Abstract
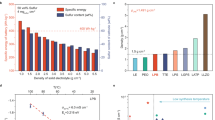
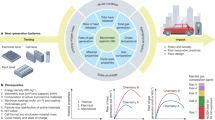
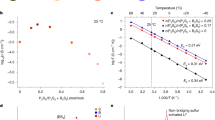
Sulfide all solid-state batteries represent a promising next generation energy storage technology. However, their presumed safety is challenged by the risk of thermal runaway initiating at unexpectedly low temperatures. This critical issue stems from the unstable chemical interface between the positive electrode and thiophosphate solid electrolyte, a factor often overlooked in favor of electrochemical studies. Here we demonstrate that this electrochemically formed interphase is the primary trigger for catastrophic failure, not the bulk materials. Our investigation reveals a universal two stage degradation mechanism. The first stage involves intense exothermic reactions at the interface below 160 °C, releasing heat and gases. This initiates a second stage of propagating reactions leading to thermal runaway. Crucially, we show this hazardous process can be suppressed by interface engineering. We design a stable interfacial layer using a germanium sulfur chemistry, specifically lithium germanium sulfide. This modification delivers improved thermal safety without sacrificing battery performance. Our findings have the potential to establish a forward-looking safety paradigm, shifting the focus from bulk material compatibility to interfacial stability, and provide a vital design principle for future safe solid-state batteries.
Similar content being viewed by others
Data availability
Source data are provided with this paper. Source data have been deposited in Figshare under the accession code DOI link https://doi.org/10.6084/m9.figshare.30461468. The computational configurations have been deposited as Supplementary Data 1.
References
Lu, P. et al. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Advanced Materials 33, 2100921 (2021).
Kato, Y., Hori, S. & Kanno, R. Li10 GeP2 S12 -type superionic conductors: synthesis, structure, and ionic transportation. Advanced Energy Materials 10, 2002153 (2020).
Zhou, L., Minafra, N., Zeier, W. G. & Nazar, L. F. Innovative approaches to li-argyrodite solid electrolytes for all-solid-state lithium batteries. Acc. Chem. Res. 54, 2717–2728 (2021).
Wu, J. et al. All-solid-state lithium batteries with sulfide electrolytes and oxide positive electrodes. Electrochem. Energ. Rev. 4, 101–135 (2021).
Hu, L. et al. Fusion bonding technique for solvent-free fabrication of all-solid-state battery with ultrathin sulfide electrolyte. Advanced Materials 36, 2401909 (2024).
Wang Z., et al. Soft carbon-thiourea with fast bulk diffusion kinetics for solid-state lithium metal batteries. Advanced Materials, 2310395 (2023).
Ma, T. et al. High-areal-capacity and long-cycle-life all-solid-state battery enabled by freeze drying technology. Energy Environ. Sci. 16, 2142–2152 (2023).
Ge, S. et al. Quantification of lithium battery fires in internal short circuit. ACS Energy Lett. 9, 5747–5755 (2024).
Yang, S.-J. et al. Oxygen-induced thermal runaway mechanisms of Ah-level solid-state lithium metal pouch cells. eTransportation 18, 100279 (2023).
Darmet, N., Charbonnel, J., Reytier, M., Broche, L. & Vincent, R. First experimental assessment of all-solid-state battery thermal runaway propagation in a battery pack. ACS Appl. Energy Mater. 7, 4365–4375 (2024).
Vishnugopi, B. S., Hasan, M. T., Zhou, H. & Mukherjee, P. P. Interphases and electrode crosstalk dictate the thermal stability of solid-state batteries. ACS Energy Lett. 8, 398–407 (2023).
Rui, X. et al. Distinct thermal runaway mechanisms of sulfide-based all-solid-state batteries. Energy Environ. Sci. 16, 3552–3563 (2023).
T. Kim, et al, Critical Factors Contributing to the Thermal Runaway of Thiophosphate Solid Electrolytes for All-Solid-State Batteries. Adv Funct Materials, 2404806 (2024).
Kim, T. et al. Thermal Runaway Behavior of Li6 PS5 Cl Solid Electrolytes for LiNi0.8 Co0.1 Mn0.1 O2 and LiFePO4 in All-Solid-State Batteries. Chem. Mater. 34, 9159–9171 (2022).
Wang, S. et al. Thermal stability between sulfide solid electrolytes and oxide positive electrode. ACS Nano 16, 16158–16176 (2022).
Xiao, Y. et al. Understanding interface stability in solid-state batteries. Nat. Rev. Mater. 5, 105–126 (2019).
Ren, F. et al. The nature and suppression strategies of interfacial reactions in all-solid-state batteries. Energy Environ. Sci. 16, 2579–2590 (2023).
Tan, D. H. S. et al. Elucidating Reversible Electrochemical Redox of Li6 PS5 Cl Solid Electrolyte. ACS Energy Lett. 4, 2418–2427 (2019).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Banik, A. et al. Can substitutions affect the oxidative stability of lithium argyrodite solid electrolytes? ACS Appl. Energy Mater. 5, 2045–2053 (2022).
Liang, Y. et al. Challenges, interface engineering, and processing strategies toward practical sulfide-based all-solid-state lithium batteries. InfoMat 4, e12292 (2022).
Tsukasaki, H. et al. Exothermal behavior and microstructure of a LiNi1/3Mn1/3Co1/3O2 electrode layer using a Li4SnS4 solid electrolyte. Journal of Power Sources 479, 228827 (2020).
Dong, Y. & Li, J. Oxide positive electrodes: functions, instabilities, self healing, and degradation mitigations. Chem. Rev. 123, 811–833 (2023).
Zhu, Y., He, X. & Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces. 7, 23685–23693 (2015).
Zhang, W. et al. The Detrimental Effects of Carbon Additives in Li10 GeP2 S12 -Based Solid-State Batteries. ACS Appl. Mater. Interfaces. 9, 35888–35896 (2017).
Yoon, K., Kim, J.-J., Seong, W. M., Lee, M. H. & Kang, K. Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in all-solid-state lithium battery. Sci. Rep. 8, 8066 (2018).
Deng, S. et al. Eliminating the Detrimental Effects of Conductive Agents in Sulfide-Based Solid-State Batteries. ACS Energy Lett. 5, 1243–1251 (2020).
Negi, R. S. et al. Insights into the positive effect of post-annealing on the electrochemical performance of Al2 O3 -Coated Ni-Rich NCM positive electrodes for lithium-ion batteries. ACS Appl. Energy Mater. 4, 3369–3380 (2021).
Negi, R. S. et al. A Dry-Processed Al2 O3 /LiAlO2 Coating for Stabilizing the Positive electrode/Electrolyte Interface in High-Ni NCM-Based All-Solid-State Batteries. Adv Materials Inter. 9, 2101428 (2022).
Lu, P. et al. Realizing long-cycling all-solid-state Li-In||TiS2 batteries using Li6+xMxAs1-xS5I (M=Si, Sn) sulfide solid electrolytes. Nat. Commun. 14, 4077 (2023).
Zhao, J. et al. Size-Dependent Chemomechanical Failure of Sulfide Solid Electrolyte Particles during Electrochemical Reaction with Lithium. Nano Lett. 22, 411–418 (2022).
Wenzel, S. et al. Direct Observation of the Interfacial Instability of the Fast Ionic Conductor Li10 GeP2 S12 at the Lithium Metal Negative electrode. Chem. Mater. 28, 2400–2407 (2016).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys Rev B Condens Matter 49, 14251–14269 (1994).
Nedjemeddine, B., Abderrahim, B. & Farida, B. Structure, microstructure and ionic conductivity of the solid solution LiTi2−xSnx(PO4)3. Physica B 407, 403–407 (2012).
John, P., Kieron, B. & Matthias, E. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Ou, X., Liu, T. & Zhong, W. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat. Commun. 13, 2319 (2022).
Yi, Z., He, X. & Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A. 4, 3253–3266 (2016).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant No. 52572279, received by L. H., U22A20440, received by G. C.), the Emerging Industry Cultivation Plan of Qingdao Future Industry Cultivation Project (No. 24-1-4-xxgg-7-gx, received by G. C.), the Key R&D Program of Shandong Province, China (No. 2023CXGC010302, received by G. C.), and the Taishan Scholars of Shandong Province (Nos. ts201511063, received by G. C.)
Author information
Authors and Affiliations
Contributions
G. C. supervised this work. L. H. and G. C. designed this work. Y. W., Y. S. carried out the electrochemical experiments and the material characterizations. Shu. Z. performed the theoretical calculations. J. X., C. L., Shanshan. Z., Z. J., T. G., L. G., L. C., T. L. and J. J. did the data analysis. Y. W. and L. H. wrote the paper. G. C., Shu. Z., T. L. and J. J. revised the manuscript. All authors commented on the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Janghyuk Moon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, Y., Zhang, S., Sun, Y. et al. Electrochemical initiation and chemical reaction cascades in dual-stage thermal runaway in sulfide-based all-solid-state batteries. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69472-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69472-3