Abstract
This study assesses the safety and efficacy of metformin administration in older adult burn patients, a rapidly growing demographic with substantially poorer outcomes. This is a single-centre cohort study of older adults (≥60 years) admitted to a provincial burn center over 15 years. Clinical outcomes, laboratory measures, inflammatory markers, and adipose tissue single-nuclei RNA sequencing (SnRNA-seq) were compared among metformin-treated and non-treated controls. A total of 50 metformin-treated and 262 control older burn patients met the eligibility criteria. Despite pre-admission comorbidities, metformin-treated patients showed improved survival, no significant differences in the number of hypoglycemic episodes, a lower incidence of lactic acidosis, and reduced circulating levels of organ damage markers. SnRNA-Seq further revealed that metformin may exert its beneficial effects by local restoration of immune and inflammatory responses. In older burn patients, metformin was linked with improved outcomes and no adverse effects, underscoring its safety and efficacy in this population.
Similar content being viewed by others
Introduction
Since its introduction by Greet van den Berghe in 2001, the concept of glucose control has emerged as an important adjunct in the modern-day care of severely burned patients1,2. Indeed, stress-induced diabetes with insulin resistance and hyperglycemia is a hallmark of severely burned patients associated with many adverse outcomes after injury, such as increased infections and sepsis, increased incidence of pneumonia, profoundly increased catabolism and hypermetabolism, and most importantly, an overall increase in post-burn mortality3,4. Clinically, the gold standard to treat insulin resistance and hyperglycemia in burn patients has been insulin therapy, with over 73% of American Burn Association (ABA) verified burn centers now employing intensive insulin practices to maintain glucose levels within the upper limit target range of 5–8 mmol/L (90–140 mg/dL)1,5,6. While tight glycemic control has been shown in various studies to be beneficial for burn patients, it quickly became apparent that insulin is a therapy with inherent risks—namely, hypoglycemia, which not only negates the beneficial effects of insulin, but even worse, is independently associated with increased risk of death, cardiovascular death, and death due to infections7,8,9,10,11,12. Consequently, new alternative treatments have subsequently been introduced.
Metformin is a long-established hypoglycemic agent that is not commonly used in the burn field because of the potential risks of lactic acidosis13,14. This is based on the fact that burn patients inherently have high lactate levels and experience significant acidosis throughout the acute hospitalization phase, particularly the first 3 days after injury15,16. However, a randomized controlled trial in 2015 demonstrated that metformin can be a safe and effective agent to control hyperglycemia in burn patients without causing hypoglycemia or any lactic acidosis17. One of the most critical findings of this trial was that metformin also had additional beneficial effects on insulin resistance, fat metabolism, and inflammatory responses after injury when compared with insulin controls17. Despite strong clinical evidence supporting the safety and utility of metformin in burns, little is known about the benefits in older burned patients—a rapidly growing demographic with significantly poorer outcomes18. Notably, older adults have a greater incidence of pre-existing diabetes, and studies have shown that, even immediately after a burn, older patients are more hyperglycemic with profoundly increased insulin resistance, organ dysfunction, morbidity, and mortality after injury19,20. Interestingly, there is strong evidence that metformin, in addition to its effects on glucose metabolism, also exerts protective effects on longevity and health span by modulating metabolic and cellular processes associated with biological aging, including inflammation, mitochondrial dysfunction, and cellular senescence21,22. Thus, we suggest that metformin can be translated to the geriatric burn population with great potential. However, there is still hesitation in using metformin in older burn patients due to previous FDA recommendations regarding safety and renal concerns, although these guidelines have since been updated after a review of scientific literature, with the FDA concluding that metformin is safe for patients with mild to moderate kidney impairment23.
Therefore, in this study, we conducted a large-scale investigation to assess the safety and efficacy of metformin administration in a clinical cohort of older adult burn patients. We hypothesized that metformin is safe to use in the older adult burn population and does not cause any drug toxicity-related adverse effects on survival, i.e., hypoglycemia, lactic acidosis, worsening of renal function, etc.
Results
Patient characteristics and outcomes
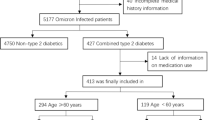
Between 2006 and 2021, 738 older adult patients were admitted and assessed for eligibility. A total of 312 older burn patients met the eligibility criteria for this study and were included in the final analysis (Supplementary Fig. 1). Out of this population, 50 (16%) patients received metformin during their stay in the burn unit, whereas the remaining 262 (84%) did not (controls). Demographics, injury characteristics, and pre-admission comorbidities are summarized in Table 1. Notably, there were no significant differences in age or sex between the two groups. Likewise, burn size, Revised Baux Score, and incidence of inhalation injury were similar between the control and metformin groups. In terms of pre-admission comorbidities, however, we found that the number of patients with hypertension was significantly lower in the control group compared to the metformin group (p < 0.001). Similarly, a greater proportion of metformin-treated patients had pre-existing diabetes when compared to controls (p < 0.001). Given the high prevalence of diabetes in the metformin group, which could potentially skew the demographic characteristics and outcomes, we compared the diabetic and non-diabetic patients within this group (Supplementary Table 1). The only notable differences were that diabetic patients had a slightly lower median Revised Baux Score (p < 0.05) and a higher prevalence of hypertension (p < 0.01), the latter of which is expected given its common association with diabetes.
In terms of clinical outcome data, we found that hospital LOS (length of stay) and LOS/TBSA (total body surface area) were similar between metformin and control patients (Table 1). Additionally, there were no significant differences between the two groups in the incidence of various in-hospital complications, including acute kidney injury (AKI) (p = 0.48), sepsis (p = 0.70), pneumonia (p = 1.0), and wound infection (p = 0.16) (Fig. 1A). However, the incidence of graft failure was significantly higher in the metformin group than that of controls (25 (9.5%) vs 11 (22.0%), p < 0.05), likely due to the fact a greater proportion of metformin-treated patients had pre-existing diabetes.
A Incidence of acute kidney injury (AKI), sepsis, pneumonia, graft loss, and wound infection of older burn patients in the metformin and control groups. B Kaplan-Meier curve demonstrating improved survival among older burn patients in the metformin group relative to controls at 40 days post-injury. Values are presented as mean ± standard error. *p < 0.05.
Remarkably, despite the increased incidence of graft loss and pre-admission comorbidities, both of which are typically associated with poor outcomes after burn injury, older adult burn patients who received metformin showed significantly increased probability of survival at 40 days post-injury when compared to those who did not (96% vs. 74%, p < 0.05) (Fig. 1B), suggesting metformin administration is not only safe in older burn patients but may also be associated with improved survival after injury. Intriguingly, adjusting for TBSA%, using the Cox proportional hazards model, had minimal impact on the hazard ratio for metformin (adjusted HR = 0.15, 95% CI = 0.02–1.07, p = 0.059 vs. unadjusted HR = 0.13, 95% CI = 0.02–0.93, p = 0.043), suggesting that that the survival benefit associated with metformin treatment is not primarily driven by differences in burn severity between groups.
Metabolic profile: glucose, lactate, and pH
There were no significant differences in the number of hypoglycemic episodes between the metformin and control groups (Table 1). Glucose levels were significantly higher among the older burn patients who received metformin when compared to those who did not at 1- (7.4 ± 0.1 vs 9.0 ± 0.5, p < 0.01) and 2-weeks (7.1 ± 0.1 vs 8.2 ± 0.4, p < 0.05) post-injury, presumably due to increased incidence of pre-existing diabetes (Supplementary Fig. 2). Interestingly, however, there were significant differences when comparing the incidence of lactic acidosis between groups (Table 1). While lactic acidosis occurred in 25 (10%) patients in the control group, none (0%) of the metformin patients showed signs of lactic acidosis (p < 0.05). This was further demonstrated by no differences in blood lactate and pH at 1- (p = 0.54), 2- (p = 0.78), 3- (p = 0.25) and 4- (p = 0.58) weeks post-injury (Supplementary Fig. 2B, C). Together, these results support our hypothesis that metformin use is safe in older burn patients and does not cause any adverse effects on survival, namely hypoglycemia and metabolic lactic acidosis.
Organ function biomarkers
In addition to metformin-associated lactic acidosis (MALA), another classic side effect of metformin is exacerbation of renal failure in cases of prerenal failure24,25. To assess the effect of metformin administration on organ function in older burn patients, we next compared circulating levels of organ biomarkers between groups (Fig. 2). Despite no changes in blood urea nitrogen (BUN) and alanine aminotransferase (ALT) levels, we found that metformin-treated patients have significantly decreased troponin T (153.8 ± 56.7 vs 34.4 ± 8.2, p < 0.05) at 2 weeks post-injury, significantly decreased creatine kinase (CK) at 1- (374.4 ± 78.8 vs 157.1 ± 49.4, p < 0.05) and 2- (141.7 ± 24.6 vs 73.8 ± 13.4, p < 0.05) weeks post-injury, significantly decreased creatinine (89.5 ± 6.5 vs 67.8 ± 6.2, p < 0.05) at 3-weeks post-injury, and significantly decreased bilirubin levels at 2- (13.5 ± 2.7 vs 5.8 ± 1.4, p < 0.05) and 3- (9.7 ± 2.1 vs 4.7 ± 0.9, p < 0.05) weeks post-injury when compared to controls, collectively indicating an unexpected protective effect of metformin on organ function in older adults after injury.
Inflammatory and immune responses
To gain further insight into the mechanisms underlying metformin’s beneficial effects on survival and organ function in older adult burn patients, we compared inflammatory profiles and immune cell responses between groups. In the cytokine subgroup, the metformin and control groups showed no significant differences in demographic characteristics or clinical outcomes, except a higher prevalence of diabetes in the metformin group (12% vs 61%; P < 0.001), which reflects the distribution observed in the overall population (Supplementary Table 2). No significant differences were observed in circulating levels of various cytokines and chemokines at several time points during hospitalization, with a notable exception of granulocyte-macrophage colony-stimulating factor (GM-CSF), which was significantly decreased in metformin patients only at 0–3 days post-injury (35.2 ± 4.1 vs 21.9 ± 4.8, p < 0.05) (Supplementary Fig. 3). Surprisingly, however, single-nuclei RNA (SnRNA) profiling of excised subcutaneous adipose tissue (sWAT), or site of injury, taken from control and treated older burn patients revealed a unique and significant shift in immune cell-specific populations and inflammatory profiles with metformin administration at the tissue level (Supplementary Table 3).
As illustrated in Fig. 3A, B, initial integrated clustering was manually annotated based on the nuclei expression pattern of signature genes consistent with macrophages (MRC1), endothelial cells (VWF), adipose progenitor cells (APCs; PDGFRA), T cells (CD247), adipocytes (PLIN1), lymphatic endothelial cells (MMRN1), smooth muscles cells (SMCs; ACTA2), mesenchymal stem cells (MScs; KIT), and fibroblast-like cells (HMGA2). We first discovered that the sWAT of the metformin-treated patient had a greater proportion of T cells and macrophages than the control, indicative of an enhanced immune response (Fig. 3C). Indeed, we found that the percent expression of genes associated with an M2-like anti-inflammatory macrophage phenotype (RPBJ, CD163, MRC1, CD86, IL-10) and, to a lesser extent, an M1-like proinflammatory macrophage phenotype (NFKB1, EREG, COL4A1, IL1B, IL-6) were increased in the metformin-treated sample (Fig. 3D). These data are in line with previous work from our lab indicating that burn injuries provoke an increase in macrophage migration and polarization to sWAT, and suggest that metformin can restore these immune responses in older adults26,27. In fact, these findings were further supported by pathway analysis of upregulated genes, which further revealed remarkable enrichment in several immune-related pathways, including leukocyte migration, phagocytosis, leukocyte chemotaxis and differentiation, among other immune effector processes (Fig. 3E, F). Interestingly, the production and regulation pathways for the IL-6 cytokine, a key mediator of post-burn immune and metabolic responses, was also significantly enriched in the sWAT of the metformin group despite no difference in serum IL-6 levels (Fig. 3F; Supplementary Fig. 3B). Collectively, these findings suggest that metformin can reverse post-burn immune senescence in older adults and enhance inflammatory responses at the tissue level, which are diminished with age following injury, thereby potentially improving patient outcomes.
A UMAP plot of nuclei sub-populations combined from metformin-treated and control older adult burn patient subcutaneous white adipose tissues. B UMAP plots of signature genes used for classifying cell populations as adipose progenitor cells (PDGFRA), smooth muscle cells (ACTA2), adipocytes (PLIN1), T cells (CD247), macrophages (MRC1), endothelial cells (VWF), lymphatic endothelial cells (MMRN1), fibroblast-like cells (HMGA2) and mesenchymal stem cells (MSCs; KIT). C Proportion of cell populations between metformin-treated and non-treated control (D) Dot plot of the expression of genes related to a proinflammatory/M1-like phenotype (PPARG, NFKB1, EREG, COL1A1, IL1B, IL-6) or an anti-inflammatory/M2-like phenotype (RBJ, CD163, MRC1, CD86, IL-10) in the macrophage sub-population between metformin-treated and control older adult burn patients (E) Volcano plots showing differential gene expression in metformin-treated and control older adult burn patients, with (F) respective biological pathway gene ontology over-representation analyses (GO-BP ORA). n = 1 patient per condition.
Discussion
Older adult burn patients continue to have an unacceptably high mortality rate that has not changed over the last three decades19. This is due to a lack of understanding as to why older adults have profoundly increased morbidity and mortality and, consequently, lack of effective interventions. Emerging evidence suggests that insulin resistance and hyperglycemia are critical clinical problems underlying poor outcomes in older adult burn patients19. To that effect, metformin is an attractive agent to use in older adults due to a low risk of hypoglycemia, a serious side effect of insulin treatment associated with a 9-fold increase in mortality in burn patients, as well as its recently uncovered anti-aging properties1,7,17,28. Despite its therapeutic potential, however, the safety of metformin administration in older adult burn patients has been questioned because of a perceived risk of life-threatening lactic acidosis, though recent studies have not supported this concern17. Indeed, the possibility of MALA is the primary concern with metformin use, particularily in older adults and patients with compromised liver and kidney function14,29,30. Therefore, the present study aimed to address the safety and outcomes associated with metformin administration in a large clinical cohort of older adult burn patients.
We investigated whether older burn patients who received metformin suffered any adverse events or detrimental effects that could be documented. Surprisingly, we found no evidence of hypoglycemia or metabolic lactic acidosis in older adult burn patients receiving metformin. On the contrary, we found the incidence of lactic acidosis was significantly lower in older burn patients treated with metformin relative to the controls. The probability of survival was also drastically higher in older adult burn patients who received metformin at 40 days post-injury when compared to those who did not, despite having a higher incidence of pre-existing diabetes and hypertension. These remarkable clinical findings might seem contradictory in the context of studies that have associated comorbid conditions with adversely affecting post-burn outcomes31,32. However, it is important to note that there is growing evidence that metformin can prolong healthspan and lifespan. For instance, one study demonstrated that metformin increased healthy lifespan by 4–6% in different mouse breeds21. Mortality benefits associated with metformin have also been described in a multitude of clinical studies, such as those supported by the UK Clinical Practice Research Datalink service, reporting that diabetic patients treated with metformin live longer than matched non-diabetic controls, even though the diabetic patients were more obese and had greater co-morbidities at baseline33.
Although the precise mechanisms underlying metformin’s protective effects in older burn patients are currently unclear and await further clarification, a recent study showed that increased mortality in older adults following burn injury is associated with an increased incidence of multi-organ failure when compared to adults19. Notably, multi-organ dysfunction (MODS), typically involving hepatic, renal, and cardiovascular dysfunction, is a common and detrimental post-brun complication in patients with severe burns, with several reports showing 27–45% of burn patients develop MODS, which increased mortality rates from 29% to 86%34,35,36,37,38,39. Interestingly, we previously demonstrated that metformin treatment improves hepatic organ dysfunction in aged mice subjected to thermal injury by alleviating age-related mitochondrial dysfunction through AMP-activated protein kinase (AMPK) activation40. In this regard, one can postulate that the significant improvement in survival and lower incidence of lactic acidosis observed in the metformin group in this study may be attributed to its ability to improve organ function, which enhances metabolic efficiency and reduces lactate production and/or accumulation, thereby mitigating the risk of complications such as lactic acidosis. In direct support of this notion, we show here that systematic levels of several organ damage markers, including troponin T, creatine kinase, creatinine, and bilirubin, were significantly reduced in metformin-treated older burn patients at various time points after injury.
While we did not directly assess the effect of metformin on metabolic responses and mitochondrial bioenergetics in this study, we did observe notable effects on post-burn inflammatory and immune responses, which are severely impaired in older adults after injury, predisposing them to a greater risk of mortality and morbidity41,42,43,44. For example, one study showed that, in contrast to adult burn patients who exhibit a hyperinflammatory response during the acute phase post-burn (first 96 hours), older adults are hypo-inflammatory with markedly lower levels of inflammatory markers, including IL-6, MCP-1, MCP-3, and G-CSF41. Another genome-wide study in older adult and adult burn patients further demonstrated that older adult patients express a downregulation of several immune-related pathways, ubiquitination, and proteasome degradation42. Fascinatingly, metformin administration appeared to rescue and restore aging-impaired immune responses and inflammatory profiles at the tissue level, as demonstrated by our SnRNA seq data, which revealed an increase in the proportion of various immune populations, including T cells and macrophages, in adipose tissue from the metformin group relative to control, indicative of immunostimulatory effects. Additionally, in accordance with metformin’s well-established anti-inflammatory properties, we also observed a shift in the polarization of macrophages towards a predominately M2 phenotype, which was accompanied by a slight decrease in circulating levels of GM-CSF, a cytokine responsible for the polarization of monocytes to M1 macrophages17,45,46. These findings are consistent with previous reports highlighting metformin’s therapeutic effects on inflammatory and autoimmune diseases, virus infection, aging, and cancers by improving immune responses and reducing inflammation, mainly through promoting the formation of M2 macrophages and T regulatory and CD8+ memory T cells via AMPK47.
We further found that the production and regulation pathways for IL-6 were enriched in the sWAT of metformin-treated patients relative to controls, suggesting a partial restoration in inflammatory responses at the tissue level. Of note, both M2 macrophages and IL-6 have been implicated as key immunological regulators of post-burn white adipose tissue browning, a major hallmark of the hypermetabolic response to burn trauma recently discovered to be impaired in older adult burn patients27. In fact, this failure of the browning response was associated with reduced whole-body metabolism and decreased survival compared to younger counterparts after injury27. Mechanistically, the adipose of both aged patients and aged mice after burn trauma showed impairments in M2 macrophage infiltration and IL-627. Thus, given that these aging-impaired immune and inflammatory factors were restored in older burn patients treated with metformin, our findings suggest that metformin may also enhance metabolic function and rescue key post-burn hypermetabolic responses, ultimately improving outcomes in older adults after injury. However, this remains to be further explored.
That said, we were quite surprised by the lack of a systemic inflammatory response. Despite local immune modulation in adipose tissue, the absence of significant systemic cytokine alterations may be due to several factors. Indeed, as previously noted, burn injuries disrupt the systemic inflammatory response, and when combined with the heightened pro-inflammatory state associated with inflammaging in older adults, this may obscure the more nuanced systemic immunomodulatory effects of metformin19,48. Moreover, the effects of metformin may be more pronounced at the site of injury, where increased blood flow and cellular recruitment occur as part of the body’s attempt to promote wound healing. This localized environment creates a unique metabolic and immune niche that may amplify metformin’s effects, particularly within adipose tissue, which plays an active role in both inflammation and tissue repair49,50. The close interplay between adipose tissue’s immunometabolic activity and cutaneous repair processes may enhance metformin’s local efficacy, even without observable systemic changes. However, it is also possible that the lack of systemic changes is partially due to limited sample sizes. Future studies should incorporate larger cohorts, protein-level validation, and longitudinal cytokine profiling to better elucidate the mechanistic underpinnings of metformin’s tissue-specific immune modulation and its minimal impact at the systemic level.
In summary, in this large clinical cohort of 312 older adult burn patients, metformin administration was associated with no episodes of lactic acidosis and several beneficial effects, including improved organ function, immune responses, and survival, despite a higher incidence of pre-existing comorbidities. As the first intervention shown to improve survival in older adult burn patients, we surmise metformin administration is not only safe but may also serve as a potentially powerful adjunct therapy to improve the adverse outcomes of this highly vulnerable demographic. Nevertheless, while the current study highlights several important findings, we would like to point out that the observational design precludes causal inference. To fully indicate its scientific validity, a larger randomized clinical trial of metformin administration in older adult burn patients is required. Moreover, we conducted SnRNA-seq analysis on a highly limited number of adipose tissue samples (n = 1 per group), which represents a major limitation of this study. While this approach provides valuable insights into cell-type-specific transcriptomic changes, the findings should be interpreted with caution due to the lack of biological replicates. Future studies are needed to validate these observations using complementary techniques, such as bulk RNA-seq or qPCR for gene expression confirmation, as well as protein quantification assays to assess translational relevance. Additionally, functional assays will be crucial to determine whether the observed transcriptomic alterations translate into meaningful changes in adipose tissue biology. We also acknowledge that a limitation of the study is the absence of data on metformin dosage, duration of use, and HbA1c measurements, which limits our ability to perform a dose-response analysis and fully assess glucose regulation in our study groups.
Finally, it is currently unclear why the metformin group had a higher graft loss incidence than controls. A possible explanation could be a higher incidence of diabetes in the metformin group, as diabetic burn patients are more prone to delayed and impaired wound healing, graft loss, and infection following injury51,52. However, metformin has been shown to have both beneficial and detrimental effects on wound healing, depending on the context. While metformin has been shown to improve angiogenesis, reduce oxidative stress, and enhance tissue repair in some contexts, it has also been associated with impaired fibroblast function and delayed wound closure in diabetic models53,54,55. Therefore, it will also be necessary for future studies to determine whether metformin is protective or detrimental to wound healing and graft survival in older adult burn patients and the diverse mechanisms underlying its effects. Additionally, graft location is a critical but unaccounted factor in this analysis. Lower extremity grafts, for example, are known to have higher failure rates due to compromised vascular supply, mechanical stress, and greater susceptibility to infection56. Without detailed anatomical data, it remains uncertain whether graft placement contributed to the observed differences.
Notwithstanding these limitations, many of the beneficial effects linked to metformin use in older adults reported in this study are not only observed in burns but have also been reported in a multitude of aging-related diseases, such as degenerative skeletal diseases, cardiovascular diseases, neurodegenerative diseases, obesity, and related-metabolic abnormalities57. As such, not only are these findings pertinent to older adult burn patients, but may also be translated to other age-related conditions, including hypermetabolic conditions like cancer, diabetes mellitus, frailty, and other traumatic injuries, all of which have also proposed a promising perspective for metformin in aging-related clinical applications28. With a rapidly aging global population, our efforts to transform care and outcomes for older adults are visionary and necessary to reduce the healthcare burden in the imminent future.
Materials and methods
Study design, participants and approval
This single-centre retrospective cohort study involved 312 burn patients admitted to the Ross Tilley Burn Centre at Sunnybrook Health Sciences Centre between 2006–2021. Approval for this study was obtained from the Research Ethics Board of Sunnybrook Health Sciences Centre (#307-2015; #194-2010) and by the Hamilton Integrated Research Ethics Board (#15833; #16705).
Subjects for this study were selected based on meeting the following criteria: patients ≥60 years of age; >5% total body surface area (TBSA) burn; admitted to our burn unit within 120 hours (5 days) following injury. Older burn patients were excluded if they died within 4 days of admission, had previous existing renal failure or severe liver disease, had contraindications to metformin, or received propranolol during their admission. Eligible patients were then divided into two groups based on whether they received metformin administration during their acute hospital stay. Patients might have received metformin for several reasons, including clinical indications due to acute hyperglycemia, continuation of a pre-existing prescription upon admission, or as part of a study protocol. Written informed consent was received from all participants for prospective blood and tissue collection before study inclusion.
Clinical outcomes including mortality
Demographics and clinical outcomes were prospectively recorded by the burn team, including the attending burn and critical care staff, and were entered into a database. Outcomes included mortality during hospitalization, infections, pneumonia, and septic episodes as defined by the ABA guidelines58. These include demographic data, burn size, presence or absence of inhalation injury, pre-admission morbidities, and in-hospital complications. Length of stay (LOS) was defined as the days elapsed between admission and discharge from the burn unit. The Revised Baux Score was calculated for all patients as described by Osler et al.59.
Metabolic and organ biomarkers
Blood glucose, lactate, and pH levels were continuously measured during hospitalization. Hypoglycemia was defined as blood glucose below the normal range (4.0–8.0 mmol/L). Patients with at least 1 glucose measure recorded <4.0 mmol/L during hospitalization were classified as having a hypoglycemic event. Patients with at least 1 incidence of serum lactate concentrations >4 mmol/L with an accompanying blood pH <7.35 were classified as having lactic acidosis (metabolic acidosis due to an accumulation of lactic acid in the blood).
Using standard laboratory assays, organ biomarkers were measured twice weekly until discharge, and more frequently when organ dysfunction was suspected. These markers include troponin T for cardiac function; creatine kinase (CK), creatinine, and blood urea nitrogen (BUN) for renal function; and bilirubin and alanine aminotransferase (ALT) for hepatic function.
Inflammatory profile
The inflammatory cytokine data was collected from a representative subgroup of the overall population (n = 57 control; n = 18 metformin). Circulating cytokines and chemokines were measured in the serum of burn patients, both those treated with metformin and those without, and were collected at various timepoints throughout hospitalization. Cytokine profiling of select mediators was determined with the Milliplex MAP human cytokine/chemokine/growth factor panel A (cat#HCYTA-60K) using the Luminex 200 instrument (EMD Millipore). The cytokines analyzed in this study are as follows: Interleukin-1α (IL-1α), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin-15 (IL-15), Interleukin-1β (IL-1β), Tumor necrosis factor-α (TNF-α), Macrophage chemoattractant protein-1 (MCP-1), Interferon-γ (IFN-γ) and Granulocyte-macrophage colony-stimulating factor (GM-CSF).
Isolation of nuclei and single-nuclei RNA sequencing (SnRNA-seq) analysis on frozen subcutaneous white adipose tissue (sWAT) from burn patients with and without metformin treatment.
sWAT was collected from burn patients as discarded tissue during surgeries. To isolate nuclei, frozen adipose tissues were cut into 1-2 mm3 pieces over dry ice and transferred into lysis buffer (1 M sucrose, 1 M CaCl2, 1 M Mg(Ac)2. 1 M Tris-HCl, 0.1% Triton X-100, 0.5 M EDTA, 40 U/mL RNase Inhibitor and H2O) for 5 min while homogenizing tissues over ice using glass douncers. The nuclei quality was assessed under a microscope using SYBR green (ThermoFisher Scientific #S7564). Samples were then centrifuged at 800 g for 10 min and washed with 2 mL of wash buffer (1X PBS, 10% BSA, 0.2U/uL RNase Inhibitor) three times before suspending in 1 mL of wash buffer and filtering nuclei through a 40 uM Flowmi cell strainer (Sigma-Aldrich #BAH136800040). Nuclei were stained with 7AAD and sorted by FACS with a BD cell sorter for 7-AAD positive nuclei to exclude any debris or nuclei aggregates. Finally, nuclei were centrifuged at 800 g for 10 min, resuspended in cold wash buffer, counted, and immediately processed with the 10X Genomics platform following the Chromium Single Cell 3′ v3.1 kit protocol. A library was generated and sequenced on an Illumina NovaSeq 6000 with a sequencing depth of 50,000 reads per nuclei. Reads were aligned to the GRCh38-2020-A human transcriptome reference and nuclei were de-multiplexed, filtered for background noise, and counted using 10X CellRanger version 6.1.2.
The filtered and preprocessed data was uploaded to and analyzed using the Seurat V5 package in R for quality control and downstream analysis. Cells were then filtered in a custom pipeline based on gene expression (>200 and <5000 features) and mitochondrial content (<5%). Next, the dataset was normalized and scaled. Unsupervised cell clustering was performed using the FindNeighbors and FindClusters functions at a resolution of 0.5 and based on 20 principal components (PC). The Harmony package was used for batch correction and dataset integration to account for the heterogeneity between datasets from different patients. Cell-type annotations were assigned through gene marker analysis, where cell-type signature genes were identified and annotated for the clusters. Moreover, the cellular composition within different cell types was evaluated to assess variation among different conditions. ClusterProfiler package in R was used to conduct the gene ontology over-representation analyses (GO-BP ORA) of upregulated genes. The volcano plot was generated using the EnhancedVolcano package in R to show differentially expressed genes (DEG) between conditions. The expression of specific genes was visualized using the DotPlot function in Seurat to compare between conditions.
Statistical analysis
Data are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement60. Continuous variables were analyzed using the Mann–Whitney U-test. Fisher’s exact test was used to analyze categorical data. All tests were 2-tailed, with a P-value of <0.05 considered statistically significant. Analyses were performed using SPSS Statistics version 29.0 (IBM Corporation, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC). Figures were generated using GraphPad Prism version 10 (GraphPad Software Incorporated, San Diego, CA).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Jeschke, M. G. et al. Intensive insulin therapy in severely burned pediatric patients. Am. J. Respir. Crit. Care Med. 182, 351–359 (2010).
Van den Berghe, G. et al. Intensive insulin therapy in critically Ill patients. N. Engl. J. Med. 345, 1359–1367 (2001).
Gore, D. C. et al. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit. Care Med. 30, 2438–2442 (2002).
Gore, D. C. et al. Association of hyperglycemia with increased mortality after severe burn injury. J. Trauma 51, 540–544 (2001).
Jeschke, M. G. et al. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann. Surg. 252, 521–7 (2010).
Mann, E. A. et al. The impact of intensive insulin protocols and restrictive blood transfusion strategies on glucose measurement in American burn association (ABA) verified burn centers. J. Burn Care Res. 29, 718–723 (2008).
Jeschke, M. G., Pinto, R., Herndon, D. N., Finnerty, C. C. & Kraft, R. Hypoglycemia is associated with increased post-burn morbidity and mortality in pediatric patients. Crit. Care Med. 42, 1221–1231 (2014).
Rehou, S., Mason, S., Burnett, M. & Jeschke, M. G. Burned adults develop profound glucose intolerance. Crit. Care Med. 44, 1059–1066 (2016).
Cochran, A., Davis, L., Morris, S. E. & Saffle, J. R. Safety and efficacy of an intensive insulin protocol in a burn-trauma intensive care unit. J. Burn Care Res. 29, 187–191 (2008).
Pham, T. N. et al. Impact of tight glycemic control in severely burned children. J. Trauma 59, 1148–1154 (2005).
Jeschke, M. G., Klein, D. & Herndon, D. N. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann. Surg. 239, 553–560 (2004).
Klein, D., Schubert, T., Horch, R. E., Jauch, K.-W. & Jeschke, M. G. Insulin treatment improves hepatic morphology and function through modulation of hepatic signals after severe trauma. Ann. Surg. 240, 340–349 (2004).
Blough, B., Moreland, A. & Mora, A. Metformin-induced lactic acidosis with emphasis on the anion gap. Bayl. Univ. Med. Cent. Proc. 28, 31–33 (2015).
DeFronzo, R., Fleming, G. A., Chen, K. & Bicsak, T. A. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metab. Clin. Exp. 65, 20–29 (2016).
Cochran, A., Edelman, L. S., Saffle, J. R. & Morris, S. E. The relationship of serum lactate and base deficit in burn patients to mortality. J. Burn Care Res. 28, 231–240 (2007).
Mokline, A. et al. Lactate: prognostic biomarker in severely burned patients. Ann. Burns Fire Disasters 30, 35–38 (2017).
Jeschke, M. G., Abdullahi, A., Burnett, M., Rehou, S. & Stanojcic, M. Glucose control in severely burned patients using metformin. Ann. Surg. 264, 518–527 (2016).
Pham, T. N. et al. Epidemiology and outcomes of older adults with burn injury: an analysis of the national burn repository. J. Burn Care Res. 30, 30–36 (2009).
Jeschke, M. G. et al. Pathophysiologic Response to Burns in the Elderly. EBioMedicine 2, 1536–1548 (2015).
Dolp, R., Rehou, S., Pinto, R., Trister, R. & Jeschke, M. G. The effect of diabetes on burn patients: a retrospective cohort study. Crit. Care 23, 28 (2019).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Anisimov, V. N. Metformin: Do we finally have an anti-aging drug?. Cell Cycle 12, 3483–3489 (2013).
Research, C. for D. E. and. FDA Drug Safety Communication. FDA Revises Warnings Regarding Use Of The Diabetes Medicine Metformin In Certain Patients With Reduced Kidney Function. https://www.modahealth.com/medical/drug_warnings/2016/metformin.shtml?fat_select=ID (2025).
Peters, N. et al. Metformin-associated lactic acidosis in an intensive care unit. Crit. Care 12, R149 (2008).
Hung, S.-C. et al. Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diab. Endocrinol. 3, 605–614 (2015).
Abdullahi, A. et al. Alternatively activated macrophages drive browning of white adipose tissue in burns. Ann. Surg. 269, 554–563 (2019).
Abdullahi, A. et al. Adipose browning response to burn trauma is impaired with aging. JCI Insight 6, e143451 (2021).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Seidowsky, A., Nseir, S., Houdret, N. & Fourrier, F. Metformin-associated lactic acidosis: a prognostic and therapeutic study. Crit. Care Med. 37, 2191–2196 (2009).
Gosmanova, E. O., Shahzad, S. R., Sumida, K., Kovesdy, C. P. & Gosmanov, A. R. Metformin is associated with increase in lactate level in elderly patients with type 2 diabetes and CKD stage 3: a case-control study. J. Diab. Complicat. 34, 107474 (2020).
Lundgren, R. S. et al. Influence of comorbidities and age on outcome following burn injury in older adults. J. Burn Care Res. 30, 307–314 (2009).
Thombs, B. D., Singh, V. A., Halonen, J., Diallo, A. & Milner, S. M. The effects of preexisting medical comorbidities on mortality and length of hospital stay in acute burn injury: evidence from a national sample of 31,338 adult patients. Ann. Surg. 245, 629 (2007).
Bannister, C. A. et al. Can people with type 2 diabetes live longer than those without? a comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diab. Obes. Metab. 16, 1165–1173 (2014).
Feng, J.-Y. et al. Predictors of early onset multiple organ dysfunction in major burn patients with ventilator support: experience from a mass casualty explosion. Sci. Rep. 8, 10939 (2018).
Cumming, J., Purdue, G. F., Hunt, J. L. & O’Keefe, G. E. Objective estimates of the incidence and consequences of multiple organ dysfunction and sepsis after burn trauma. J. Trauma 50, 510–515 (2001).
Chen, J. et al. Characteristics of burn deaths from 2003 to 2009 in a burn center: a retrospective study. Burns Trauma 1, 80–86 (2013).
Saffle, J. R., Sullivan, J. J., Tuohig, G. M. & Larson, C. M. Multiple organ failure in patients with thermal injury. Crit. Care Med. 21, 1673 (1993).
Kallinen, O., Maisniemi, K., Böhling, T., Tukiainen, E. & Koljonen, V. Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 33, 206–211 (2012).
Khalaf, F. et al. Decoding burn trauma: biomarkers for early diagnosis of burn-induced pathologies. Biomark. Res. 12, 160 (2024).
Auger, C. et al. Metformin adapts its cellular effects to bioenergetic status in a model of metabolic dysfunction. Sci. Rep. 8, 5646 (2018).
Rehou, S., Shahrokhi, S., Thai, J., Stanojcic, M. & Jeschke, M. G. Acute phase response in critically ill elderly burn patients. Crit. Care Med. 47, 201–209 (2019).
Dreckmann, S. C., Amini-Nik, S., Tompkins, R. G., Vojvodic, M. & Jeschke, M. G. Genome-wide comparisons of gene expression in adult versus elderly burn patients. PLoS One 14, e0226425 (2019).
Khalaf, F., Ricciuti, Z., Barayan, D., Wojtowicz-Piotrowski, S. & Jeschke, M. G. Post-burn endocrine-immune dynamics and ageing considerations. Nat. Rev. Endocrinol. https://doi.org/10.1038/s41574-024-01018-3 (2024).
Khalaf, F., Barayan, D., Saldanha, S. & Jeschke, M. G. Metabolaging: a new geroscience perspective linking aging pathologies and metabolic dysfunction. Metab. Clin. Exp. 166, 156158 (2025).
Nassif, R. M. et al. Metformin inhibits ROS production by human M2 macrophages via the activation of AMPK. Biomedicines 10, 319 (2022).
Nagdy, B., Kassem, H. A., Abdel-Ghaffar, A.-R. B., Seoudi, D. M. & Kassem, N. M. The clinicopathological impact of granulocyte-macrophage colony-stimulating factor gene expression and different molecular prognostic biomarkers in egyptian acute myeloid leukemia patients. Asian Pac. J. Cancer Prev. 21, 1993–2001 (2020).
Schuiveling, M., Vazirpanah, N., Radstake, T. R. D. J., Zimmermann, M. & Broen, J. C. A. Metformin, a new era for an old drug in the treatment of immune mediated disease?. Curr. Drug Targets 19, 945–959 (2018).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590 (2018).
Cai, J. et al. The browning and mobilization of subcutaneous white adipose tissue supports efficient skin repair. Cell Metab. 36, 1287–1301.e7 (2024).
Shook, B. A. et al. Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell 26, 880–895.e6 (2020).
Tebby, J. et al. Outcomes of polytrauma patients with diabetes mellitus. BMC Med. 12, 111 (2014).
Brem, H. & Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 117, 1219–1222 (2007).
Gillespie, Z. E. et al. Metformin induces the AP-1 transcription factor network in normal dermal fibroblasts. Sci. Rep. 9, 5369 (2019).
Ochoa-Gonzalez, F. et al. Metformin induces cell cycle arrest, reduced proliferation, wound healing impairment in vivo and is associated to clinical outcomes in diabetic foot ulcer patients. PLoS ONE 11, e0150900 (2016).
Han, X. et al. Metformin accelerates wound healing in type 2 diabetic db/db mice. Mol. Med. Rep. 16, 8691–8698 (2017).
Reddy, S. et al. The incidence and risk factors for lower limb skin graft failure. Dermatol. Res. Pr. 2014, 582080 (2014).
Padki, M. M. & Stambler, I. Targeting aging with metformin (TAME). In Encyclopedia of Gerontology and Population Aging (eds. Gu, D. & Dupre, M. E.) 4908–4910 (Springer International Publishing, Cham, 2021).
The American Burn Association Consensus Conference on Burn Sepsis and Infection Group et al. American Burn association consensus conference to define sepsis and infection in burns. J. Burn Care Res. 28, 776–790 (2007).
Osler, T., Glance, L. G. & Hosmer, D. W. Simplified estimates of the probability of death after burn injuries: extending and updating the baux score. J. Trauma 68, 690–697 (2010).
von Elm, E. et al. Burn associatiothe strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studiesn consensus conference to define sepsis and infection in burns. Lancet 370, 1453–1457 (2007).
Acknowledgements
This work was supported by the Juravinski Research Institute and grants from the National Institutes of Health (R01AG080040-01A1; 1R01GM133961-01A1). DB is a recipient of the Frederick Banting and Charles Best Canada Graduate Scholarship (Canadian Institutes of Health Research—Institute of Aging #181563). FK and DJT are recipients of the Canada Graduate Scholarship—Master’s (Canadian Institutes of Health Research). AA is a recipient and supported by the Banting Postdoctoral Fellowships program through the Canadian Institutes of Health Research. The authors would like to thank all staff and patients at the Ross Tilley Burn Centre at Sunnybrook Health Sciences Centre.
Author information
Authors and Affiliations
Contributions
D.B., S.R., and M.G.J. conceived the project and conducted the study design. F.K. prepared and performed experiments, analyzed data, and made figures. D.J.T. collected clinical data, coordinated sample acquisition, and made figures. P.B. and G.P. analyzed experimental and clinical data. All authors were involved in interpreting the data, drafting the article, and revising it critically for content, and all authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclosure Statement
The authors have nothing to disclose
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Barayan, D., Khalaf, F., Rehou, S. et al. Metformin administration improves adverse outcomes in older adult burn patients: a single-centre cohort study. npj Aging 11, 43 (2025). https://doi.org/10.1038/s41514-025-00224-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41514-025-00224-1
This article is cited by
-
Beyond diabetes: harnessing the power of metformin in burn care
Critical Care (2025)