Abstract
Our understanding of the emergence of mountain floras rests on our ability to infer how orogeny, landscape dynamics and climate change altered their evolutionary trajectories. Here we reconstruct the assembly of the diverse sky-island flora of the European Alps and test the impact of key geo-climatic events. We use a dated 5,231-species phylogeny, including 96% of the sky-island flora. The assembly of this flora occurred through the colonization of over a thousand distinct lineages, of which 46% speciated from their lowland or non-Alpine ancestor and 6% underwent in situ cladogenesis. The young ages of extant sky-island lineages show that their accumulation was decoupled from ancient geo-climatic events but accelerated throughout the Plio-Pleistocene. The sky-island vegetation therefore assembled through recent lineage turnover, which was triggered, rather than impeded, by Pleistocene glacial intensification. This perspective challenges previous assumptions and highlights the complex interplay of geo-climatic factors in shaping the intricate tapestry of alpine floras.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data needed to replicate the findings of this study are available on figshare at https://doi.org/10.6084/m9.figshare.25459135 (ref. 80). All raw reads data generated by the PhyloAlps and PhyloNorway projects were submitted to the EMBL-EBI Short Read Archive under the following bioprojects: PhyloAlps (PRJEB30497, PRJEB48874, PRJEB50489, PRJEB82787), PhyloNorway (PRJEB43865, PRJEB48693, https://www.ebi.ac.uk/ena/data/view/PRJEB50550). An online database of genome skimming of Arctic–Alpine plants, representing all information regarding samples, extraction, sequencing and reconstructed genomic data for the PhyloAlps and PhyloNorway projects can be found at https://phyloalps.osug.fr/main/home (ref. 81). Source data are provided with this paper.
References
Hoorn, C. et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 (2010).
Feijó, A. et al. Mammalian diversification bursts and biotic turnovers are synchronous with cenozoic geoclimatic events in asia. Proc. Natl Acad. Sci. USA 119, e2207845119 (2022).
Antonelli, A. et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 11, 718–725 (2018).
Sklenár, P., Hedberg, I. & Cleef, A. M. Island biogeography of tropical alpine floras. J. Biogeogr. 41, 287–297 (2014).
McCormack, J. E., Huang, H. & Knowles, L. L. in Encyclopedia of Islands (eds Gillespie, R. G. & Clague, D.) 839–843 (Univ. Chicago Press, 2009).
Ebersbach, J., Schnitzler, J., Favre, A. & Muellner-Riehl, A. Evolutionary radiations in the species-rich mountain genus Saxifraga L. BMC Evol. Biol. 17, 119 (2017).
Ding, W.-N., Ree, R. H., Spicer, R. A. & Xing, Y.-W. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 369, 578–581 (2020).
Boschman, L. M. & Condamine, F. L. Mountain radiations are not only rapid and recent: ancient diversification of South American frog and lizard families related to Paleogene Andean orogeny and Cenozoic climate variations. Glob. Planet. Change 208, 103704 (2022).
Sedano, R. E. & Burns, K. J. Are the Northern Andes a species pump for neotropical birds? Phylogenetics and biogeography of a clade of Neotropical tanagers (Aves: Thraupini). J. Biogeogr. 37, 325–343 (2010).
Lagomarsino, L. P., Condamine, F. L., Antonelli, A., Mulch, A. & Davis, C. C. The abiotic and biotic drivers of rapid diversification in Andean bellflowers (Campanulaceae). New Phytol. 210, 1430–1442 (2016).
Kandziora, M. et al. The enigmatic tropical alpine flora on the african sky islands is young, disturbed, and unsaturated. Proc. Natl Acad. Sci. USA 119, e2112737119 (2022).
Nevado, B., Contreras-Ortiz, N., Hughes, C. & Filatov, D. A. Pleistocene glacial cycles drive isolation, gene flow and speciation in the high-elevation Andes. New Phytol. 219, 779–793 (2018).
Zhang, J. et al. Evolutionary history of the Arctic flora. Nat. Commun. 14, 4021 (2023).
Vasconcelos, T. N. C. et al. Fast diversification through a mosaic of evolutionary histories characterizes the endemic flora of ancient neotropical mountains. Proc. R. Soc. B Biol. Sci. 287, 20192933 (2020).
Kadereit, J. W. The role of in situ species diversification for the evolution of high vascular plant species diversity in the European Alps—a review and interpretation of phylogenetic studies of the endemic flora of the Alps. Perspect. Plant Ecol. Evol. Syst. 26, 28–38 (2017).
Ball, J. On the origin of the flora of the European Alps. Proc. R. Geogr. Soc. 1, 564–589 (1879).
Ozenda, P. La végétation de la chai^ne alpine dans l’espace montagnard européen (Masson, 1985).
Ozenda, P. On the genesis of the plant population in the Alps: new or critical aspects. C. R. Biol. 332, 1092–1103 (2009).
Kadereit, J. W., Licht, W. & Uhink, C. H. Asian relationships of the flora of the European Alps. Plant Ecol. Divers. 1, 171–179 (2008).
Favre, A. et al. Out of Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae). J. Biogeogr. 43, 1967–1978 (2016).
Comes, H. P. & Kadereit, J. W. Spatial and temporal patterns in the evolution of the flora of the European Alpine system. TAXON 52, 451–462 (2003).
Hughes, C. E. & Atchison, G. W. The ubiquity of alpine plant radiations: from the Andes to the Hengduan mountains. New Phytol. 207, 275–282 (2015).
Boucher, F. C., Zimmermann, N. E. & Conti, E. Allopatric speciation with little niche divergence is common among Alpine Primulaceae. J. Biogeogr. 43, 591–602 (2016).
Smyčka, J. et al. Tempo and drivers of plant diversification in the European mountain system. Nat. Commun. 13, 2750 (2022).
Fauquette, S. et al. Quantifying the Eocene to Pleistocene topographic evolution of the southwestern Alps, France and Italy. Earth Planet. Sci. Lett. 412, 220–234 (2015).
Gradwohl, G., Stüwe, K., ROBL, J. & Liebl, M. in Geodynamics of the Alps Vol. 1 (Rosenberg, C. L. & Bellahsen, N.) 115–160 (ISTE, 2023).
Krsnik, E. et al. Miocene high elevation in the Central Alps. Solid Earth 12, 2615–2631 (2021).
Fox, M., Herman, F., Willett, S. D. & Schmid, S. M. The exhumation history of the European Alps inferred from linear inversion of thermochronometric data. Am. J. Sci. 316, 505–541 (2016).
Herwegh, M. et al. Late stages of continent–continent collision: timing, kinematic evolution, and exhumation of the Northern rim (Aar Massif) of the Alps. Earth Sci. Rev 200, 102959 (2020).
Winterberg, S. & Willett, S. D. Greater Alpine river network evolution, interpretations based on novel drainage analysis. Swiss J. Geosci. 112, 3–22 (2019).
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Favre, A. et al. The role of the uplift of the Qinghai Tibetan Plateau for the evolution of Tibetan biotas. Biol. Rev. 90, 236–253 (2015).
Hagen, O. et al. Mountain building, climate cooling and the richness of cold-adapted plants in the Northern Hemisphere. J. Biogeogr. 46, 1792–1807 (2019).
Sternai, P. et al. Present-day uplift of the European Alps: evaluating mechanisms and models of their relative contributions. Earth Sci. Rev. 190, 589–604 (2019).
Valla, P. G., Sternai, P. & Fox, M. How climate, uplift and erosion shaped the Alpine topography. Elements 17, 41–46 (2021).
Winkworth, R. Evolution of the New Zealand mountain flora: origins, diversification and dispersal. Org. Divers. Evol. 5, 237–247 (2005).
Flantua, S. G., O’Dea, A., Onstein, R. E., Giraldo, C. & Hooghiemstra, H. The flickering connectivity system of the North Andean páramos. J. Biogeogr. 46, 1808–1825 (2019).
Schonswetter, P., Stehlik, I., Holderegger, R. & Tribsch, A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol. Ecol. 14, 3547–3555 (2005).
Alvarez, N. et al. History or ecology? Substrate type as a major driver of spatial genetic structure in Alpine plants. Ecol. Lett. 12, 632–640 (2009).
Thiel-Egenter, C. et al. Break zones in the distributions of alleles and species in alpine plants: break zones in allele and species distributions. J. Biogeogr. 38, 772–782 (2011).
Taberlet, P. et al. Genetic diversity in widespread species is not congruent with species richness in Alpine plant communities. Ecol. Lett. 15, 1439–1448 (2012).
Paun, O., Schönswetter, P., Winkler, M., Consortium, I. & Tribsch, A. Historical divergence vs. contemporary gene flow: evolutionary history of the calcicole Ranunculus alpestris group (Ranunculaceae) in the European Alps and the Carpathians. Mol. Ecol. 17, 4263–4275 (2008).
Zhang, L., Comes, H. P. & Kadereit, J. W. The temporal course of quaternary diversification in the European high mountain endemic Primula sect. Auricula (Primulaceae). Int. J. Plant Sci. 165, 191–207 (2004).
Parisod, C. Plant speciation in the face of recurrent climate changes in the Alps. Alp. Bot. 132, 21–28 (2022).
Boucher, F. C. et al. Discovery of cryptic plant diversity on the rooftops of the Alps. Sci. Rep. 11, 11128 (2021).
Alsos, I. G. et al. The treasure vault can be opened: large-scale genome skimming works well using herbarium and silica gel dried material. Plants 9, 432 (2020).
Wang, Y. et al. Late quaternary dynamics of Arctic biota from ancient environmental genomics. Nature 600, 86–92 (2021).
Lavergne, S. et al. Towards a comprehensive barcoding and phylogenomic reference for the European Arctic–alpine flora. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-6136147/v1 (2025).
Aeschimann, D., Lauber, K., Moser, D. M. & Theurillat, J.-P. Flora Alpina Vols 1–3 (Haupt, 2004).
Smith, S. A. & O’Meara, B. C. treePL: divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 28, 2689–2690 (2012).
Ramírez-Barahona, S., Sauquet, H. & Magallón, S. The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 4, 1232–1238 (2020).
Hörandl, E. & Emadzade, K. The evolution and biogeography of alpine species in Ranunculus (Ranunculaceae): a global comparison. TAXON 60, 415–426 (2011).
Wagner, N. D., He, L. & Hörandl, E. The evolutionary history, diversity, and ecology of willows (Salix L.) in the European Alps. Diversity 13, 146 (2021).
Carruthers, T. et al. Repeated upslope biome shifts in Saxifraga during Late-Cenozoic climate cooling. Nat. Commun. 15, 1100 (2024).
Valente, L. M., Phillimore, A. B. & Etienne, R. S. Equilibrium and non equilibrium dynamics simultaneously operate in the Galápagos Islands. Ecol. Lett. 18, 844–852 (2015).
Mosbrugger, V., Favre, A., Muellner-Riehl, A. N., Päckert, M. & Mulch, A. in Mountains, Climate and Biodiversity (eds Hoorn, C. et al.) Ch. 28 (Wiley-Blackwell, 2018).
Muellner Riehl, A. N. et al. Origins of global mountain plant biodiversity: testing the ‘mountain geobiodiversity hypothesis’. J. Biogeogr. 46, 2826–2838 (2019).
MacArthur, R. H. & Wilson, E. O. The Theory of Island Biogeography (Princeton Univ. Press, 1967).
Legrain, N., Stüwe, K. & Wölfler, A. Incised relict landscapes in the Eastern Alps. Geomorphology 221, 124–138 (2014).
Muttoni, G. et al. Onset of major Pleistocene glaciations in the Alps. Geology 31, 989–992 (2003).
Steinbauer, M. J. et al. Topography-driven isolation, speciation and a global increase of endemism with elevation: topographic isolation and endemism. Glob. Ecol. Biogeogr. 25, 1097–1107 (2016).
Aguilée, R., Claessen, D. & Lambert, A. Adaptive radiation driven by the interplay of eco-evolutionary and landscape dynamics. Evolution 67, 1291–1306 (2013).
Thomas, A. et al. Multiple origins of mountain biodiversity in New Zealand’s largest plant radiation. J. Biogeogr. 50, 947–960 (2023).
Dullinger, S. et al. Post-glacial migration lag restricts range filling of plants in the European Alps: range filling of alpine plants. Glob. Ecol. Biogeogr. 21, 829–840 (2012).
Janzen, D. H. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 (1967).
Qian, H. & Sandel, B. Phylogenetic structure of regional angiosperm assemblages across latitudinal and climatic gradients in North America. Glob. Ecol. Biogeogr. 26, 1258–1269 (2017).
Gehrke, B. Staying cool: preadaptation to temperate climates required for colonising tropical alpine-like environments. PhytoKeys 96, 111–125 (2018).
Qian, H., Ricklefs, R. E. & Thuiller, W. Evolutionary assembly of flowering plants into sky islands. Nat. Ecol. Evol. 5, 640–646 (2021).
Pouchon, C. et al. Orthoskim: in silico sequence capture from genomic and transcriptomic libraries for phylogenomic and barcoding applications. Mol. Ecol. Resour. 22, 2018–2037 (2022).
Katoh, K. & Standley, D. M. MAFFTmultiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimal: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Minh, B. Q. et al. Iq-tree 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Li, H.-T. et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 5, 461–470 (2019).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
Salisbury, B. A. & Kim, J. Ancestral state estimation and taxon sampling density. Syst. Biol. 50, 557–564 (2001).
Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems 2nd edn (Springer, 2011).
Westerhold, T. et al. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 369, 1383–1387 (2020).
Lindeløv, J. K. mcp: an R package for regression with multiple change points. OSF Preprints https://doi.org/10.31219/osf.io/fzqxv (2020).
Vehtari, A., Gelman, A. & Gabry, J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat. Comput. 27, 1413–1432 (2017).
Wootton, L. M. et al. The late rise of sky-island vegetation in the European Alps: supplementary data. figshare https://doi.org/10.6084/m9.figshare.25459135 (2025).
PhyloAps & PhyloNorway. A Genome Skim Database for the Arctic-Alpine Flora (Arctic-Alpine PhyloSkims, accessed 1 February 2024); https://phyloalps.osug.fr/main/home
Acknowledgements
This paper is dedicated to the memory of Prof. Serge Aubert (1966–2015) who devoted his career to the study of alpine plants and was an invaluable source of motivation for the PhyloAlps project. This work was supported by the French National Research Agency in the framework of the ‘Investissements d’avenir’ program (ANR-15-IDEX-02). The research was funded by the joint ANR-SNF project Origin-Alps (ANR-16-CE93-0004, SNF-310030L_170059, S.L. and N.E.Z.) and the OSUG@2020 labex (ANR10 LABX56, S.L.). The sequencing was performed within the framework of the PhyloAlps project, funded by France Génomique (ANR-10-INBS-09-08, P.W.), and the PhyloNorway project funded by the Research Council of Norway (226134/F50, I.G.A.) and the Norwegian Biodiversity Information Centre (14-14, 70184209, I.G.A.). Bioinformatics and statistical analyses were carried out with the GRICAD infrastructure (https://gricad.univ-grenoble-alpes.fr). The sampling campaign and preliminary genomic analyses were partly funded by the European Research Council under the European Community’s Seventh Framework Programme FP7/2007-2013 grant agreement 281422 (TEEMBIO, W.T.) and by SNF grant 31003A_149508 (N.E.Z.). M.R. was supported by the Polish National Science Centre project no. NCN 2020/37/B/NZ8/03307. Some samples were also provided by G. Coldea, J. Sibik, R. Geremia, D. Pozarova, T. Figura, B. Offerhaus, M. Pires, Y. Morvant, G. Pellet, A. Jacotot, C. Chambrey, A. Gérard, A. Masswadeh, C. Bornand, F. Hoffer-Mallard, J. Détraz-Méroz, M. Leibundgut, M. Jutzi, P. Juillerat, S. Birrer, L. Filipaş, A. Coste, D. Suteu, A. Plecenikova, A. Guttova, J. Kucera, E. Stubnova, J. Kochjarova, I. Hodalova, S. Spaniel, R. Letz, S. Wróbel, J. Pilatova, M. Kolarova, M. Smyckova, H. Marx, R. Quesada, A. R. Frattaroli, L. Bartha, G. Sramko, V. A. Molnar, N. Passalacqua, J. L. Desrayaud, R. Giordano, B. Pont, M. Frei, F. Ojeda, J. Engel, O. Tostain, P. Anastasiu, G. Casazza, A. Hilpold, P. Koutecký, G. Schneeweiss and P. Schönswetter. Sample materials were kindly contributed from museum collections of the Jaca Herbarium, National Herbarium of Venezuela, University of Alaska Fairbanks Museum, University of Idaho Museum, Oslo Museum of Natural History, Royal Botanic Garden of Edinburgh, and the Berlin Museum of Natural History. We thank R. Ree, J. (A.) de Leeuw and H. Morlon for discussions during earlier stages of the project.
Author information
Authors and Affiliations
Consortia
Contributions
L.M.W., F.C.B., C. Pouchon, C.R., E.C., I.G.A., P.G.V., L.H., M.B., C. Perrier, R.D., M.R., J.-G.V., N.E.Z., P.W., W.T., J.R. and S.L. conceived and designed the experimental/analytical approach. C.R., A.A., F.D., P.W., S.L., PhyloAlps Consortium and PhyloNorway Consortium performed the experiments/conducted fieldwork. L.M.W., F.C.B., C. Pouchon, E.C., A.A., F.D., P.W., J.R. and S.L. analysed the data. C. Pouchon, C.R., E.C., I.G.A., C. Perrier, R.D., M.R., J.-G.V., N.E.Z., S.L., PhyloAlps Consortium and PhyloNorway Consortium contributed materials/analysis tools. L.M.W., F.C.B., W.T. and S.L. wrote the paper with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
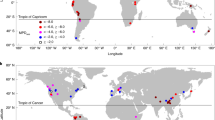
Extended Data Fig. 1 Map of the PhyloAlps and PhyloNorway sampling.
A geographically-constrained map of the PhyloAlps and PhyloNorway sampling effort. While sampling was focused on the Alps and the Arctic, the data set contains many samples from other European mountain system ranges, such as the Pyrenees, Carpathians, Balkans and Apennines. To visualise the full data set, and explore the data further, please visit the PhyloAlps website (https://phyloalps.osug.fr/).
Extended Data Fig. 2 A schematic of the decision-making process for defining colonisation, single speciation and cladogenetic speciation.
Examples of the decision process for defining assembly processes. For each clade we combined two ancestral state reconstructions, shown here on two adjacent trees: one of elevational distribution (red and blue tip states) and the other of geographic distribution (yellow and green tip states). The ancestral state reconstructions were run on 35 Myr time slices of the phylogeny, with the state of stem node forced to be either low-elevation or non-EAS as relevant. a. A colonisation event is defined as the arrival of a lineage in the Alpine sky-islands. We consider that a sky-island species has originated via colonisation if its most recent common ancestor (MRCA) occurred at low elevations or in non-EAS regions, and its current distribution includes non-EAS regions, as illustrated by species D. b. We define a single speciation event as the generation of a new species due to a lineage splitting across the boundaries of the Alpine sky-islands. Species A, B, and D are all considered single-speciation species. Species A originated via single speciation as it has speciated across both elevational and geographic boundaries, its MRCA occurring at low elevations in non-EAS regions. Species B split across elevational boundaries, likely speciating during a transition from lower elevation zones to sky-islands within the EAS. Conversely, species D had a high-elevation ancestor, but likely underwent geographic divergence after arriving from a non-EAS mountain range. c. We define a cladogenetic speciation event as lineage splitting within the boundaries of the Alpine sky-island. In the left-hand example, species B, C, and D are considered a sky-island group that originated through cladogenetic speciation, as their MRCAs are all reconstructed to occur in the sky-islands and the EAS. In this scenario, species B is assumed to have originated in the sky-island and since extended its range to include non-EAS regions. Species E is not considered part of the group, as it does not occur in the sky-islands. Although species E has sky-island and EAS ancestors, its non-sky-island distribution suggests that speciation occurred across the treeline rather than within the sky-island boundaries. Species A is not included in the group as it does not have sky-island ancestry. In the right-hand example, all five species have sky-island ancestry. Non-EAS ancestry is reconstructed at the starred node however, suggesting that at this node lineage splitting did not occur in a sky-island context. Therefore, we circumscribe two groups, the first containing species A and B, and the second containing species D and E. Species C is then considered a single speciation event.
Extended Data Fig. 3 A worked example for defining assembly processes.
A worked example for defining assembly processes within Viola. The coloured squares on the tips represent the current elevational and geographic states for each species. The pie charts on the internal nodes display the likelihoods of each state based on ancestral state reconstructions. All species that occur in the sky-islands (denoted by red squares) are considered sky-island species. Endemism status (green and yellow squares) allow us to differentiate between species that have assembled in the sky-islands through colonisation or through single speciation. Blue text on tip labels represent colonist species (for example V. palustris). Colonists are not endemic to the EAS (green squares) and their ancestors occurred at low elevations or in non-EAS regions. Orange text on tip labels represent species that originated via single speciation (for example V. thomasiana). Single-speciation species are endemic to the EAS (yellow squares) and their ancestors occurred at low elevations or in non-EAS regions, but they are not (yet) part of an EASI clade. Red tip labels represent cladogenetic species (for example V. cenisia). Cladogenetic species are endemic to the EAS (yellow squares) and their ancestors occurred at high-elevations and were EAS endemics. The size of a sky-island clade is determined by the node depth to which high-elevation and EAS endemism is reconstructed. In this example, all nodes between the tips and the starred node are reconstructed as high-elevation EAS endemics, and therefore all species below the starred node (with the exceptions of V. declinata and V. dacica) are considered to be part of a single clade that diversified in situ. V. declinata and V. dacica do not occur in the sky-islands, and thus are not considered part of the sky-island clade. The speciation events leading to their formation are not used in our calculation of cladogenetic rate. All other branching events within the clade are, however, used in the calculation of cladogenetic rate.
Extended Data Fig. 4 Distribution of the ages of the fossils used to calibrate the dating analysis.
Distribution of the ages of fossils used to calibrate the dating analysis. In total, 130 fossils were used, spanning 45 different orders and 96 different families. All fossils were sourced from the database of [51].
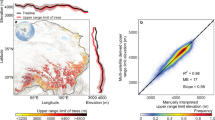
Extended Data Fig. 5 Comparison of node ages recovered in the dating analysis of this study to those sourced from previously published phylogenies.
Comparison of selected node ages (n = 29) recovered in the dating analysis of this study to those sourced from dated phylogenies across 19 different articles from the literature search described in the section 5.3 of the methods. Each article is represented by 1-5 points in the figure. The dashed grey line represents a 1:1 reference line.
Extended Data Fig. 6 EASI colonization events per genus.
Number of times each sky-island genus has independently entered the sky-islands.
Extended Data Fig. 7 Distribution of clade sizes in the EASI flora.
Distribution of clade sizes in the EASI flora, demonstrating that cladogenetic speciation in the sky-islands has occurred predominately in small clades of 2-3 species.
Extended Data Fig. 8 Examples of EASI plant species.
Examples of Alpine plant species occurring in the sky-islands which originated through colonisation (left column, top to bottom: Allium oleraceum, Primula farinosa, Eleocharis quinqueflora, Lilium martagon, Laserpitium latifolium), single speciation (centre column, top to bottom: Artemisia glacialis, Potentilla delphinensis, Trifolium alpinum, Myosotis alpestris, Soldanella alpina), and cladogenesis (right column, top to bottom: Scabiosa lucida, Homogyne alpina, Phyteuma scheuchzeri, Festuca alpina, Rhinanthus glacialis). All photographs copyright Jardin du Lautaret.
Supplementary information
Supplementary Information
Supplementary Tables 1–6, Fig. 1, Extended Methods, Fossil information and Biogeographic literature search references.
Source data
Source Data Fig. 2
Dated phylogeny.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wootton, L.M., Boucher, F.C., Pouchon, C. et al. The late rise of sky-island vegetation in the European Alps. Nat. Plants 11, 1142–1153 (2025). https://doi.org/10.1038/s41477-025-02001-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02001-0