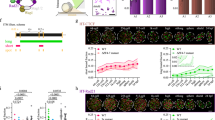
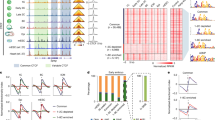
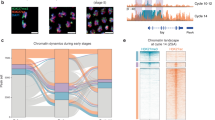
Abstract
The initiation of gene expression during development, known as zygotic genome activation (ZGA), is accompanied by massive changes in chromosome organization. However, the earliest events of chromosome folding and their functional roles remain unclear. Using Hi-C on zebrafish embryos, we discovered that chromosome folding begins early in development with the formation of fountains, distinct elements of chromosome organization. Emerging preferentially at enhancers, fountains show an initial accumulation of cohesin, which later redistributes to CTCF sites at TAD borders. Knockouts of pioneer transcription factors driving ZGA enhancers cause a specific loss of fountains, establishing a causal link between enhancer activation and fountain formation. Polymer simulations demonstrate that fountains may arise as sites of facilitated cohesin loading, requiring two-sided but desynchronized loop extrusion, potentially caused by cohesin collisions with obstacles or internal switching. Moreover, we detected cohesin-dependent fountain patterns at enhancers in mouse cells and found them reemerging with cohesin loading after mitosis. Altogether, fountains represent enhancer-specific elements of chromosome organization and suggest that chromosome folding during development and after cell division starts with facilitated cohesin loading. Observations in multiple systems further support facilitated loading at enhancers as a widespread phenomenon.
Similar content being viewed by others
Data availability
Raw and processed Hi-C data for zebrafish embryogenesis is available at GEO at (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE195609). The list of zebrafish fountains is available in Supplementary Dataset 1, Xenopus and medaka fountains in Supplementary Dataset 2, zebrafish TAD boundaries in Supplementary Dataset 3, zebrafish initiation zones at 4.3 hpf in Supplementary Dataset 4, and fountains group assignment based on chromatin accessibility change in mutants is available in Supplementary Dataset 5. The detailed list of the sequencing datasets is available in Supplementary Dataset 6. Additional datasets (including intermediary processed files) are available at OSF at (https://osf.io/mt4vf). Interactive HiGlass views are available at Resgen at (https://resgen.io/galitsyna/Zebrafish_embryogenesis/). Source data are provided with this paper.
Code availability
Fontanka tool for fountain calling (with examples) is available at (https://github.com/agalitsyna/fontanka) (v0.2, version available at https://doi.org/10.5281/zenodo.18018594). Simulations of facilitated loop extrusion are available at (https://github.com/agalitsyna/polychrom_workbench) under the directory targeted_extrusion. TAD calling for zebrafish is available at (https://github.com/encent/danio-2022). Code for reproducing the figures from the paper (main Figures and Supplementary Figs.) is available at (https://github.com/agalitsyna/embryonic-chromatin).
References
Vastenhouw, N. L., Cao, W. X. & Lipshitz, H. D. The maternal-to-zygotic transition revisited. Development 146, dev161471 (2019).
Larson, E. D., Marsh, A. J. & Harrison, M. M. Pioneering the developmental frontier. Mol. Cell 81, 1640–1650 (2021).
Hug, C. B., Grimaldi, A. G., Kruse, K. & Vaquerizas, J. M. Chromatin architecture emerges during zygotic genome activation independent of transcription. Cell 169, 216–228.e19 (2017).
Wike, C. L. et al. Chromatin architecture transitions from zebrafish sperm through early embryogenesis. Genome Res. 31, 981–994 (2021).
Pecori, F. & Torres-Padilla, M.-E. Dynamics of nuclear architecture during early embryonic development and lessons from liveimaging. Dev. Cell 58, 435–449 (2023).
Niu, L. et al. Three-dimensional folding dynamics of the Xenopus tropicalis genome. Nat. Genet. 53, 1075–1087 (2021).
Du, Z. et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 547, 232–235 (2017).
Ke, Y. et al. 3D chromatin structures of mature gametes and structural reprogramming during mammalian embryogenesis. Cell 170, 367–381.e20 (2017).
Nakamura, R. et al. CTCF looping is established during gastrulation in medaka embryos. Genome Res. 31, 968–980 (2021).
Chen, X. et al. Key role for CTCF in establishing chromatin structure in human embryos. Nature 576, 306–310 (2019).
Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Fudenberg, G. et al. Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016).
Flyamer, I. M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544, 110–114 (2017).
Gabriele, M. et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science 6583, eabn (2022).
Mach, P. et al. Cohesin and CTCF control the dynamics of chromosome folding. Nat. Genet. 54, 1907–1918 (2022).
Kane, L. et al. Cohesin is required for long-range enhancer action at the Shh locus. Nat. Struct. Mol. Biol. 29, 891–897 (2022).
Rinzema, N. J. et al. Building regulatory landscapes reveals that an enhancer can recruit cohesin to create contact domains, engage CTCF sites and activate distant genes. Nat. Struct. Mol. Biol. 29, 563–574 (2022).
Gschwind, A. R. et al. An encyclopedia of enhancer-gene regulatory interactions in the human genome. Preprint bioRxiv https://doi.org/10.1101/2023.11.09.563812 (2023).
Rekaik, H. et al. Sequential and directional insulation by conserved CTCF sites underlies the Hox timer in stembryos. Nat. Genet. 55, 1164–1175 (2023).
Adams, N. M. et al. Cohesin-mediated chromatin organization controls the differentiation and function of dendritic cells. Sci. Immunol. 11, eadx4622 (2026).
Lee, M. T. et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 503, 360–364 (2013).
Leichsenring, M., Maes, J., Mössner, R., Driever, W. & Onichtchouk, D. Pou5f1 transcription factor controls zygotic gene activation in vertebrates. Science 341, 1005–1009 (2013).
Onichtchouk, D. Evolution and functions of Oct4 homologs in non-mammalian vertebrates. Biochim. Biophys. Acta 1859, 770–779 (2016).
Veil, M., Yampolsky, L. Y., Grüning, B. & Onichtchouk, D. Pou5f3, SoxB1, and Nanog remodel chromatin on high nucleosome affinity regions at zygotic genome activation. Genome Res. 29, 383–395 (2019).
Miao, L. et al. The landscape of pioneer factor activity reveals the mechanisms of chromatin reprogramming and genome activation. Mol. Cell 82, 986–1002.e9 (2022).
Pálfy, M., Schulze, G., Valen, E. & Vastenhouw, N. L. Chromatin accessibility established by Pou5f3, Sox19b and Nanog primes genes for activity during zebrafish genome activation. PLoS Genet 16, e1008546 (2020).
Meier, M. et al. Cohesin facilitates zygotic genome activation in zebrafish. Development 145, (2018).
Guo, Y. et al. Chromatin jets define the properties of cohesin-driven in vivo loop extrusion. Mol. Cell https://doi.org/10.1016/j.molcel.2022.09.003 (2022).
Liu, N. Q. et al. Extrusion fountains are restricted by WAPL-dependent cohesin release and CTCF barriers. Nucleic Acids Res 53, gkaf549 (2025).
Lüthi, B. N. et al. Cohesin forms fountains at active enhancers in C. elegans. Nat. Commun. 17, 681 (2026).
Kim, J., Wang, H. & Ercan, S. Cohesin organizes 3D DNA contacts surrounding active enhancers in C. elegans. Genome Res. 35, 1108–1123 (2025).
Shao, W. et al. The jet-like chromatin structure defines active secondary metabolism in fungi. Nucleic Acids Res. 52, 4906–4921 (2024).
Wang, D. et al. Promoter capture Hi-C identifies promoter-related loops and fountain structures in Arabidopsis. Genome Biol. 25, 324 (2024).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Kaaij, L. J. T., van der Weide, R. H., Ketting, R. F. & de Wit, E. Systemic Loss and Gain of Chromatin Architecture throughout Zebrafish Development. Cell Rep. 24, 1–10.e4 (2018).
Gassler, J. et al. A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J. 36, 3600–3618 (2017).
Polovnikov, K. E. et al. Crumpled polymer with loops recapitulates key features of chromosome organization. Phys. Rev. X. 13, 041029 (2023).
Open2C et al. Cooltools: enabling high-resolution Hi-C analysis in Python. PLoS Comput. Biol. 20, e1012067 (2024).
Fudenberg, G., Abdennur, N., Imakaev, M., Goloborodko, A. & Mirny, L. A. Emerging Evidence of Chromosome Folding by Loop Extrusion. Cold Spring Harb. Symp. Quant. Biol. 82, 45–55 (2017).
Siefert, J. C., Georgescu, C., Wren, J. D., Koren, A. & Sansam, C. L. DNA replication timing during development anticipates transcriptional programs and parallels enhancer activation. Genome Res. 27, 1406–1416 (2017).
Baranasic, D. et al. Multiomic atlas with functional stratification and developmental dynamics of zebrafish cis-regulatory elements. Nat. Genet. 54, 1037–1050 (2022).
Gao, M. et al. Pluripotency factors determine gene expression repertoire at zygotic genome activation. Nat. Commun. 13, 788 (2022).
Lunde, K., Belting, H.-G. & Driever, W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr. Biol. 14, 48–55 (2004).
Veil, M. et al. Maternal Nanog is required for zebrafish embryo architecture and for cell viability during gastrulation. Development 145, (2018).
Riesle, A. J. et al. Activator-blocker model of transcriptional regulation by pioneer-like factors. Nature Communications https://doi.org/10.1038/s41467-023-41507-z (2023).
Hiller, M. et al. Computational methods to detect conserved non-genic elements in phylogenetically isolated genomes: application to zebrafish. Nucleic Acids Res. 41, e151 (2013).
Yang, H. et al. A map of cis-regulatory elements and 3D genome structures in zebrafish. Nature 588, 337–343 (2020).
Banigan, E. J. et al. Transcription shapes 3D chromatin organization by interacting with loop extrusion. Proc. Natl. Acad. Sci. Usa. 120, e2210480120 (2023).
Vierstra, J. et al. Global reference mapping of human transcription factor footprints. Nature 583, 729–736 (2020).
Davidson, I. F. et al. CTCF is a DNA-tension-dependent barrier to cohesin-mediated loop extrusion. Nature 616, 822–827 (2023).
Dequeker, B. J. H. et al. MCM complexes are barriers that restrict cohesin-mediated loop extrusion. Nature 606, 197–203 (2022).
Vian, L. et al. The Energetics and Physiological Impact of Cohesin Extrusion. Cell 175, 292–294 (2018).
Wang, X., Brandão, H. B., Le, T. B. K., Laub, M. T. & Rudner, D. Z. Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 355, 524–527 (2017).
Brandão, H. B., Ren, Z., Karaboja, X., Mirny, L. A. & Wang, X. DNA-loop-extruding SMC complexes can traverse one another in vivo. Nat. Struct. Mol. Biol. 28, 642–651 (2021).
Kagey, M. H. et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467, 430–435 (2010).
Liu, N. Q. et al. WAPL maintains a cohesin loading cycle to preserve cell-type-specific distal gene regulation. Nat. Genet. 53, 100–109 (2021).
Nitzsche, A. et al. RAD21 cooperates with pluripotency transcription factors in the maintenance of embryonic stem cell identity. PLoS One 6, e19470 (2011).
Hsieh, T.-H. S. et al. Enhancer–promoter interactions and transcription are largely maintained upon acute loss of CTCF, cohesin, WAPL or YY1. Nat. Genet. 54, 1919–1932 (2022).
Zhang, H. et al. Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature 576, 158–162 (2019).
Abramo, K. et al. A chromosome folding intermediate at the condensin-to-cohesin transition during telophase. Nat. Cell Biol. 21, 1393–1402 (2019).
Nelson, A. C. et al. In vivo regulation of the zebrafish endoderm progenitor niche by T-box transcription factors. Cell Rep. 19, 2782–2795 (2017).
Tosic, J. et al. Eomes and Brachyury control pluripotency exit and germ-layer segregation by changing the chromatin state. Nat. Cell Biol. 21, 1518–1531 (2019).
Djeghloul, D. et al. Loss of H3K9 trimethylation alters chromosome compaction and transcription factor retention during mitosis. Nat. Struct. Mol. Biol. 30, 489–501 (2023).
Schooley, A. et al. Interphase chromosome conformation is specified by distinct folding programmes inherited through mitotic chromosomes or the cytoplasm. Nat. Cell. Biol. 28, 82–97 (2026).
Schmidt, D. et al. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 20, 578–588 (2010).
Faure, A. J. et al. Cohesin regulates tissue-specific expression by stabilizing highly occupied cis-regulatory modules. Genome Res 22, 2163–2175 (2012).
Schaaf, C. A. et al. Genome-wide control of RNA polymerase II activity by cohesin. PLoS Genet 9, e1003382 (2013).
Pherson, M., Misulovin, Z., Gause, M. & Dorsett, D. Cohesin occupancy and composition at enhancers and promoters are linked to DNA replication origin proximity in Drosophila. Genome Res 29, 602–612 (2019).
Council of Europe. European convention for the protection of vertebrate animals used for experimental and other scientific purposes. European Treaty Series No. 123. Strasbourg: Council of Europe, Section des Publications. https://rm.coe.int/168007a67b (1986) updated by Directive 2010/63/EU ISSN 1725-2555 OJ L 276, 20.10.2010, 33–79 https://doi.org/10.3000/17252555.L_2010.276.eng (2010)
Westerfield, M. A guide for the laboratory use of zebrafish (Danio rerio) (2000).
Ulianov, S. V. et al. Suppression of liquid-liquid phase separation by 1,6-hexanediol partially compromises the 3D genome organization in living cells. Nucleic Acids Res. 49, 10524–10541 (2021).
Scharr, H. et al. Optimal operators in digital image processing. PhD thesis, Ruprecht Karl University of Heidelberg https://doi.org/10.11588/heidok.00000962 (2000).
Scharr, H. et al. Optimal Filters for Extended Optical Flow. in Complex Motion 14–29 (Springer Berlin Heidelberg, 2007).
Li, C. H. & Tam, P. K. S. An iterative algorithm for minimum cross entropy thresholding. Pattern Recognit. Lett. 19, 771–776 (1998).
van der Walt, S. et al. scikit-image: image processing in Python. PeerJ 2, e453 (2014).
Abdennur, N. et al. Bioframe: operations on genomic intervals in Pandas dataframes. Bioinformatics 40, btae088 (2024).
Abdennur, N. & Mirny, L. A. Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics 36, 311–316 (2020).
Eastman, P. et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 13, e1005659 (2017).
M. Imakaev, A. G. Polychrom: Polymer Simulations of Chromosomes and Generating ‘in Silico’ Hi-C Maps. (Github, https://github.com/open2c/polychrom, https://doi.org/10.5281/zenodo.3579473 2019).
Goloborodko, A., Marko, J. F. & Mirny, L. A. Chromosome Compaction by Active Loop Extrusion. Biophys. J. 110, 2162–2168 (2016).
Goloborodko, A., Imakaev, M. V., Marko, J. F. & Mirny, L. Compaction and segregation of sister chromatids via active loop extrusion. Elife 5, e14864 (2016).
Mölder, F. et al. Sustainable data analysis with Snakemake. F1000Res. 10, 33 (2021).
Crane, E. et al. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature 523, 240–244 (2015).
Xu, C. et al. Nanog-like regulates endoderm formation through the Mxtx2-Nodal pathway. Dev. Cell 22, 625–638 (2012).
McLean, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Sethi, A. et al. Supervised enhancer prediction with epigenetic pattern recognition and targeted validation. Nat. Methods 17, 807–814 (2020).
ENCODE Project Consortium et al Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 583, 699–710 (2020).
Weiss, M. J., Yu, C. & Orkin, S. H. Erythroid-cell-specific properties of transcription factor GATA-1 revealed by phenotypic rescue of a gene-targeted cell line. Mol. Cell. Biol. 17, 1642–1651 (1997).
Acknowledgements
We are grateful to Edward Banigan for proofreading the text and to all members of the Mirny lab for many productive discussions. We thank Damir Baranasic and Chris Sansam for sharing unpublished data. A.G. thanks Max Imakaev, Nezar Abdennur, and Anton Goloborodko for early ideas for the polymer simulations; Henrik Pinholt, Simon Grosse-Holz, and Emily Navarrete for ideas on the model and figures; Antoine Coulon, Mark Pownall, and Christopher Bohrer for the thorough discussions of the results and critical assessment of the hypotheses; students Alexey Shkolikov (FBB MSU) and Eli Rybnikova (PRIMES MIT) for their preliminary analysis of fountains. D.O. is grateful to Prof. Lev Yampolsky for the consultation about statistical analysis. This work was supported by DFG-ON86/5-1, DFG ON86/6-1 and DFG-EXC2189 for D.O., by NIH GM114190 to L.M., by RSF 23-14-00136 to M.S.G., and by RSF 21-64-00001-P to S.R.; A.G. and L.M. were also supported by NIH R01 AI164728 and NIH R01 NS113929.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.U.: Hi-C libraries; A.G.: Hi-C and genomic analysis, polymer simulations; M.B.: replication timing data analysis, N.B., K.P., M.S.G.: bioinformatics; S.U., M.V., M.G., D.O.: zebrafish mutants; D.O.: study design; S.R., D.O.: supervision of the wet part, funding acquisition; M.S.G., L.M.: supervision of Hi-C and genomic analysis and simulations. A.G. wrote the first draft, and S.U., S.R., L.M., and D.O. developed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Julia Horsfield and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galitsyna, A., Ulianov, S.V., Bazarevich, M. et al. Extrusion fountains are hallmarks of chromosome organization emerging upon zygotic genome activation. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69105-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69105-9