Abstract
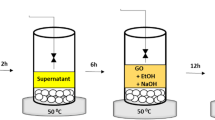
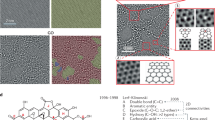
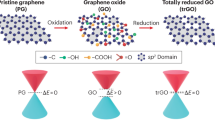
We report a scalable and sustainable method for synthesizing graphene oxide (GO) via a non-thermal atmospheric nano-second pulsed plasma (NSPP) process, using methane as the carbon source and water as the substrate. Unlike conventional chemical vapor deposition (CVD), which demands high temperatures, low pressures, and inert gases, this approach operates at ambient conditions without additional gas inputs. The plasma decomposes methane directly on or near the water surface, producing high-purity, single-layer GO with tunable oxygen content and flake size. Gas chromatography confirms substantial hydrogen generation and minimal greenhouse gas emissions. Atomic Force Microscopy (AFM) analysis verifies single-layer morphology. Scaling the process with a four-gap reactor yields 5 g of GO per day, exceeding conventional CVD output while reducing cost and environmental impact. This plasma-driven strategy provides an energy-efficient route for large-scale GO production, with potential applications in electronics, energy storage, coatings, and concrete composites.
Similar content being viewed by others
Data availability
References
Liu, Z., Robinson, J. T., Sun, X. & Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 130, 10876–10877 (2008).
Zhang, L., Xia, J., Zhao, Q., Liu, L. & Zhang, Z. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 6, 537–544 (2010).
Yang, K. et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 33, 2206–2214 (2012).
Esmaeili, Y., Bidram, E., Zarrabi, A., Amini, A. & Cheng, C. Graphene oxide and its derivatives as promising In-vitro bio-imaging platforms. Sci. Rep. 10, 18052 (2020).
Akhavan, O. & Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4, 5731–5736 (2010).
Hu, W. et al. Graphene-based antibacterial paper. ACS Nano 4, 4317–4323 (2010).
Liu, Y., Dong, X. & Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 41, 2283–2307 (2012).
Wang, L. et al. A graphene–conjugated oligomer hybrid probe for light-up sensing of Lectin and Escherichia Coli. Adv. Mater. 23, 4386–4391 (2011).
Sitko, R. et al. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 42, 5682–5689 (2013).
Bong, J. et al. Dynamic graphene filters for selective gas-water-oil separation. Sci. Rep. 5, 14321 (2015).
Li, X. et al. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. 113, 13953–13958 (2016).
Zhu, M., Chen, P. & Liu, M. Graphene oxide enwrapped Ag/AgX (X = Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano 5, 4529–4536 (2011).
Li, B. & Cao, H. ZnO@graphene composite with enhanced performance for the removal of dye from water. J. Mater. Chem. 21, 3346–3349 (2011).
Wang, M. et al. All-Solid-State Reduced Graphene Oxide Supercapacitor with Large Volumetric Capacitance and Ultralong Stability Prepared by Electrophoretic Deposition Method. ACS Appl. Mater. Interfaces 7, 1348–1354 (2015).
Gao, X., Li, J., Xie, Y., Guan, D. & Yuan, C. A multilayered silicon-reduced graphene oxide electrode for high performance lithium-ion batteries. ACS Appl. Mater. Interfaces 7, 7855–7862 (2015).
Zhang, L. L. et al. Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors. Nano Lett. 12, 1806–1812 (2012).
Qian, L., Thiruppathi, A. R., van der Zalm, J. & Chen, A. Graphene oxide-based nanomaterials for the electrochemical sensing of isoniazid. ACS Appl. Nano Mater. 4, 3696–3706 (2021).
Zhuang, W. et al. Enhancing electrochemical sensing through molecular engineering of reduced graphene oxide–solution interfaces and remote floating-gate FET Analysis. ACS Appl. Mater. Interfaces 16, 27961–27968 (2024).
Soni, M., Kumar, P., Pandey, J., Sharma, S. K. & Soni, A. Scalable and site specific functionalization of reduced graphene oxide for circuit elements and flexible electronics. Carbon 128, 172–178 (2018).
Chang, D. et al. Reversible fusion and fission of graphene oxide–based fibers. Science 372, 614–617 (2021).
Brisebois, P. P. & Siaj, M. Harvesting graphene oxide – years 1859 to 2019: a review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C. 8, 1517–1547 (2020).
Shen, J. et al. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 21, 3514–3520 (2009).
Hummers, W. S. Jr. & Offeman, R. E. Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339–1339 (1958).
Pan, S. & Aksay, I. A. Factors controlling the size of graphene oxide sheets produced via the graphite oxide route. ACS Nano 5, 4073–4083 (2011).
Chen, J., Yao, B., Li, C. & Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64, 225–229 (2013).
Sun, J. et al. Fully converting graphite into graphene oxide hydrogels by preoxidation with impure manganese dioxide. ACS Appl. Mater. Interfaces 7, 21356–21363 (2015).
Chen, J., Li, Y., Huang, L., Li, C. & Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 81, 826–834 (2015).
Hu, Y., Song, S. & Lopez-Valdivieso, A. Effects of oxidation on the defect of reduced graphene oxides in graphene preparation. J. Colloid Interface Sci. 450, 68–73 (2015).
Yu, C., Wang, C.-F. & Chen, S. Facile access to graphene oxide from ferro-induced oxidation. Sci. Rep. 6, 17071 (2016).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. chemischen Ges. 31, 1481–1487 (1898).
Ikram, R., Jan, B. M. & Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 9, 11587–11610 (2020).
Sinitskii, A. et al. Kinetics of Diazonium functionalization of chemically converted graphene nanoribbons. ACS Nano 4, 1949–1954 (2010).
Kosynkin, D. V. et al. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458, 872–876 (2009).
Sun, L. & Fugetsu, B. Mass production of graphene oxide from expanded graphite. Mater. Lett. 109, 207–210 (2013).
Parvez, K. et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 136, 6083–6091 (2014).
Utkan, G., Yumusak, G., Tunali, B. C., Ozturk, T. & Turk, M. Production of reduced graphene oxide by using three different microorganisms and investigation of their cell interactions. ACS Omega 8, 31188–31200 (2023).
Huang, H.-H., De Silva, K. K. H., Kumara, G. R. A. & Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 8, 6849 (2018).
Zafar, M. A. & Jacob, M. V. Plasma-based synthesis of graphene and applications: a focused review. Rev. Mod. Plasma Phys. 6, 37 (2022).
Yu, X. Z. et al. New synthesis method for the growth of epitaxial graphene. J. Electron Spectrosc. Relat. Phenom. 184, 100–106 (2011).
Muñoz, R. & Gómez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 19, 297–322 (2013).
Boyd, D. A. et al. Single-step deposition of high-mobility graphene at reduced temperatures. Nat. Commun. 6, 6620 (2015).
Dato, A. Graphene synthesized in atmospheric plasmas—A review. J. Mater. Res. 34, 214–230 (2019).
Tatarova, E. et al. Microwave plasmas applied for the synthesis of free standing graphene sheets. J. Phys. D: Appl. Phys. 47, 385501 (2014).
Dato, A., Radmilovic, V., Lee, Z., Phillips, J. & Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 8, 2012–2016 (2008).
Zielinski, T. & Kijenski, J. Plasma carbon black—the new active additive for plastics. Compos. A: Appl. Sci. Manuf. 36, 467–471 (2005).
Wu, A. et al. Conversion of coalbed methane surrogate into hydrogen and graphene sheets using rotating gliding arc plasma. Plasma Sci. Technol. 21, 115501 (2019).
Moreno-Couranjou, M., Monthioux, M., Gonzalez-Aguilar, J. & Fulcheri, L. A non-thermal plasma process for the gas phase synthesis of carbon nanoparticles. Carbon 47, 2310–2321 (2009).
Gonzalez-Aguilar, J., Moreno, M. & Fulcheri, L. Carbon nanostructures production by gas-phase plasma processes at atmospheric pressure. J. Phys. D: Appl. Phys. 40, 2361 (2007).
Melero, C. et al. Scalable graphene production from ethanol decomposition by microwave argon plasma torch. Plasma Phys. Control. Fusion 60, 014009 (2018).
Münzer, A., Xiao, L., Sehlleier, Y. H., Schulz, C. & Wiggers, H. All gas-phase synthesis of graphene: Characterization and its utilization for silicon-based lithium-ion batteries. Electrochim. Acta 272, 52–59 (2018).
Abdolhosseinzadeh, S., Asgharzadeh, H. & Seop Kim, H. Fast and fully-scalable synthesis of reduced graphene oxide. Sci. Rep. 5, 10160 (2015).
Wang, K. et al. Electric fuel conversion with hydrogen production by multiphase plasma at ambient pressure. Chem. Eng. J. 433, 133660 (2022).
Li, M. et al. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 3, 1600003 (2016).
Yuan, X. et al. A kinetic study of nonthermal plasma pyrolysis of methane: Insights into hydrogen and carbon material production. Chem. Eng. J. 499, 156396 (2024).
Kado, S. et al. Reaction mechanism of methane activation using non-equilibrium pulsed discharge at room temperature. Fuel 82, 2291–2297 (2003).
Lefkowitz, J. K., Guo, P., Rousso, A. & Ju, Y. Species and temperature measurements of methane oxidation in a nanosecond repetitively pulsed discharge. Philos. Trans. R. Soc. A: Math., Phys. Eng. Sci. 373, 20140333 (2015).
Wu, A. et al. Co-generation of hydrogen and carbon aerosol from coalbed methane surrogate using rotating gliding arc plasma. Appl. Energy 195, 67–79 (2017).
Gorbanev, Y., O’Connell, D. & Chechik, V. Non-thermal plasma in contact with water: the origin of species. Chem. – A Eur. J. 22, 3496–3505 (2016).
Bai, M., Bai, X., Zhang, Z., Bai, M. & Yang, B. Treatment of red tide in ocean using non-thermal plasma based advanced oxidation technology*. Plasma Chem. Plasma Process. 25, 539–550 (2005).
Gaydon, A. G. The Identification of Molecular Spectra. Vol. VIII (Springer Dordrecht, 1976).
Bruggeman, P. J. et al. Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci. Technol. 25, 053002 (2016).
Li, N. et al. Nucleation and growth dynamics of graphene grown by radio frequency plasma-enhanced chemical vapor deposition. Sci. Rep. 11, 6007 (2021).
Araújo, M. P., Soares, O. S. G. P., Fernandes, A. J. S., Pereira, M. F. R. & Freire, C. Tuning the surface chemistry of graphene flakes: new strategies for selective oxidation. RSC Adv. 7, 14290–14301 (2017).
Seon Lee, Y. et al. Effects of high-temperature thermal reduction on thermal conductivity of reduced graphene oxide polymer composites. Appl. Surf. Sci. 650, 159140 (2024).
Zhao, J. et al. Mild thermal reduction of graphene oxide as a lubrication additive for friction and wear reduction. RSC Adv. 7, 1766–1770 (2017).
Gómez-Navarro, C. et al. Atomic structure of reduced graphene oxide. Nano Lett. 10, 1144–1148 (2010).
Lee, B. et al. Membrane of functionalized reduced graphene oxide nanoplates with Angstrom-level channels. Sci. Rep. 6, 28052 (2016).
David Staack, H. B. J. Kunpeng Wang. Conductive liquid hydrocarbon gas plasma for material and chemical synthesis and transformation. USA patent (2023).
Bhuiyan, S. I. et al. Greenhouse gas emission reduction and energy impact of electrifying upgraders in refineries using plasma processing technology. Sustain. Energy Fuels 7, 2178–2199 (2023).
Wang, K. S. et al. Heavy oil cracking device scaleup with multiple electrical discharge modules USA patent (2025).
Dreyer, D. R., Park, S., Bielawski, C. W. & Ruoff, R. S. The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240 (2010).
Cote, L. J., Kim, F. & Huang, J. Langmuir−Blodgett assembly of graphite oxide single layers. J. Am. Chem. Soc. 131, 1043–1049 (2009).
Park, S. & Ruoff, R. S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 4, 217–224 (2009).
Nishina, Y. Mass production of graphene oxide beyond the laboratory: bridging the gap between academic research and industry. ACS Nano 18, 33264–33275 (2024).
C. T. Inc. Reduced Graphene Oxide, https://www.cheaptubes.com/product/reduced-graphene-oxide/ (2025).
Sigma-Aldrich. Graphene oxide (15–20 sheets, 4–10 % edge-oxidized), https://www.sigmaaldrich.com/US/en/substance/grapheneoxide1234598765?srsltid=AfmBOooVxIFZ0X8wBAhjLhmWaAI6DSjgQW6uwtW8qzou3pzjQmD5MHeG&utm_source=chatgpt.com (2025).
Eigler, S., Dotzer, C., Hof, F., Bauer, W. & Hirsch, A. Sulfur species in graphene oxide. Chem. – A Eur. J. 19, 9490–9496 (2013).
Beloin-Saint-Pierre, D. & Hischier, R. Towards a more environmentally sustainable production of graphene-based materials. Int. J. Life Cycle Assess. 26, 327–343 (2021).
Serrano-Luján, L. et al. Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl. Sci. 1, 179 (2019).
Acknowledgements
LTEOIL provided funding at TAMU. The authors would like to thank the Materials Characterization Facility (RRID: SCR022202) and Microscopy and Imaging Center (RRID: SCR_022128) at Texas A&M University for their XPS, SEM, TEM, EDS, and AFM setups. The authors would like to acknowledge the assistance of Adam T. Ronderos for performing the EDS measurements and Cameron Stoltz for conducting the Zeta potential measurements. The authors would also like to acknowledge the use of the vacuum tube furnace setup at Texas A&M University Soft Matter Facility (RRID: SCR_022482). The authors thank BioRender.com for providing tools used to create the graphical schematics of Figs. 1, 2a.
Author information
Authors and Affiliations
Contributions
R.B.: Writing – original draft, methodology, investigation, thermal reduction experiments, all material characterization, schematics, and visualization. Y.Z.: investigation. M.A.: thermal reduction experiments and Optical emission spectroscopy measurements. S.T.U.: investigation. S.D.: investigation. J.D.L.: investigation. M.P.: circuit design for a reactor. A.S.S.: investigation. H.B.J.: project administration, funding acquisition. R.S.: Review & editing, project administration, M.J.G.: Writing – review & editing, supervision, project administration, funding acquisition. K.W.: Methodology, investigation, project administration, and conceptualization. D.S.: Writing – review & editing, supervision, project administration, funding acquisition, conceptualization.
Corresponding authors
Ethics declarations
Competing interests
LTEOIL authors (JDL, HBJ, RS, KW) acknowledge intellectual property holdings on the synthesis process referenced here, and all other authors declare no competing interests. LTEOIL funded much of the work carried out on this topic at TAMU.
Peer review
Peer review information
Nature Communications thanks Yuming Chen, Albert Dato, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Banavath, R., Zhang, Y., Akhter, M. et al. Graphene oxide synthesis at a nonthermal plasma-water interface. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69831-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69831-0