriLyt. Virology 230, 22–34 (1997).
Lu, F. et al. Defective Epstein-Barr virus genomes and atypical viral gene expression in B-cell lines derived from multiple myeloma patients. J. Virol. 95, e0008821 (2021).
Rowe, M. et al. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. USA 104, 3366–3371 (2007).
van Gent, M. et al. Silencing the shutoff protein of Epstein-Barr virus in productively infected B cells points to (innate) targets for immune evasion. J. Gen. Virol. 96, 858–865 (2015).
van Gent, M. et al. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 186, 1694–1702 (2011).
Bruce, J. P. et al. Whole-genome profiling of nasopharyngeal carcinoma reveals viral-host co-operation in inflammatory NF-kappaB activation and immune escape. Nat. Commun. 12, 4193 (2021).
Abou Harb, M., Meckes, D. G. Jr & Sun, L. Epstein-Barr virus LMP1 enhances levels of large extracellular vesicle-associated PD-L1. J. Virol. 97, e0021923 (2023).
Choi, I. K. et al. Mechanism of EBV inducing anti-tumour immunity and its therapeutic use. Nature 590, 157–162 (2021).
Jiang, S. et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 53, D1670–D1676 (2025).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445.e18 (2019).
Sollid, L. M. Epstein-Barr virus as a driver of multiple sclerosis. Sci. Immunol. 7, eabo7799 (2022).
Lunemann, A., Rowe, M. & Nadal, D. Innate immune recognition of EBV. Curr. Top. Microbiol. Immunol. 391, 265–287 (2015).
Wu, J. et al. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18, 333–344 (2015).
Alberti, S. & Dormann, D. Liquid-liquid phase separation in disease. Annu. Rev. Genet. 53, 171–194 (2019).
Zhao, M. et al. The stress granule protein G3BP1 promotes pre-condensation of cGAS to allow rapid responses to DNA. EMBO Rep. 23, e53166 (2022).
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles 13, e12404 (2024).
Hong, M. et al. Immunotherapy for EBV-associated nasopharyngeal carcinoma. Crit. Rev. Oncog. 23, 219–234 (2018).
Zhang, B. & Choi, I. K. Facts and hopes in the relationship of EBV with cancer immunity and immunotherapy. Clin. Cancer Res. 28, 4363–4369 (2022).
Kalra, M. et al. Epstein-Barr Virus (EBV)-derived BARF1 encodes CD4- and CD8-restricted epitopes as targets for T-cell immunotherapy. Cytotherapy 21, 212–223 (2019).
Ahmed, W., Tariq, S. & Khan, G. Tracking EBV-encoded RNAs (EBERs) from the nucleus to the excreted exosomes of B-lymphocytes. Sci. Rep. 8, 15438 (2018).
Higuchi, H. et al. Role of exosomes as a proinflammatory mediator in the development of EBV-associated lymphoma. Blood 131, 2552–2567 (2018).
Sato, Y. et al. Epstein-Barr virus tegument protein BGLF2 in exosomes released from virus-producing cells facilitates de novo infection. Cell Commun. Signal 20, 95 (2022).
Teow, S. Y., Liew, K., Khoo, A. S. B. & Peh, S. C. Pathogenic role of exosomes in Epstein-Barr virus (EBV)-associated cancers. Int J. Biol. Sci. 13, 1276–1286 (2017).
Zhao, M., Nanbo, A., Sun, L. & Lin, Z. Extracellular vesicles in Epstein-Barr virus’ life cycle and pathogenesis. Microorganisms 7, 48 (2019).
Middeldorp, J. M. & Pegtel, D. M. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol. 18, 388–396 (2008).
Pai, S. & Khanna, R. Role of LMP1 in immune control of EBV infection. Semin Cancer Biol. 11, 455–460 (2001).
Niemeyer, C. M., Adler, M. & Wacker, R. Detecting antigens by quantitative immuno-PCR. Nat. Protoc. 2, 1918–1930 (2007).
Acknowledgements
National Key R&D Program grant 2022YFC3400900 (MSZ). National Natural Science Foundation of China grant U22A20322 (QZ), 81830090 (MSZ), 81621004 (MSZ), 82072982 (QZ), 82203230 (ZLH). Program for Guangdong Introducing Innovative and Entrepreneurial Teams grant 2019BT02Y198 (MSZ). Guangdong Science and Technology Department grant 2020B1212030004 (MSZ). Natural Science Foundation of Guangdong Province grant 2023A1515011790 (QZ). Guangdong Basic and Applied Basic Research Foundation grant 2021A1515111157 (ZLH). Sun Yat-sen University Talent Program grant 22yklj08 (QZ). Young Talents Program of Sun Yat-sen University Cancer Center grant YTP-SYSUCC-0090 (QZ). Cancer Innovative Research Program of Sun Yat-sen University Cancer Center grant CIRP-SYSUCC-0006 (MSZ).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.S.Z., Q.Z., and Z.L.H. Methodology: Z.Q.L., Z.L.H., Y.W., Y.L.L., and W.P.G. Investigation: Z.Q.L., Z.L.H., Y.W., Y.L.L., N.M., G.L.B., W.P.G., L.L.Z., S.X.L., X.W.K., X.Y.F., Q.L.W., R.K.H., Z.Z., Z.Y.J., G.X.Z., and R.X.J. Visualization: Z.Q.L. and Z.L.H. Funding acquisition: M.S.Z., Q.Z., and Z.L.H. Project administration: M.S.Z., Q.Z., Z.L.H., and Z.Q.L. Supervision: M.S.Z. and Q.Z. Writing–original draft: Z.Q.L. Writing–review & editing: Q.Z., M.S.Z., Z.Q.L., and Y.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Aurelio Lorico, who co-reviewed with Mark Santos; Shunbin Ning and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, ZL., Li, ZQ., Wang, Y. et al. Extracellular vesicles derived EBV tegument protein BRRF2 suppresses cGAS phase separation to promote anti-viral innate immune evasion. Nat Commun 16, 9015 (2025). https://doi.org/10.1038/s41467-025-64037-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64037-2