Abstract
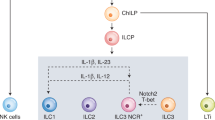
Innate lymphoid cells type 2 (ILC2s) are key regulators of tissue homeostasis and inflammation. In cancer, ILC2s can exhibit pro-tumoral functions by increasing the myeloid derived suppressor cells (MDSC)/T-cell ratio. Nevertheless, the upstream ILC2 triggers remain poorly defined. Here, we identify nerve growth factor (NGF) as the driver of ILC2 pro-tumoral functions in patients with bladder cancer. We show that ILC2s express the NGF receptor TrkA and respond to NGF by secreting type-2 cytokines. In the tumor microenvironment, NGF-producing mast cells accumulate and activate ILC2s to induce regulatory T cells (Tregs), ultimately fostering tumor growth. In patients, NGF levels inversely correlate with survival in ILC2-rich tumors, underscoring the clinical significance of this axis. In vivo administration of a selective TrkA inhibitor improves survival in orthotopic tumor-bearing female mice and sensitizes them to immune checkpoint blockade (ICB). Overall, we identify NGF as an ILC2 activator that shapes pro-tumoral ILC2 functions. The blockade of TrkA+ ILC2s might represent a targetable strategy to improve survival, particularly in ICB-resistant patients.
Similar content being viewed by others
Data availability
The RNAseq data of human ILC2s generated in this study have been deposited in NCBI’s Gene Expression Omnibus database under the GEO accession number GSE311046. The TCGA Bladder Urothelial Carcinoma (BLCA) RNA sequencing dataset is available from the National Institutes of Health’s (NIH) dbGaP (Database of Genotypes and Phenotypes) database under accession number phs000178 [https://portal.gdc.cancer.gov/projects/TCGA-BLCA]. Microarray gene expression datasets are available from the National Center for Biotechnology Information (NCBI)‘s GEO database under accession numbers GSE31684 and GSE48075. - Human ILCs: ArrayExpress accession E-MTAB-8494 (bulk RNA sequencing data) [https://www.ebi.ac.uk/biostudies/ArrayExpress/studies/E-MTAB-8494], GSE112591 (bulk RNA sequencing data) [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE112591], GSE150050 (single-cell RNA sequencing, Smart-Seq2 protocol) [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150050] The remaining data are available within the article, Supplementary information or Source Data file and/or from the corresponding author upon request. Source data are provided with this paper.
References
Saginala, K. et al. Epidemiology of Bladder Cancer. Med. Sci. (Basel) 8, https://doi.org/10.3390/medsci8010015 (2020).
Zlotta, A. R., Fleshner, N. E. & Jewett, M. A. The management of BCG failure in non-muscle-invasive bladder cancer: an update. Can. Urol. Assoc. J. 3, S199–S205 (2009).
Lopez-Beltran, A. et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel) 13, https://doi.org/10.3390/cancers13010131 (2021).
Shi, S., Ma, T. & Xi, Y. Characterization of the immune cell infiltration landscape in bladder cancer to aid immunotherapy. Arch. Biochem Biophys. 708, 108950 (2021).
Kang, H. W., Kim, W.-J. & Yun, S. J. The role of the tumor microenvironment in bladder cancer development and progression. Transl. Cancer Res. S 744, S758 (2017).
Chevalier, M. F. et al. ILC2-modulated T cell-to-MDSC balance is associated with bladder cancer recurrence. J. Clin. Invest. 127, 2916–2929 (2017).
Spits, H. & Mjosberg, J. Heterogeneity of type 2 innate lymphoid cells. Nat. Rev. Immunol. 22, 701–712 (2022).
Halim, T. Y. et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40, 425–435 (2014).
Bernink, J. H., Germar, K. & Spits, H. The role of ILC2 in pathology of type 2 inflammatory diseases. Curr. Opin. Immunol. 31, 115–120 (2014).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Ebbo, M., Crinier, A., Vely, F. & Vivier, E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 17, 665–678 (2017).
Simoni, Y. & Newell, E. W. Dissecting human ILC heterogeneity: more than just three subsets. Immunology 153, 297–303 (2018).
Mjosberg, J. & Spits, H. Human innate lymphoid cells. J. Allergy Clin. Immunol. 138, 1265–1276 (2016).
Bahhar, I. et al. The IL-25/ILC2 axis promotes lung cancer with a concomitant accumulation of immune-suppressive cells in tumors in humans and mice. Front Immunol. 14, 1244437 (2023).
Trabanelli, S. et al. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat. Commun. 8, 593 (2017).
Wu, L. et al. Mesenchymal PGD(2) activates an ILC2-Treg axis to promote proliferation of normal and malignant HSPCs. Leukemia 34, 3028–3041 (2020).
Schuijs, M. J. et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 21, 998–1009 (2020).
Ercolano, G. et al. PPARɣ drives IL-33-dependent ILC2 pro-tumoral functions. Nat. Commun. 12, 2538 (2021).
Xu, X. et al. Group-2 innate lymphoid cells promote HCC progression through CXCL2-neutrophil-induced immunosuppression. Hepatology 74, 2526–2543 (2021).
Maresca, D. C. et al. Circulating innate lymphoid cells are dysregulated in patients with prostate cancer. Cell Mol. Biol. Lett. 30, 48 (2025).
Moral, J. A. et al. ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020).
Jacquelot, N. et al. Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat. Immunol. 22, 851–864 (2021).
Qi, J. et al. Single-cell transcriptomic landscape reveals tumor specific innate lymphoid cells associated with colorectal cancer progression. Cell Rep. Med. 2, 100353 (2021).
Wen, J. et al. Group 2 innate lymphoid cells boost CD8(+) T-cell activation in anti-tumor immune responses. Oncoimmunology 12, 2243112 (2023).
Amisaki, M. et al. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature 638, 1076–1084 (2025).
Olguin-Martinez, E., Ruiz-Medina, B. E. & Licona-Limon, P. Tissue-specific molecular markers and heterogeneity in type 2 innate lymphoid Cells. Front Immunol. 12, 757967 (2021).
Yano, H. & Artis, D. Neuronal regulation of innate lymphoid cell responses. Curr. Opin. Immunol. 76, 102205 (2022).
Klose, C. S. & Artis, D. Neuronal regulation of innate lymphoid cells. Curr. Opin. Immunol. 56, 94–99 (2019).
Cardoso, F. et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 597, 410–414 (2021).
Yin, Z., Zhou, Y., Turnquist, H. R. & Liu, Q. Neuro-epithelial-ILC2 crosstalk in barrier tissues. Trends Immunol. 43, 901–916 (2022).
Liu, H. T. & Kuo, H. C. Intravesical botulinum toxin A injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 70, 463–468 (2007).
Bjorling, D. E. et al. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int. 87, 697–702 (2001).
Jacobs, B. L. et al. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. Can. J. Urol. 17, 4989–4994 (2010).
Griffin, N., Faulkner, S., Jobling, P. & Hondermarck, H. Targeting neurotrophin signaling in cancer: the renaissance. Pharm. Res. 135, 12–17 (2018).
Meldolesi, J. Neurotrophin trk receptors: new targets for cancer therapy. Rev. Physiol. Biochem Pharm. 174, 67–79 (2018).
Yin, T. et al. Breaking NGF-TrkA immunosuppression in melanoma sensitizes immunotherapy for durable memory T cell protection. Nat. Immunol. 25, 268–281 (2024).
Cancer Genome Atlas & Research, N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Riester, M. et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin. Cancer Res. 18, 1323–1333 (2012).
Choi, W. et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 25, 152–165 (2014).
Meakin, S. O. & Shooter, E. M. The nerve growth factor family of receptors. Trends Neurosci. 15, 323–331 (1992).
Ochodnicky, P., Cruz, C. D., Yoshimura, N. & Cruz, F. Neurotrophins as regulators of urinary bladder function. Nat. Rev. Urol. 9, 628–637 (2012).
Quatrini, L., Vivier, E. & Ugolini, S. Neuroendocrine regulation of innate lymphoid cells. Immunol. Rev. 286, 120–136 (2018).
Ercolano, G. et al. Distinct and shared gene expression for human innate versus adaptive helper lymphoid cells. J. Leukoc. Biol. 108, 723–737 (2020).
Li, S. et al. Gene expression signatures of circulating human type 1, 2, and 3 innate lymphoid cells. J. Allergy Clin. Immunol. 143, 2321–2325 (2019).
Mazzurana, L. et al. Tissue-specific transcriptional imprinting and heterogeneity in human innate lymphoid cells revealed by full-length single-cell RNA-sequencing. Cell Res. 31, 554–568 (2021).
Moretta, A. et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 (2001).
Wu, R., Li, K., Yuan, M. & Luo, K. Q. Nerve growth factor receptor increases the tumor growth and metastatic potential of triple-negative breast cancer cells. Oncogene 40, 2165–2181 (2021).
Molloy, N. H., Read, D. E. & Gorman, A. M. Nerve growth factor in cancer cell death and survival. Cancers (Basel) 3, 510–530 (2011).
Surace, L. et al. Dichotomous metabolic networks govern human ILC2 proliferation and function. Nat. Immunol. 22, 1367–1374 (2021).
Leon, A. et al. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA. 91, 3739–3743 (1994).
Nilsson, G. et al. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur. J. Immunol. 27, 2295–2301 (1997).
Chen, F., Zhang, G., Cao, Y., Hessner, M. J. & See, W. A. MB49 murine urothelial carcinoma: molecular and phenotypic comparison to human cell lines as a model of the direct tumor response to bacillus Calmette-Guerin. J. Urol. 182, 2932–2937 (2009).
Oliphant, C. J. et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 41, 283–295 (2014).
Schneider, C. et al. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity 50, 1425–1438 e1425 (2019).
Vocanson, M. et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. J. Allergy Clin. Immunol. 126, 280–289 (2010).
Halim, T. Y. F. et al. Tissue-restricted adaptive type 2 immunity is orchestrated by expression of the costimulatory molecule OX40L on group 2 innate lymphoid cells. Immunity 48, 1195–1207 e1196 (2018).
Molofsky, A. B. et al. Interleukin-33 and interferon-gamma counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 43, 161–174 (2015).
Skapenko, A., Kalden, J. R., Lipsky, P. E. & Schulze-Koops, H. The IL-4 receptor alpha-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25-CD4+ precursors. J. Immunol. 175, 6107–6116 (2005).
Singh, R. et al. TRAF4-mediated ubiquitination of NGF receptor TrkA regulates prostate cancer metastasis. J. Clin. Invest. 128, 3129–3143 (2018).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Vizzard, M. A. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp. Neurol. 161, 273–284 (2000).
Fowler, C. J., Griffiths, D. & de Groat, W. C. The neural control of micturition. Nat. Rev. Neurosci. 9, 453–466 (2008).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018).
Stabile, A. M. et al. Long term effects of cigarette smoke extract or nicotine on nerve growth factor and its receptors in a bronchial epithelial cell line. Toxicol. Vitr. 53, 29–36 (2018).
Freedman, N. D., Silverman, D. T., Hollenbeck, A. R., Schatzkin, A. & Abnet, C. C. Association between smoking and risk of bladder cancer among men and women. JAMA 306, 737–745 (2011).
Alam, A. et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 40, 153–167 e111 (2022).
O’Keefe, R. N. et al. A tuft cell - ILC2 signaling circuit provides therapeutic targets to inhibit gastric metaplasia and tumor development. Nat. Commun. 14, 6872 (2023).
Bonini, S., Rasi, G., Bracci-Laudiero, M. L., Procoli, A. & Aloe, L. Nerve growth factor: neurotrophin or cytokine? Int Arch. Allergy Immunol. 131, 80–84 (2003).
Aloe, L., Rocco, M. L., Balzamino, B. O. & Micera, A. Nerve growth factor: role in growth, differentiation and controlling cancer cell development. J. Exp. Clin. Cancer Res. 35, 116 (2016).
Di Donato, M. et al. Targeting the nerve growth factor signaling impairs the proliferative and migratory phenotype of triple-negative breast cancer cells. Front Cell Dev. Biol. 9, 676568 (2021).
Adriaenssens, E. et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 68, 346–351 (2008).
Di Donato, M., Cernera, G., Migliaccio, A. & Castoria, G. Nerve Growth Factor Induces Proliferation and Aggressiveness In Prostate Cancer Cells. Cancers (Basel) 11, https://doi.org/10.3390/cancers11060784 (2019).
Ratner, V. Mast cell activation syndrome. Transl. Androl. Urol. 4, 587–588 (2015).
Kritas, S. K. et al. Nerve growth factor interactions with mast cells. Int J. Immunopathol. Pharm. 27, 15–19 (2014).
Kurashima, Y. et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 3, 1034 (2012).
Komi, D. E. A. & Redegeld, F. A. Role of mast cells in shaping the tumor microenvironment. Clin. Rev. Allergy Immunol. 58, 313–325 (2020).
Lowe, D., Fletcher, C. D. & Gower, R. L. Tumour-associated eosinophilia in the bladder. J. Clin. Pathol. 37, 500–502 (1984).
Popov, H., Donev, I. S. & Ghenev, P. Quantitative analysis of tumor-associated tissue Eosinophilia in recurring bladder cancer. Cureus 10, e3279 (2018).
Chen, Q. et al. ICOS signal facilitates Foxp3 transcription to favor suppressive function of regulatory T cells. Int. J. Med. Sci. 15, 666–673 (2018).
Morita, H. et al. An interleukin-33-mast cell-interleukin-2 axis suppresses papain-induced allergic inflammation by promoting regulatory T cell numbers. Immunity 43, 175–186 (2015).
Haist, M., Stege, H., Grabbe, S. & Bros, M. The functional crosstalk between myeloid-derived suppressor cells and regulatory T cells within the immunosuppressive tumor microenvironment. Cancers (Basel) 13, https://doi.org/10.3390/cancers13020210 (2021).
Fallegger, A. et al. TGF-beta production by eosinophils drives the expansion of peripherally induced neuropilin(-) RORgammat(+) regulatory T-cells during bacterial and allergen challenge. Mucosal Immunol. 15, 504–514 (2022).
Wang, T., Yu, D. & Lamb, M. L. Trk kinase inhibitors as new treatments for cancer and pain. Expert Opin. Ther. Pat. 19, 305–319 (2009).
Jiang, T. et al. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 11, 355–372 (2021).
Rouanne, M. et al. Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J. Urol. 36, 1727–1740 (2018).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529 (2021).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Colaprico, A. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25, 1463–1465 (2009).
Sternberg, S. R. Biomedical image processing. Computer 16, 22–34 (1983).
Acknowledgements
We thank patients and healthy donors for participating in this study. We thank the genomic and flow cytometry facilities of the Universities of Lausanne and Geneva for their excellent technical assistance. We thank Prof Tim Halim (University of Cambridge, CRUK Cambridge Institute, Cambridge, UK) for the insightful discussions on the project and Prof Andrew Mackenzie (MRC Laboratory of molecule biology, Cambridge, UK) for providing the ILC2KO animals. We thank Nataniele Piol (MD), Anatomia Patologica Universitaria, IRCCS Ospedale Policlinico San Martino, Genova, Italy who kindly provided us some of the bladder cancer patient sections. This work was supported by the Ludwig Institute for Cancer Research, by grants from Swiss National Science Foundation (PRIMA PR00P3_179727), the IDEAL grant from Debiopharm, the INNOGap grant from UNIGE, a generous donor, advised by Carigest SA to C.J., the Fond’Action contre le cancer grant to H.E.A., AIRC grant (Fondazione AIRC IG 2021, Id. 26037) to E.M. and AIRC grant (Fondazione AIRC MFAG 2021, Id. 26002) to G.E., the Swiss Cancer League (KLS-4836-08-2019) to C.S., the Geneva Cancer League (2106) to C.S., the EU ITN (813284, INTEGRATA) to C.S., and the Swiss National Science Foundation MD-PhD fellowship to D.G.; B.F is supported by a PhD fellowship from the ISREC Foundation. L.T.J. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 818806).
Author information
Authors and Affiliations
Contributions
M.F., G.E., S.T. and C.J. conceived the project. M.F., H.E.A., A.G-C., Z.S., A.C., T.W., B.K., R.P., K.F., P.W., B.F., I.S., N.K., D.G., D.C.M., S.P., S.C., G.E., S.T. and C.J. performed experiments and data analyses. M.K., R.M., S.H., L.J., E.M., C.S. and P.T. assisted in data analyses and interpretation. J-C.T., M.M.L., K.M., K.G., J.D., M.C., C.P.B., E.G-J., G.V., D.M., M.A. and D.B. provided study material. M.F., G.E., S.T. and C.J. wrote the manuscript. All authors interpreted the data and contributed valuable feedback for the improvement of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.T.J. co-founder, holding equity of Cimeio Therapeutics AG (Cimeio). Cimeio board member. Sponsored research agreement with Cimeio. Inventor on granted patents and patent applications related to immune cell engineering and antibodies. Received speaker fees from Novartis. Paid consultant for Kyowa Kirin. No competing interests directly affecting this study. The other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Communications thanks Elena Jachetti, Neelam Mukherjee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Falquet, M., El Ahanidi, H., Gomez-Cadena, A. et al. Mast-cell derived nerve growth factor drives ILC2 pro-tumoral functions in bladder cancer. Nat Commun (2026). https://doi.org/10.1038/s41467-026-69841-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-026-69841-y