Abstract
Water loss and carbon gain are balanced by stomatal control1, a trade-off that has allowed trees to survive and thrive under fluctuating environmental conditions2,3,4. During periods of lower water availability, stomatal closure prevents excess water loss5. Various strategies of stomatal control have been found among tree species6,7, but the trigger for this behaviour remains elusive. We found a uniform pre-dawn water potential threshold (−1.2 MPa) for stomatal closure across species, which coincided with stem-growth cessation. Meanwhile, midday water potentials at stomatal closure were more variable across species and stomatal control did not follow species-specific thresholds of hydraulic failure, a commonly adopted theory in plant biology8,9,10, and often used in predictive water-use modelling11,12. This indicates that nocturnal rehydration, rather than daytime hydraulic safety is an optimization priority for stomatal closure in trees13. We suggest that these processes are critical for forecasting the global carbon cycle dynamics.
Similar content being viewed by others
Main
Plants must balance water loss through stomatal pores on the leaf surface with carbon uptake via photosynthesis. In the short term—daily or seasonally—plants can reduce water loss to the atmosphere by reducing stomatal conductance (gs) to water vapour. While stomatal closure decreases transpiration during periods of low water availability, it also constrains carbon uptake1. Understanding the environmental and physiological thresholds for stomatal closure in mature trees is crucial, as forests are estimated to absorb around a fourth of the annual anthropogenic carbon emissions14. However, different tree species have been reported to reduce gs at varying environmental thresholds, creating uncertainty in predictions of global water and carbon cycles6. Explaining gs responses to low water availability is of particular importance, as global observations indicate significant variability in gs and photosynthesis responses to environmental variables3,7,15.
Stomatal closure occurs when adjacent guard cells lose turgor either through passive diffusion of water or via active metabolic pathways16. This process responds to a decrease in the internal water status of tissues, typically quantified with leaf water potential measurements (Ψleaf)5. As Ψleaf decreases, gs also decreases. A common explanation for species-specific gs responses to midday Ψleaf is that trees optimize carbon uptake against the risk of lethal hydraulic conductance loss in the xylem which occurs at low Ψleaf (refs. 2,9,17), known as the ‘safety-efficiency trade-off’. Earth system models use this principle12, and recent studies supported this theorem in juvenile trees8. These models assume that species-specific stomatal sensitivity to reduced water availability is guided by the species-specific point of loss of plant hydraulic conductivity4. However, recent observations in mature trees challenge this trade-off and suggest that maintaining turgor within growing tissues during night-time is the optimization priority for gs across temperate tree species13,18. Tree growth occurs mainly at night19 and depends on the nocturnal water status. Delayed stem rehydration after a day of transpiration due to low water availability in the soil can hamper cambial turgor recovery20. Conversely, very negative Ψleaf values, which cause lethal embolisms10, typically occur around midday. To test whether trees optimize gs primarily to avoid daytime embolisms or are more conservative and induce stomatal closure already around the point of night-time cambial turgor loss, we can compare the uniformity of pre-dawn and midday Ψleaf thresholds for stomatal closure across species. Yet, empirical evidence for such a test is lacking as long-term in situ measurements on tall trees are rare, limiting our general understanding of species-specific gs responses to diel Ψleaf dynamics.
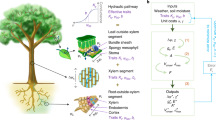
We collected unique empirical evidence to characterize the water status conditions under which mature tall trees (~20–35 m in height and 80–150 years of age; Supplementary Tables 1 and 2) of various species reduce gs, considering diel Ψleaf dynamics and growth (Fig. 1). Over 3 years of intensive canopy and stem monitoring of 95 trees from 9 common temperate tree species, we made observations across a wide range of environmental conditions (Supplementary Fig. 1). This natural climatic variability allowed us to cover a unique hydration spectrum in the target species. The hydration status of the trees ranged from well hydrated, early in the season (pre-dawn Ψleaf −0.22 to −0.69 MPa; Fig. 1b and Supplementary Table 3), to pre-dawn Ψleaf values below −1.5 MPa (Supplementary Table 3), close to the expected turgor loss points of leaves for these species (Supplementary Table 4). We observed a wide range of gs values, from fully open stomata to stomatal closure (Fig. 1a). Using these data, we tested whether stomatal closure is better explained by pre-dawn or midday Ψleaf conditions and if stomatal closure occurs under more uniform pre-dawn or midday Ψleaf conditions across species. We defined the point of stomatal closure (Pst) by analysing the relationship between stomatal conductance (gs) and leaf water potential (Ψleaf). The Pst was identified as the Ψleaf point where gs transitions from a negative exponential decrease to low values21 that followed a slight linear decline10 (Fig. 1c)22.
a, Mean species-specific midday gs measured on leaves from the top of the canopy, shown with large, coloured circles. Open circles indicate measurement dates where Ψleaf was below −1.2 MPa. Grey dots represent raw measurements. b, Pre-dawn (04:00–06:00 CET) Ψleaf, with coloured dots indicating the species mean. In 2022 an exceptional drought caused all Ψleaf to drop below −1.2 MPa (shown with open circles). c, Theoretical example of how the point of stomatal closure (Pst) was defined. Pst was assumed when the negative exponential behaviour of gs changed to a linear decrease (Supplementary Methods). Midday Ψleaf values are presented in Supplementary Fig. 3. J through D, months of January through December.
We first examined how well pre-dawn and midday Ψleaf values can explain the in situ gs measurements (Fig. 2). The pre-dawn Ψleaf showed a high explanatory power for the gs response across species (R2 = 0.58, P < 0.01), which was greater than that of midday Ψleaf (R2 = 0.41, P < 0.01). As pre-dawn Ψleaf largely reflects soil water availability dynamics, the strong relationship between pre-dawn Ψleaf and gs suggests that stomatal responses are influenced by insufficient tissue rehydration caused by drying soils, with stomata remaining closed unless the soil is adequately wet. Thus, pre-dawn Ψleaf appears to be a primary control mechanism for stomatal closure in temperate trees. Including vapour pressure deficit (VPD) in the analysis substantially increased the goodness-of-fit, particularly under wetter conditions with less-negative pre-dawn Ψleaf (R2 = 0.68, P < 0.01 in Supplementary Table 5). This suggests a secondary control mechanism or an association between pre-dawn Ψleaf and the hidden variable controlling stomatal conductance (for example, turgor in the guard cells).
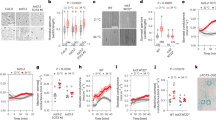
a, The relationship between normalized midday gs and pre-dawn Ψleaf for all studied species at the SCCII site. The point of stomatal closure (Pst) was quantified for each species individually. The across-species mean Pst is indicated by a bold line, with the 50th and 95th percentile ranges shown as grey shading (Supplementary Fig. 4). b, Instantaneous response conditions between midday gs and midday Ψleaf, presented as in a. The across-species mean, as well as the 50th and 95th percentile ranges for Pst, are depicted as for a. c, Growth probability and rate responses to pre-dawn Ψleaf, obtained from weekly band dendrometer readings. Zero growth rates versus non-zero growth rates were analysed to determine the growth probability using a mixed-effect model with a binomial distribution. Axes labelled (-) indicate unitless values, resulting from normalization to the maximum value. The grey shading around the line represents the 95% confidence interval.
We quantified the pre-dawn and midday Pst thresholds across species. For these thresholds, we both determined the point where gs response to Ψleaf approaches linearity (Fig. 1c) and the point where gs crosses a fixed lower gs threshold (Supplementary Fig. 4), to confirm the robustness of our Pst quantification. Importantly, the variance in the estimated point of stomatal closure (Pst) across different species was much smaller for pre-dawn than midday Ψleaf (s.d. of 0.2 versus 0.7 MPa, respectively; Fig. 2). This indicates not only that pre-dawn Ψleaf is a primary control for stomatal closure in temperate trees, but also that a uniform pre-dawn Ψleaf threshold for stomatal closure of approximately −1.2 MPa across species exists.
Our data shed light on several basic aspects of tree water relations and their control mechanisms. Pst occurred at substantially more negative Ψleaf when considering midday Ψleaf (−2.3 ± 0.7 MPa, Fig. 2b) compared to pre-dawn Ψleaf (1.2 ± 0.2 MPa, Fig. 2a), indicating that if the soil is sufficiently wet, trees allow more negative midday water potentials. Moreover, Pst coincided with the inflection point of the nonlinear relationship between pre-dawn and midday Ψleaf (ref. 23) (referred to as the hydroscape), indicating an important hydraulic threshold at which we defined the Pst (ref. 22) (Supplementary Fig. 5). Furthermore, although it is commonly assumed that Pst should occur at the point of turgor loss in the leaves (Tlp; refs. 23,24), for most species Pst approached less-negative values than the minimum Tlp for the species (Supplementary Figs. 6 and 7). Finally, Pst did not correspond to species-specific sensitivity to embolism formation (Supplementary Table 4 and Supplementary Figs. 6 and 7). We conclude that stomatal closure might only occur close to the Tlp under well-hydrated pre-dawn conditions (Supplementary Fig. 4), and the pre-dawn water status modulates commonly used gs responses to midday Ψleaf and VPD7, particularly when pre-dawn Ψleaf values are more negative (Supplementary Fig. 8).
To test whether stomatal control is optimized to facilitate growth by sustaining turgor within the cambium18, we examined whether growth rates of the monitored trees at the Swiss Canopy Crane II (SCCII) site would halt at similar pre-dawn water status conditions as stomatal closure. Weekly manual readings of stem diameters, using band dendrometers, allowed us to relate tree-specific pre-dawn Ψleaf to daily growth rates driven by xylem and bark cell production and expansion25. Our findings showed that all species reduced stem growth rates to nearly zero after reaching −1.2 MPa pre-dawn Ψleaf (Fig. 2c), which corresponds to the water status at which midday stomatal closure occurs20. Additionally, the probability of growth across species rapidly decreased after −1.2 MPa, with only a 5% probability for any stem growth to occur after −1.5 MPa (Fig. 2c, P < 0.01). Indeed, growth cessation in herbaceous plants is often observed at less-negative water potentials26 than those reported in this study. One possible explanation is that acclimation through osmotic adjustments within cambial tissues may enable growth at more negative water potentials27, although this hypothesis warrants further investigation.
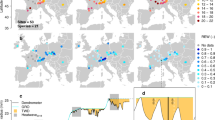
To analyse whether pre-dawn Ψleaf plays a critical role in regulating whole-canopy gs, we compared the constraints imposed by pre-dawn and midday Ψleaf on whole-tree transpiration across multiple monitoring sites in Europe (Supplementary Table 5). We confirmed a strong relationship across species, showing that a more negative water status (that is, pre-dawn Ψleaf more negative than −1.2 MPa) significantly decreased sap flux density (R2 = 0.32, P < 0.0001; Fig. 3a,b). In contrast, midday Ψleaf did not predict a consistent or significant reduction in daily maximum sap flux density (R2 = 0.01, P = 0.218; Supplementary Fig. 9). Moreover, we again found a clear change in the hydroscape behaviour (or the relationship between pre-dawn and midday Ψleaf), where a less steep decrease in midday Ψleaf with pre-dawn Ψleaf was found below −1.2 MPa (Supplementary Fig. 10), suggesting a clear shift in the whole-tree hydraulic response beyond this threshold. The importance of pre-dawn Ψleaf was also observed across four common tree genera growing in Australia along a steep precipitation gradient (278–1,705 mm of annual precipitation; Fig. 3c,d and Supplementary Table 6). Although full halt of conductance (gs < 0.05 mol m−2 s−1) was not measured in these species, we nevertheless found again a shift in their stomatal behaviour and pre-dawn Ψleaf relationship at −1.2 MPa (ref. 22) (Supplementary Fig. 10). Despite expectations of gs adjustment to drier conditions28, we found that across species gs was substantially reduced with decreasing pre-dawn Ψleaf (R2 = 0.28, P < 0.0001), particularly when it approached −1.2 MPa.
a, Pre-dawn Ψleaf measurements related to daily maximum sap flux density (expressed as a percentage of the maximum sap flux density of the tree). Species are distinguished by different colours. The line represents a significant log-transformed relationship, with species considered as a random effect to generate an across-species response curve. The grey dotted line is shown at −1.2 MPa for reference. b, Locations of the sites included in this analysis, as detailed in Supplementary Table 5. c,d, Relationship between pre-dawn Ψleaf and gs for Australian tree species (c), distinguishable by colours as presented in the location map (d).
Our data demonstrated that decreasing nocturnal water status of the entire tree plays a crucial role in inducing stomatal closure. Across natural forest sites, we found that pre-dawn Ψleaf is of primary importance for predicting gs response, often forcing stomatal closure long before the midday water status reaches Tlp of leaves (Supplementary Fig. 7). Until now the −1.2 MPa pre-dawn Ψleaf threshold was solely reported in potted juvenile trees and shrubs22,29, without considering this in the context of midday Ψleaf or whether it would be a similar threshold for mature trees. The combined results we report here clearly indicate that pre-dawn water status is critically important for stomatal control across tree species, age classes and ecosystems. Here, we do, however, need to note that we still do not fully understand the exact interplay between midday VPD and pre-dawn Ψleaf on gs (compare Supplementary Figs. 11 and 12).
Our results highlight that stomatal closure behaviour is not explained by the variance in vulnerability to xylem embolisms across species8,17. Instead, trees were observed to be conservative with their water usage, allowing stomata to remain open until the Tlp only when well hydrated in the morning (pre-dawn Ψleaf > −1.2 MPa; Supplementary Table 4). This behaviour is probably not easily detected in small trees, which are exposed to reduced water availability8 as a result of their smaller water-storage capacity, compared to tall trees, which makes the time between Tlp and the occurrence of xylem embolisms shorter. Larger trees probably benefit from greater storage capacity, which decreases the colinear behaviour between pre-dawn and midday water status20. The occurrence of stomatal closure when a mature tree stem cannot rehydrate over night can be physiologically explained, as negative pressures on the phloem hinder both sugar transport in the phloem and growth, and unconstrained water use until Tlp could cascade into ever-increasing water loss13. However, this does not diminish the importance of daytime conditions. Under well-hydrated pre-dawn conditions, mature trees appear to optimize stomatal closure according to species-specific osmotic adjustments in the leaf tissue23.
It is well-known that plant hormones such as abscisic acid (ABA) regulate stomatal closure5,16. However, the signalling pathways trees use to propagate hormonal response to a pre-dawn water status to control midday gs response—as our data suggest—remain unclear. Leaf turgor has been identified as a key sensor within the signalling pathway30, yet this does not explain why stomata close long before leaf turgor is lost (Supplementary Fig. 7). Declining turgor associated with decreasing water potential of the leaves could still be important31. Alternative signalling could come from surrounding tissues, such as the mesophyll or phloem cells17, possibly through circadian processes that make these tissues more sensitive during night-time conditions. There is experimental evidence for high foliar ABA production in 8-year-old vines when the pre-dawn water status begins to decline32. However, the exact mechanisms by which tall trees perceive their water status in other tissues, such as the cambium of the stem, and at earlier times of day, remain unknown. The greater importance of pre-dawn water status reported here could explain why some studies on smaller plants emphasize the significance of root–soil connectivity for stomatal control33, as this is the primary process affecting rehydration in plants with lower water-storage capacity.
Water potential thresholds at which mature tree species close stomata are theorized to optimize carbon assimilation versus water availability in the living tissues, affecting other physiological functions such as growth. Current investigations of these thresholds for different tree species often focus on the instantaneous, daytime cost of turgor loss in the leaf tissue8,23. However, our findings suggest that pre-dawn water status in mature trees is probably more important. This underscores the necessity for trees to conserve water during the day to avoid compromising turgor-driven growth in the following night. Moreover, the inhibition of tissue formation at relatively high water potentials, closer to our observed pre-dawn threshold of −1.2 MPa than to the threshold of hydraulic failure, supports the importance of carbon sink activity in modulating stomatal conductance26. This dynamic diel controlling mechanism is currently not considered in most gs modelling approaches, which typically simulate an instantaneous midday stomatal response (but see ref. 13). Our observed pre-dawn thresholds should thus be integrated into these models to avoid overestimating carbon assimilation at the landscape scale and beyond, particularly during drought events.
Methods
Study sites for detailed monitoring of gas exchange and sap flow
The majority of measurements were conducted in a mature mixed temperate forest at the SCCII site in Hӧlstein, Switzerland (47.439° N, 7.776° E, 500 m above sea level). Since 2018, the site has included a 50-m-tall canopy crane with a 62.5-m jib (Supplementary Fig. 2), allowing access to the canopies of over 300 trees via a manned gondola. Ecophysiological measurements were collected during 35 diurnal campaigns from 2020 until 2022, for a total of 95 trees (Supplementary Table 1). The tallest trees within the range of the crane from each of the nine species were selected as target trees. Diurnal campaigns were typically conducted from May until October, where each campaign included pre-dawn sampling before sunrise (04:00–06:00 Central European Time (CET)) and midday sampling (12:00–14:00 CET). In addition to the SCCII site, we included multiple European sites which have data on concurrent pre-dawn and midday Ψleaf combined with high-resolution sap flow measurements (Supplementary Table 6). Finally, we included a unique study along a North Australian climate gradient which collected gs and both midday and pre-dawn Ψleaf for the genera Eucalyptus, Corymbia and Acacia (Supplementary Methods).
Ecophysiological measurements and meteorological data
At the SCCII site, point measurements of gs were made from branches in the upper canopy of the target trees. We used a LI-6800 Portable Photosynthesis System (LI-COR Biosciences), where for broadleaved trees, we selected healthy, sun-exposed leaves, whereas for conifers, we chose healthy sun-exposed second-year ramets to ensure fully developed leaves throughout the growing season. In Australia, point measurements of gs were recorded using a Li-Cor 1600 Steady-State-Porometer (LI-COR).
Sap flow data collected from European forest sites (Supplementary Table 3) used thermal dissipation sap flow sensors, including either the SFS2‐M sensors (UP GmbH) or self-made sensors (for data processing see Supplementary Methods). All sensors were installed at a height of 1.5 m on the northeast side of the main tree bole, where dead bark was removed without damaging the phloem tissue. Raw measurements were obtained from either Hӧlstein or the corresponding authors of the published data (Supplementary Table 3), providing ΔT time series spanning periods where concurrent pre-dawn and midday Ψleaf measurements were performed.
At all sites, measurements of Ψleaf were performed by using a Scholander-type pressure chamber (PMS Instrument Company). All measurements were conducted immediately after sampling of sun-exposed apical branches with multiple healthy leaves attached to each one. In June and August of 2023, we sampled additional branches to generate pressure–volume curves with which we established tree-specific leaf turgor loss points (Tlp).
At the SCCII site, band dendrometers (D1 tree girth band, Meter GmbH) were mounted at breast height (1.3 m above the ground) on the stems of all target trees (Supplementary Table 1). Before mounting, the stem surface was cleaned of other vegetation and uneven parts of the outer bark. Approximately weekly readings were performed manually throughout the monitoring years to record the diameter at breast height (DBH in cm). Moreover, to confirm the accuracy of the band dendrometers (ZN11-T-WP type, Natkon), for some of the target trees point dendrometers were installed which automatically recorded radial changes in a higher precision and a temporal resolution of 10 min.
On-site air temperature (Ta in °C), relative humidity (%), solar irradiance (W m−2) and precipitation (mm) were monitored using a weather station placed in a forest gap at the SCCII site (Davis Vantage Pro 2, Scientific Sales). Additional measurements of solar irradiance (W m−2) and precipitation (mm) were collected from the top of the crane using the same climate station, where precipitation patterns were averaged over all sensors.
Data treatment and statistical analyses
The relationship between Ψleaf and gs was tested using linear mixed-effect modelling. This process involved model selection, model assumption testing, and finally, post hoc tests and model application. Absolute gs values vary between species and can introduce issues when performing analyses that include multiple species. For this reason, we normalized our gs data to the maximum species-specific gs values. We performed a log transformation on both the gs measurements and VPD (measured by the LI-6800) to better describe the nonlinear behaviour and avoid violating normality assumptions for the residuals. Tree number was included as a random effect, while species was added as a fixed effect.
To determine the point of stomatal closure (Pst) due to Ψleaf, we used the linear mixed-effect model to predict the linear part of the data after stomatal closure (at more negative Ψleaf values), where VPD conditions are set to high values (VPD = 2.4 kPa; Supplementary Fig. 5). When the data points approach the 95% confidence interval of this projection under high VPD conditions, we define this as the Pst as the behaviour becomes statistically indistinguishable from the approached linear decrease (schematically shown in Fig. 1c). To generalize the behaviour of all data, we used a generalized additive mixed-effect model (GAMM) function. The intercept between the 95% confidence interval and the GAMM model was used to represent the point when stomatal behaviour reaches an inflection point, transitioning from active closure to approaching gs reduction due to loss of conductivity to soil water reservoirs. Moreover, we tested the robustness of the Pst by using the fitted GAMM to quantify the Ψleaf point at which gs was <0.05 mol m−2 s−1, a common stomatal closure threshold used in literature21 (Supplementary Fig. 4). We modelled the stomatal response to pre-dawn Ψleaf for both sap flow-derived stomatal conductance and gs measurements from Australia using a linear mixed-effects model with log-transformed dependent and independent variables, treating species and site as random effects.
Growth rates were calculated by determining the difference in DBH between each monitoring session and dividing this by the number of days between measurements. We applied the zero-growth concept to the DBH data before calculating the radius growth rates, to ensure that no negative growth will be included in the analyses (due to drought induced shrinkage). For the analyses we only considered June, July and August, to avoid the inclusion of growth halt due to winter dormancy of the cambium. A generalized linear mixed-effect model with a binomial distribution was applied to assess the probability of growth occurrence across species, using species and tree nested in species as random effects. See Supplementary Fig. 13 for the results from the daily point dendrometer measurements. More detailed information on the statistical methods is provided in the Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data are available in the main text and Supplementary Information. The gas exchange, sap flow, leaf water potential and growth data used in this study are available via Zenodo at https://doi.org/10.5281/zenodo.14852038 (ref. 34). Source data are provided with this paper.
References
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003).
Franklin, O., Fransson, P., Hofhansl, F., Jansen, S. & Joshi, J. Optimal balancing of xylem efficiency and safety explains plant vulnerability to drought. Ecol. Lett. 26, 1485–1496 (2023).
Liang, X. et al. Stomatal responses of terrestrial plants to global change. Nat. Commun. 14, 2188 (2023).
Anderegg, W. R. L. et al. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl Acad. Sci. USA 113, 5024–5029 (2016).
Buckley, T. N. How do stomata respond to water status? New Phytol. 224, 21–36 (2019).
Wolz, K. J., Wertin, T. M., Abordo, M., Wang, D. & Leakey, A. D. B. Diversity in stomatal function is integral to modelling plant carbon and water fluxes. Nat. Ecol. Evol. 1, 1292–1298 (2017).
Novick, K. A. et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Change 6, 1023–1027 (2016).
Joshi, J. et al. Towards a unified theory of plant photosynthesis and hydraulics. Nat. Plants 8, 1304–1316 (2022).
Henry, C. et al. A stomatal safety–efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 10, 3398 (2019).
Arend, M. et al. Rapid hydraulic collapse as cause of drought-induced mortality in conifers. Proc. Natl Acad. Sci. USA 118, e2025251118 (2021).
Wolf, A., Anderegg, W. R. L. & Pacala, S. W. Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proc. Natl Acad. Sci. USA 113, E7222–E7230 (2016).
Kennedy, D. et al. Implementing plant hydraulics in the Community Land Model, Version 5. J. Adv. Model. Earth Syst. 11, 485–513 (2019).
Potkay, A. & Feng, X. Do stomata optimize turgor-driven growth? A new framework for integrating stomata response with whole-plant hydraulics and carbon balance. New Phytol. 238, 506–528 (2023).
Friedlingstein, P. et al. Global Carbon Budget 2023. Earth Syst. Sci. Data 15, 5301–5369 (2023).
Stocker, B. D. et al. Drought impacts on terrestrial primary production underestimated by satellite monitoring. Nat. Geosci. 12, 264–270 (2019).
Brodribb, T. J. & McAdam, S. A. M. Passive origins of stomatal control in vascular plants. Science 331, 582–585 (2011).
Choat, B. et al. Triggers of tree mortality under drought. Nature 558, 531–539 (2018).
Peters, R. L. et al. Daytime stomatal regulation in mature temperate trees prioritizes stem rehydration at night. New Phytol. 239, 553–546 (2023).
Zweifel, R. et al. Why trees grow at night. New Phytol. 231, 2174–2185 (2021).
Peters, R. L. et al. Turgor—a limiting factor for radial growth in mature conifers along an elevational gradient. New Phytol. 229, 213–229 (2021).
Xu, G. Q., Farrell, C. & Arndt, S. K. Climate of origin has no influence on drought adaptive traits and the drought responses of a widely distributed polymorphic shrub. Tree Physiol. 42, 86–98 (2022).
Knipfer, T. et al. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 184, 881–894 (2020).
Hartmann, H., Link, R. M. & Schuldt, B. A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiol. 41, 901–905 (2021).
Bartlett, M. K., Scoffoni, C. & Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol. Lett. 15, 393–405 (2012).
Hilty, J., Muller, B., Pantin, F. & Leuzinger, S. Plant growth: the what, the how, and the why. New Phytol. 232, 25–41 (2021).
Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 25, 107–114 (2015).
Matthews, M. A., Van Volkenburgh, E. & Boyer, J. S. Acclimation of leaf growth to low water potentials in sunflower. Plant Cell Environ. 7, 199–206 (1984).
Steger, D. N. et al. Site matters—canopy conductance regulation in mature temperate trees diverges at two sites with contrasting soil water availability. Agric. For. Meteorol. 345, 109850 (2024).
Arend, M., Brem, A., Kuster, T. M. & Günthardt-Goerg, M. S. Seasonal photosynthetic responses of European oaks to drought and elevated daytime temperature. Plant Biol. 15, 169–176 (2013).
Huber, A. E., Melcher, P. J., Piñeros, M. A., Setter, T. L. & Bauerle, T. L. Signal coordination before, during and after stomatal closure in response to drought stress. New Phytol. 224, 675–688 (2019).
Rodriguez-Dominguez, C. M. et al. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ. 39, 2014–2026 (2016).
Tombesi, S. et al. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 5, 12449 (2015).
Carminati, A. & Javaux, M. Soil rather than xylem vulnerability controls stomatal response to drought. Trends Plant Sci. 25, 868–880 (2020).
Peters, R. L. et al. Data from: Uniform regulation of stomatal closure across temperate tree species to sustain nocturnal turgor and growth [Data set]. Zenodo https://doi.org/10.5281/zenodo.14852038 (2025).
Acknowledgements
We thank A. Kühne, L. Rizzelli and N. ten Cate for crane operation and D.N. Steger and D. Basler for support in the field. We are also grateful to N. Zosso, A. Fos, J. Weibel and Y. Rudin for performing the band dendrometer readings. We thank C. Körner for discussion on the initial results. Funds for this research came from the University of Basel and the Swiss Federal Office for the Environment (FOEN). C.Z. received funding from the Swiss National Science Foundation (SNSF, project nr. 31003A_182538 to G.H.).
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Contributions
R.L.P. and A.K. conceived the study and wrote the first draft of the manuscript. M.A. conceived the experimental design to perform pre-dawn and midday measurements and organized the first 2 years of data collection, including the band dendrometer readings. R.L.P., M.A., G.H., C.Z. and T.Z. collected the data at the SCCII site. Other data included in the manuscript were generated by A.K., S.K.A., L.A.C. and R.P. R.L.P. performed the data analysis and prepared the figures. All authors discussed the manuscript and supported the writing process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Samuel Cordeiro Vitor Martins and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–14 and Tables 1–6.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peters, R.L., Arend, M., Zahnd, C. et al. Uniform regulation of stomatal closure across temperate tree species to sustain nocturnal turgor and growth. Nat. Plants 11, 725–730 (2025). https://doi.org/10.1038/s41477-025-01957-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-01957-3