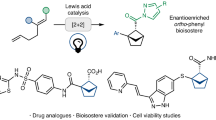
Abstract
Three-dimensional molecules have drawn tremendous attention due to their pivotal roles in drug discovery as saturated bioisosteres of benzenoids. The direct construction of these scaffolds from simple and readily available one-dimensional building blocks is highly attractive but challenging. This study presents a concise synthesis of bicyclo[2.2.1]heptanones and bicyclo[3.2.1]octanones via a photoinduced decatungstate-catalyzed bicyclization of internal alkynes with aldehydes. The reaction enables simultaneous formation of four chemical bonds and two carbocycles, demonstrating excellent site-, regio-, and diastereoselectivity. Experimental and theoretical investigations suggest that the initial cyclization produces a cyclopentanone intermediate with poor diastereoselectivity, and an uncommon dynamic kinetic resolution enabled by hydrogen atom transfer-mediated C-H epimerization yields bicyclic products with excellent diastereoselectivity. This method represents an in situ concurrent editing of skeleton and stereochemistry, which exhibits great potentials for increasing molecular diversity and complexity and changing the way to assemble biologically important compounds.
Similar content being viewed by others
Data availability
Detailed experimental procedures and characterization of new compounds can be found in the Supplementary Information. Source data of cartesian coordinates of computed structures are provided with this paper. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre as CCDC 2355780 (31). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Crystal data are also provided in Supplementary Information. Further relevant data are available from the authors upon request. Source data are provided with this paper.
References
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discov. Today 14, 1011–1020 (2009).
Lovering, F. Escape from flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Boger, D. L., Lener, R. A. & Cravatt, B. F. Synthesis of a functionalized rigid Bicyclo[2.2.1]heptane: a useful hapten for eliciting catalytic antibodies. J. Org. Chem. 59, 5078–5079 (1994).
Ikeuchi, K., Sasage, T., Yamada, G., Suzuki, T. & Tanino, K. Synthesis of a bicyclo[2.2.1]heptane skeleton with two oxy-functionalized bridgehead carbons via the Diels–Alder reaction. Org. Lett. 23, 9123–9127 (2021).
Staveness, D., Collins III, J. L., McAtee, R. C. & Stephenson, C. R. J. Exploiting imine photochemistry for masked N-centered radical reactivity. Angew. Chem., Int. Ed. 58, 19000–19006 (2019).
Staveness, D. et al. Providing a new aniline bioisostere through the photochemical production of 1-aminonorbornanes. Chem 5, 215–226 (2019).
Zhao, J. et al. Intramolecular crossed [2+2] photocycloaddition through visible light-induced energy transfer. J. Am. Chem. Soc. 139, 9807–9810 (2017).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Rigotti, T. & Bach, T. Bicyclo[2.1.1]hexanes by visible light-driven intramolecular crossed [2 + 2] photocycloadditions. Org. Lett. 24, 8821–8825 (2022).
Reinhold, M., Steinebach, J., Golz, C. & Walker, J. C. L. Synthesis of polysubstituted bicyclo[2.1.1]hexanes enabling access to new chemical space. Chem. Sci. 14, 9885–9891 (2023).
Posz, J. M. et al. Synthesis of borylated carbocycles by [2 + 2]-cycloadditions and photo-ene reactions. J. Am. Chem. Soc. 146, 10142–10149 (2024).
Bear, B. R., Sparks, S. M. & Shea, K. J. The type 2 intramolecular Diels–Alder reaction: synthesis and chemistry of bridgehead alkenes. Angew. Chem. Int. Ed. 40, 820–849 (2001).
Parvatkar, P. T., Kadam, H. K. & Tilve, S. G. Intramolecular Diels–Alder reaction as a key step in tandem or sequential processes: a versatile tool for the synthesis of fused and bridged bicyclic or polycyclic compounds. Tetrahedron 70, 2857–2888 (2014).
Min, L., Hu, Y.-J., Fan, J.-H., Zhang, W. & Li, C.-C. Synthetic applications of type II intramolecular cycloadditions. Chem. Soc. Rev. 49, 7015–7043 (2020).
Miura, T., Sasaki, T., Nakazawa, H. & Murakami, M. Ketone synthesis by intramolecular acylation of organorhodium(I) with ester. J. Am. Chem. Soc. 127, 1390–1391 (2005).
Hu, C. et al. Gold(I)-catalyzed cycloisomerization of 3-alkoxyl-1,6-diynes: A facile access to bicyclo[2.2.1]hept-5-en-2-ones Angew. Chem. Int. Ed. 59, 8522–8526 (2020).
Combs-Walker, L. A. & Hill, C. L. Use of excited-state and ground-state redox properties of polyoxometalates for selective transformation of unactivated carbon-hydrogen centers remote from the functional group in ketones. J. Am. Chem. Soc. 114, 938–946 (1992).
Wang, Y. et al. Epimerization of tertiary carbon centers via reversible radical cleavage of unactivated C(sp3)–H Bonds. J. Am. Chem. Soc. 140, 9678–9684 (2018).
Zhang, Y.-A. et al. Stereochemical editing logic powered by the epimerization of unactivated tertiary stereocenters. Science 378, 383–390 (2022).
Li, X., Zhang, Z. & Wu, J. Photocatalytic stereo-chemical editing for the concise syntheses of (25s)-δ7-dafachronic acid, demissidine, and smilagenin. Angew. Chem. Int. Ed. 64, e202500341 (2025).
Shin, N. Y., Ryss, J. M., Zhang, X., Miller, S. J. & Knowles, R. R. Light-driven deracemization enabled by excitedstate electron transfer. Science 366, 364–369 (2019).
Wang, Y., Carder, H. M. & Wendlandt, A. E. Synthesis of rare sugar isomers through site-selective epimerization. Nature 578, 403–408 (2020).
Carder, H. M., Wang, Y. & Wendlandt, A. E. Selective axial-to-equatorial epimerization of carbohydrates. J. Am. Chem. Soc. 144, 11870–11877 (2022).
Carder, H. M. et al. The sugar cube: network control and emergence in stereoediting reactions. Science 385, 456–463 (2024).
Shen, Z. et al. General light-mediated, highly diastereoselective piperidine epimerization: from most accessible to most stable stereoisomer. J. Am. Chem. Soc. 143, 126–131 (2021).
Shen, Z., Vargas-Rivera, M. A., Rigby, E. L., Chen, S. & Ellman, J. A. Visible light-mediated, diastereoselective epimerization of morpholines and piperazines to more stable isomers. ACS Catal. 12, 12860–12868 (2022).
Meinhardt, J. M. et al. Light-driven crystallization-induced dynamic resolution of amines. J. Am. Chem. Soc. 147, 25851–25857 (2025).
Zhang, J. et al. Photocatalytic hydrogenation of quinolines to form 1,2,3,4-tetrahdyroquinolines using water as the hydrogen atom donor. Angew. Chem. Int. Ed. 64, e202502864 (2025).
Kazerouni, A. M. et al. Visible light-mediated, highly diastereoselective epimerization of lactams from the most accessible to the more stable stereoisomer. ACS Catal. 12, 7798–7803 (2022).
Freund, P. et al. Photochemical deracemization of 4,7-diaza-1-isoindolinones by unidirectional hydrogen atom shuttling. J. Am. Chem. Soc. 147, 1434–1439 (2025).
Yan, X., Pang, Y., Zhou, Y., Chang, R. & Ye, J. Photochemical deracemization of lactams with deuteration enabled by dual hydrogen atom transfer. J. Am. Chem. Soc. 147, 1186–1196 (2025).
Dai, L., Shen, C., Wang, J., Li, Y. & Dong, K. Visible light-driven deracemization of cyclic sulfonamides by quinuclidine and chiral arylthiol catalysis. Angew. Chem. Int. Ed. 64, e202505719 (2025).
Oswood, C. J. & MacMillan, D. W. C. Selective isomerization via transient thermodynamic control: dynamic epimerization of trans to cis diols. J. Am. Chem. Soc. 144, 93–98 (2022).
Zhang, Y.-A., Gu, X. & Wendlandt, A. E. A change from kinetic to thermodynamic control enables trans-selective stereochemical editing of vicinal diols. J. Am. Chem. Soc. 144, 599–605 (2022).
Dang, H.-S. & Roberts, B. P. Strategy for contra-thermodynamic radical-chain epimerisation of 1,2-diols using polarity-reversal catalysis. Tetrahedron Lett. 41, 8595–8599 (2020).
Tan, G. & Glorius, F. Stereochemical editing. Angew. Chem. Int. Ed. 62, e202217840 (2023).
Wang, P.-Z., Xiao, W.-J. & Chen, J.-R. Light-empowered contra-thermodynamic stereochemical editing. Nat. Rev. Chem. 7, 35–50 (2023).
Liu, Y., Yang, B., He, H. & Gao, S. Development and application of radical-mediated stereochemical epimerization in natural product synthesis. Angew. Chem. Int. Ed. 64, e202516814 (2025).
Le, S. et al. [3+2] Cycloaddition of alkyl aldehydes and alkynes enabled by photoinduced hydrogen atom transfer. Nat. Commun. 13, 4734 (2022).
Marie, N., Ma, J.-A., Tognetti, V. & Cahard, D. Photocatalyzed cascade hydrogen atom transfers for assembly of multi-substituted α-SCF3 and α-SCF2H cyclopentanones. Angew. Chem. Int. Ed. 63, e202407689 (2024).
Fu, J.-G., Shan, Y.-F., Sun, W.-B., Lin, G.-Q. & Sun, B.-F. An asymmetric approach to bicyclo[2.2.1]heptane-1-carboxylates via a formal [4 + 2] cycloaddition reaction enabled by organocatalysis. Org. Biomol. Chem. 14, 5229–5232 (2016).
Carlson, P. R., Burns, A. S., Shimizu, E. A., Wang, S. & Rychnovsky, S. D. Silacycle-templated intramolecular Diels–Alder cyclizations for the diastereoselective construction of complex carbon skeletons. Org. Lett. 23, 2183–2188 (2021).
Call, A. et al. Carboxylic acid directed γ-lactonization of unactivated primary C–H bonds catalyzed by Mn complexes: application to stereoselective natural product diversification. J. Am. Chem. Soc. 144, 19542–19558 (2022).
Sennari, G., Yamagishi, H. & Sarpong, R. Remote C–H amination and alkylation of camphor at C8 through hydrogen-atom abstraction. J. Am. Chem. Soc. 146, 7850–7857 (2024).
Mahdy, A.-H. S., Zayed, S. E., Abo-Bakr, A. M. & Hassan, E. A. Camphor: synthesis, reactions and uses as a potential moiety in the development of complexes and organocatalysts. Tetrahedron 121, 132913 (2022).
Ravelli, D., Fagnoni, M., Fukuyama, T., Nishikawa, T. & Ryu, I. Site-selective C–H functionalization by decatungstate anion photocatalysis: synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 8, 701–713 (2018).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Chatgilialoglu, C. et al. 5-Endo-trig radical cyclizations: disfavored or favored processes? J. Am. Chem. Soc. 124, 10765–10772 (2002).
Deposition Number 2355780 (31) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Lemay, M., Aumand, L. & Ogilvie, W. W. Design of a conformationally rigid hydrazide organic catalyst. Adv. Synth. Catal. 349, 441–447 (2007).
Top, B., Lane, L. & Cheshire, W. Electroluminescent materials. WO 2009087364 Al, https://patents.google.com/patent/WO2009087364A1 (2008).
Chemel, B. R., Roth, B. L., Armbruster, B., Watts, V. J. & Nichols, D. E. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology 188, 244–251 (2006).
Zhou, Y., Qin, Y., Wang, Q., Zhang, Z. & Zhu, G. Photocatalytic sulfonylcarbocyclization of alkynes using SEt as a traceless directing group: access to cyclopentenes and indenes. Angew. Chem. Int. Ed. 61, e202110864 (2022).
Hong, Y. et al. Tetrafluoroisopropylation of alkenes and alkynes enabled by photocatalytic consecutive difluoromethylation with CF2HSO2Na. Nat. Commun. 15, 5685 (2024).
For a recent review, see: Zhou, Y. et al. Recent advances of 5-endo-trig radical cyclization: promoting strategies and applications. Chem. Commun. 60, 10098–10111 (2024).
Acknowledgements
We thank the Natural Science Foundation of Zhejiang Province (LJHSD26B020001 to G.Z. and LY23B020004 to H.Z.), National Natural Science Foundation of China (22371262 to G.Z., 22203076 to H.Z., and 22071218 to G.Z.), Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2022R01007), and Jinhua Science and Technology Bureau (2026-1-014 to G.Z.) for financial support. This work is dedicated to the 70th anniversary of Zhejiang Normal University.
Author information
Authors and Affiliations
Contributions
G.Z. conceived the idea. Z.G. and T.Z. conducted the experiments. H.Z. performed the density functional computations. Z.Y., H.Z., and G.Z. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hui Li, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, Z., Zeng, T., Yuan, Z. et al. Photocatalytic Stereoselective Editing of Alkynes to 3D Molecules via Hydrogen Atom Transfer-Mediated Dynamic Epimerization. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69219-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69219-0