Abstract
Dairy farms are major reservoirs of zoonotic bacterial pathogens, which harbor antimicrobial resistance genes (ARGs), and raise critical questions about their dissemination on and off the farm environment. Here, we investigated the role of coprophagous muscid flies (Diptera: Muscidae) as carriers of zoonotic pathogens and antimicrobial resistance. We collected cow manure and flies on a dairy farm and used shotgun metagenomics to identify the presence of clinically relevant bacteria, virulence factors, and ARGs in both environments. Our results reveal that, although the fly microbiome is largely composed of manure-associated taxa, they also harbor specific insect-associated bacteria, which may be involved in nutrient provisioning to the host. Furthermore, we identifed shared ARGs, virulence factors, and zoonotic pathogens enriched within the fly gastrointestinal tract (GIT). Our study illustrates the potential flow of pathogenic microorganisms from manure to coprophagous flies, suggesting that flies may pose an important zoonotic threat on dairy farms.
Similar content being viewed by others
Introduction
Emerging infectious diseases (EID), the majority of which are zoonotic in origin, represent a significant threat to the global economy and food system1. Dairy farms and other cattle livestock systems are major reservoirs of economically significant zoonotic pathogens, including Escherichia coli O157, Salmonella enterica, Listeria monocytogenes, and Coxiella burnetii2,3. Antibiotic usage and the horizontal transfer of ARGs can contribute to the emergence of antimicrobial resistant pathogens on farms4, further highlighting the need to better understand the ecology of agricultural microbiomes. On dairy farms, past studies have focused primarily on the direct transmission of microorganisms from cattle to farm workers and through the food system2,5. In contrast, far less is known about the interactions between pathogenic microbes and other animals cohabiting on livestock facilities.
Dairy farms support a large community of synanthropic pests, including birds, rodents, and insects, which regularly interact with both cattle and farm workers. Muscid flies (Diptera: Muscidae) are globally ubiquitous, coprophagous pests that include members of the genera Musca (house and face flies) and Neomyia (false greenbottle flies). Large fly populations are sustained by a near constant supply of manure – a protein and carbohydrate rich source of nutrition for both immature fly larvae and adult flies6,7,8. Bovine manure also serves as an important source of animal-borne pathogens, which may colonize and proliferate in the fly digestive tract following ingestion9,10. These bacteria can be disseminated by flies via regurgitation and defecation, increasing the potential transmission risk of pathogenic microorganisms throughout the environment10,11,12.
Prior studies utilizing 16S rRNA amplicon sequencing and culture-dependent methodologies have shown that flies commonly harbor medically important bacterial species including some within the Staphylococcus and Enterobacteriaceae13,14,15,16,17,18,19. Flies are also carriers of antimicrobial resistant bacteria, which often carry extrachromosomal ARGs on plasmids, transposable elements, or other mobile genetic elements20,21. This is of great concern given the continuous usage of antibiotics to treat bovine diseases such as mastitis and metritis22,23, resulting in selective pressures that maintain ARGs within the bovine intestinal microbiome, which, by extension, persist in bovine feces.
Studies using 16S rRNA gene amplicon sequencing, while informative, cannot be used to identify specific shared transmitted genetic features (ARGs, virulence factors, etc.) or for strain level analyses13,14,15,16,17. Despite the known epidemiological significance, only a handful of studies have examined the potential for transfer of genomic features and/or bacterial strains between flies and agricultural environments24,25,26, but were either limited to culture-based approaches and Whole Genome Sequencing (WGS) of isolates or used metagenomic approaches only on flies and not on environmental or manure samples. However, approaches such as shotgun metagenomic sequencing allow for the assembly of bacterial community genomes, which can be used to identify the distribution of functional genetic features like virulence factors and ARGs, in addition to mobile genetic elements27,28,29.
Previously, we performed a metagenomic analysis on individual dairy cow fecal samples to construct a manure-associated genome database and characterized both microbial diversity and virulence factors across this environment30. Here, we expand upon our work by analyzing the metagenomes of muscid flies feeding on these same manure samples to explore the transmission of microorganisms through the farm environment. We hypothesize that coprophagous muscid flies can acquire fecal-associated bacteria and their associated genomic features (virulence factors and ARGs). Our results show that many highly abundant metagenome-assembled genomes (MAGs) and reference bacterial genomes in the fly GIT microbiome can be traced to manure, and that pathogenic microbes (Coxiella, E. coli pathotypes) and virulence genes may persist in the fly gut. We further identified insect-associated microbes (Frischella, Entomomonas, Apilactobacillus) in the GIT, which we hypothesize may provide mutualistic benefits to the fly. This study is the first to offer functional predictions using metagenomic sequencing data from environmental reservoirs (cattle manure) and the Neomyia fly carriers interacting with them in real-time.
Results
Sample collection, metagenome sequencing, and construction of fly-derived MAGs
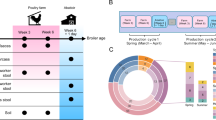
A total of 29 flies were collected from a dairy farm in Saint-Genès-Champanelle, France over the course of two consecutive days. Metagenomic sequencing of the dissected GITs from these flies generated a total of 3.52 billion reads, representing ~528 Gbp of sequence data that averaged 18.2 ± 2.4 Gbp per sample. The metagenomes and their associated sequencing metrics for the 48 cow manure samples also used in this study have been previously reported30.
Shotgun metagenomic sequences from our fly GIT microbiomes were assembled into >37 million contigs. Taxonomic classification of these contigs using the NCBI’s non-redundant protein database showed that the vast majority were not represented in publicly available databases (ca. 78%), while most classifiable contigs belonged to Bacteria (>15%) and Eukaryotes (>5%), with minor contributions from Archaea (<0.4%) and Viruses (<0.5%) (Supplementary Table 1). Of note, 96% of the eukaryotic contigs belonged to the Arthropoda and Euglenozoa, with the former being the most abundant (>71% mean abundance) in almost every sample and the latter ranging from 0 to 56% per sample. A total of 506 fly GIT MAGs were constructed, of which 42 MAGs passed our filtering threshold of >85% completeness and <5% contamination (Supplementary Table 2). These 42 MAGs were resolved to 15 different bacterial genera, which included Prevotella (n = 7), Frischella (n = 6), Fibrobacter (n = 5), and Rumminococcus (n = 3) as the most prevalent (Supplementary Table 3). We constructed a phylogeny by concatenating all shared orthologs between the 42 fly GIT MAGs, closely related manure-derived MAGs30, and closely related RefSeq MAGs. This phylogenetic analysis revealed the presence of closely related MAGs shared between flies and manure (Supplementary Fig. 1).
Fly genotyping
The vast majority of collected flies were morphologically identified as belonging to the common green fly, which consists of several greenbottle fly species that are abundant in farms across Western Europe8,31. We genotyped each fly by identifying the mitochondrial cytochrome oxidase subunit I (COI) gene in their respective metagenome and comparing it against the published mitochondrial COI genes for Musca domestica, Myospila meditabunda, and Neomyia cornicina. A total of 27 out of 29 samples were found to contain near-complete dipteran mitochondrial COI genes (2287 bp) that matched with >99% sequence identity to the published mitochondrial COI gene from N. cornicina (MW592695.1), which belongs to the Muscidae family. For the two samples that did not yield near-complete mitochondrial COI genes, a partial sequence was obtained that matched most closely to the reported mitochondrial COI gene for My. meditabunda (KJ510632 & FJ025642), which also belongs to the Muscidae. All sequences and their closest matches were then aligned and used to construct a maximum likelihood phylogenetic tree (Supplementary Fig. 2). Of note, 26 of the 29 mitochondrial COI gene sequences formed a single clade with N. cornicina mitochondrial COI gene sequences reported from Europe (Belgium, France, Poland, and Portugal; KF919034, KU932144, MN868909 respectively) and China32. Of the other three sequences, one formed a sister clade with several other published N. cornicina sequences, while the other two formed a distant clade with sequences from My. meditabunda.
Fly GIT and cow manure microbiome structure
We next sought to characterize fly GIT bacterial communities in relation to manure to identify the potential for microbial transmission in the dairy environment. To accomplish this, we performed a read mapping recruitment analysis of the 29 fly GIT and the 48 manure metagenomes against a reference MAG database containing the 42 MAGs derived from our fly GIT metagenomes and the 2114 MAGs derived from cow manure30. For each sample, we determined the CLR transformed abundances of MAGs across each bacterial order. Notably, the bacterial orders Bacteroidales, Burkholderiales, and Enterobacterales were relatively abundant across the fly microbiome (Supplementary Fig. 3). Bacteroidales was also one of the most abundant bacterial orders across manure samples (Supplementary Fig. 4). Beta-diversity analysis (Achitson distance) further revealed clustering of bacterial order abundance by sample type, indicating that the fly GIT community structure is distinct from that of cow manure (Supplementary Fig. 5).
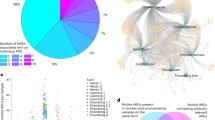
We next determined the 50 most abundant MAGs (CLR transformed counts) in both the fly GIT and cow manure samples to identify the potential microbial flow through the environment. We found that 34 MAGs from 17 different genera were among the 50 most common MAGs shared between both sample types, suggesting that the potential transfer of these MAGs from manure to flies via feeding is common (Fig. 1). To identify potentially enriched MAGs in the fly GIT and manure samples, we performed an indicspecies analysis, which revealed a total of 41 MAGs enriched in manure samples and 21 MAGs enriched in the fly GIT (Supplementary Fig. 6; Supplementary Table 4). This included highly enriched fly GIT MAGs belonging to the genera Frischella, Enterococcus, Entomomonas, Dysgonomonas, Flavobacterium, Prevotella, Apilactobacillus, and Acinetobacter. In contrast, the most enriched cow manure MAGs belonged to the Lachnospiraceae, Selenomonas, Bifidobacterium, and Flintibacter.
Chord diagrams depict the 50 most abundant MAGs found in flies (A) and manure (B) with their corresponding abundances in both manure (blue arc) and fly GITs (red arc) shown by connecting lines/chords. The thickness of each line/chord is proportional to the corresponding MAG’s abundance, which was calculated as the centered log ratio normalized proportion of the total counts successfully mapped to the MAG divided by the estimated number of genomes in each sample (see methods). MAGs with <20% prevalence across all samples were removed from the dataset prior to CLR transformation and downstream analysis.
Using data from both the CLR transformed counts of highly abundant MAGs (Fig. 1) and our enrichment analysis (Supplementary Fig. 6), we selected a set of MAGs representative of the fly GIT, cow manure, and taxa shared between the two groups. We functionally annotated these MAGs using the G database to determine KEGG orthologs (KOs) both unique to and common between groups (Fig. 2A). We next analyzed three intersections of KOs: KOs shared between the fly GIT and the common group, unique KOs to the fly GIT, and unique KOs to cow manure (Supplementary Table 5). Analysis of each KO’s associated Clusters of Orthologous Group (COGs) annotations showed that KOs related to transcription, cell motility and trafficking, secretion and vesicular transport (COG groups K, N & U) were significantly higher (Fisher Exact Test, p < 0.05) in the KOs unique to the fly GIT, relative to either common or unique to manure groups (Fig. 2B). We also found the intersection of the fly and common groups to have significantly higher proportion of housekeeping KOs involved in cell division (D), translation (J), replication (L), and posttranscriptional modification (O) as compared to the unique fly and manure groups. We further identified complete metabolic pathways encoded within the MAGs unique to and common between the fly GIT and cow manure microbiomes (Fig. 2C). We found that a portion of the fly group MAGs, which included the highly enriched Frischella and Entomomonas MAGs, encoded pathways related to the metabolism of cofactors and vitamins (Lipoic acid biosynthesis and Pyridoxal-P biosynthesis). We also identified several pathways involved in amino acid biosynthesis, energy metabolism, carbohydrate metabolism, and drug resistance, which were unique to the fly-specific MAGs (Fig. 2C).
A Comparison of shared and unique KEGG orthologs (KOs) in the 21 representative fly-selected, manure-selected, and common MAGs. Genus identification of each MAG is shown below the Venn-diagram, with parentheses indicating when multiple MAGs were assigned to the same genus. Daggers (†) indicate MAGs without a specific genus identification. B Bar graph showing the distribution of clusters of orthologous groups of proteins (COGs) associated with the common transmissible KOs (top bar, orange), unique KOs to flies (middle bar, red), and unique KOs to manure (bottom bar, blue). Lines indicate comparisons where the proportion of counts is significantly different (Fisher’s exact test, p < 0.05). C Overview of complete KEGG metabolic pathways in the fly-selected, manure-selected, and common MAG groups. The ternary plot shows the relative proportion of MAGs in each group encoding complete metabolic pathways. The position of the pathway on the ternary plot represents the prevalence in each group, with pathways equally common between groups in the center. The color of each point indicates the metabolic class the pathway belongs to.
Comparison of virulence factors between the fly and manure microbiomes
We examined virulence factors present in the fly GIT and cow manure metagenomes by mapping reads against the VFdb database. Our analysis revealed a total of 1934 virulence factors shared between the fly GIT and cow manure microbiomes (Fig. 3A). We repeated the same comparison for those VFs present in at least 50% of each group and found a total of 43 VFs shared between the fly GIT and cow manure. Shared VFs were primarily related to adherence and immune modulation (encoding for surface antigens and capsules). Notably, we also identified a gene coding for a Shiga toxin (stx1vB, VFG043752) that was present in at least 50% of fly and cow manure samples, with an additional Shiga toxin (stxB, VFG001829) present in 50% of the cow manure samples. We also identified 18 VFs associated with Coxiella present in at least 50% of the fly samples (Supplementary Table 7). We then performed an additional filtering step that retained VF genes for which mapped reads covered at least 90% of the gene length and were also present in at least 50% of the samples. Using these criteria, we identified seven VFs associated with the fly GIT microbiome, which were classified as belonging to Escherichia coli and Coxiella burnetii and included six VFs related to secretion and one to adherence (stgB) (Supplementary Table 7). The CLR transformed counts suggested that the abundance of espL4, espX5, CBUK_RS01150, and coxFIC1 was significantly higher (Wilcoxon test, p < 0.05) across fly GIT samples as compared to cow manure (Fig. 3B).
A Venn diagrams showing in descending order all common and unique virulence (left) and antimicrobial resistance (right) features, features that were present in at least 50% of fly and cow samples (50% prevalence), and features corresponding to genes for which the reads covered ≥90% of the gene length and were present in at least half of the samples (≥50% prevalence & ≥90% coverage). Left circle (blue) corresponds to the manure microbiomes; right circle (red) corresponds to the fly GIT microbiomes. B Box plot showing the relative abundances (centered normalized log ratios) of 6 VFs with ≥50% prevalence and ≥90% coverage in fly samples. C Box plot showing the relative abundance of corresponding ARGs with at least 50% prevalence and 90% coverage in either fly or manure samples. Top bars (red) depict fly abundances; bottom bars (blue) depict manure abundances. VFs were identified through mapping against the VFdb and ARGs were identified through mapping against the Resfinder database. Asterisks indicate comparisons with a significantly different abundance between fly and manure samples (p < 0.05, Wilcoxon signed rank test).
Comparison of antibiotic resistance genes between the fly and manure microbiomes
We similarly identified ARGs across our fly GIT and cow manure metagenomes through read mapping against the ResFinder Database. We found 86 ARGs common across all samples and, after removing ARGs present in less than 50% of the groups, only 40 ARGs were retained (Fig. 3A). Using a method similar to our VF analysis, we examined how many ARGs would be retained after filtering for those genes present in ≥50% of the samples with reads mapping to ≥90% of the gene length (Fig. 3A). We identified 18 ARGs common between both groups: 1 unique to the fly GIT and 13 unique to cow manure (Supplementary Table 7). We then examined the CLR transformed abundances of these 32 ARGs (Fig. 3C), which notably showed higher relative abundances (Wilcoxon test, p < 0.05) of a beta-lactam blaOXA gene (ACWG01000053), three tetracycline resistance genes, and an aminoglycoside resistance gene (KF864551) in the fly GIT as compared to cow manure. Conversely, we found CLR transformed abundances of a macrolide resistance gene (AY928180) and three other tetracycline genes to be significantly higher in manure samples (Wilcoxon test, p < 0.05). We then compiled the antimicrobial usage (AMU) records on the farm from the last 5 years to examine the potential correlation of ARGs discovered with antibiotic administration. We found that macrolides, aminoglycosides, tetracyclines, and beta-lactam antibiotics were all commonly administered to sick animals and ARGs linked to these antibiotics were commonly found in manure and fly metagenomes (Fig. 3, Supplementary Table 7). However, formal statistical evaluation showed no significant correlation between antibiotic administration and ARG presence (co-inertia p > 0.05).
Tracing fly-manure interactions and identification of pathogens in the dairy barn environment
As noted in our sampling approach, flies were collected standing on or hovering above cow manure, which urged us to examine whether we could identify traces of the bovine COI gene in the fly GIT. We found single alleles of the bovine COI gene in 23/29 fly samples, which we presume is correlated to the most recent manure from which they fed. Comparison of these sequences showed that recovered Bos taurus COI genes from flies formed three distinct clusters (Supplementary Fig. 7). We then used these data to identify potential zoonotic pathogens shared between fly GIT and cow manure metagenomes belonging to the same COI cluster, which would suggest the potential acquisition of these pathogens from cow manure pools. We identified 47 bacterial genomes and plasmids associated with VF genes that were shared between our fly GIT and cow manure samples with at least 90% coverage (Fig. 3). Sequencing reads were then mapped to the retrieved full genome (chromosomal and plasmid) sequences and were resolved to 8 pathogen genomes that were found in at least one fly GIT sample and one cow manure sample with >10% total genome coverage. These pathogens included 4 E. coli, 2 Shigella, and 2 Coxiella genomes, which were shared between the fly GIT and cow manure microbiomes. When comparing the carriage of each of these pathogen genomes to the bovine mitochondrial COI gene (Fig. 4), we found specific instances where both fly GIT and cow manure metagenomes harboring highly similar bovine COI genes also shared the same pathogens. We found that, not only were these pathogens more commonly identified in the fly GIT samples, but that they often could be mapped with a higher coverage and at a greater relative abundance as compared to their cow manure counterparts (Supplementary Fig. 8).
Cow and fly samples were plotted on a PCA based on the dissimilarity of the recovered Bos taurus COI sequences. Fly samples are represented by red dots and manure samples are represented by blue dots. Samples with at least 10% total coverage against the respective reference pathogen genome are labeled. Corresponding coverage and CLR transformed abundance for each pathogen are shown in Supplementary Fig. 8.
Discussion
In this study, we used deep metagenomic sequencing to investigate the microbiome of muscid flies in relation to bovine manure on a pasture-based dairy farm in France. Bovine manure represents a major reservoir of zoonotic pathogens, including E. coli O157, Salmonella spp., and C. burnetti2,3; however, few studies have attempted to trace the dissemination of manure-associated microbes through the dairy farm environment. As the microbiota of dipteran insects is often derived from environmental bacteria33,34, we hypothesized that coprophagous flies could acquire manure-associated microbes and pathogens. Our analysis identified genomic signatures (VFs, ARGs, pathogen genomes) shared between the fly GIT and the cow manure microbiome, which suggests the potential for bacteria to passage from cow manure to flies. Collectively, our results indicate that Neomyia flies may serve as underrecognized carriers of zoonotic pathogens and may contribute to disease transmission on dairy farms.
Dairy farms support large populations of ecologically diverse fly taxa, which regularly interact with cattle hosts, farm workers, and bovine manure. While most synanthropic muscid flies utilize manure as a preferred oviposition site, adult flies will feed on different nutritional sources including manure (Musca domestica, Neomyia spp.), cattle blood (Stomoxys spp., Haematobia spp.), or cattle eye secretions (Musca autumnalis)35. To date, most studies on the muscid fly microbiota have been focused on M. domestica, whose gut microbiome is often dominated by Lactobacillales, Enterobacterales, and Bacillales16,17,18,19, and has been shown to participate in the carriage of zoonotic pathogens in both field and laboratory settings10,12,36,37,38. Recent 16S rRNA gene amplicon sequencing studies of M. domestica and Stomoxys flies collected from livestock facilities have shown that the majority of the fly microbiota can be identified in nearby manure samples13,16,19, indicating that the microbiota of flies is shaped by interactions with the animal feces or other elements of the local environment. Likewise, whole genome sequencing of E. coli isolates has revealed the presence of highly similar bacterial strains shared between M. domestica and animal manure samples24,25. In this study, we contribute to the growing body of fly-microbe literature through parallel metagenomic sequencing of flies and manure on a pasture based dairy farm. Nearly all flies in this study were molecularly identified as Neomyia flies, which although common to pasture based dairy farms in Europe8,39, remain highly understudied in comparison to other synanthropic muscid flies. Our study represents, to our knowledge, the first culture-independent analysis of the Neomyia fly gastrointestinal microbiota. Comparing the taxonomic and functional characteristics of the fly microbiome to bovine manure revealed commonly shared MAGs between fly and manure samples, which included bacterial taxa (Fibrobacter, Sutteralla, and various Bacteroidales) associated with the bovine GIT microbiome30. While termites and other social insects harbor specialized lineages of cellulose degrading Fibrobacter40,41, phylogenetic analysis revealed that the fly-derived Fibrobacter MAGs are closely related to Fibrobacter genomes constructed from manure samples collected at the same facility. Our analysis also revealed that, despite the presence of shared taxa, microbial community composition is still largely determined by sample type. This supports the hypothesis that, while flies acquire a substantial portion of their microbiota through the consumption of manure13,42, physiological conditions (pH, enzymatic activity, oxygen levels), environmental factors, and competition between resident microorganisms in the fly GIT all likely contribute to the microbial diversity we identified in the flies we sampled42. For example, our finding that rumen-associated obligate anaerobes, such as Bifidobacterium and members of the Lachnospiraceae family30,43,44, were enriched in manure microbiomes but not in the fly GIT suggests that these bacteria likely cannot effectively colonize the fly GIT. However, some sequences enriched in the fly GIT microbiome included taxa (Apilactobacillus, Entomomonas, and Frischella) commonly associated with nectarivorous insects, which may have been acquired through feeding on sugar substrates or sources of plant matter42,45,46,47. Frischella, the most highly enriched MAG in our fly samples, has been previously identified as a honeybee symbiont that can colonize a spatially restricted niche in the bee GIT and alter scab formation immune responses48,49. While Frischella has also been identified in fruit flies (Diptera: Tephritidae)50, this is the first report, to our knowledge, of Frischella in coprophagous flies and further studies are needed to determine if Frischella represents a specialized bacterial symbiont in the fly GIT.
Our functional metabolic predictions of MAGs suggest a potential role in providing mutualistic services to the host. A comparison of identified complete metabolic pathways in the common, unique to manure, or unique to fly GIT group of MAGs showed that the latter encoded several complete pathways (Fig. 2C). This included pathways involved in the metabolism of co-factors/vitamins (Vitamin B6/Pyridoxial-P biosynthesis, Lipoic acid biosynthesis) and amino acids (Valine/Isoleucine biosynthesis), which were attributed to Frischella. The biosynthesis of pyridoxial-P is particularly significant as insects must derive B vitamins from the diet or from microbial symbionts51. This pathway was present in several MAGs unique to manure and the fly GIT, including Frischella, a bacterium which may represent an important source of B-vitamin and amino acid provisioning due to its ubiquitous presence across fly samples.
The next objective of our study was to determine if the fly GIT can serve as a reservoir for manure-associated pathogenic microbes. We identified a set of VFs common to the fly microbiome, which included genes originating from both C. burnettii, the causative agent of Q fever52, as well as various E. coli pathotypes. Our analysis further revealed VFs that mapped with high coverage and were highly prevalent in the fly GIT. These included E. coli T3SS and C. burnetti T4SS effectors, which were significantly enriched in the fly GIT microbiome compared to cow manure (Fig. 3B; Supplementary Table 7). Genes related to secretion systems represented a substantial proportion of highly prevalent VFs in flies but were comparatively less prevalent in the manure microbiome (twenty-nine secretion system related VF genes were found in the prevalent fly GIT features but only six in the respective manure group features). Expression of secretion systems has been shown to promote colonization in other invertebrate hosts53,54, which could contribute to the ability of these bacterial pathogens to transition into the fly GIT niche and may help explain the high prevalence and coverage of the observed E. coli and Coxiella pathotypes in the fly GIT environment.
Beyond the mere presence of VFs across fly GIT microbiomes, we also found specific cooccurrence patterns suggesting potential cow manure to fly transmission through our bovine COI gene analysis. By mapping reads from the fly and manure metagenomes to the pathogen genomes associated with the VFs identified (Fig. 3, Supplementary Table 7), we identified four Escherichia, two Shigella, and two Coxiella genomes, which could be mapped to flies and manure samples that shared the same bovine COI (Fig. 4). We found that the relative pathogen abundance and coverage was normally higher in flies compared to manure samples (Supplementary Fig. 8), which suggests that manure-associated bacteria may be able to proliferate in the fly GIT13,42. Although we found multiple pairs of manure and fly samples with similar bovine COI genes that carried sequences associated with these pathogens, we also found instances of pathogen carriage where there was no overlap in COI gene similarity (i.e., flies with bovine COI sequences that were not similar to the bovine COI sequences from the manure samples), suggesting that the flies may have acquired these pathotypes elsewhere (e.g., previous feeding) but persisted in the fly GIT. Alternatively, the presence of pathotypes in the flies’ guts but not in the manure sample could be related to a temporal delay, if the animals may have shed some of these pathogens at some point before the collections took place, which the flies then acquired via feeding.
Our finding of C. burnetti strains from one manure sample in multiple flies could potentially indicate the ability of this pathogen to radiate across environments. The widespread persistence of C. burnetti RSA493 in flies is of significant concern due to the extremely low infectious dose required to cause disease symptoms52,55, and suggests an increased risk for Q fever among farm workers through fly to human transmission. The factors aiding to its wide dispersal could be related to both the multiple secretion systems present and the presence of a near-complete Pyridoxal biosynthesis pathway, which may be important for fly host physiology (see above). While this hypothesis is at present based only on the occurrence of predicted enzymes of predicted bacterial genomes (and thus remains unsubstantiated), future studies are warranted to explore whether the presence of human or animal pathogens in insect vectors may be facilitated by their ability to produce useful (by)products for their insect host.
We also found that manure and fly samples with similar bovine COI genes also harbored sequences mapped to potential E. coli and Shigella pathogens. The presence of E. coli or other Enterobacteriaceae in the fly GIT microbiome is not surprising, as these are known commensals in dipteran insects33,34, but our finding of large genomic sequences belonging to enterohemorrhagic E. coli O111 and O157 as well as the opportunistic urinary tract pathogen E. coli VR50 in both fly GIT and cow manure microbiomes is of concern, given that these strains are known to cause illness in humans56,57. Further comparative studies are needed to understand if manure-associated insects carry higher rates of virulent strains of E. coli or other zoonotic pathogens.
In addition to the acquisition of zoonotic pathogens in the fly GIT microbiome, there is also growing concern regarding the selection for ARGs through antimicrobial use on dairy farms. In Europe, ceftiofur, tetracyclines, and lincosamides are commonly used on dairy farms58, and this was confirmed through our examination of antimicrobial use records from our research farm. We found that antibiotic usage for the 48 animals included administration of antibiotics containing cephalosporins and tetracyclines as active compounds (data not shown). Our corresponding analysis of ARGs in the cow manure and fly GIT microbiomes revealed the presence of both tet and blaOXA genes, which are associated with resistance to tetracycline and beta-lactams, respectively59. While the congruency of these findings suggest that long-term use of these antibiotics may have resulted in the selection for these specific ARGs, we found no evidence of such a relationship, and we acknowledge that AMR emergence and dissemination are linked to various factors, including environment and ecology, and not solely to AMU60. However, it is important to note that our findings of the same ARGs in both cow manure and fly GIT microbiomes suggests the transfer of these genes across environments and would thus pose a significant risk for opportunistic pathogens in the fly GIT that could acquire these ARGs via horizontal transfer.
To our knowledge, this study is the first to offer metagenomic insight for the potential transmission, and persistence of specific microbes from cow manure to muscid (Neomyia) flies. Our study identifies a microbiome that is specific to the muscid fly GIT with genomic features that overlap with cow manure. Importantly, fly GIT and cow manure microbiomes share numerous VFs and ARGs belonging to known zoonotic pathogens. This suggests that these genomic features may be acquired by the fly during feeding, but more functional experiments and a wider sampling campaign, including more samples from multiple farms will be needed to confirm these findings. Furthermore, considering how the microbial content of manure potentially changes across seasons and years, longitudinal studies will be needed to examine how the transmission potential may vary depending on temporal change. Follow-up studies will also help formulate hypotheses about the environmental or physiological constraints preventing some microbes from transmitting to the fly GIT (e.g., physiological, ecological or related to the virulence of some microbes). Such studies may also elucidate the roles of flies in the dissemination of ARGs on active livestock facilities, as has been previously observed under laboratory conditions38,42,61.
We found strong specificity for pathogens like C. burnetti, which were recovered from multiple flies consuming the same cow manure. This suggests that some pathogens may more easily transmitted across environments, which presents significant occupational hazards for farm workers who are frequently exposed to these potential vectors. As such, flies could serve as a potential route of zoonotic transmission off of farms, given that house flies in agricultural settings can travel over three kilometers within 24 h62. Transmission of these pathogenic microbes to humans and other animals can occur through the contamination of food as flies disseminate GIT-associated bacteria when feeding via defecation and regurgitation of salivary fluids10,11,12. Our findings are further compounded by antibiotic use in the agricultural industry, which represents one of the largest users of antibiotics and is a known contributor to the rise of antibiotic resistance. Indeed, our study found the presence of beta-lactam resistance genes in known pathogens both in the fly GIT and in cow manure, which coincided with the usage of antibiotics containing these active compounds on our research farm. Overall, our study highlights the need for increased scrutiny of farm management practices to consider the role of flies and other potential vectors as risk factors for the dissemination of zoonotic pathogens both on and off of farm environments.
Methods
Sample collection
Twenty-nine flies, which were either standing on or hovering above freshly defecated cow manure, were collected using a fly net at an INRAE experimental dairy farm in Saint-Genès-Champanelle, France over the course of two days (15 and 17 September 2020). Following collection with a mosquito net, flies were placed on ice (which rendered them uncoscious immediately) for approximately four hours. They were then transferred (on ice) and dissected under a stereoscope on a petri dish filled with ice to keep them anaesthetized. Using sterile forceps, their GITs were dissected in a drop of sterile phosphate-buffered solution and placed into sterile Eppendorf tubes, which were stored at −80 °C until DNA extraction. The treatment of cow fecal samples collected the same day from the field has been described before30.
DNA extraction, library preparation, and sequencing
Genomic DNA was extracted from dissected fly GITs using a Qiagen DNeasy Blood and Tissue Kit (Qiagen, Germany) following manufacturer’s instructions. DNA quality and quantity were determined using a Nanodrop (ThermoFischer Scientific, Waltham, MA) and then sent to Novogene (Singapore) for sequencing. Library construction was performed using the PCR-free NEBNext Ultra II DNA Library Prep Kit, and all samples were sequenced in a single run of an Illumina NovaSeq 6000 using a 2 × 150 bp paired-end sequencing kit, generating a minimum of 60 million reads per sample. Sequencing of the 48 cow manure samples used in this study were performed using identical parameters, as described in Teseo et al.30. Manure sample reads are available under BioProject accession number PRJNA681986.
Data filtering, contig assembly, and MAG building
Following shotgun metagenomic sequencing, we performed data filtering, assembly, and bin construction using a pipeline established previously30. Briefly, raw reads were trimmed and filtered using Trimmomatic and assembled using megahit (v1.1.1) with the “−meta-large” option and a minimum of 300 bp contig length63,64. Autometa was used to map filtered reads to the assembled contigs and create a coverage table65. Prodigal was used to identify open reading frames (ORFs) and the resulting predicted proteins were used to compare against the NCBI non-redundant (nr) protein database (downloaded December 2020)66,67. Taxonomic classification of assembled contigs was performed through sequence comparison against the NCBI prokaryotic genome database68. Contigs greater than 1000 base pairs were binned by both taxonomy and sequence characteristics. MAGs were then constructed using the Autometa pipeline65. MAG completeness was determined using CheckM69, which resulted in a total of 42 non-redundant fly-derived MAGs with >85% completeness and <5% contamination.
Fly genotyping
To genotype the collected flies and build a host phylogeny, we first identified existing mitochondrial COI genes from the metagenome of each sample using KMA v1.3.11 against three partial reference sequences of the Neomyia cornicina COI (MW592695.1, KF919034.1, KU932144.2)70,71. The identified 29 COI sequences were compared to the NCBI’s non-redundant (nr) database (January 2023) using BLASTN, and the closest published sequences were obtained and used to build a phylogeny. For samples W15 and W20, for which the COI gene was more similar to the COI gene for Myospila meditabunda, we ran the KMA algorithm using the published M. meditabunda COI genes (KJ510632.1 and FJ025642.2). All sequences were aligned using MUSCLE and gaps were trimmed using trimAl72,73. Phylogenies were constructed using PhyML implemented in Seaview with 100 bootstraps, and the tree was further edited in Inkscape74,75,76.
Phylogenomic analysis of fly-derived MAGs
Phylogenomic trees were constructed for the 42 non-redundant fly GIT MAGs, the closest Refseq genomes used to identify these MAGs through Autometa, and the closest MAGs from the 2114 cow manure MAGs derived in Teseo et al.30. Single copy orthologs for MAGs within each dataset were identified using orthofinder and alignments were built using MUSCLE v3.8.3173,77. We removed all positions with gaps in 10% or more of the sequences using TrimAl unless this resulted in <50% of the original sequences and alignments were further tested for recombination using the Phipack software72,78. Concatenated alignments were built using AMAS, and model selection was performed with Prottest79,80. Maximum likelihood (ML) phylogenomic trees were built using RaxML v889 using the rapid Bootstrap analysis algorithm with 1,000 bootstraps, and the best scoring ML tree from each run was imported into R (Version 4.2.3) and further modified using ggtree81,82,83.
Analysis of microbial community structure
A recruitment analysis was performed on reads from all fly GIT and cow manure metagenomes against a combined database of the 42 non-redundant MAGs from the fly GIT metagenome and the 2114 non-redundant MAGs from our previous study30. To accomplish this, we first built a combined database of MAGs derived from the 29 fly GIT metagenomes (as described above) and a collection of metagenomes (N = 436) from cow manure samples that included a subset (N = 48) collected from the same farm and during the period of fly collection30. This combined database of MAGs was dereplicated using dRep with default options (primary clusters cutoff: 0.9, secondary clusters cutoff: 0.99) to generate a non-redundant MAG database84. Using KMA (k-mer alignment) a reference template was constructed from this non-redundant MAG database and the filtered reads from the 29 fly GIT and the 48 cow manure metagenomes were aligned to create a count table of contigs/MAGs in each sample. Sample counts were normalized by estimating genome equivalents using MicrobeCensus and used to create relative abundance proportion tables, which were used for all downstream analyses85. Abundance tables were further imputed to adjust 0 counts and MAGs with <20% prevalence across all samples were removed. Tables were transformed to centered-log ratios (CLR) using the zComposition package in R when relevant86. The 50 most abundant MAGs in the fly and manure samples were visualized as chord diagrams using the circulize package in R87. CLR transformed abundances of bacterial orders (MAGs were merged by taxonomy) were visualized using ggplot288. Sample dissimilarity based on the Aitchison distances of bacterial order abundances was calculated using vegan package in R and visualized using PCA89.
Enrichment and functional analysis of MAGs
To determine if specific MAGs from our non-redundant MAG database were enriched in either the fly GIT or cow manure samples, we performed a differential abundance test on normalized proportions of MAG abundance counts using the indicspecies package in R (Version 1.7.14, 1000 permutations)90, with p-values adjusted for false discovery rates using the “fdr” multiple testing correction. This showed that 21 MAGs were highly specific to the fly-samples and 41 to the cow manure samples (FDR corrected p < 0.05). For the cow manure group, out of the 41 which were statistically significant enriched, we chose the 21 that showed the biggest specificity to the cow-manure group (association value > 0.86). Finally, a set of MAGs defined as common between the fly GIT and cow manure metagenomes was determined as the intersection of the 100 most abundant MAGs in the fly GIT and cow manure dataset, again by picking the top 21, in order to create three groups with equal MAG numbers.
For each group of MAGs, we then extracted the functional annotation information, to identify common and unique orthologs. In short, predicted protein sequences were used to perform a diamond BLAST (v2.0.5.143) against the eggNOG protein database (v2020-11-12) with a 1e-05 E-value cutoff, and the best match of each query was then used as input for eggNOG-mapper (version 2.0.4)91,92. The resulting unique and shared KEGG KOs as well as the associated clusters of orthologous groups (COGs) were visualized using ggplot2 and ggvenndiagram in R88,93. Differences in COG counts were statistically evaluated using a Fisher’s exact test and Bonferroni correction for multiple comparisons. Complete functional pathways encoded by MAGs were determined through comparison against the KEGG Modules database.
Virulence factor and antimicrobial resistance gene annotation
Reads from the 29 fly GIT and 48 cow manure metagenomes were aligned against a KMA reference template constructed from nucleotide sequences obtained from the VFdb (October 2022) and the Resfinder (October 2022) databases94,95. Count tables were then normalized and transformed using MicrobeCensus and the zComposition package, as described above. Differences in CLR transformed abundances between fly and manure samples were determined using two-sided Wilcoxon signed-rank tests, with p-values adjusted for multiple comparisons using a Bonferroni correction based on the number of CLR transformed genes in each set (N = 399 VFs; N = 57 ARGs). To examine the correlation between ARGs and antimicrobial use on the farm, we first obtained records for all antimicrobial usage from 2017 to 2021. We then performed a coinertia analysis using the ‘ade4’ package in R to correlate antimicrobial use against the ARGs identified in our metagenomes96.
Identifying bovine mitochondrial COI genes from fly GIT metagenomes
To link individual flies to specific manure pools, we identified and compared mitochondrial COI gene sequences found in the fly GIT and manure metagenomes against the Bos taurus genome. This was accomplished by mapping the sequence reads of each metagenome against the mitochondrial COI (NC_006853.1) genes from the Bos taurus genome (GCF_002263795. 1_ARS-UCD1.2) using KMA. Although not all fly GIT metagenomes contained a mitochondrial COI gene sequence that mapped to Bos taurus (23/29 recovered), most of the ones that did have, presented a > 99% sequence identity. Comparisons of these sequences with the mitochondrial COI genes recovered from the cow manure metagenomes revealed sequence identities ranging mostly from 98.6 to 99.9% across the majority of the gene alignment (range: 73–100%, median: 99%).
We then examined whether multiple mitochondrial COI alleles could be identified in our fly GIT metagenomes, indicating that a given fly consumed cow manure that originated from more than one manure sample. To accomplish this, we identified the consensus mitochondrial COI gene sequences from each of the 48 cow manure metagenomes and the 23 fly GIT metagenomes for which mitochondrial COI genes were identified. Alignment of these mitochondrial COI genes using bowtie2 showed that for each fly GIT sample, only a single allele could be identified (allele frequency = 0.99)97. For both the fly samples and the combined fly-manure dataset, distance matrixes for the bovine COI gene alignments were calculated using an F81 model with the phangorn package in R98. Principal component analysis (PCA) suggested that the first component of the analysis could explain the most variation, and based on sample clustering, three groups were assigned.
Identification of pathogen genomes across samples
We next sought to determine if specific pathogen genomes could be linked to the identified bovine COI clusters, which could suggest transmission of microbial pathogens from manure to fly samples. A pathogen genome reference set (chromosomes and plasmids) was constructed by retrieving NCBI genomes associated with virulence factor genes that were identified as shared between fly and manure samples with at least 90% coverage of their gene length. The fly GIT and manure metagenomes were mapped using KMA to the retrieved genomes (excluding results with <10% genome coverage), and CLR transformed abundance was determined as described above.
Data availability
Raw Illumina reads from the 48 manure samples associated with Teseo et al.30 are available in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under BioProject accession number PRJNA681986; reads from the 29 fly samples generated in this study are available under BioProject accession number PRJNA911623. Scripts used for analysis and figure generation are available at https://forgemia.inra.fr/sapountzis0454medis/Neomyia_microbiome. Supplementary Tables 5 and 7 are available at https://github.com/kcoonlab/neomyia-metagenomics. All other data generated by this study are available as Supplementary Information herein.
References
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Guo, Y. et al. Association of common zoonotic pathogens with concentrated animal feeding operations. Front. Microbiol. 12, 810142 (2022).
McDaniel, C. J. et al. Humans and cattle: a review of bovine zoonoses. Vector-Borne Zoonotic Dis. 14, 1–19 (2014).
Call, D. R., Davis, M. A. & Sawant, A. A. Antimicrobial resistance in beef and dairy cattle production. Anim. Health Res. Rev. 9, 159–167 (2008).
Mahmud, B. et al. Longitudinal dynamics of farmer and livestock nasal and faecal microbiomes and resistomes. Nat. Microbiol. 9, 1007–1020 (2024).
Hansens, E. J. Fly populations in dairy barns. J. Econ. Entomol. 56, 842–844 (1963).
Meyer, J. A. & Petersen, J. J. Characterization and seasonal distribution of breeding sites of stable flies and house flies (Diptera: Muscidae) on eastern nebraska feedlots and dairies. J. Econ. Entomol. 76, 103–108 (1983).
Wall, R., Anderson, E. & Lee, C. M. Seasonal abundance and reproductive output of the dung flies Neomyia cornicina and N. viridescens (Diptera: Muscidae). Bull. Entomol. Res. 98, 397–403 (2008).
Manyi-Loh, C. et al. An overview of the control of bacterial pathogens in cattle manure. IJERPH 13, 843 (2016).
Sasaki, T., Kobayashi, M. & Agui, N. Epidemiological potential of excretion and regurgitation by Musca domestica (Diptera: Muscidae) in the dissemination of Escherichia coli O157: H7 to Food. J. Med. Entomol. 37, 945–949 (2000).
Butler, J. F. et al. Recontamination of food after feeding a 32P food source to biting muscidae. J. Med. Entomol. 13, 567–571 (1977).
Wasala, L. et al. Transfer of Escherichia coli O157:H7 to Spinach by House Flies, Musca domestica (Diptera: Muscidae). Phytopathology 103, 373–380 (2013).
Sommer, A. J., Kettner, J. E. & Coon, K. L. Stable flies are bona fide carriers of mastitis-associated bacteria. mSphere 9, e00336–24 (2024).
Bahrndorff, S. et al. Integrated genome-wide investigations of the housefly, a global vector of diseases reveal unique dispersal patterns and bacterial communities across farms. BMC Genom. 21, 66 (2020).
Khamesipour, F. et al. A systematic review of human pathogens carried by the housefly (Musca domestica L). BMC Public Health 18, 1049 (2018).
Neupane, S. & Nayduch, D. Effects of habitat and sampling time on bacterial community composition and diversity in the gut of the female house fly, Musca domestica Linnaeus (Diptera: Muscidae). Med Vet. Entomol. 36, 435–443 (2022).
Park, R. et al. Microbial communities of the house fly Musca domestica vary with geographical location and habitat. Microbiome 7, 147 (2019).
Bahrndorff, S. et al. Bacterial communities associated with houseflies (Musca domestica L.) Sampled within and between Farms. Wilson BA (ed.). PLoS ONE 12, e0169753 (2017).
Poudel, A., et al. Comparison of microbiota, antimicrobial resistance genes and mobile genetic elements in flies and the feces of sympatric animals. FEMS Microbiol. Ecol. 96, fiaa027 (2020).
Onwugamba, F. C. et al. The role of ‘filth flies’ in the spread of antimicrobial resistance. Travel Med. Infect. Dis. 22, 8–17 (2018).
Von Wintersdorff, C. J. H. et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 7, 173 (2016).
Gonçalves, J. L. et al. Incidence and treatments of bovine mastitis and other diseases on 37 dairy farms in Wisconsin. Pathogens 11, 1282 (2022).
Krömker, V. & Leimbach, S. Mastitis treatment—Reduction in antibiotic usage in dairy cows. Reprod. Domest. Anim. 52, 21–29 (2017).
Behrens, W. et al. Bacterial genome sequencing tracks the housefly-associated dispersal of fluoroquinolone- and cephalosporin-resistant Escherichia coli from a pig farm. Environ. Microbiol. 25, 1174–1185 (2023).
Hickman, R. A. et al. Dissemination of resistant Escherichia coli among wild birds, rodents, flies, and calves on dairy farms. Front. Microbiol. 13, 838339 (2022).
Junqueira, A. C. M., et al. The microbiomes of blowflies and houseflies as bacterial transmission reservoirs. Sci. Rep. 7, 16324 (2017).
Brito, I. L. & Alm, E. J. Tracking strains in the microbiome: insights from metagenomics and models. Front. Microbiol. 7, 712 (2016).
Douglas, G. M. & Langille, M. G. I. Current and promising approaches to identify horizontal gene transfer events in metagenomes (ed Dagan T.). Genome Biol. Evol. 11:2750–2766 (2019).
Handelsman, J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 68, 669–685 (2004).
Teseo, S., et al. A global phylogenomic and metabolic reconstruction of the large intestine bacterial community of domesticated cattle. Microbiome 10, 155 (2022).
McAlpine, J. F. et al. Manual of Nearctic Diptera (Research Branch Agriculture Canada, 1987).
Pi, Z. et al. The complete mitochondrial genome of Neomyia cornicina (Diptera: Muscidae). Mitochondrial DNA Part B 6, 2691–2692 (2021).
Engel, P. & Moran, N. A. The gut microbiota of insects – diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735 (2013).
Arellano, A. A., Sommer, A. J. & Coon, K. L. Beyond canonical models: why a broader understanding of Diptera-microbiota interactions is essential for vector-borne disease control. Evol. Ecol. 37, 165–188 (2023).
Moon, R. D. Medical and Veterinary Entomology (Elsevier, 2019)
Alam, M. J. & Zurek, L. Association of Escherichia coli O157:H7 with Houseflies on a Cattle Farm. Appl. Environ. Microbiol. 70, 7578–7580 (2004).
Ahmad, A., Nagaraja, T. G. & Zurek, L. Transmission of Escherichia coli O157:H7 to cattle by house flies. Prevent. Vet. Med. 80, 74–81 (2007).
Fleming, A. et al. Temporospatial fate of bacteria and immune effector expression in house flies fed GFP - Escherichia coli O157:H7. Med. Vet. Entomol. 28, 364–371 (2014).
Papp, L. & Garzó, P. Flies (Diptera) of pasturing cattle: some new data and new aspects. Folia Entomol. hungarica 46, 153–168 (1985).
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
Jewell, K. A. et al. A phylogenetic analysis of the phylum Fibrobacteres. Syst. Appl. Microbiol. 36, 376–382 (2013).
Nayduch, D. & Burrus, R. G. Flourishing in filth: house fly–microbe interactions across life history. Ann. Entomol. Soc. Am. 110, 6–18 (2017).
Hungate, R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol. Rev. 14, 1–49 (1950).
Creevey, C. J. et al. Determining the culturability of the rumen bacterial microbiome. Microb. Biotechnol. 7, 467–479 (2014).
Lysyk, T. J. Effects of Temperature, Food, and Sucrose Feeding on Longevity of the House Fly (Diptera: Muscidae). Environ. Entomol. 20, 1176–1180 (1991).
Holley, J. C. et al. Carpenter Bees (Xylocopa) harbor a distinctive gut microbiome related to that of honey bees and bumble bees (ed Rudi, K.). Appl. Environ. Microbiol. 88, e00203–e00222 (2022).
Neupane, S. et al. Sex-specific feeding behavior of adult house flies, Musca domestica L. (Diptera: Muscidae) (ed. Trout Fryxell R.). J. Med. Entomol. 60, 7–13 (2023).
Engel, P., Kwong, W. K. & Moran, N. A. Frischella perrara gen. nov., sp. nov., a gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int. J. Syst. Evolut. Microbiol. 63, 3646–3651 (2013).
Engel, P., Bartlett, K. D. & Moran, N. A. The Bacterium Frischella perrara causes scab formation in the gut of its honeybee host (ed. McFall-Ngai M. J.). mBio 6, e00193–15 (2015).
Ren, X. et al. Gut symbiotic bacteria are involved in nitrogen recycling in the tephritid fruit fly Bactrocera dorsalis. BMC Biol. 20, 201 (2022).
Serrato-Salas, J. & Gendrin, M. Involvement of microbiota in insect physiology: focus on B vitamins. (eds Weiss B., Yount J.). mBio 14, e02225–22 (2023).
Eldin, C. et al. From Q Fever to Coxiella burnetii Infection: a paradigm change. Clin. Microbiol. Rev. 30, 115–190 (2017).
Preston, G. M. Metropolitan microbes: type III secretion in multihost symbionts. Cell Host Microbe 2, 291–294 (2007).
Ganesan, R. et al. How It all begins: bacterial factors mediating the colonization of invertebrate hosts by beneficial symbionts. Microbiol. Mol. Biol. Rev. 86, e00126–21 (2022).
Jones, R. M. et al. The Infectious Dose of Coxiella Burnetii (Q Fever). Appl. Biosaf. 11, 32–41 (2006).
Beatson, S. A. et al. Molecular analysis of asymptomatic bacteriuria Escherichia coli strain VR50 reveals adaptation to the urinary tract by gene acquisition (ed McCormick B. A.). Infect Immun. 83, 1749–1764 (2015).
Ogura, Y. et al. Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc. Natl. Acad. Sci. USA 106, 17939–17944 (2009).
De Briyne, N. et al. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 175, 325–325 (2014).
Oliver, S. P., Murinda, S. E. & Jayarao, B. M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog. Dis. 8, 337–355 (2011).
Lin, Z. et al. Impact factors of the accumulation, migration and spread of antibiotic resistance in the environment. Environ. Geochem. Health 43, 1741–1758 (2021).
Gan, D., et al. Housefly gut microbiomes as a reservoir and facilitator for the spread of antibiotic resistance. ISME J. 18, wrae128 (2024).
Pickens, L. G. et al. Dispersal Patterns and Populations of the House Fly Affected by Sanitation and Weather in Rural Maryland. J. Econ. Entomol. 60, 1250–1255 (1967).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, D. et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Miller, I. J. et al. Autometa: automated extraction of microbial genomes from individual shotgun metagenomes. Nucleic Acids Res. 47, e57–e57 (2019).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 11, 119 (2010).
Pruitt, K. D., Tatusova, T. & Maglott, D. R. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35, D61–D65 (2007).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Parks, D. H. et al. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Bernasconi, M. V. et al. Phylogenetic relationships among Muscoidea (Diptera: Calyptratae) based on mitochondrial DNA sequences. Insect Mol. Biol. 9, 67–74 (2000).
Clausen, P. T. L. C., Aarestrup, F. M. & Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 19, 307 (2018).
Capella-Gutiérrez, S., Silla-Martínez, J. M. & Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973 (2009).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Galtier, N., Gouy, M. & Gautier, C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Bioinformatics 12, 543–548 (1996).
The Inkscape Team. “Inkscape: Open Source Scalable Vector Graphics Editor,” 2003. https://inkscape.org/.
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Bruen, T. C., Philippe, H. & Bryant, D. A simple and robust statistical test for detecting the presence of recombination. Genetics 172, 2665–2681 (2006).
Borowiec, M. L. AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ 4, e1660 (2016).
Abascal, F., Zardoya, R. & Posada, D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21, 2104–2105 (2005).
R Core Team. R: A language and environment for statistical computing. 2021.
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Yu, G. et al. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. (ed McInerny, G.). Methods Ecol. Evol. 8, 28–36 (2017).
Olm, M. R. et al. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Nayfach, S. & Pollard, K. S. Average genome size estimation improves comparative metagenomics and sheds light on the functional ecology of the human microbiome. Genome Biol. 16, 51 (2015).
Palarea-Albaladejo, J. & Martín-Fernández, J. A. zCompositions — R package for multivariate imputation of left-censored data under a compositional approach. Chemometrics Intell. Lab. Syst. 143, 85–96 (2015).
Gu, Z. et al. circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014).
Wickham H., Chang W. Package ‘ggplot2’. create elegant data visualisations using the grammar of graphics. https://doi.org/10.1007/978-0-387-98141-3.
Dixon, P. VEGAN, a package of R functions for community ecology. J. Vegetation Sci. 14, 927–930 (2003).
Cáceres, M. D. & Legendre, P. Associations between species and groups of sites: indices and statistical inference. Ecology 90, 3566–3574 (2009).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314 (2019).
Gao, C. -H., Yu, G. & Cai, P. ggVennDiagram: an intuitive, easy-to-use, and highly Customizable R Package to Generate Venn Diagram. Front Genet 12, 706907 (2021).
Liu, B. et al. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 50, D912–D917 (2022).
Zankari, E. et al. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644 (2012).
Dolédec, S. & Chessel, D. Co-inertia analysis: an alternative method for studying species–environment relationships. Freshw. Biol. 31, 277–294 (1994).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Schliep, K. P. phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 (2011).
Acknowledgements
We would like to thank Sabine Leroy and the entire farm staff at the INRAE research farm in Saint-Genès-Champanelle, France for their help in sampling and animal management, and the Mésocentre Clermont Auvergne University and AuBi platform for providing support regarding computation and storage resources. We would also like to thank the MEDIS, Coon and Suen laboratories for their support and insightful discussions. This study was supported by a MICA starting grant to P.S., two United States Department of Agriculture (USDA) National Institute of Food and Agricultura (NIFA) Hatch grants (#WIS06036 and #WIS04039 to K.L.C. and G.S., respectively), a UW Dairy Innovation Hub Short-Term, High-Impact award (to K.L.C.) and a presidential postdoctoral Fellowship from the Nanyang Technological University of Singapore to S.T. A.J.S. was additionally supported by a USDA AFRI Education and Workforce Development Predoctoral Fellowship (#2023-67011-40337).
Author information
Authors and Affiliations
Contributions
S.T. and P.S. conceived the study and collected samples. All authors contributed to the design of the analysis. A.J.S. and P.S. led the analyses and wrote the initial paper draft, while J.H.S., S.O., G.S., and K.L.C. contributed to revisions. All authors read and approved the final manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study exclusively involved coprophagous muscid flies (Diptera: Muscidae) and did not involve any vertebrates or higher invertebrates. All procedures, including anesthesia and dissection, were conducted in accordance with standard ethical guidelines for invertebrate research. As Muscidaeare not classified as higher invertebrates or regulated animals under institutional or national guidelines, no formal ethical approval was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sommer, A.J., Skarlupka, J.H., Teseo, S. et al. Genomic evidence for flies as carriers of zoonotic pathogens on dairy farms. npj Biofilms Microbiomes 11, 111 (2025). https://doi.org/10.1038/s41522-025-00685-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00685-y