Abstract
The intestinal microbiota (IM) plays a role in the severity of alcohol-associated liver disease. Modifying severe alcohol-associated hepatitis (AH) dysbiosis improves liver injury through tryptophan (Trp) metabolites and the aryl hydrocarbon receptor (AhR). However, Trp’s effect on the IM in alcohol use disorder (AUD) patients remains unclear. Here, we used an in vitro microbiota modeling system named Minibioreactor arrays (MBRAs). Feces from AUD patients with or without AH were treated with low, normal, or high Trp concentrations, with subsequent treatment with alcohol. Microbiota composition and AhR activity were studied. We showed that microbial communities from donors were maintained in MBRAs. High and low Trp increased the abundance of pathogen Escherichia Shigella. In the absence of alcohol, Trp changed more bacteria in AUD IM compared to AH IM. Normal Trp increased the AhR activity. Overall, maintaining normal Trp levels may prevent dysbiosis in AUD or AH, pending in vivo confirmation.
Similar content being viewed by others
Introduction
Alcohol-associated liver disease (ALD) is a broad term encompassing a spectrum of liver pathologies that result from excessive and chronic alcohol intake, named alcohol use disorder (AUD). In ALD patients, liver injury progresses from steatosis, inflammation (hepatitis), fibrosis, cirrhosis, and its complications, including liver cancer1. The therapeutic options in both AUD and ALD, notably to avoid liver disease progression, are limited and mainly depend on alcohol withdrawal, which is often unsuccessful in the majority of patients2,3. The disease progression and the severity of lesions are tightly linked to the gut-liver axis. This includes alterations in the composition and function of the gut microbiota and a gut barrier dysfunction characterized by a disruption of the homeostasis between intestinal epithelial cells, mucosal immune cells, and IM, inducing an increase in gut permeability (leaky gut)4,5,6. Both the sensitivity to and the protection from ALD are transmissible from patients to mice by fecal transplantation, emphasizing the critical role of IM in this condition7. In ALD, the improvement of the leaky gut induced by alcohol consumption is an essential feature to improve liver injury8,9. Through fecal microbiota transplantation (FMT), it was discovered that the IM of patients with AH plays a crucial role in liver injury, while pectin can improve IM and liver damage by activating the indole/AhR pathway10,11.
Tryptophan (Trp), an α-amino acid, can be metabolized by intestinal bacteria to produce indole12. Supplementation with indole-3-acetic acid (IAA), an indole ligand, could ameliorate intestinal and liver injury by inducing the expression of IL22 and Reg3 in mice13. IL22 prevents intestinal epithelial injury, promotes intestinal epithelial regeneration, and restores intestinal immunity13. Reg3 lectins reduce mucosa-associated bacteria and decrease bacterial translocation14. Some studies have shown that different concentrations of Trp could separate the IM cluster and activate AhR signaling in mice with autoimmune diseases and weaned piglets15,16,17. However, a recent clinical study on healthy individuals found that oral administration of Trp does not change the IM composition but can activate the AhR pathway18. However, the impact of Trp as a precursor of indole ligands on ALD IM has not been thoroughly investigated.
Long-term alcohol consumption alters the intestinal environment in healthy individuals, but only approximately 15–20% of AUD patients progress to AH, with a central role played by the IM in such disease progression19. We have previously reported that the IM of AUD and AH patients exhibit significant differences and are closely linked to liver injury11. However, the direct effect of alcohol on AUD and AH IM has not been studied yet. Moreover, compared to AUD patients, AH patients show markedly reduced levels of tryptophan, kynurenine, and AhR agonists in their serum11, while it remains unclear whether Trp supplementation could affect AUD and AH IMs. Hence, we used here an in vitro microbiota modeling system, named minibioreactor arrays (MBRAs), which allows stable cultivation of human IM and to investigate the direct modulatory role played by Trp and alcohol20,21. We assessed the direct effects of Trp and alcohol on IM from AUD and AH patients, as well as the activation of AhR by IM metabolites.
Results
MBRA is stable to keep each individual fecal community
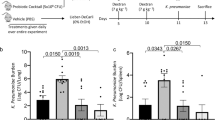
Gut microbiota samples from two AUD patients (AUD1, AUD2), and two AH patients (AH3, AH4) were inoculated into MBRA and treated with different concentrations of Trp in the culture medium, as described previously20. During cultivation, alcohol was added to the medium by two pulses of alcohol per day in order to simulate alcohol intake (Fig. 1a). The clinical characteristics of donors with sAH are detailed in Supplementary Table 1. To assess the stability of MBRA, beta diversity was conducted on all samples at all time points. Principal coordinate analysis (PCoA) showed that MBRA communities from each fecal donor (AUD1, AUD2, AH3, AH4) formed distinct clusters while exhibiting a shift compared to the starting fecal inoculum using different metrics (Jaccard Fig. 1b, Bray_Curtis, unweighted_Unifrac and weighted_Unifrac distances were shown in Supplementary Fig. 2, PERMANOVA analysis, p = 0.001). Moreover, Escherichia shigella exhibited lower levels in the fecal inoculum of AUD patients but significantly higher levels in AH patients (Fig. 1c). Furthermore, this difference persisted at 24 h (Fig. 1d). However, by 72 h, this distinction was only evident in the group with normal Trp concentration (Supplementary Fig. 2). The IM of all the patients was similar to the inoculum and stable over time, suggesting the MBRA allows the study of the impact of Trp and alcohol on the composition of human IM.
AUD, alcohol use disorder; AH, alcohol-related hepatitis. a Experimental design: fecal samples from two AUD patients and two AH patients were transferred to MBRA chambers in three different tryptophan mediums (8 mg/L, 24 mg/L and 72 mg/L) through 13 days. Ethanol was introduced into the medium on Day 4, 50 mM per day, and eliminated on Day 9. Fecal samples and supernatants were collected at 24 h, 72 h, 96 h, 120 h, 192 h and 264 h. Fecal samples were subjected to 16S rRNA sequencing and the supernatant was used to process HT-29-Lucia AhR cells and HepG2-Lucia AhR cells. Microbiota analysis: b PCoA plot, showing the Jaccard distance (PERMANOVA analysis, p = 0.001, 6 samples per patient, per time point). c, d The level of Escherichia Shigella in 4 individual inocula and corresponding samples in 24 h (n = 6 for each group, Statistical analyses were performed using Kruskal–Wallis tests and significant differences were recorded as *p < 0.05, **p < 0.01, and ***p < 0.001.).
Effect of Tryptophan on IM varies by IM compositions
We next examined the extent to which different concentrations of Trp, including low, normal, and high concentrations of Trp, might affect the IM composition in AUD and AH samples. Alpha-diversity analysis using several metrics (Shannon entropy: Fig. 2a, Simpson, Pielou_evenness and Faith’s phylodiversity: Supplementary Fig. 3) revealed that there was no difference in the IM alpha-diversity between the three concentrations of Trp. Through comparing the Bray–Curtis distance, there was no significant impact of low and high Trp concentrations on microbiota composition compared to the normal Trp condition (Fig. 2b, c, and Supplementary Fig. 3). However, upon comparing the differential bacteria between the different Trp concentrations in AUD samples, it was observed that low Trp concentration treatment resulted in differences with 14 genera, and 2 phyla compared to normal Trp concentration (Fig. 2d). In contrast, only 4 genus differed between the normal and high Trp concentration conditions (Fig. 2e). Both high and low concentrations of Trp increased the levels of Escherichia Shigella. For the remaining differential bacteria, normal and high Trp concentrations had similar effects, distinct from the impact observed with the low Trp treatment. Low Trp concentration significantly reduced the levels (p < 0.05, MaAsLin2 analysis) of Alistipes, Stutterella, Bacteroides, Ruminococcaceae_UBA1819, Flavonifractor_plautii, Bacteroides_intestinalis, Bacteroidota and Cyanobacteria and increased the level of Escherichia shigella, Tissierella, Hungatella, Clostridium_scindes, Dorea, Acetanaerobacterium, Acidaminococcus, and Firmicutes (Fig. 2f, g). For AH IM, the differential bacterial species between low Trp concentration treatment and normal Trp concentration treatment are 10 genera and 2 phyla. However, the differential bacteria between high Trp concentration treatment and normal Trp concentration treatment are more in AH samples (10 genera) than in AUD samples. Interestingly, the trends in changes observed for Escherichia Shigella in AH microbiota are consistent with those in AUD samples, with both showing lower levels in the normal Trp group. In addition, low Trp concentration markedly increased the abundance of some bacteria such as Bilophila, Blautia, Prevotella, Proteobacteria, Desulfobacterota (Fig. 2j, k). Thus, in the absence of alcohol, Trp is able to change the relative abundance of several bacteria species in both AUD- and AH-associated IMs, with donor-specific effects.
Trp, tryptophan; IM, intestinal microbiota; a Shannon alpha-diversity index at 72 h among low, normal and high Trp groups. b, c Bray–Curtis distance separating samples from different groups at (b) 24 h and (c) 72 h. d, e, h, i Volcano plots show differential bacteria between Normal Trp and Low Trp in AUD IM (d) and AH IM (h), and between Normal Trp and High Trp in AUD IM (e) and AH IM (i) in 72 h (MaAsLin2 analysis p < 0.05 and fold change > 1.5). f, g Differential bacteria shown in different levels including genus (f, j) and phylum (g, k). (n = 4/group for the Shannon index, and n = 6–16/group for Bray–Curtis distance. Significant results for *p < 0.05, **p < 0.01, and ***p < 0.001 were determined by MaAsLin2 analysis unless stated otherwise.).
Alcohol induces greater changes in AUD microbiota composition as compared AH
Next, to investigate the effect of alcohol on AUD and AH microbiota, we compared IMs before and after the addition of alcohol, culturing them for five days post-alcohol addition (Fig. 1a). Alcohol addition to the medium did not alter the alpha diversity (Shannon entropy) and beta diversity (Bray–Curtis distance) of the IM in individuals with AUD and AH in normal tryptophan conditions (Fig. 3a, b, other alpha and beta diversity indices were shown in Supplementary Fig. 4). However, alcohol induced changes in the relative abundance of members of the AUD microbiota community (7 genera, 2 phyla) compared to those in AH (4 genera) (Fig. 3c, f). In AUD IM with normal Trp concentration, alcohol increased the levels of pathobionts, including Escherichia Shigella and Paeniclostridium, while decreasing the levels of bacteria such as Bacteroides (Fig. 3d, e). In AH IM with normal Trp concentration, alcohol altered a small subset of gut bacteria, including an increase in Dialister, Blautia, and Paeniclostridium, and a decrease in Parasutterella (Fig. 3g).
a, b Shannon alpha-diversity index (n = 4) (a) and Bray–Curtis distances (n = 6–16) (b) between 72 h samples and 192 h samples in Normal Trp group. c–e Volcano plots show differential bacteria between 192 h samples and 72 h samples in AUD Normal Trp group (d), and differential bacteria are displayed in bar charts (d, e). f, g Volcano plots show differential bacteria between 192 h samples (n = 4) and 72 h samples (n = 4) in AH Normal Trp group (f), and differential bacteria are displayed in bar charts (g).
We also analyzed the effects of alcohol on the IM of AUD and AH under conditions of low and high Trp concentrations. Under conditions of low Trp concentration, in AUD IM, alcohol reduced the levels of Hungatella, Ruminococcaceae_UBA1819, Acidaminococcus, Lachnospiraceae_uncultured, and Dorea, and increased the levels of Alistipes, Sutterella, Gastranaerophilales, and Cyanobacteria (Supplementary Fig. 5a). In AH IM under low Trp concentration conditions, alcohol reduced the level of Bilophila, Lachnoclostridium, Phascolarctobacterium, Acidaminococcus, and Scciniclasticum, and increased the levels of Tissierella (Supplementary Fig. 5b). Under conditions of high Trp concentration, alcohol also modified some bacteria from AUD individuals (7 genera) and AH individuals (7 genera), both including an increase in Actinobacteriota, and decreasing the levels of Ruminococcaceae_UBA1819 (Supplementary Fig. 5c, d). Therefore, alcohol still affects the IM of AUD patients, increasing the expression of some pathobionts, but its impact on AH IM appears minimal in normal Trp conditions.
Tryptophan has limited effects on AUD and AH IM in the presence of alcohol
To explore the effects of different concentrations of Trp on AUD and AH IM under alcohol exposure, we compared the differential microbial communities between different concentrations of Trp at timepoint 192 h. In order to remove the bias induced by MBRA and alcohol exposure on the microbiome, we excluded the differential taxa observed in intra-groups comparisons between the time points 72 and 192 h (before and after alcohol supplementation) (Fig. 1a). With alcohol exposure, only a few bacteria are affected by Trp concentration in AUD and AH IM (Fig. 4a–f). Low Trp concentration significantly increased the abundance of Desulfovibrio and Megasphaera in AUD IM, while reducing the abundance of Bilophila and CAG.352 (Fig. 4a). High Trp concentration did not change any bacteria in AUD IM (Fig. 4b). For AH IM, Low Trp concentration increased levels of Actinobacteriota and Enterobacteriaceae, and decreased the level of Alistipes, Erysipelatoclostridium and Ruminococcaceae_UBA1819. High Trp concentration decreased the abundance of Alistipes, Anaerostipes and Erysipelatoclostridium (Fig. 4f). When alcohol and Trp are added simultaneously, the influence of different Trp concentrations on IM is minimal.
a–c Volcano plots show differential bacteria between Normal Trp (n = 4) and Low Trp (n = 4) (a), and between Normal Trp and High Trp (n = 4) (b) in 72 h AUD samples, and display these differential bacteria in bar charts (c). d–f Volcano plots show differential bacteria between Normal Trp and Low Trp (d), and between Normal Trp and High Trp (e) in 72 h AH samples, and display these differential bacteria in bar charts (f).
Dynamics of the IM upon alcohol withdrawal
To further assess the impact of alcohol on the AUD and AH microbiota, we removed alcohol from the culture medium and conducted a 4-day recovery period culture. Subsequently, we compared the IM after recovery (264 h) with that before alcohol supplementation (72 h) to determine whether the IM returned to its initial state after alcohol removal. Low and high concentrations of Trp reduced the alpha diversity of AUD and AH IM, but statistical significance was observed only in low concentrations of Trp in AUD IM (Fig. 5a, b). Normal Trp concentrations did not alter the diversity of the IM (Fig. 5a, b). For the overall IM composition, there were no differences between the IM during the recovery period after alcohol removal and the initial state (Fig. 5c, other alpha and beta diversity indices were shown in Supplementary Fig. 6). However, the analysis of differential bacteria revealed that under normal tryptophan concentration, the AUD IM still exhibited numerous differential bacteria compared to the initial IM community. Conversely, in the AH IM, a few differential bacteria were found after alcohol withdrawal as compared to the initial states (Fig. 5d). After alcohol withdrawal, Escherichia-Shigella, Paenibacillus_lautus, Tissierella, Firmicutes and Desulfobacterota remained at high levels in AUD IM under normal Trp concentration. But the relative abundance of Bacteroides, Proteobacteria, Intestinimonas, Verrucomicrobiota, Ruminococcaceae_UBA1819, Campilobacterota, and Sutterella, in the AUD microbiota decreased (Fig. 5d–h). In conclusion, AUD IM did not return to its pre-alcohol state after alcohol withdrawal under normal Trp concentration. Moreover, low Trp decreased alpha diversity of AUD IM.
a, b Shannon alpha-diversity index between 72 h samples (n = 4) and 264 h samples (n = 4) in AUD samples (A) and AH samples (b). c Bray–Curtis distance separating samples from different treatments in AUD and AH samples at 264 h (n = 6–16). d–h Differential bacteria between 264 h samples and 72 h samples in AUD (d) and AH (e) Normal Trp groups. And related differential bacteria in AUD samples display in bar charts (f–h).
The impact of Trp supplementation on Aryl-hydrocarbon receptor activity
Our previous research reported that microbiota-derived Trp metabolism can activate AhR and improve liver damage. We collected fecal supernatant from MBRAs to treat HT-29 (Fig. 6a–d) and HepG2 (Fig. 6e–h) cells to test the activation of AhR. At 24 h, the AhR activity in the AH group is significantly reduced compared to the AUD group (p < 0.0001, Mann–Whitney test, Fig. 6a, e). After treatments with different concentrations of tryptophan, normal levels of Trp can significantly activate AhR activity in both the AUD and AH groups (*p < 0.05, **p < 0.01, ***p < 0.001, Kruskal–Wallis test), more specifically in AH where normal Trp activated AhR while high Trp didn’t have any statistically significant impact (Fig. 6b, f). However, alcohol supplementation led to increased AhR activity in AUD under high Trp (*p < 0.05, **p < 0.01, ***p < 0.001, Kruskal–Wallis test). For the AH microbiota, the activation of AhR by normal and high Trp concentrations with alcohol is similar (Fig. 6c, g). After alcohol withdrawal, high Trp activated HT-29 AhR, but not in HepG2 in the AUD group. For the AH microbiota, we observed that high Trp concentrations in AH could no longer activate AhR (Fig. 6d, h).
Collected fecal supernatants from different time points in MBRA for treatments of HT-29 Lucia-AhR cells (a–d) and HepG2 Lucia-AhR cells to test the activity of AhR (e–h). (n = 4–14, Significant results for *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 were determined by Kruskal–Wallis test).
To conclude, metabolites of AH IM decreased AhR activity in HT-29 cells and hepatocytes but can be reversed by normal concentrations of Trp in the presence of alcohol or not. Conversely, activation of AhR by AUD IM seems to depend on alcohol and Trp concentrations.
Discussion
Alcohol induces alterations in the intestinal microbiota in both AUD and ALD patients19. Moreover, the causal role of the IM in the development, progression, and severity of AH has been largely documented11,22,23,24. Among the microbiome-related mechanisms involved in ALD, it has been described that bacterial-produced indoles from dietary tryptophan, including AhR ligands, were decreased in patients with more severe injury11,25. In addition, the use of AhR agonists11 and AhR agonists-producing bacteria13 improves alcohol-induced liver injury in mice models of ALD. Conversely, deficient mice for AhR demonstrated a worsening of the alcohol-induced liver injury26 and the loss of the protective effect of pectin on the liver injury11.
Chronic alcohol consumption is associated with malnutrition in both AUD and ALD patients with protein and fiber intake below the recommended daily intake25, leading to lower levels of tryptophan and AhR agonists11. However, Trp supplementation could be risky as an excessive Trp diet could be carcinogenic and increase inflammation27,28 and different intestinal environments may respond differently to the Trp diet29. In the present study, we addressed the impact of Trp on the IM from AUD and AH patients by an in vitro approach by using MBRA to test how a diet with low, normal, and high contents of Trp could modulate the IM and the AhR ligands production.
This study showed that MBRA communities from four independent microbiota donors (AUD1, AUD2, AH3, AH4) formed distinct clusters and evolved independently as compared to the starting fecal inoculum. The shift may arise from IM adaptation in MBRA and is in line with previous research21. However, these similar trends suggest that the microbiome communities from different donors maintain their characteristics and thus enabling the possibility of in vitro IM cultivation. Although the IM compositions are driven mostly by the different donors, rather than the underlying pathologies (AUD and AH), we were still able to observe changes in the IM composition between AUD and AH.
Interestingly, the level of Escherichia Shigella is lower in the AUD inoculum as compared to the AH inoculum, and this change is stable in the first 24 h. Furthermore, the levels of Escherichia Shigella were influenced by the concentration of Trp. Increasing or decreasing the concentration of Trp in the media resulted in an increase in Escherichia Shigella in AUD as well as in AH. Indeed, we found that in AUD IM, high concentration and low concentration of Trp increased the level of Escherichia Shigella. Conversely, normal Trp concentration did not change Escherichia Shigella levels, maintaining the lower Escherichia Shigella level found in the IM inoculum of AUD compared to AH. In AH IM, low Trp also induced an increase in the levels of Escherichia Shigella and Proteobacteria, while high Trp only increased Escherichia Shigella. The intestinal pathogen Escherichia-Shigella, associated with various diseases, is considered pro-inflammatory and is related to metabolic disorders30,31. Escherichia shigella and Escherichia coli (E.coli) both belong to the genus Escherichia. In fact, Escherichia shigella is more closely related to enteroinvasive E. coli than to non-invasive E. coli32. However, their genetic sequences differ by less than 1%, making it difficult to precisely distinguish between Escherichia shigella and E. coli using 16S rRNA sequencing33. The high levels of Escherichia shigella in AH compared to AUD are consistent with previous studies, which have shown that E.coli O antibody levels are significantly higher in patients with combined alcoholic hepatitis and cirrhosis compared to those with non-alcoholic fatty liver disease34. Overall, we found that both high and low concentrations of Trp elevated pathogenic bacteria in AUD and AH IM suggesting that a normal Trp concentration might be essential to prevent the expansion of potential pathobionts and the evolution to ALD.
Alcohol could induce dysbiosis per se in the intestine35. Therefore, we explored the effects of alcohol on AUD and AH IM in our in vitro system. We observed that in AUD patients without liver disease, alcohol increased the levels of pathobionts such as Escherichia Shigella and decreased Bacteroides and Agathobacter.
Low Bacteroides levels are associated with increased susceptibility to ALD10, and increasing Bacteroides levels through pectin administration in ALD models alcohol-induced liver and gut lesions can be prevented by increasing indole derivatives, activating AhR and increasing IL22 and REG3γ expression11. Moreover, studies have shown that short-chain fatty acids (SCFAs) are involved in the ALD metabolism, and as the disease progresses, SCFAs are gradually depleted36. Interestingly, Agathobacter has been identified as a SCFAs-producing bacterium37, and its reduction in AUD IM by alcohol addition may exacerbate the burden of ALD.
To explore the potential therapeutic effects of Trp on alcohol drinkers, we then studied whether different concentrations of Trp still affected AUD and AH IM in the presence of alcohol. We found that the effect of Trp on IM is independent of its concentrations on both AUD and AH microbiota after alcohol addition. In AUD, the differences in gut microbiota between different concentrations of Trp are limited under alcohol conditions. However, a normal concentration of Trp decreased the level of Enterobacteriaceae._in AH IM, relative to low Trp groups. Although normal and high concentrations of Trp had a limited effect on the alteration of AUD and AH in the presence of alcohol, normal Trp reduced potentially harmful bacteria in the absence of alcohol. However, if Trp supplementation did not restore the levels of Bacteroides and Agathobacter, high Trp concentrations were able to increase the AhR activity in the presence of alcohol suggesting that the overall changes in the IM composition can restore this defective metabolic capacity.
As abstinence from alcohol is currently the most effective treatment for ALD, we removed alcohol to simulate and study IM changes. We observed that the effect of alcohol withdrawal on IM is dependent on the IM donors. Normal Trp had limited effects and did not fully restore the IM in AUD while low Trp decreased the IM diversity in all samples, especially in AUD IM. Low concentrations of Trp could disrupt the IM diversity and may be harmful to patients with AUD and AH. Interestingly, the levels of pathogenic bacteria Escherichia Shigella remained high after removing the alcohol in AUD IM suggesting that, the effects of alcohol on AUD IM may persist after its removal and would not recover, at least for a short period independent of Trp concentration.
IM could metabolize Trp to produce AhR ligands, activating AhR to improve intestinal inflammation and liver damage11,12,38,39. IM metabolites of AH were not effective in activating AhR compared to those in AUD. We observed that a normal level of Trp can restore AhR activity in AH. However, high Trp in AUD was more effective and normal Trp in AH had more function in activating AhR when alcohol was added, which persisted even after alcohol withdrawal.
As AH patients have low Trp levels11, supplementation of Trp to a normal concentration could be enough in these patients to increase AhR ligands and this aspect should be further tested in patients with ALD. Trp as one of essential amino acids with the lowest tissue stock in the body requires a consistent daily intake. The recommended Trp intake for adults is 250 mg/day to 425 mg/day, which is about 3.5–6.0 mg/kg of body weight per day40. This could be easily achieved through consuming some daily foods such as milk (0.42 mg/g), eggs (1.65 mg/g), potatoes (0.28 mg/g), and beef (2.3 mg/g) by giving precise suggestions to patients41.
One limitation of this study was the absence of a control group without alcohol treatment compared to manipulating IM with alcohol. Although we solved this by exploring the effects of alcohol on the IM by comparing IM before and after alcohol addition, the impact of MBRA on IM during the stable period may have been overlooked due to the lack of a control group. A second limitation is that some MBRA supernatants’ samples are missing in low Trp groups at 72 h and 264 h timepoints. Moreover, MBRA cultivation could not entirely preserve and simulate the entire IM environment, necessitating further in vivo experiments.
In conclusion, our study modeled the evolution of IM from patients with AUD and AH in vitro and showed that the AUD IM’s is the more dynamic in terms of changes as compared to AH. Therefore, with the advantage of MBRA in stabilizing IM and the exclusion of other interfering factors, our study suggests that Trp supplementation to restore the recommended nutritional intake in AUD and AH patients may be beneficial and set the basis for further studies in clinical settings.
Methods
Fecal samples collection
Human fecal samples from two AUD patients without liver injury and two AH patients were collected. An anaerobiosis generator (Anaerocult, Merck, Darmstadt, Germany) was added to samples to favor the preservation of anaerobic bacteria. The samples were processed within 24 h and frozen for bacterial analysis7. The fecal sample was homogenized and aliquoted into 50 mL Falcon tubes, storing them at -80 °C until use. Clinical characteristics of AUD and AH patients are presented in Supplemental Table 1. This study protocol was approved by the local ethics committee of the Hospital with the approval number “CPP IDF VII; C0-09-016”. All the patients provided informed consent.
MBRAs system
We performed an in vitro experiment in a MBRAs system, as described in previous studies20,42. Briefly, the MBRAs system, operated in an anaerobic chamber at 37 °C, consisted of 24 minireactors containing 15 mL Bioreactor Medium. These chambers are placed on a magnetic stirrer to simulate intestinal peristalsis, ensuring continuous homogenization of the contents. All treatment media must be prestored in the small anaerobic chambers for at least 48 h before entering the MBRA system (Supplementary Fig. 1).
Inoculation, sample processing and collection
Fecal samples from four patients were inoculated into MBRA chambers, as previously described43. Each patient’s fecal sample was placed in three sets of tryptophan culture media of varying concentrations, with one replicate per group. The composition of the culture media is detailed in Supplemental Table 2. All components are consistent with the previous description, except for the tryptophan that is available in three different concentrations, low (8 mg/L), medium (24 mg/L), and high (72 mg/L)20. Following a three-day stabilization phase, we added 50 mM ethanol in 2 pulses daily to the culture medium for 5 days. This concentration of ethanol, equivalent to ~5 g/L, falls within the 5-30 g/L range known to inhibit intestinal amino acid and glucose transport while stimulating triglyceride synthesis44. Afterwards, the culture was continued for an additional 5 days following the removal of alcohol. The chamber contents and supernatants were collected at different time points (24 h, 72 h, 96 h, 120 h, 192 h, and 264 h) (Fig. 1a). Samples were stored at −80°C until further use.
Bacteria DNA extraction
Bacterial DNA was extracted according to the manufacturer’s guideline of the QIAamp 96 PowerFecal QIAcube HT Kit (Qiagen, Venlo, Netherlands), and then using the QIAcube High-robot, as previously reported42.
16S rRNA gene sequencing
Extracted DNA were amplified by targeting the 16S rRNA gene, V4 hypervariable region and then confirmed by electrophoresis on a 2% agarose gel. The amplified 16S rRNA sequencing was performed by using the Illumina MiSeq sequencer (paired-end reads, 2 × 250 bp), as previously reported42.
16S rRNA sequencing data analysis
16S rRNA sequences were analyzed by using QIIME2-version 202445. 16S rRNA sequences were demultiplexed and quality filtered using DADA2 method46 with default QIIME2 parameters to detect and correct illumina amplicon sequencing data, resulting in the generation of a QIIME2 artifact table. A phylogenetic tree was subsequently created for phylogenetic diversity analysis using the align-to-tree-mafft-fast tree plugin, while alpha and beta diversity analyses were performed with the core-metrics-phylogenetic command, with rarefaction applied at a sampling depth of 27000 reads. To assess the variation between experimental groups (beta diversity), Principal Coordinate Analysis (PCoA) plots were utilized, and the PERMANOVA command was applied to determine statistical significance. By using the feature-classifier classify-sklearn command with the silva-138-99-515-806-nb-classifier47, an Amplicon Sequence Variant (ASV) table was generated to classify the sequences. The relative abundance of bacteria was used to calculate the expression of differential microbial communities between treatments through MaAsLin2 analysis48 (absolute fold change > 1.5 and p-value < 0.05). The bacterial abundance was visualized using relative abundance and plotted using GraphPad Prism 10.
HT-29 and HepG2 cells cultured and the AhR activity test
HT29-LuciaTM AhR cells (Catalog code: ht2l-ahr) and HepG2-LuciaTM AhR cells (Catalog code: hpgl-ahr) were purchased from commercial InvivoGen. HT29-LuciaTM AhR cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), 4.5 g/l glucose, 2 mM L-glutamine, 100 ug/ml NormocinTM and 100 ug/ml Pen-Strep. HepG2-LuciaTM AhR cells were cultured in Eagle’s Minimal Essential Medium (EMEM) with 10% FBS, 1 × non-essential amino acids (NEAA), 100 μg/ml NormocinTM and 100 μg/ml Pen-Strep. Cells were cultured at 37 °C in a 5% CO2 incubator. We seeded 5000 HT29 cells and 20000 HepG2 cells in 96-well plates with flat-bottom. After 24 h of cell adherence, cells were treated with water (control), IAA 500 μM and 100 μM (positive control), and supernatant diluted to an equal volume. After 24 h and 48 h, QUANTI-LucTM reagent was added, and a reading value was obtained using a luminometer without injectors.
Statistical analysis
Statistical analyses were performed by RStudio (version 2023.06.1 + 524) and GraphPad Prism 10. The statistical analysis of differential bacteria was performed using MaAsLin2 in Rstudio (absolute fold changed > 1.5 and p-value < 0.05), and then volcano plots and bar graphs were generated in GraphPad Prism 10. Statistical significances for alpha diversity and AhR activity were calculated by using the one-way ANOVA with the Kruskal–Wallis test. All results were presented as the mean ± standard deviation (SD). Significant results are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Data availability
The sequencing data for 16S rRNA are available in NCBI Sequence Read Archive (SRA) under the accession number PRJNA1163693.
References
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011).
Mackowiak, B., Fu, Y., Maccioni, L. & Gao, B. Alcohol-associated liver disease. J. Clin. Invest. 134, https://doi.org/10.1172/jci176345 (2024).
Mirijello, A. et al. Identification and management of alcohol withdrawal syndrome. Drugs 75, 353–365 (2015).
Rao, R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50, 638–644 (2009).
Bishehsari, F. et al. Alcohol and gut-derived inflammation. Alcohol. Res. 38, 163–171 (2017).
Maccioni, L. et al. Alcohol-associated bowel disease: new insights into pathogenesis. eGastroenterology 1, https://doi.org/10.1136/egastro-2023-100013 (2023).
Llopis, M. et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65, 830–839 (2016).
Albillos, A., de Gottardi, A. & Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72, 558–577 (2020).
Xu, Q. et al. Propionate ameliorates alcohol-induced liver injury in mice via the gut-liver axis: focus on the improvement of intestinal permeability. J. Agric. Food Chem. 70, 6084–6096 (2022).
Ferrere, G. et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 66, 806–815 (2017).
Wrzosek, L. et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 70, 1299–1308 (2021).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Hendrikx, T. et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 68, 1504–1515 (2019).
Wang, L. et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 19, 227–239 (2016).
Lamas, B. et al. Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation. Sci. Transl. Med. 12, https://doi.org/10.1126/scitranslmed.aba0624 (2020).
Choi, S. C. et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci. Transl. Med. 12, https://doi.org/10.1126/scitranslmed.aax2220 (2020).
Liang, H. et al. Dietary L-tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front Microbiol. 9, 1736 (2018).
Rueda, G. H. et al. Oral tryptophan activates duodenal aryl hydrocarbon receptor in healthy subjects: a crossover randomized controlled trial. Am. J. Physiol. Gastrointest. Liver Physiol. https://doi.org/10.1152/ajpgi.00306.2023 (2024).
Bajaj, J. S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 235–246 (2019).
Auchtung, J. M., Robinson, C. D., Farrell, K. & Britton, R. A. MiniBioReactor Arrays (MBRAs) as a tool for studying C. difficile physiology in the presence of a complex community. Methods Mol. Biol. (Clifton, N. J.) 1476, 235–258 (2016).
Auchtung, J. M., Robinson, C. D. & Britton, R. A. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs). Microbiome 3, 42 (2015).
Hsu, C. L. & Schnabl, B. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733 (2023).
Leclercq, S. et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 111, E4485–E4493 (2014).
Wolstenholme, J. T. et al. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat. Commun. 13, 6198 (2022).
Amadieu, C. et al. Dietary fiber deficiency as a component of malnutrition associated with psychological alterations in alcohol use disorder. Clin. Nutr. 40, 2673–2682 (2021).
Qian, M. et al. Aryl hydrocarbon receptor deficiency in intestinal epithelial cells aggravates alcohol-related liver disease. Cell. Mol. Gastroenterol. Hepatol. 13, 233–256 (2021).
Chung, K. T. & Gadupudi, G. S. Possible roles of excess tryptophan metabolites in cancer. Environ. Mol. Mutagen 52, 81–104 (2011).
Hu, D. et al. Tryptophan intake, not always the more the better. Front Nutr. 10, 1140054 (2023).
Gao, J. et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect. Microbiol. 8, 13 (2018).
Zhao, J. et al. Expansion of escherichia-shigella in gut is associated with the onset and response to immunosuppressive therapy of IgA nephropathy. J. Am. Soc. Nephrol. 33, 2276–2292 (2022).
Kong, C., Gao, R., Yan, X., Huang, L. & Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 60, 175–184 (2019).
van den Beld, M. J. & Reubsaet, F. A. Differentiation between Shigella, enteroinvasive Escherichia coli (EIEC) and noninvasive Escherichia coli. Eur. J. Clin. Microbiol Infect. Dis. 31, 899–904 (2012).
Devanga Ragupathi, N. K., Muthuirulandi Sethuvel, D. P., Inbanathan, F. Y. & Veeraraghavan, B. Accurate differentiation of Escherichia coli and Shigella serogroups: challenges and strategies. N. Microbes N. Infect. 21, 58–62 (2018).
Staun-Olsen, P., Bjørneboe, M., Prytz, H., Thomsen, A. C. & Orskov, F. Escherichia coli antibodies in alcoholic liver disease. Correlation to alcohol consumption, alcoholic hepatitis, and serum IgA. Scand. J. Gastroenterol. 18, 889–896 (1983).
Engen, P. A., Green, S. J., Voigt, R. M., Forsyth, C. B. & Keshavarzian, A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 37, 223–236 (2015).
Ganesan, R. et al. Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease. Hepatol. Int. 18, 486–499 (2024).
Rodríguez-García, A. et al. Short-chain fatty acid production by gut microbiota predicts treatment response in multiple myeloma. Clin. Cancer Res. 30, 904–917 (2024).
Michaudel, C. et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 72, 1296–1307 (2023).
Yamazaki, T. et al. Serum aryl hydrocarbon receptor activity is associated with survival in patients with alcohol-associated hepatitis. Hepatology https://doi.org/10.1097/hep.0000000000000777 (2024).
Richard, D. M. et al. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. 2, 45–60 (2009).
Kałużna-Czaplińska, J., Gątarek, P., Chirumbolo, S., Chartrand, M. S. & Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 59, 72–88 (2019).
Naimi, S., Viennois, E., Gewirtz, A. T. & Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 9, 66 (2021).
Bonazzi, E. et al. Individualized microbiotas dictate the impact of dietary fiber on colitis sensitivity. Microbiome 12, 5 (2024).
Halsted, C. H., Robles, E. A. & Mezey, E. Distribution of ethanol in the human gastrointestinal tract. Am. J. Clin. Nutr. 26, 831–834 (1973).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Bokulich, N. et al. bokulich-lab/RESCRIPt: 2023.5.0. Zenodo https://doi.org/10.5281/zenodo.8004808 (2023).
Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17, e1009442 (2021).
Acknowledgements
This work was supported by INSERM, Université Paris-Sud, “Fondation pour la recherche médicale” (FRM), the National French Society of Gastroenterology (SNFGE), “Association Française pour l’Etude du Foie” (AFEF), and the national transversal program “Microbiote” from INSERM, and ANR grant TIMPAH (ANR-22-CE17-0056-01). W.H. received a PhD funding from CSC (Chinese Scholarship Council). B.C. laboratory is supported by a Starting Grant from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. ERC-2018-StG- 804135), an award from the Fondation de l’avenir (AP-RM-21-032), ANR grants EMULBIONT (ANR-21-CE15-0042-01) and DREAM (ANR-20-PAMR-0002), and the national program “Microbiote” from INSERM. The authors thank Arthi Rajasekaran for preparing the experimental medium.
Author information
Authors and Affiliations
Contributions
S.N. and B.C. samples generation. W.H., S.N. and N.T. experimental procedures. W.H. data analysis and interpretation, drafting of the manuscript. V.L.L.M. helps in technical support and study concepts. D.C. and G.P.: recruitment of AUD patients. A.M.C., B.C., and D.C.: study concept, design, and supervision, analysis and interpretation of the data, drafting of the manuscript, and funding raising.
Corresponding authors
Ethics declarations
Competing interests
D.C. received travel funds from Biocodex and Gilead, lecture fees from Gilead, and royalties from John Libbey Eurotext. G.P. received travel funds from Janssen and Gilead, consulting fees from Bayer, Biocodex, Roche, Gilead, Pierre Fabre, and Servier, and royalties from Elsevier-Masson, Solar, Flammarion/Versilio, and John Libbey Eurotext. A.M.C. received royalties from Elsevier-Masson, Solar, Flammarion/Versilio, and John Libbey Eurotext. All other authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, W., Naimi, S., Trainel, N. et al. Minibioreactor arrays to model microbiome response to alcohol and tryptophan in the context of alcohol-associated liver disease. npj Biofilms Microbiomes 10, 132 (2024). https://doi.org/10.1038/s41522-024-00602-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-024-00602-9