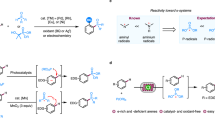
Abstract
Organic electrosynthesis is a versatile and evergreen tool for constructing chemical compounds. However, the study of highly active electrodes has not received enough attention, which limits the further development of organic electrosynthesis. This work introduces a bottom-up route to prepare chitin-derived composite carbon aerogel electrodes (CCAEs), which can be directly used as electrodes in organic electrosynthesis systems. Various metal nanoparticles, such as Pt, Pd, RuO2, Cu and Ni, are well confined in these free-standing and porous CCAEs (M-CCAEs). The linear sweep voltammetry and in-situ Raman tests under electrochemical conditions show that RuO2-CCAEs possess good electrochemical oxidation ability for chlorine anions and good stabilizing effect on the generated chlorine radicals, which can serve as a mediator for the electrochemical C(sp3)-H activation. The combination of M-CCAEs with mediators achieves a series of electrochemical oxidative C(sp3)-H chlorination, bromination, nitration and etherification. Moreover, M-CCAEs promote the electrochemical hydrogen isotope exchange reaction of some important drug molecule structures, such as Ibuprofen, Diclofenac and Zolpidem.
Similar content being viewed by others
Data availability
All data needed to support the findings of this study are included in the main text or in the Supplementary Information. Data supporting the findings of this manuscript are also available from the corresponding author upon request.
References
Gomollón-Bel, F. IUPAC’s 2023 top ten emerging technologies in chemistry. Chem. Int. 45, 14–22 (2023).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Huang, X., Zhang, Q., Lin, J., Harms, K. & Meggers, E. Electricity-driven asymmetric Lewis acid catalysis. Nat. Catal. 2, 34–40 (2018).
Zhang, L. et al. Photoelectrocatalytic arene C-H amination. Nat. Catal. 2, 266–373 (2019).
Mo, Y. et al. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 368, 1352–1357 (2020).
Dong, X., Roeckl, J. L., Waldvogel, S. R. & Morandi, B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 371, 507–514 (2021).
Chen, H. et al. One-pot bioelectrocatalytic conversion of chemically inert hydrocarbons to imines. J. Am. Chem. Soc. 144, 4047–4056 (2022).
Pulignani, C. et al. Rational design of carbon nitride photoelectrodes with high activity toward organic oxidations. Angew. Chem. Int. Ed. 61, e202211587 (2022).
Han, C. et al. Electrocatalytic hydrogenation of alkenes with Pd/carbon nanotubes at an oil–water interface. Nat. Catal. 5, 1110–1119 (2022).
Kim, J., Jang, J., Hilberath, T., Hollmann, F. & Park, C. B. Photoelectrocatalytic biosynthesis fuelled by microplastics. Nat. Syn. 1, 776–786 (2022).
Luo, L. et al. Selective photoelectrocatalytic glycerol oxidation to dihydroxyacetone via enhanced middle hydroxyl adsorption over a Bi2O3-incorporated catalyst. J. Am. Chem. Soc. 144, 7720–7730 (2022).
Sun, G. Q. et al. Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations. Nature 615, 67–72 (2023).
Leech, M. C., Garcia, A. D., Petti, A., Dobbs, A. P. & Lam, K. Organic electrosynthesis: from academia to industry. React. Chem. Eng. 5, 977–990 (2020).
Leow, W. R. et al. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 368, 1228–1233 (2020).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Wang, Q. et al. Electrocatalytic methane oxidation greatly promoted by chlorine intermediates. Angew. Chem. Int. Ed. 60, 17398–17403 (2021).
Fagnani, D. E., Kim, D., Camarero, S. I., Alfaro, J. F. & McNeil, A. J. Using waste poly(vinyl chloride) to synthesize chloroarenes by plasticizer-mediated electro(de)chlorination. Nat. Chem. 15, 222–229 (2023).
The Njarðarson Group. “Top 200 small molecule drugs by sales in 2023”. https://bpb-us-e2.wpmucdn.com/sites.arizona.edu/dist/9/130/files/2024/07/2023Top200SmallMoleculePosterV6.pdf (2023).
Yang, Z., Shi, W., Alhumade, H., Yi, H. & Lei, A. Electrochemical oxidative C(sp3)–H cross-coupling with hydrogen evolution. Nat. Syn. 2, 217–230 (2023).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C-H oxidation. Nature 533, 77–81 (2016).
Kawamata, Y. et al. Scalable, electrochemical oxidation of unactivated C-H bonds. J. Am. Chem. Soc. 139, 7448–7451 (2017).
Rafiee, M., Wang, F., Hruszkewycz, D. P. & Stahl, S. S. N-hydroxyphthalimide-mediated electrochemical iodination of methylarenes and comparison to electron-transfer-initiated C-H functionalization. J. Am. Chem. Soc. 140, 22–25 (2018).
Huang, H., Strater, Z. M. & Lambert, T. H. Electrophotocatalytic C-H functionalization of ethers with high regioselectivity. J. Am. Chem. Soc. 142, 1698–1703 (2020).
Xu, P., Chen, P. Y. & Xu, H. C. Scalable photoelectrochemical dehydrogenative cross-coupling of heteroarenes with aliphatic C-H bonds. Angew. Chem. Int. Ed. 59, 14275–14280 (2020).
Meyer, T. H., Samanta, R. C., Del Vecchio, A. & Ackermann, L. Mangana(III/IV)electro-catalyzed C(sp3)-H azidation. Chem. Sci. 12, 2890–2897 (2020).
Liu, Y. et al. Time-resolved epr revealed the formation, structure, and reactivity of N-centered radicals in an electrochemical C(sp3)-H arylation reaction. J. Am. Chem. Soc. 143, 20863–20872 (2021).
Zhang, L. et al. Ritter-type amination of C(sp3)-H bonds enabled by electrochemistry with SO42-. Nat. Commun. 13, 4138 (2022).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, 4998 (2017).
Heard, D. M. & Lennox, A. J. J. Electrode materials in modern organic electrochemistry. Angew. Chem. Int. Ed. 59, 18866–18884 (2020).
Wu, X., Wang, Y. & Wu, Z.-S. Design principle of electrocatalysts for the electrooxidation of organics. Chem 8, 2594–2629 (2022).
Huang, Y., Chong, X., Liu, C., Liang, Y. & Zhang, B. Boosting hydrogen production by anodic oxidation of primary amines over a nise nanorod electrode. Angew. Chem. Int. Ed. 57, 13163–13166 (2018).
Huang, C., Huang, Y., Liu, C., Yu, Y. & Zhang, B. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni2P bifunctional electrode. Angew. Chem. Int. Ed. 58, 12014–12017 (2019).
Liu, C., Han, S., Li, M., Chong, X. & Zhang, B. Electrocatalytic deuteration of halides with D2O as the deuterium source over a copper nanowire arrays cathode. Angew. Chem. Int. Ed. 59, 18527–18531 (2020).
Wu, Y., Liu, C., Wang, C., Lu, S. & Zhang, B. Selective transfer semihydrogenation of alkynes with H2O (D2O) as the H (D) source over a Pd-P cathode. Angew. Chem. Int. Ed. 59, 21170–21175 (2020).
Kurimoto, A., Sherbo, R. S., Cao, Y., Loo, N. W. X. & Berlinguette, C. P. Electrolytic deuteration of unsaturated bonds without using D2. Nat. Catal. 3, 719–726 (2020).
Bu, F. et al. Electrocatalytic reductive deuteration of arenes and heteroarenes. Nature 634, 592–599 (2024).
Fang, Z., Li, P. & Yu, G. Gel electrocatalysts: an emerging material platform for electrochemical energy conversion. Adv. Mater. 32, 2003191 (2020).
Fu, G. et al. Boosting bifunctional oxygen electrocatalysis with 3D graphene aerogel-supported Ni/MnO particles. Adv. Mater. 30, 1704609 (2018).
Miao, Z. et al. Atomically dispersed Fe-Nx/C electrocatalyst boosts oxygen catalysis via a new metal-organic polymer supramolecule strategy. Adv. Energy Mater. 8, 1801226 (2018).
Meng, J., Cheng, C., Wang, Y., Yu, Y. & Zhang, B. Carbon support enhanced mass transfer and metal-support interaction promoted activation for low-concentrated nitric oxide electroreduction to ammonia. J. Am. Chem. Soc. 146, 10044–10051 (2024).
Wu, K. et al. An iron-decorated carbon aerogel for rechargeable flow and flexible Zn-air batteries. Adv. Mater. 32, 2002292 (2020).
Chen, Z. et al. Iron single atom catalyzed quinoline synthesis. Adv. Mater. 33, 2101382 (2021).
Xu, C., Nasrollahzadeh, M., Selva, M., Issaabadi, Z. & Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio(nano)materials. Chem. Soc. Rev. 48, 4791–4822 (2019).
Yan, N. & Chen, X. Sustainability: don’t waste seafood waste. Nature 524, 155–157 (2015).
Liu, T. et al. Chitin-induced dimerization activates a plant immune receptor. Science 336, 1160–1164 (2012).
Suginta, W., Khunkaewla, P. & Schulte, A. Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113, 5458–5479 (2013).
Lu, L. et al. Carbon nanofibrous microspheres promote the oxidative double carbonylation of alkanes with CO. Chem 4, 2861–2871 (2018).
Manker, L. P. et al. Sustainable polyesters via direct functionalization of lignocellulosic sugars. Nat. Chem. 14, 976–984 (2022).
Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 31, 603–632 (2006).
Pei, X. et al. Size-controllable ultrafine palladium nanoparticles immobilized on calcined chitin microspheres as efficient and recyclable catalysts for hydrogenation. Nanoscale 10, 14719–14725 (2018).
Sadeghi, M. C. sp3)−H functionalization using chlorine radicals. Adv. Synth. Catal. 366, 2898–2918 (2024).
Shields, B. J. & Doyle, A. G. Direct C(sp3)-H cross coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc. 138, 12719–12722 (2016).
Shi, A., Xie, P., Wang, Y. & Qiu, Y. Photoelectrocatalytic Cl-mediated C(sp3)-H aminomethylation of hydrocarbons by BiVO4 photoanodes. Nat. Commun. 16, 2322 (2025).
Terao, J. & Kambe, N. Cross-coupling reaction of alkyl halides with Grignard reagents catalyzed by Ni, Pd, or Cu complexes with π-carbon ligand(s). Acc. Chem. Res. 41, 1545–1554 (2008).
Liu, W. & Groves, J. T. Manganese porphyrins catalyze selective C−H bond halogenations. J. Am. Chem. Soc. 132, 12847–12849 (2010).
Quinn, R. K. et al. Site-selective aliphatic C-H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc. 138, 696–702 (2016).
Lv, X. L. et al. A base-resistant metalloporphyrin metal-organic framework for C-H bond halogenation. J. Am. Chem. Soc. 139, 211–217 (2017).
Zhao, M. & Lu, W. Visible light-induced oxidative chlorination of alkyl sp3 C-H bonds with NaCl/Oxone at room temperature. Org. Lett. 19, 4560–4563 (2017).
Lan, J. et al. Efficient electrosynthesis of formamide from carbon monoxide and nitrite on a Ru-dispersed Cu nanocluster catalyst. Nat. Commun. 14, 2870 (2023).
Gao, X. et al. Membrane-free water electrolysis for hydrogen generation with low cost. Angew. Chem. Int. Ed. 64, e202417987 (2025).
Tomat, R. & Rigo, A. Electrochemical oxidation of aliphatic hydrocarbons promoted by inorganic radicals. II. NO3 radicals. J. Appl. Electrochem. 16, 8–14 (1986).
Patra, S., Mosiagin, I., Giri, R., Nauser, T. & Katayev, D. Electron-driven nitration of unsaturated hydrocarbons. Angew. Chem. Int. Ed. 62, e202300533 (2023).
Huang, R., Yu, C. & Patureau, F. W. Electrochemical dehydrogenative acetalization protection of alcohols with tetrahydrofuran. ChemElectroChem 8, 3943–3946 (2021).
Schmidt, C. First deuterated drug approved. Nat. Biotechnol. 35, 493–494 (2017).
Keam, S. J. & Duggan, S. Donafenib: first approval. Drugs 81, 1915–1920 (2021).
Mullard, A. First de novo deuterated drug poised for approval. Nat. Rev. Drug Discov. 21, 623–625 (2022).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Li, R. et al. One-pot H/D exchange and low-coordinated iron electrocatalyzed deuteration of nitriles in D2O to α,β-deuterio aryl ethylamines. Nat. Commun. 13, 5951 (2022).
Behera, N. et al. Electrochemical hydrogen isotope exchange of amines controlled by alternating current frequency. Faraday Discuss. 247, 45–58 (2023).
Gao, Q. et al. Electroselective C(sp3)–H deuteration of isoindolinones. Org. Chem. Front. 10, 6212–6218 (2023).
Acknowledgements
This work was supported by the National Key R&D Program of China (grant no. 2021YFA1500100, A.L.), the National Natural Science Foundation of China (grant no. 22031008, A.L.), the Science Foundation of Wuhan (grant no. 2020010601012192, A.L.), and the Cultivation Project of Nanchang University (F.L.). The authors would like to acknowledge the Center for Electron Microscopy at Wuhan University for their substantial supports to TEM work. The authors would like to acknowledge Prof. Abhishek Dutta Chowdhury from Wuhan University for advice on the manuscript. The authors would like to acknowledge Prof. Lina Zhang from Wuhan University for advice on this work. Mrs. Lina Zhang sadly passed away on October 17th, 2020, as a result of illness. She proposed an alkali urea system and made a great contribution to the dissolving biomass renewable energy.
Author information
Authors and Affiliations
Contributions
L.L., Y.L., and A.L. conceived and designed the project. L.L. and H.L. performed initial discovery and optimization of reaction conditions. Y.L., X.J., and X.P. prepared the catalysts. L.L. and H.L. performed the study of substrate scope. L.L., Y.L., and H.L. performed mechanistic studies. D.Y., Y.-C.C., and J.-L.C. performed the XAS test and analyzed the data. L.L., Y.L., F.L., and A.L. wrote the manuscript with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hyotaik Kang, Youai Qiu, and Chao Yan for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, L., Li, Y., Li, H. et al. Electrocatalytic C(sp3)-H bond functionalization using biomass-derived electrodes. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69274-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69274-7