Abstract
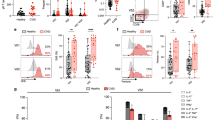
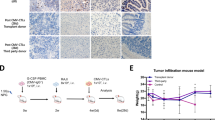
γδ T cells show promise for anti-tumoral therapies but have yet to be evaluated to treat infectious diseases. In this preclinical study, we assess a Vδ1 + γδ T cell-based adoptive cell therapy, named Delta One T cells, to treat cytomegalovirus (CMV) infection in high-risk transplant recipients. Even when expanded from CMV-naïve healthy donors, Delta One T cells efficiently control CMV dissemination in vitro. CMV recognition is independent of the γδTCR but requires LFA-1 co-stimulation. In an in vivo model, adoptive transfer of mouse γδ T cells recapitulating Delta One T cell features protects mice against lethal murine CMV infection. Importantly, CMV-reactive Delta One T cells can be successfully generated from kidney transplant recipients undergoing refractory CMV infections and maintain their functionality in the presence of immunosuppressive drugs. These findings broaden the scope of γδ T cell therapies to infectious diseases and uncover a universal adoptive T cell therapy to treat refractory CMV infections.
Similar content being viewed by others
Data availability
All datasets generated and analyzed during this study are available in the Source Data file provided. Source data are provided with this paper.
References
Humar, A. et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transplant. 10, 1228–1237 (2010).
Witzke, O. et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation 93, 61–68 (2012).
Kliem, V. et al. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am. J. Transplant. 8, 975–983 (2008).
Kotton, C. N. et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102, 900–931 (2018).
Ljungman, P. et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e260–e272 (2019).
Natori, Y. et al. Recurrence of CMV infection & the effect of prolonged antivirals in organ transplant recipients. Transplantation 101, 1449–1454 (2017).
Ljungman, P., Hakki, M. & Boeckh, M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol. Oncol. Clin. North Am. 25, 151–169 (2011).
Couzi, L. et al. High incidence of anticytomegalovirus drug resistance among D+R-kidney transplant recipients receiving preemptive therapy. Am. J. Transplant. 12, 202–209 (2012).
Avery, R. K. et al. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial. Clin. Infect. Dis. 75, 690–701 (2022).
Chou, S. et al. Drug resistance assessed in a phase 3 clinical trial of maribavir therapy for refractory or resistant cytomegalovirus infection in transplant recipients. J. Infect. Dis. 229, 413–421 (2024).
Chemaly, R. F. et al. Cytomegalovirus (CMV) cell-mediated immunity and cmv infection after allogeneic hematopoietic cell transplantation: the REACT study. Clin. Infect. Dis. 71, 2365–2374 (2020).
Nicholson, E. & Peggs, K. S. Cytomegalovirus-specific T-cell therapies: current status and future prospects. Immunotherapy 7, 135–146 (2015).
Houghtelin, A. & Bollard, C. M. Virus-specific T cells for the immunocompromised patient. Front. Immunol. 8, 1272 (2017).
Prockop, S. E. et al. Third-party cytomegalovirus-specific T cells improved survival in refractory cytomegalovirus viremia after hematopoietic transplant. J. Clin. Investig. 133, e165476 (2023).
Khoury, R. et al. Third-party virus specific T cells for the treatment of double stranded DNA viral reactivation and PTLD after solid organ transplant. Am. J. Transplant. 24, 1634–1643 https://doi.org/10.1016/j.ajt.2024.04.009. (2024).
Smith, C. et al. Autologous adoptive T-cell therapy for recurrent or drug-resistant cytomegalovirus complications in solid organ transplant recipients: a single-arm open-label phase I clinical trial. Clin. Infect. Dis. 68, 632–640 https://doi.org/10.1093/cid/ciy549. (2018).
Smith, C. et al. T cell repertoire remodeling following post-transplant T cell therapy coincides with clinical response. J. Clin. Investig. 129, 5020–5032 (2019).
Kaminski, H. et al. Understanding human γδ T cell biology toward a better management of cytomegalovirus infection. Immunol. Rev. 298, 264–288 (2020).
Shen, L. et al. Fast-acting γδ T-cell subpopulation and protective immunity against infections. Immunol. Rev. 298, 254–263 (2020).
Pamplona, A. & Silva-Santos, B. γδ T cells in malaria: a double-edged sword. FEBS J. 288, 1118–1129 (2021).
Prinz, I. et al. Donor Vδ1+ γδ T cells expand after allogeneic hematopoietic stem cell transplantation and show reactivity against CMV-infected cells but not against progressing B-CLL. Exp. Hematol. Oncol. 2, 2–5 (2013).
Déchanet, J. et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J. Clin. Investig. 103, 1437–1449 (1999).
Couzi, L. et al. Gamma-delta T cell expansion is closely associated with cytomegalovirus infection in all solid organ transplant recipients. Transpl. Int. 24, e40–e42 (2011).
Pitard, V. et al. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood 112, 1317–1324 (2008).
Halary, F. et al. Shared reactivity of Vδ2 neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578 (2005).
Couzi, L. et al. Antibody-dependent anti-cytomegalovirus activity of human gd T cells expressing CD16 (FcgRIIIa). Blood 119, 1418–1427 (2012).
Kaminski, H. et al. Surveillance of γδ T cells predicts cytomegalovirus infection resolution in kidney transplants. J. Am. Soc. Nephrol. 27, 637–645 (2016).
Khairallah, C. et al. γδ T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog 11, 1–22 (2015).
Sell, S. et al. Control of murine cytomegalovirus infection by γδ T cells. PLoS Pathog 11, 1–21 (2015).
Almeida, A. R. et al. Delta one T cells for immunotherapy of chronic lymphocytic leukemia: clinical-grade expansion/differentiation and preclinical proof of concept. Clin. Cancer Res. 22, 5795–5804 (2016).
Di Lorenzo, B. et al. Broad cytotoxic targeting of acute myeloid leukemia by polyclonal delta one T cells. Cancer Immunol. Res. 7, 552–558 (2019).
Sánchez Martínez, D. et al. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J. Immunother. Cancer 10, e005400 (2022).
Mensurado, S. et al. CD155/PVR determines Acute Myeloid Leukemia targeting by Delta One T cells. Blood J. 143 https://doi.org/10.1182/blood.2023022992. (2024)
Gattinoni, L. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Investig. 115, 1616–1626 (2005).
Berger, C. et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Investig. 118, 294–305 (2008).
Kang, S., Brown, H. M. & Hwang, S. Direct antiviral mechanisms of interferon-gamma. Immune Netw 18, e33 (2018).
Guerville, F. et al. TCR-dependent sensitization of human γδ T cells to non-myeloid IL-18 in cytomegalovirus and tumor stress surveillance. OncoImmunology 4, 1–13 (2015).
Kaminski, H. et al. Characterization of a unique γδ T-cell subset as a specific marker of cytomegalovirus infection severity. J. Infect. Dis. https://doi.org/10.1093/infdis/jiaa400. (2020).
Willcox, C. R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl. Acad. Sci. USA 114, 3163–3168 (2017).
Simões, A. E., Di Lorenzo, B. & Silva-Santos, B. Molecular determinants of target cell recognition by human γδ T cells. Front. Immunol. 9, 1–7 (2018).
Amini, L. et al. Comprehensive characterization of a next-generation antiviral T-cell product and feasibility for application in immunosuppressed transplant patients. Front. Immunol. 10, 1148 (2019).
Ferrandiz, I. et al. Impact of early blood transfusion after kidney transplantation on the incidence of donor-specific anti-LA Antibodies. Am. J. Transplant. 16, 2661–2669 (2016).
Varis, T., Kaukonen, K.-M., Kivistö, K. T. & Neuvonen, P. J. Plasma concentrations and effects of oral methylprednisolone are considerably increased by itraconazole*. Clin. Pharmacol. Ther. 64, 363–368 (1998).
Jarque, M. et al. Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: a prospective, interventional, multicenter clinical trial. Clin. Infect. Dis. 1, 1–11 (2020).
Hanley, P. J. et al. Erratum for the research article: “CMV-specific T cells generated from naïve T cells recognize atypical epitopes and may be protective in vivo” by P. J. Hanley, J. J. Melenhorst, S. Nikiforow, P. Scheinberg, J. W. Blaney, G. Demmler-Harrison, C. R. Cruz. Sci. Transl. Med. 8, 321er1–321er1 (2016).
Jackson, S. E., Mason, G. M., Okecha, G., Sissons, J. G. P. & Wills, M. R. Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T Cells. J. Virol. 88, 10894–10908 (2014).
Houldcroft, C. J. et al. Assessing Anti-HCMV cell mediated immune responses in transplant recipients and healthy controls using a novel functional assay. Front. Cell. Infect. Microbiol. 10, 1–21 (2020).
Watanabe, M., Ito, M., Kamiya, H. & Sakurai, M. Adherence of peripheral blood leukocytes to cytomegalovirus-infected fibroblasts. Microbiol. Immunol. 40, 519–523 (1996).
Waldman, W. J., Knight, D. A. & Huang, E. H. An in vitro model of T cell activation by autologous cytomegalovirus (CMV)-infected human adult endothelial cells: contribution of CMV-enhanced endothelial ICAM-1. J. Immunol. 160, 3143–3151 (1998).
Leong, C. C. et al. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatibility complex and a viral class I homolog. J. Exp. Med. 187, 1681–1687 (1998).
Siegers, G. M. Integral roles for integrins in γδ T. Front. Immunol. 9, 521 (2018).
van de Berg, P. J. et al. Human cytomegalovirus induces systemic immune activation characterized by a Type 1 Cytokine signature. J. Infect. Dis. 202, 690–699 (2010).
Karaba, A. H. et al. Interleukin-18 and tumor necrosis factor-α are elevated in solid organ transplant recipients with possible cytomegalovirus end-organ disease. Transpl. Infect. Dis. 23, e13682 (2021).
Dalton, J. E., Pearson, J., Scott, P. & Carding, S. R. The interaction of γδ T cells with activated macrophages is a property of the Vγ1 subset. J. Immunol. 171, 6488–6494 (2003).
Hirsh, M. I. & Junger, W. G. Roles of heat shock proteins and γδT cells in inflammation. Am. J. Respir. Cell Mol. Biol. 39, 509–513 (2008).
Drobyski, W. R., Majewski, D. & Hanson, G. Graft-facilitating doses of ex vivo activated γδ T cells do not cause lethal murine graft-vs.-host disease. Biol. Blood Marrow Transplant. 5, 222–230 (1999).
Bachelet, T. et al. Cytomegalovirus-responsive gd T Cells: novel effector cells in antibody-mediated kidney allograft microcirculation lesions. J. Am. Soc. Nephrol. 25, 2471–2482 (2014).
Charmetant, X. et al. T cells’ role in donor-specific antibody generation: insights from transplant recipients and experimental models. Transpl. Int. 38, 12859 (2025).
Yared, N. et al. Long-lived central memory γδ T cells confer protection against murine cytomegalovirus reinfection. PLOS Pathog 20, e1010785 (2024).
Kaminski, H. et al. mTOR inhibitors prevent CMV infection through the restoration of functional ab and gd T cells in kidney transplantation. J. Am. Soc. Nephrol. 33, 121–137 (2022).
Concordet, J. P. & Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46, W242–W245 (2018).
Haeussler, M. et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148 (2016).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Tycko, J. et al. Mitigation of off-target toxicity in CRISPR-Cas9 screens for essential non-coding elements. Nat. Commun. 10, 4063 (2019).
Oh, S. A., Seki, A. & Rutz, S. Ribonucleoprotein transfection for CRISPR/Cas9-mediated gene knockout in primary T cells. Curr. Protoc. Immunol. 124, e69 (2019).
Satchell, S. C. et al. Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int. 69, 1633–1640 (2006).
Baer, A. & Kehn-Hall, K. Viral concentration determination through plaque assays: using traditional and novel overlay systems. J. Vis. Exp. 1–10 https://doi.org/10.3791/52065. (2014).
Acknowledgements
This study was supported by research funding from Fondation de Bordeaux (grant number: FB201903 to L.C.), Fondation du Rein (FRM 9428 Prix Jeune Chercheur 2019 FDR_SFNDT to M.C.) and Agence de la Biomédecine (Appel d’Offres Recherche “Recherche et Greffe” 2021, grant number 21GREFFE004, to L.C.). The authors thank the staff of the Animal Facility A2 (University of Bordeaux, France) and notably Benoit Rousseau for their expertise and assistance regarding mouse experiments. The authors also thank the staff of Vect’UB and CRISP’edit technology platforms (INSERM US005/CNRS UAR3427 TBM Core, University of Bordeaux, France), as well as Daniel Correia (iMM) and Amandine Demestre (ImmunoConcEpT) for technical assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization, G.M., J.D.-M., and L.C.; Methodology, G.M., C.G., V.P., V.P.-M., B.T., B.S.-S, H.K., M.Ca., J.D.-M., and L.C.; Investigation, G.M., C.G., M.C., C.T., A.C., S.C.-C., V.B., J.I., V.P., A.Z., I.G., M.Ca., and V.P.-M.; Writing: Original Draft, G.M., C.G., J.D.-M., and L.C.; Writing: Review & Editing, G.M., C.G., J.D.-M., L.C., H.K., V.P.-M., B.T., E.F., P.M., B.S.-S., and M.Ca.; Funding Acquisition, M.C., J.D-M., and L.C.; Resources, D.A.; Supervision, J.D-M. and L.C.
Corresponding authors
Ethics declarations
Competing interests
B.S.-S. was a co-inventor of the DOT cell technology now owned by Takeda, which supports his work via a sponsored research agreement. The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Leila Amini, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marsères, G., Gentil, C., Tinevez, C. et al. Adoptive γδ T cell therapy controls cytomegalovirus infection in preclinical transplantation models. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69538-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69538-2