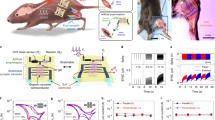
Abstract
Limb loss causes severe sensorimotor deficits and often necessitates prosthetic devices, particularly in lower-limb amputees. Although direct neural recording from residual nerves offers a biomimetic route for prosthetic control, low signal amplitudes and challenges in nerve interfacing have limited adoption. Intraneural multichannel electrodes provide a potential solution by enabling access to motor signals from muscles lost after amputation. Here, we report intraneural recordings from two transfemoral amputees using transversal intrafascicular multichannel electrodes implanted in distal branches of the sciatic nerve. We identified multiunit activity associated with volitional phantom movements of the knee, ankle, and toes, exhibiting joint- and direction-specific modulation distributed across electrodes. A Spiking Neural Network–based decoder outperformed conventional methods in predicting attempted movements, with further gains achieved by integrating intraneural and intermuscular signals. Motor and sensory maps showed minimal overlap, indicating early segregation within the sciatic nerve. These findings pave the way for bidirectional, neurally-controlled prosthetic systems.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary files. Individual de-identified participant data experimental data supporting the findings are immediately and indefinitely available at https://github.com/rossicecilia/intraneural_phantom_leg.git for anyone who wishes to access the data for any purpose. Source data are also provided in this paper. Any additional requests for information can be directed to and will be fulfilled by the corresponding authors. Protocol for human clinical trials is given as part of the reporting summary. Source data are provided with this paper.
Code availability
Custom code used for analysis is publicly available through https://github.com/rossicecilia/intraneural_phantom_leg.git. Code used for data collection can be made available upon request to the study PIs.
References
Kim, Y. C., Park, C. I., Kim, D. Y., Kim, T. S. & Shin, J. C. Statistical analysis of amputations and trends in Korea. Prosthet. Orthot. Int. 20, 88–95 (1996).
Ziegler-Graham, K., MacKenzie, E. J., Ephraim, P. L., Travison, T. G. & Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 89, 422–429 (2008).
Waters, R. L., Perry, J., Antonelli, D. & Hislop, H. J. Energy cost of walking of amputees: the influence of level of amputation. The journal of bone and joint surgery. American ume 58, 42–46 (1976).
Raspopovic, S., Valle, G. & Petrini, F. M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 1–15 https://doi.org/10.1038/s41563-021-00966-9 (2021).
Johansson, J. L., Sherrill, D. M., Riley, P. O., Bonato, P. & Herr, H. A clinical comparison of variable-damping and mechanically passive prosthetic knee devices. Am. J. Phys. Med. Rehabil. 84, 563 (2005).
Cimolato, A. et al. EMG-driven control in lower limb prostheses: a topic-based systematic review. J. Neuroeng. Rehabil. 19, 43 (2022).
Hefferman, G. M., Zhang, F., Nunnery, M. J. & Huang, H. Integration of surface electromyographic sensors with the transfemoral amputee socket: a comparison of four differing configurations. Prosthet. Orthot. Int. 39, 166–173 (2015).
Grison, A. et al. Multidimensional motoneuron control using intramuscular microelectrode arrays in tetraplegic spinal cord injury. Preprint at https://doi.org/10.1101/2025.07.17.25331429 (2025).
Pasquina, P. F. et al. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand. J. Neurosci. Methods 244, 85–93 (2015).
Ferrante, L. et al. Implanted microelectrode arrays in reinnervated muscles allow separation of neural drives from transferred polyfunctional nerves. Nat. Biomed. Eng 1–16 https://doi.org/10.1038/s41551-025-01537-y (2025).
Shu, T., Herrera-Arcos, G., Taylor, C. R. & Herr, H. M. Mechanoneural interfaces for bionic integration. Nat. Rev. Bioeng. 2, 374–391 (2024).
Vu, P. P. et al. A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees. Sci. Transl. Med. 12, eaay2857 (2020).
Kuiken, T. A. et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 301, 619–628 (2009).
Ortiz-Catalan, M., Mastinu, E., Sassu, P., Aszmann, O. & Brånemark, R. Self-contained neuromusculoskeletal arm prostheses. N. Engl. J. Med. 382, 1732–1738 (2020).
Hargrove, L. J. et al. Robotic leg control with EMG decoding in an amputee with nerve transfers. N. Engl. J. Med. 369, 1237–1242 (2013).
Clites, T. R. et al. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 10, eaap8373 (2018).
Song, H. et al. Continuous neural control of a bionic limb restores biomimetic gait after amputation. Nat. Med. 30, 2010–2019 (2024).
Shu, T. et al. Tissue-integrated bionic knee restores versatile legged movement after amputation. Science 389, eadv3223 (2025).
Srinivasan, S. S. et al. Agonist-antagonist myoneural interface amputation preserves proprioceptive sensorimotor neurophysiology in lower limbs. Sci. Transl. Med. 12, eabc5926 (2020).
Perry, S. D., McIlroy, W. E. & Maki, B. E. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 877, 401–406 (2000).
Roll, R., Kavounoudias, A. & Roll, J.-P. Cutaneous afferents from human plantar sole contribute to body posture awareness. Neuroreport 13, 1957–1961 (2002).
Kim, D., Triolo, R. & Charkhkar, H. Plantar somatosensory restoration enhances gait, speed perception, and motor adaptation. Sci. Robot. 8, eadf8997 (2023).
Charkhkar, H., Christie, B. P. & Triolo, R. J. Sensory neuroprosthesis improves postural stability during sensory organization test in lower-limb amputees. Sci. Rep. 10, 6984 (2020).
Petrini, F. M. et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 25, 1356–1363 (2019).
Petrini, F. M. et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 11, eaav8939 (2019).
Valle, G. et al. Mechanisms of neuro-robotic prosthesis operation in leg amputees. Sci. Adv. 7, eabd8354 (2021).
Preatoni, G., Valle, G., Petrini, F. M. & Raspopovic, S. Lightening the perceived weight of a prosthesis with cognitively integrated neural sensory feedback. Curr. Biol. 31, 1–7 (2021).
Valle, G. et al. Biomimetic computer-to-brain communication enhancing naturalistic touch sensations via peripheral nerve stimulation. Nat. Commun. 15, 1151 (2024).
Davis, T. S. et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 13, 036001 (2016).
Petrini, F. M. et al. Microneurography as a tool to develop decoding algorithms for peripheral neuro-controlled hand prostheses. Biomed. Eng. Online 18, 44 (2019).
Cracchiolo, M. et al. Computational approaches to decode grasping force and velocity level in upper-limb amputee from intraneural peripheral signals. J. Neural Eng. 18, 055001 (2021).
Cracchiolo, M. et al. Decoding of grasping tasks from intraneural recordings in trans-radial amputee. J. Neural Eng. https://doi.org/10.1088/1741-2552/ab8277 (2020).
Wendelken, S. et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah slanted electrode arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14, 121 (2017).
Donati, E. & Indiveri, G. Neuromorphic bioelectronic medicine for nervous system interfaces: from neural computational primitives to medical applications. Prog. Biomed. Eng. 5, 013002 (2023).
Donati, E. & Valle, G. Neuromorphic hardware for somatosensory neuroprostheses. Nat. Commun. 15, 556 (2024).
Pillastrini, P., Marchetti, M. & Abbruzzese, G. Neurofisiologia del movimento. Anatomia, biomeccanica, chinesiologia, clinica, 2nd edn (Piccin-Nuova Libraria, Padova, 2020).
Rigoard, P. Atlas of Anatomy of the Peripheral Nerves: The Nerves of the Limbs–Expert Edition (Springer International Publishing, 2020).
Herdin, M., Czink, N., Ozcelik, H. & Bonek, E. Correlation matrix distance, a meaningful measure for evaluation of non-stationary MIMO channels. In Proc. 2005 IEEE 61st Vehicular Technology Conference Vol. 1, 136–140 (IEEE, 2005).
Yavuz, U. Ş, Negro, F., Diedrichs, R. & Farina, D. Reciprocal inhibition between motor neurons of the tibialis anterior and triceps surae in humans. J. Neurophysiol. 119, 1699–1706 (2018).
Bian, S., Donati, E. & Magno, M. Evaluation of encoding schemes on ubiquitous sensor signal for spiking neural network. IEEE Sens. J. 24, 35008–35018 (2024).
Sava, R., Donati, E. & Indiveri, G. Feed-forward and recurrent inhibition for compressing and classifying high dynamic range biosignals in spiking neural network architectures. In Proc. IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–5, https://doi.org/10.1109/BioCAS58349.2023.10388963 (2023).
Hong, K.-S., Aziz, N. & Ghafoor, U. Motor-commands decoding using peripheral nerve signals: a review. J. Neural Eng. 15, 031004 (2018).
Goncharova, I. I., McFarland, D. J., Vaughan, T. M. & Wolpaw, J. R. EMG contamination of EEG: spectral and topographical characteristics. Clin. Neurophysiol. 114, 1580–1593 (2003).
Schone, H. R. et al. Stable cortical body maps before and after arm amputation. Nat Neurosci 28, 2015–2021 (2025).
Raspopovic, S., Petrini, F. M., Zelechowski, M. & Valle, G. Framework for the development of neuroprostheses: from basic understanding by sciatic and median nerves models to bionic legs and hands. Proc. IEEE 105, 34–49 (2017).
Zelechowski, M., Valle, G. & Raspopovic, S. A computational model to design neural interfaces for lower-limb sensory neuroprostheses. J. Neuroeng. Rehabil. 17, 24 (2020).
Warchoł, Ł, Walocha, J. A., Mizia, E., Liszka, H. & Bonczar, M. Comparison of the histological structure of the tibial nerve and its terminal branches in the fresh and fresh-frozen cadavers. Folia Morphol. 80, 542–548 (2021).
Jankowska, E. & Roberts, W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J. Physiol. 222, 623–642 (1972).
Shindo, M., Harayama, H., Kondo, K., Yanagisawa, N. & Tanaka, R. Changes in reciprocal Ia inhibition during voluntary contraction in man. Exp. Brain Res. 53, 400–408 (1984).
De Luca, C. J. & Mambrito, B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J. Neurophysiol. 58, 525–542 (1987).
Dietz, V. & Sinkjaer, T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 6, 725–733 (2007).
Reilly, K. T., Mercier, C., Schieber, M. H. & Sirigu, A. Persistent hand motor commands in the amputees’ brain. Brain 129, 2211–2223 (2006).
Enoka, R. M. & Duchateau, J. Rate coding and the control of muscle force. Cold Spring Harb. Perspect. Med. 7, a029702 (2017).
Macefield, V. G., Fuglevand, A. J., Howell, J. N. & Bigland-Ritchie, B. Discharge behaviour of single motor units during maximal voluntary contractions of a human toe extensor. J. Physiol. 528, 227–234 (2000).
Barberi, F. et al. Early decoding of walking tasks with minimal set of EMG channels. J. Neural Eng. 20, 026038 (2023).
De Luca, C., Tincani, M., Indiveri, G. & Donati, E. A neuromorphic multi-scale approach for real-time heart rate and state detection. npj Unconv. Comput. 2, 6 (2025).
Baracat, F., Mazzoni, A., Micera, S., Indiveri, G. & Donati, E. Decoding gestures from intraneural recordings of a transradial amputee using event-based processing. Preprint at https://doi.org/10.36227/techrxiv.170719069.94594142/v2 (2024).
Katic, N. et al. Modeling foot sole cutaneous afferents: FootSim. iScience 26, 105874 (2023).
Delgado-Martínez, I., Badia, J., Pascual-Font, A., Rodríguez-Baeza, A. & Navarro, X. Fascicular topography of the human median nerve for neuroprosthetic surgery. Front. Neurosci. 10, 286 (2016).
Dhillon, G. S., Lawrence, S. M., Hutchinson, D. T. & Horch, K. W. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J. Hand Surg. Am. 29, 605–615 (2004).
Seyedali, M., Czerniecki, J. M., Morgenroth, D. C. & Hahn, M. E. Co-contraction patterns of trans-tibial amputee ankle and knee musculature during gait. J. Neuroeng. Rehabil. 9, 29 (2012).
Valle, G. et al. Multifaceted understanding of human nerve implants to design optimized electrodes for bioelectronics. Biomaterials 291, 121874 (2022).
Aiello, G., Valle, G. & Raspopovic, S. Recalibration of neuromodulation parameters in neural implants with adaptive Bayesian optimization. J. Neural Eng. 20, 026037 (2023).
Chen, W., Liu, X., Wan, P., Chen, Z. & Chen, Y. Anti-artifacts techniques for neural recording front-ends in closed-loop brain-machine interface ICs. Front. Neurosci. 18, 1393206 (2024).
Dosen, S., Schaeffer, M.-C. & Farina, D. Time-division multiplexing for myoelectric closed-loop control using electrotactile feedback. J. Neuroeng. Rehabil. 11, 138 (2014).
Chateaux, M. et al. New insights into muscle activity associated with phantom hand movements in transhumeral amputees. Front. Hum. Neurosci. 18, 1443833(2024).
Jarrassé, N. et al. Phantom-mobility-based prosthesis control in transhumeral amputees without surgical reinnervation: a preliminary study. Front. Bioeng. Biotechnol. 6, 164 (2018).
Čvančara, P. et al. Bringing sensation to prosthetic hands—chronic assessment of implanted thin-film electrodes in humans. npj Flex. Electron. 7, 1–14 (2023).
Rey, H. G., Pedreira, C. & Quian Quiroga, R. Past, present and future of spike sorting techniques. Brain Res. Bull. 119, 106–117 (2015).
Stimberg, M., Brette, R. & Goodman, D. F. Brian 2, an intuitive and efficient neural simulator. eLife 8, e47314 (2019).
van Schaik, A., Fragniere, E. & Vittoz, E. An analogue electronic model of ventral cochlear nucleus neurons. In Proc. of Fifth International Conference on Microelectronics for Neural Networks 52–59, https://doi.org/10.1109/MNNFS.1996.493772 (1996).
Zanghieri, M., Benatti, S., Benini, L. & Donati, E. Event-based low-power and low-latency regression method for hand kinematics from surface EMG. In Proc. 9th International Workshop on Advances in Sensors and Interfaces (IWASI) 293–298, https://doi.org/10.1109/IWASI58316.2023.10164372 (2023).
Eshraghian, J. K. et al. Training spiking neural networks using lessons from deep learning. Proc. IEEE 111, 1016–1054 (2023).
Neftci, E. O., Mostafa, H. & Zenke, F. Surrogate Gradient Learning in Spiking Neural Networks: Bringing the Power of Gradient-Based Optimization to Spiking Neural Networks. IEEE Signal Processing Magazine 36, 51–63 (2019).
Valle, G. et al. A psychometric platform to collect somatosensory sensations for neuroprosthetic use. Front. Med. Technol. 3, 8 (2021).
Acknowledgements
The authors are deeply grateful to the three subjects who freely donated months of their life for the advancement of knowledge and for a better future for leg amputees. The funder had no role in the experimental design, analysis, or manuscript preparation or submission. All authors had complete access to the data. All authors authorized the submission of the manuscript, but the final submission decision was made by the corresponding authors. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (FeelAgain grant agreement no. 759998, S.R.).
Funding
Open access funding provided by Chalmers University of Technology.
Author information
Authors and Affiliations
Contributions
C.R. analyzed the neural data, implemented and tested the SNN-based motor decoders, prepared the figures and wrote the paper; T.S. and P.C. developed the TIME and delivered technical assistance for the human implantation and explanation procedures and reviewed the manuscript; M.B. performed the human surgeries and was responsible for all the clinical aspects of the human study; S.R. designed the study, performed and supervised the human experiments and reviewed the manuscript; E.D., supervised all the analyses, and wrote the paper; G.V. developed the recording software for human experiments, performed the human experiments, supervised all the analyses, prepared the figures and wrote the paper. All authors edited and proofread the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.V. holds shares of “MYNERVA AG”, a start-up company dealing with the potential commercialization of noninvasive stimulating wearable for treating neuropathic pain. G.V. serves as a consultant for NeuroOne Medical Technologies Corporation (USA). The other authors do not have anything to disclose.
Peer review
Peer review information
Nature Communications thanks Jozina De Graaf and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rossi, C., Bumbasirevic, M., Čvančara, P. et al. Decoding phantom limb movements from intraneural recordings. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69297-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69297-0