Abstract
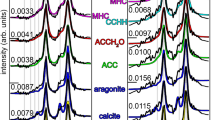
Corals form their reef-building aragonite (CaCO3) skeletons via transient precursor phases yet understanding of the dynamics of these early-stage transformations remains incomplete. Using time-independent myriad mapping (MM) at 50 nm resolution, we map five mineral phases near the skeleton surface of Stylophora pistillata corals grown in varying seawater pH. All precursors, crystalline and amorphous, exhibit a consistent exponential decay from the growth front, with a shared decay length of 0.7 ± 0.1 μm, independent of time, phase, or pH. This spatial decay, paired with the constant growth rate of the skeleton, reveals a decay time of 5.1 ± 0.5 minutes. The dominant precursor is not amorphous but crystalline: calcium carbonate hemihydrate (CCHH, CaCO₃·½H₂O). These results suggest that exponential crystallization kinetics govern coral biomineralization and may be a widespread feature in biogenic, geologic, and synthetic systems—traceable long after initial mineral deposition.
Similar content being viewed by others
Data availability
The precursor proportions generated in this work from MMs and Stylophora pistillata coral nubbin growth data have been deposited in the data.xlsx file available on Zenodo and GitHub57 with https://doi.org/10.5281/zenodo.18175786 and are source data to reproduce Figs. 2C, D, 3B, 4B, C and 5B, Supplementary Figs. 2 and 4–6, Table 1, and Supplementary Tables 2–4. The data used to produce MMs is available with no restricted access and can be obtained by contacting the corresponding author of this work at any period after publication of this work.
Code availability
GG Macros v1.0.0, Igor Pro 8, MATLAB R2023b, Python 3.11.3, Numpy 1.24.3, Pandas 2.0.1, Scipy 1.10.1, and Matplotlib 3.7.1 are used in available code, demsontrations, and software. Interactive demonstrations of the PPD code, performing exponential fits, plotting data, and the kinetic model, are publicly accessible at the following Zenodo57 and GitHub with https://doi.org/10.5281/zenodo.18175786. From available code demonstrations, all results presented in this work can be reproduced by any interested readers. Additionally, the software to produce MMs from PEEM data is available on Zenodo48 and GitHub with https://doi.org/10.5281/zenodo.17314121.
References
Cohen, A. L. McConnaughey TA. Geochemical perspectives on coral mineralization. Rev. Miner. Geochem 54, 151–187 (2003).
Von Euw, S. et al. Biological control of aragonite formation in stony corals. Science 356, 933–938 (2017).
Mass, T. et al. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 114, E7670–E7678 (2017).
Schmidt, C. A. et al. Myriad Mapping of nanoscale minerals reveals calcium carbonate hemihydrate in forming nacre and coral biominerals. Nat. Commun. 15, 1812 (2024).
Gladfelter, E. H. Skeletal Development in Acropora-Cervicornis .3. A comparison of monthly rates of linear extension and calcium-carbonate accretion measured over a year. Coral Reefs 3, 51–57 (1984).
De Yoreo, J. J. et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 (2015).
Sun, C. Y. et al. From particle attachment to space-filling coral skeletons. Proc. Natl. Acad. Sci. USA 117, 30159–30170 (2020).
Zou, Z. Y. et al. A hydrated crystalline calcium carbonate phase: calcium carbonate hemihydrate. Science 363, 396 (2019).
Tambutté, S. et al. Coral biomineralization: from the gene to the environment. J. Exp. Mar. Biol. Ecol. 408, 58–78 (2011).
Gilbert, P. et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 8, eabl9653 (2022).
De Stasio, G. et al. MEPHISTO: performance tests of a novel synchrotron imaging photoelectron spectromicroscope. Rev. Sci. Instrum. 69, 2062–2066 (1998).
Scholl, A., Ohldag, H., Nolting, F., Stöhr, J. & Padmore, H. A. X-ray photoemission electron microscopy, a tool for the investigation of complex magnetic structures (invited). Rev. Sci. Instrum. 73, 1362–1366 (2002).
De Stasio, G. et al. MEPHISTO spectromicroscope reaches 20 nm lateral resolution. Rev. Sci. Instrum. 70, 1740–1742 (1999).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA 110, 1634–1639 (2013).
Parasassi, T., Sapora, O., Giusti, A. M., De Stasio, G. & Ravagnan, G. Alterations in erythrocyte-membrane lipids induced by low-doses of ionizing-radiation as revealed by 1,6-diphenyl-1,3,5-hexatriene fluorescence lifetime. Int. J. Rad. Biol. 59, 59–69 (1991).
Scucchia, F., Sauer, K., Fara, S., Mass, T. & Zaslansky, P. 4D insights into coral biomineralization: effects of ocean acidification on the early skeleton development of a stony coral. Adv. Sci. 12, e73149 (2025).
Benzerara, K. et al. Study of the crystallographic architecture of corals at the nanoscale by scanning transmission X-ray microscopy and transmission electron microscopy. Ultramicroscopy 111, 1268–1275 (2011).
Malik, A. et al. Molecular and skeletal fingerprints of scleractinian coral biomineralization: from the sea surface to mesophotic depths. Acta Biomater. 120, 263–276 (2021).
Stolarski, J. Three-dimensional micro-and nanostructural characteristics of the scleractinian coral skeleton: a biocalcification proxy. Acta Palaeontol. Pol. 48, 497–530 (2003).
Meibom, A. et al. Vital effects in coral skeletal composition display strict three-dimensional control. Geophys. Res. Lett. 33, L11608 (2006).
Hobbie, R. K. & Roth, B. J. Exponential growth and decay. In Intermediate Physics for Medicine and Biology (eds Hobbie R. K. & Roth, B. J.) 31–47 (Springer International Publishing, 2007).
Venn, A. A. et al. Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci. Rep. 9, 2201 (2019).
Bots, P., Benning, L. G., Rodriguez-Blanco, J.-D., Roncal-Herrero, T. & Shaw, S. Mechanistic insights into the crystallization of amorphous calcium carbonate (ACC). Cryst. Growth Des. 12, 3806–3814 (2012).
Gong, Y. U. T. et al. Phase transitions in biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 109, 6088–6093 (2012).
Radha, A. V., Forbes, T. Z., Killian, C. E., Gilbert, P. U. P. A. & Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 107, 16438–16443 (2010).
Rodriguez-Blanco, J. D., Shaw, S., Bots, P., Roncal-Herrero, T. & Benning, L. G. The role of pH and Mg on the stability and crystallization of amorphous calcium carbonate. J. Alloy. Compd. 536, S477–S479 (2012).
Cohen, A. L. & Holcomb, M. Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22, 118–127 (2009).
Cohen, A. L., McCorkle, D. C., de Putron, S., Gaetani, G. A. & Rose, K. A. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem. Geophys. Geosys. 10, Q07005 (2009).
Godwin, H. Half-life of radiocarbon. Nature 195, 984–984 (1962).
Wilde, S. A., Valley, J. W., Peck, W. H. & Graham, C. M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 409, 175–178 (2001).
Politi, Y. et al. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc. Natl. Acad. Sci. USA 105, 17362–17366 (2008).
Killian, C. E. et al. Mechanism of calcite co-orientation in the sea urchin tooth. J. Am. Chem. Soc. 131, 18404–18409 (2009).
DeVol, R. T. et al. Nanoscale transforming mineral phases in fresh nacre. J. Am. Chem. Soc. 137, 13325–13333 (2015).
Schmidt, C. A. et al. Faster crystallization during coral skeleton formation correlates with resilience to ocean acidification. J. Am. Chem. Soc. 144, 1332–1341 (2022).
Turnbull, D. Kinetics of heterogeneous nucleation. J. Chem. Phys. 18, 198–203 (1950).
Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 7, 1103–1112 (1939).
Brown, M. E. The Prout-Tompkins rate equation in solid-state kinetics. Thermochim. Acta 300, 93–106 (1997).
Crank, J. The Mathematics of Diffusion (Oxford University Press, 1979).
Meldrum, F. C. & O’Shaughnessy, C. Crystallization in confinement. Adv. Mater. 32, 2001068 (2020).
Nam, H. et al. Network context and selection in the evolution to enzyme specificity. Science 337, 1101–1104 (2012).
Bezsudnova, E. Y. et al. Probing the role of the residues in the active site of the transaminase from Thermobaculum terrenum. PLoS ONE 16, e0255098 (2021).
Akiva-Tal, A. et al. In situ molecular NMR picture of bioavailable calcium stabilized as amorphous CaCO3 biomineral in crayfish gastroliths. Proc. Natl. Acad. Sci. USA 108, 14763–14768 (2011).
Al-Sawalmih, A., Li, C. H., Siegel, S., Fratzl, P. & Paris, O. On the Stability Of Amorphous Minerals In Lobster Cuticle. Adv. Mater. 21, 4011 (2009).
Stephens, C. J., Ladden, S. F., Meldrum, F. C. & Christenson, H. K. Amorphous calcium carbonate is stabilized in confinement. Adv. Funct. Mater. 20, 2108–2115 (2010).
Tambutté, E. et al. Morphological plasticity of the coral skeleton under CO-driven seawater acidification. Nat. Commun. 6, 7368 (2015).
Mergelsberg, S. T. et al. Metastable solubility and local structure of amorphous calcium carbonate (ACC). Geochim. Cosmochim. Acta 289, 196–206 (2020).
De Stasio, G., Frazer, B. H., Gilbert, B., Richter, K. L. & Valley, J. W. Compensation of charging in X-PEEM: a successful test on mineral inclusions in 4.4 Ga old zircon. Ultramicroscopy 98, 57–62 (2003).
Gilbert B. Gilbert PUPA. GG Macros. https://doi.org/10.5281/zenodo.17314121 (2025).
Coronado, I., Fine, M., Bosellini, F. R. & Stolarski, J. Impact of ocean acidification on crystallographic vital effect of the coral skeleton. Nat. Commun. 10, 2896 (2019).
Stolarski, J. et al. A modern scleractinian coral with a two-component calcite–aragonite skeleton. Proc. Natl. Acad. Sci. USA 118, e2013316117 (2021).
Moler, C. & Little, J. A history of MATLAB. Proc. ACM Program. Lang. 4, 1–67 (2020).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python (vol 33, pg 219, 2020). Nat. Methods 17, 352–352 (2020).
McKinney, W. Data structures fro statistical computing in Python. SciPy. https://doi.org/10.25080/Majora-92bf1922-00a (2010).
Marquardt, D. W. Citation Classic—algorithm for least-squares estimation of non-linear parameters. Contents/Eng. Technol. Appl. Sci. (1979).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Rechav, Z. & LeCloux, I. M. Zenodo, https://doi.org/10.5281/zenodo.18175786 (2026).
Hunter, J. D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Acknowledgements
The authors thank Aiden Gustafson and James J. De Yoreo for scientific discussions, M. Cristina Castillo Alvarez and Connor A. Schmidt for assistance during sample preparation and PEEM data acquisition, and Andreas Scholl for technical help during PEEM measurements. This work was supported by the National Science Foundation Graduate Research Fellowship Program (grant DGE-1747503) (Z.R.). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1747503. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division at the University of Wisconsin–Madison (grant DE-FG02-07ER15899) (P.G.) and at Lawrence Berkeley National Laboratory (grant FWP-FP00011135) (P.G.), and by the National Science Foundation Biomaterials Program (grant DMR-2220274) (P.G.). This research used resources of the Advanced Light Source, a U.S. Department of Energy Office of Science User Facility under Contract No. DE-AC02-05CH11231.
Author information
Authors and Affiliations
Contributions
P.G., I.L., and Z.R. conceptualized the study and carried out the investigation. E.T., S.T., and A.V. provided samples. P.G., I.L., Z.R., and B.A. performed PEEM data acquisition. M.M. production was carried out by I.L., S.A., N.B., N.C., B.D.-K., J.D., A.L., S.L., R.R., L.S., J.L.S., J.S.S., C.W., J.Y., Z.R., and P.G. I.L. and Z.R. performed the data analysis. P.G. and Z.R. acquired funding. P.G. and Z.R. wrote the original draft of the manuscript. P.G., Z.R., and all co-authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gabriela Farfan and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rechav, Z., Tambutté, E., LeCloux, I.M. et al. Exponential crystallization in corals. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69215-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69215-4