Abstract
The evolution of antibiotic resistance and the propensity of methicillin-resistant Staphylococcus aureus to form biofilms impedes antibiotic therapy, which enkindles the rummage for novel therapeutic agents like bacteriophage endolysins. This study investigates the biofilm degradation activity of novel chimeric endolysin CHAPk-SH3bk compared to single domain construct CHAPk. The in-vitro biofilm degradation assay displayed higher antibiofilm activity of CHAPk-SH3bk compared to CHAPk on glass and steel surfaces. Treatment of CHAPk-SH3bk effectively inhibited biofilm formation of hospital-associated and bovine-origin MRSA. The in-vivo results displayed a higher reduction of 24 h MRSA-biofilm using CHAPk-SH3bk compared to CHAPk in mice skin infection model. Further, confocal laser scanning microscopy, scanning electron microscopy, and immunohistochemistry confirmed the in-vivo results. The study indicated that attachment of SH3b using glycine-serine linker to CHAPk increased the catalytic domains biofilm reduction ability. The study demonstrates that construction of novel chimeric endolysins by shuffling parental endolysin domains may increase their antibiofilm activity.
Similar content being viewed by others
Introduction
Antimicrobial resistance (AMR) is a global concern associated with the emergence of resistance against antimicrobials like antibiotics, antifungals, or antivirals in microorganisms like bacteria, fungi, and viruses respectively1. AMR has been declared as a top 10 global health threat by the World Health Organization (WHO) (“WHO releases report on state of development of antibacterials,”2). The worldwide spread of AMR ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) is the major cause of mortality and morbidity in humans and animals worldwide3. Staphylococcus aureus is now established as the most opportunistic Gram-positive pathogen amongst the ESKAPE pathogens and is ubiquitously found in both humans and animals since 18814. Antimicrobial resistance was first introduced in S. aureus against antibiotic penicillin and has evolved since against antibiotics methicillin and vancomycin5. The Methicillin-resistant Staphylococcus aureus (MRSA) was first instigated in hospital settings in the 1960s6. This was followed by spreading into the community as community-associated (CA)-MRSA in the 1990s, and into animal husbandry areas as livestock-associated (LA)-MRSA in 20037. The molecular mechanism behind the progression of methicillin resistance is the presence of the mobile genetic element called Staphylococcal cassette chromosome mec (SCC mec)8. The gene mec-A present on SCC mec cassette encodes penicillin-binding protein (PBP-2a) which is responsible for resistance against almost all commercially available βeta-lactam antibiotics9. MRSA is a persistent cause of human infections like skin infections such as impetigo primary abscesses, folliculitis, secondary abscesses, soft tissue infections, bloodstream infections, endocarditis, pneumonia, and osteomyelitis10. Apart from being anthropogenic, S. aureus also causes infection as mastitis in milch animals and gangrenous dermatitis in poultry11. The S. aureus is responsible for the progression of infections due to the biofilm formation associated with these diseases12. S. aureus has the ability to form biofilm on biotic and abiotic surfaces enhancing the resistance towards antibiotics. The biofilms formed on the catheters and prosthetic devices are a major concern in hospital settings13. The presence of polymerized polysaccharide layer on biofilms restricts the activity of antibiotics on the bacterial cells growing beneath it and delays the treatment14. The S. aureus biofilms has led to chronic human skin infections and chronic cases of mastitis in cattle15. The failure of antibiotics to degrade biofilms demands usage of novel antibacterial agents like bacteriophage endolysins. Bacteriophage endolysins are peptidoglycan hydrolases encoded by bacteriophages at the end of the lytic life cycle to release phage progenies from within16. The bacteriophage endolysins encoded against Gram-positive bacteria possess a modular structure that can be manipulated to obtain novel chimeric endolysins with increased specificity, solubility, and lytic activity17. The endolysins can easily target Gram-positive peptidoglycan layer from outside, hence they make a promising antibacterial agent against planktonic and biofilm-forming bacterial cells18. Previous studies display effective bacteriolytic and biofilm-reducing activity of endolysins in their natural configurations and novel chimeric constructs against Staphylococcus aureus. The endolysins like LysCSA13, LysSYL, LysP108, and Lys109 have shown anti-staphylococcal activity against planktonic cultures and sessile biofilms formed on various biotic and abiotic surfaces19,20,21,22 While a large number of endolysins have been studied in in-vitro conditions, very few have been proceeded for in-vivo studies. The endolysins like CF-301 and SAL-200 have been studied in-vivo models and have displayed effective activity against MRSA23,24. The endolysins XZ.700, LysP108, and Staphefekt SA.100 have been studied in murine skin-infection models19,25,26. More studies are needed to study the effect of endolysins in in-vivo biofilm models. The endolysin LysK expressed by Staphylococcal bacteriophage K is composed of two enzymatically active domains (EADs); an N-terminal CHAP domain, a central Amidase_2 domain, and a C-terminal SH3b cell wall binding domain27,28. The Endolysin LysK has been explored for its antibacterial activity against S. aureus and have shown effective lytic activities in various recombinant confirmations such as LysK CHAP domain (amino acids 1–165), CHAP (amino acids 38–164)-amidase construct (amino acids 210–334), and LysK221–390 (with a deletion of amidase domain 222–343)29,30,31. In our previous study, we have constructed a novel chimeric endolysin CHAPk-SH3bk by fusing the CHAP domain (amino acid 1–165) with the SH3b domain (amino acid 409–487) of endolysin LysK using the GS linker (GSGSGS). The novel construct was evaluated for in-vitro bactericidal activity against MRSA, and the results suggested that CHAPk-SH3bk possesses an effective activity against sessile MRSA compared to planktonic MRSA32. In the present study, we explored the biofilm reduction ability of CHAPk-SH3bk on biofilms formed on abiotic surfaces (glass and steel), and biotic skin surface through ex-vivo histological studies and in in-vivo mice skin infection model.
Results
The CHAPk-SH3bk designed by fusing the CHAP domain (amino acid 1–165) with the SH3b domain (amino acid 409–487) using the GS linker (GSGSGS), and CHAPk composed of (CHAP domain (amino acid 1–165) are chimeric derivatives of Staphylococcal phage endolysin LysK. The sequencing results of the cloned genes were submitted to National Center for Biotechnology Information (NCBI) GenBank under accession numbers OR709902 and OR709903 for CHAPk and CHAPk-SH3bk construct, respectively. The chimeric constructs were expressed under 1 mM IPTG induction, followed by immobilized metal ion affinity chromatography. The purified proteins were run through SDS-PAGE and western blot to display CHAPk-SH3bk and CHAPk with discrete bands at their predicted molecular weight of 28 kDa and 22.6 kDa, respectively32.
Effect of divalent cations (Ca2+, Mg2+, Mn2+) on lytic activity of CHAPk-SH3bk
The lytic activity of the CHAPk-SH3bk was determined in the presence of divalent cations (Ca2+, Mg2+, and Mn2+). The results displayed a decrease in the bacteriolytic activity of CHAPk-SH3bk with an increase in the concentration of divalent cations (Fig. 1a). The 20 mM Mn2+ ions displayed maximum inhibition in the activity of CHAPk-SH3bk. A subsided inhibition activity of 20 mM Ca2+ and Mg2+ cations was observed against lytic activity CHAPk-SH3bk in comparison to cation Mn2+. The significant increase in the inhibitory effect of Ca2+ cations against the bacteriolytic activity of CHAPk-SH3bk was observed in more than 10 mM concentration. The (5–20 mM) of Mg2+ ions displayed the least inhibition towards the activity of CHAPk-SH3bk with minimum increase in bacterial growth. Whereas the maximum activity of the expressed endolysin was displayed in the absence of any external divalent cations and in the presence of 5 mM Ca2+ ions.
a Effect of divalent cations (Ca2+, Mg2+, and Mn2+) on the bacteriolytic ability of CHAPk-SH3bk against S. aureus (ATCC® BAA-44™). b Effect of different NaCl concentrations on the bacteriolytic ability of CHAPk-SH3bk against S. aureus (ATCC® BAA-44™). The data are presented as the means ± SD of three replicates. *P < 0.05; ***P = 0.0004; ****P < 0.0001; ns, not significant.
Effect of NaCl on lytic activity of CHAPk-SH3bk
The effect of NaCl on the bacteriolytic activity of CHAPk-SH3bk was observed using turbidity reduction assay. The results displayed an elevation in lytic activity with a decrease in NaCl concentration (Fig. 1b). The lytic activity of CHAPk-SH3bk at 25 mM NaCl did not show any significant difference compared to that of control. Significant decrease in the lytic activity of CHAPk-SH3bk was observed at 300 mM of NaCl as compared to control (p-value; α < 0.05).
Biofilm reduction activity of CHAPk and CHAPk-SH3bk on steel and glass surface
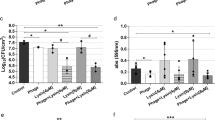
The crystal-violet staining assay was performed to analyse biofilm degradation activity of CHAPk and CHAPk-SH3bk on steel surface. The treatment of 0.05 µg/µl CHAPk-SH3bk displayed a significant reduction (Fig. 2a) on 24 h biofilm compared to CHAPk which showed a non-significant removal of biofilm preformed on steel surface. A biofilm reduction of ~25% was observed after the activity of CHAPk-SH3bk as compared to only ~14% activity of CHAPk (Fig. 2b).
a The biomass attached to the steel surface post-treatment was measured as the optical density at 570 nm. b Graph representing the percentage of biofilm reduced using 0.05 μg/μl of CHAPk and CHAPk-SH3bk from the steel surface. The data is presented as means ± SD of three replicates, *P < 0.05, ns- non-significant, **P = 0.0020; ****P < 0.0001.
The biofilm reduction ability of chimeric constructs was also studied on the 24-hour and 48-hour biofilms on glass surface. Figure 3a displays the higher biofilm reduction activity of CHAPk-SH3bk as compared to CHAPk on the preformed biofilms on glass surfaces. The CHAPk-SH3bk showed a significant biofilm reduction activity against MRSA S. aureus (ATCC® BAA-44™) as compared to the untreated negative control group. The chimeric construct CHAPk-SH3bk caused a reduction of 49% and 55% in 24 h and 48 h biofilm, respectively (Fig. 3b). While CHAPk also displayed significant activity against biofilms only a 14% and 26% depletion in 24 h and 48 h biofilms, respectively, was observed.
a The biomass attached to the glass surface post-treatment was measured as the optical density at 570 nm. b Graph representing the percentage of biofilm reduced using 0.05 μg/μl of CHAPk and CHAPk-SH3bk from the glass. c CLSM analyses of the DAPI (blue) and PI (red) stained MRSA 24 h biofilm on glass surface. The data is presented as means ± SD of three replicates, *P < 0.05, ns- non-significant, **P = 0.0020; ****P < 0.0001.
The higher biofilm reduction activity of CHAPk-SH3bk was confirmed by screening live/dead bacterial cells using CLSM on 24-hour biofilm. The staining was performed to determine cell viability and cell death using DAPI which stains DNA of live and damaged cells, and PI which only stains a dead or damaged cell. Figure 3c displays a significant increase in PI-stained red dead cells after CHAPk-SH3bk treatment as compared to negative control group. However, an increased number of DAPI-stained blue cells were observed along with few dead cells after CHAPk treatment. The maximum thickness and biomass of biofilms was analysed using COMSTAT (Table 1). The results displayed the maximum thickness of untreated S. aureus (ATCC® BAA-44™) was 19 μm, whereas CHAPk-SH3bk, and CHAPk treatment reduced thickness to 5 μm and 6 μm, respectively. The treatment of CHAPk-SH3bk reduced the biomass of bacterial cells attached to the glass by 14 units.
Biofilm formation inhibition activity of CHAPk and CHAPk-SH3bk
The biofilm inhibition assay was analysed using visual appearance in crystal-violet staining followed by measuring absorbance at OD570nm (Fig. 4a). The CHAPk-SH3bk showed significant biofilm inhibition activity against S. aureus (ATCC® BAA- 44™) and SA3 (Fig. 4b). The CHAPk-SH3bk inhibited biofilm formation by 32% and 55% in HA-MRSA and bovine-origin MRSA SA3 (Fig. 4c). The CHAPk displayed significant biofilm formation inhibition activity of 45% against bovine-origin MRSA SA3. While no significant activity of CHAPk was observed against hospital-associated S. aureus (ATCC® BAA- 44™) and bovine origin SA1, SA2, and SA4. The inhibition in the biofilm formation of MRSA S. aureus (ATCC® BAA- 44™) was confirmed using DAPI/PI live/dead staining. Figure 5 displays an increased number of PI-stained red dead cells after CHAPk-SH3bk treatment as compared to CHAPk-treated cells and untreated negative control cells. The COMSTAT analysis displayed the same maximum thickness of untreated and CHAPk-treated S. aureus (ATCC® BAA-44™). Whereas a decreased maximum thickness of 4μm was observed after CHAPk-SH3bk treatment (Table 2).
a Crystal violet staining results of biofilms after biofilm inhibition assay. b The graph represents OD570nm value for bacterial cells adhered to wells post-treatment (C- negative control, T- treated). c This graph represents the percentage of biofilm inhibition using 0.05 μg/μl of CHAPk and CHAPk-SH3bk as treatment. The data is presented as means ± SD of three replicates. *P < 0.05; ***P = 0.0006; ****P < 0.0001; ns, not significant.
Cytotoxicity of CHAPk and CHAPk-SH3bk against MDCK cell lines
The cytotoxicity of CHAPk and CHAPk-SH3bk was evaluated on MDCK cell line using MTT assay. The endolysin CHAPk and CHAPk-SH3bk used in different concentrations ranging from (0.005 µg/µl–0.5 µg/µl) were not found toxic against MDCK cells. The 100% cell viability was observed at all concentrations of CHAPk. Similarly, CHAPk-SH3bk treatment in concentration range 0.005 µg/µl–0.5 µg/µl on MDCK cells displayed 100% cell viability (Fig. S1). No cytotoxic effect was observed of 0.02 μg/μl vancomycin and low salt buffer (storage buffer of expressed endolysin) on the mammalian cell line (Fig. S2). The in-vitro cytotoxicity assay results present chimeric endolysins safe and non-toxic against mammalian cells. Therefore, CHAPK and CHAPk-SH3bk at 0.5 µg/µl were used further for in-vivo studies in animal models.
In-vivo biofilm reduction activity of CHAPk, and CHAPk-SH3bk on S. aureus infected mice skin infection model
The biofilm reduction activity of CHAPk and CHAPk-SH3bk was evaluated on the mice skin infection model. The abrasions created on mice skin were infected with MRSA S. aureus (ATCC® BAA-44™) for 24 h. The biofilm formed post 24 h on mice skin was treated with 0.5 µg/µl of CHAPk and CHAPk-SH3bk. The positive control groups were treated with 0.1 μg/μl of vancomycin. Post 20 h treatment, the 0.5 µg/µl of CHAPk-SH3bk reduced biofilm by ~0.5-log10 units in comparison to the negative control group (Fig. 6a). The vancomycin treatment reduced biofilm by ~0.8-log units as compared to the negative control group. While 0.5 µg/µl of CHAPk displayed no significant change in MRSA cell counts after treatment. The in-vivo assay displayed higher effective activity of CHAPk-SH3bk on 24-hour biofilm compared to CHAPk (Fig. 6b).
Visualization of the biofilm reduction activity of CHAPk and CHAPk-SH3bk on ex-vivo skin sample
The skin histology was studied after treating 24 h biofilm formed on the skin surface with 0.05 µg/µl of CHAPk and CHAPk-SH3bk. The histology of skin tissue without any bacterial inoculation can be observed after H&E staining and Gram-staining in Figs. 7a, S3a, where we can observe all the layers of skin without any break in the top layer and no bacterial contamination. Whereas the negative control skin sample infected with MRSA S. aureus (ATCC® BAA-44™), displays integration of bacterial colonies inside skin layers indicated with red arrows along with colonized and disintegrated top layer as shown in Figs. 7b, S3b. Remarkable changes were observed in the top layer of the CHAPk-SH3bk-treated skin samples with minimized colonization of the bacterial cells and reduced in-depth infection of MRSA (Figs. 7d, S3d). In comparison to CHAPk-SH3bk, CHAPk-treated skin tissue presented a disrupted skin layer, and a high concentration of infiltrated MRSA cells inside the skin layers indicated with red arrows in Figs. 7c, S3c. The H&E and Gram-staining results displayed the effective activity of CHAPk-SH3bk in the reduction of MRSA cells from the skin surface.
To further confirm the efficacious activity of CHAPk-SH3bk the histological sections of treated skin samples were further interrogated by immunohistochemistry using a primary antibody specific to Staphylococcus aureus and a secondary antibody with conjugate Alexa Fluor™ 488. The sections were visualized using a confocal microscope in the ranges 364 nm - 454 nm, and 499 nm - 520 nm for observing DAPI stained blue colored mammalian and Alexa Fluor™ 488 stained green colored MRSA cells, respectively. The skin tissue without MRSA inoculation displayed only DAPI-stained skin cells (Fig. 8a). While negative control skin tissue displayed migration of MRSA cells into deeper layers and colonization of bacterial cells on the top layer indicated by red arrows (Fig. 8b). The skin tissue treated with CHAPk-SH3bk displayed a thin layer of S. aureus on the top layer with no infiltration inside the tissue (Fig. 8d). Whereas, the skin tissue treated with CHAPk shows a large number of MRSA cells integrated inside the skin top layer, as indicated with red arrows in Fig. 8c.
CLSM and SEM analysis of ex-vivo skin samples
The ex-vivo treated and untreated skin tissue samples were visualized using DAPI/PI staining. The live/dead cells were screened on the ex-vivo skin surface using CLSM. The negative control tissue can be seen filled with live DAPI-stained blue cells, and only a few red dead cells are stained with PI (Fig. S4a). The CHAPk-SH3bk-treated skin tissue displayed an increased number of PI-stained red-colored dead cells compared to CHAPk-treated skin tissue (Figs. S4b-4c). The biofilm reduction ability of CHAPk-SH3bk on ex-vivo skin samples was visualized using FESEM. As shown in Fig. 9a extensive cell aggregation and polysaccharide matrix formation were observed on skin tissue after 48-hour biofilm formation. The treatment of 0.05 μg/µl CHAPk-SH3bk displayed a decrease in bacterial concentration as compared to negative control and CHAPk-treated skin tissue. The damaged and deformed MRSA cells with perturbations can be observed after CHAPk-SH3bk treatment as indicated by red arrows in Fig. 9c. The CHAPk treatment displays a decrease in polysaccharide matrix over the biofilm, but growing cells with smooth cell wall surfaces and dividing cells can be observed in Fig. 9b. The SEM images also show a higher number of primary connections forming between growing cells to form biofilm in CHAPk-treated skin.
Discussion
Staphylococcus aureus is a common commensal bacteria inhabiting almost 30% of the human population in different areas like skin, armpits, and nostrils33. With time S. aureus has developed into a nosocomial pathogen causing various infections in human and animal health sectors. The development of antibiotic resistance and biofilm formation ability of S. aureus creates trouble in eradicating and treating bacterial skin infections34. The biofilms are formed due to extracellular polysaccharide polymerization over bacterial cells attached to abiotic and biotic surfaces. The presence of this polysaccharide layer inhibits the access of antibiotics to persister cells present beneath it, thus delaying the treatment14. This study reports the antibiofilm efficacy of novel chimeric endolysin CHAPk-SH3bk in in-vitro, ex-vivo conditions, and in-vivo mice skin infection model. The presence of different divalent cations and NaCl in different biological fluids and physiological environments can impact the bacteriolytic activity of endolysins. As observed in the results the presence of divalent cation Mn2+ in concentration more than 10 mM strongly inhibited the anti-staphylococcal activity of endolysin CHAPk-SH3bk. Whereas, the presence of divalent cations Ca2+, and Mg2+ did not inhibit the CHAPk-SH3bk considerably in concentrations lower than 10 mM. In earlier studies, the activity of CHAPk was observed to decrease in the presence of calcium and magnesium ions in concentrations more than 10 mM/L35. In another study, it was observed that 5 mM of Ca2+, Mn2+, and Mg2+ cations did not influence the activity of parental endolysins LysK36. Thus, we can conclude that low concentrations of Ca2+, and Mg2+ cations do not influence the activity of CHAPk-SH3bk, whereas Mn2+, and Ca2+ ions in concentrations more than 10 mM greatly inhibited the bacteriolytic activity of novel chimeric endolysin. The calcium ions have been reported to bind CHAP domain and assist in the catalytic activity by conserving the structure of the domain37. While no such binding site of magnesium ions have been detected in CHAP domain. Hence, more studies are needed to study the mode of action of magnesium ion with CHAP domain. The results displayed that the activity of CHAPk-SH3bk decreased significantly with increased NaCl concentration. Similar results were observed for chimeric constructs of LysK CHAPk, and CHAP-amidase, a decrease in the lytic activity was observed at 300 mM NaCl31,35. The studies concluded that the activity of endolysin LysK construct CHAPk-SH3bk decreases with an increase in NaCl concentration.
The anti-staphylococcal activity of CHAPk-SH3bk in in-vitro experiments have been explored stating that this novel-chimeric construct displays bactericidal activity against HA and LA-MRSA32. The biofilm formation ability of MRSA on abiotic surfaces like glass and steel is a major concern in human health sectors. In the present study, it was observed that CHAPk-SH3bk effectively removed 24 h and 48-hour preformed biofilms from the glass surface in a low concentration of 0.05 µg/µl as compared to CHAPk. The association of HA-MRSA with medical equipment like catheters and prosthetic devices due to the biofilm formation creates hindrance in the bacterial treatment. A significant reduction of 24 h preformed biofilm from the steel surface was observed after treatment with a low dose of 0.05 µg/µl of CHAPk-SH3bk. Also, the results showed that CHAPk-SH3bk inhibits the biofilm formation of HA-MRSA by 32%. These results conclude that with an increase in dosage of CHAPK-SH3bk, a more effective reduction of biofilm can be observed on glass and steel surfaces. Hence, we hypothesize that the novel chimeric construct CHAPk-SH3bk can be incorporated in the form of antimicrobial sprays and used in hospitals to reduce and inhibit biofilm formation. The association of MRSA biofilms in skin and mastitis infections is a major concern in human and animal health sectors, respectively. The toxicity of CHAPk and CHAPk-SH3bk was analyzed on epithelial mammalian cells. No toxicity was observed against mammalian MDCK cell lines, and previous studies also display the inactivity of endolysins on mammalian cells due to the absence of cell wall outside eukaryote cells38. The activity of expressed CHAPk-SH3bk and CHAPk on MDCK cells indicates their potential for safe and effective usage in therapeutics.
The development of resistance against antibiotics and their inability to treat persister cells found in biofilms are major drawbacks of antibiotic treatment. The bactericidal activity of CHAPk and CHAPk-SH3bk was analyzed in the mice skin-infection model. The present study reports an insignificant reduction in MRSA CFU post 4-hour infection of MRSA S. aureus (ATCC® BAA-44™) using 0.5 µg/µl (10 µg) CHAPk. Whereas, in the previous study of in-vivo mice nasal infection model ~15 µg/µl of CHAPk exhibited around 2-log reduction in S. aureusXen29 post-1 h of infection39. In the ex-vivo porcine skin model, post 30 min of infection the treatment with 60 µg of CHAPk reduced ~99% of bovine-origin S. aureus DPC524640. These results demonstrate that a dose of >10 µg is required for a significant activity of CHAPk in in-vivo experiments.
Biofilm formation is a critical issue related to human skin and mucosal layer infections, and chronic bovine mastitis cases in cattle. The results of this study displayed ~0.5-log10 reduction of 24 h biofilm in mice skin-infection model post-treatment with 0.5 µg/µl of novel chimeric construct CHAPk-SH3bk. This shows that a more prominent activity of CHAPk-SH3bk can be observed by increasing the dose of this novel chimeric construct. This study reports a non-significant activity of 0.5 µg/µl CHAPk in biofilm reduction of 24 h biofilm in in-vivo skin infection model. Previous in-vitro studies have shown that CHAPk is more effective against planktonic cells as compared to sessile MRSA cells, and a high concentration of CHAPk is required to cause an effective degradation of MRSA biofilm. Additionally, it was observed that when CHAPk was attached to the SH3b domain of lysostaphin, it significantly increased the anti-biofilm activity of the CHAPk domain41. Another chimeric endolysin XZ.700 carrying SH3b domain of endolysin Ply2638 has also displayed effective activity against S. aureus in the form of cream formulation in skin-infection model26. This exhibits the importance of the cell wall binding domain in enhancing the biofilm-reducing ability of the CHAPk catalytic domain. The enhanced activity of CHAPk domain when associated with SH3b domain of LysK was observed even at the concentration of 0.05 µg/µl. The ex-vivo histology studies confirmed the effective anti-biofilm activity of 0.05 µg/µl CHAPk-SH3bk compared to CHAPk. This study presents the novel chimeric endolysin CHAPk-SH3bk as a prominent anti-biofilm agent. This study indicates the novel chimeric endolysin CHAPk-SH3bk as a prominent anti-biofilm agent. We propose that the novel chimeric endolysin CHAPk- SH3bk has more potential to reduce formed biofilm in both in-vitro and in-vivo conditions than the single domain construct CHAPk. Moreover, the higher activity of CHAPk-SH3bk was also observed against biofilms formed on steel, glass, and skin surfaces. This study recommends the usage of chimeric construct CHAPk-SH3bk for the depletion of biofilms associated with hospital-related medical equipment and livestock-associated MRSA infections. Further, the anti-biofilm activity of CHAPk-SH3bk needs detailed exploration in the in-vivo mice mastitis model to subject this novel endolysin as a potential antimicrobial for treating biofilm formation in the bovine udder.
In conclusion, the present study reveals the activity of novel chimeric endolysin CHAPK-SH3bk against biofilms formed on biotic and abiotic surfaces. The CHAPk-SH3bk cleared MRSA biofilms from glass, steel, and skin surfaces more effectively compared to CHAPk. The study also provides evidence of the effective activity of CHAPk-SH3bk in inhibiting the formation of biofilms by HA-MRSA and LA-MRSA. The in-vivo anti-biofilm assay and the ex-vivo histological studies confirmed that the addition of cell wall binding domain SH3bk to catalytic domain CHAPk enhanced its anti-biofilm activity. Altogether, the study provides a promising anti-staphylococcal activity of CHAPk-SH3bk in an antibiotic-exempted environment. Further dose-ranging studies are obligatory to explore the role of the CHAPk domain against HA-MRSA. Additional studies are required to investigate the ability of chimeric endolysin CHAPk-SH3bk in in-vivo blood-stream infection, osteomyelitis, mastitis, and endocarditis models for therapeutic usage in MRSA-associated infections in humans and livestock.
Methods
Bacterial strains
The Escherichia coli strains used for gene cloning and protein expression include E. coli (DH5α) and E. coli BL21 (DE3). The hospital-associated Staphylococcus aureus strain used in this study comprises the MRSA S. aureus (ATCC® BAA-44™) purchased from HiMedia, India. The bovine-origin MRSA isolates used in this study were manually isolated from mastitis-infected milk samples. Isolation of S. aureus was carried out as explained in the previous publication42. Briefly, the yellow-colored colonies obtained after streaking the infected milk samples on mannitol salt agar (MSA) plates (GMH118, HiMedia, India) supplemented with 4 μg/ml oxacillin were collected and stored as glycerol stocks. The bacterial cultures were activated from glycerol stock and subjected to DNA isolation followed by polymerase chain reaction (PCR). The methicillin resistance gene mecA and virulence genes (coa, and nuc) were detected using PCR. The primer details are mentioned in Table S1. The mannitol-fermenting yellow-colored colonies, positive for genes (mecA, coa, and nuc) and phenotypically resistant to oxacillin were confirmed as MRSA.
Cloning of chimeric constructs (CHAPk-SH3bk, and CHAPk), Expression, and Purification
The chimeric proteins CHAPk and CHAPk-SH3bk were cloned in expression vector pET22b(+), expressed, and purified as described previously32. Briefly, the synthetic E. coli optimized gene constructs of CHAPk and CHAPk-SH3bk were obtained from GenScript, USA. The gene sequences were subcloned into a pET-22b (+) expression vector inclusive of C-terminal 6 ×His-tag and pelB signal sequence. The cloned gene sequences of CHAPk and CHAPk-SH3bk were subjected to Sanger sequencing using T7 terminator and promoter primer. The plasmids positive for CHAPk and CHAPk-SH3bk, respectively, were transformed into E. coli BL21 (DE3) competent cells and protein expression was induced using IPTG (Thermo Scientific™ IPTG, dioxane-free, R0392), added to a final concentration of 1 mM. The expressed protein located in the periplasmic space of E. coli was derived after conferring the cells with cold osmotic shock. The SDS-PAGE analysis followed by a western blot was performed to detect the expressed proteins. Expressed proteins were subjected to Ni-IDA affinity chromatography to obtain purified proteins using the G-His protein purification kit (GDH01A, GCC BIOTECH (INDIA) PVT. LTD.). The purified proteins were maintained in low salt buffer (10 mM Tris-HCl, 150 mM NaCl, 1% glycerol; pH 7.5) and quantified using the Bradford assay.
Characterization of endolysin CHAPk-SH3bk: Effect of divalent cations and NaCl
The impact of divalent cations on the lytic activity of endolysin CHAPk-SH3bk was determined as described previously with modifications43. Briefly, the overnight culture of MRSA S. aureus (ATCC® BAA-44™) was prepared in Brain Heart Infusion (BHI) broth (SRL, 87864, India). The bacterial cells were harvested and resuspended in 1X PBS (pH = 7.4) to adjust the OD600nm = 0.1. Different concentrations of CaCl2, MgCl2, and MnCl2 (0 mM, 5 mM, 10 mM, 15 mM, and 20 mM) were prepared in 1X PBS (pH= 7.4). The 5 μg of endolysin CHAPk-SH3bk was incubated in 50 μl of CaCl2, MgCl2, and MnCl2 solution for 30 min at 37 °C. The treated endolysins were added to the 50 μl of MRSA S. aureus (ATCC® BAA-44™) culture and incubated for 30 min at 37 °C. The MRSA cell concentration post 30 min of incubation was determined by measuring OD at 600 nm. The lytic activity of CHAPk-SH3bk was also analyzed in the presence of NaCl. The NaCl was prepared in 1X PBS (pH= 7.4) in various concentrations (0 mM, 25 mM, 50 mM, 150 mM, 300 mM). The 5 μg of chimeric construct CHAPk-SH3bk was incubated in 50 µl NaCl prepared in different concentrations for 30 min at 37 °C. The NaCl-treated CHAPk-SH3bk was added to 50 μl of MRSA S. aureus (ATCC® BAA-44™) culture. The thoroughly mixed cultures were incubated for 30 min at 37 °C, and post-treatment OD of bacterial culture was measured at 600 nm.
Biofilm reduction activity on steel surface
The biofilm reduction ability of endolysin CHAPk and CHAPk-SH3bk was observed against preformed biofilms on steel surfaces as previously mentioned with slight modifications21. Briefly, the steel surgical blades (3.1 cm long, type: No. 15) were sterilized by submerging them in 100% ethanol followed by autoclaving. The overnight culture of MRSA S. aureus (ATCC® BAA-44™) prepared in Tryptone Soya Broth (TSB) (LQ009A, Himedia, India) supplemented with 0.25% D- (+) -glucose (G8644, Sigma) were diluted (1:50) to obtain OD ~ 0.1 at 600 nm. The steel blades were placed in 50 mm microplates and incubated in 200 μl of bacterial culture dissolved in 3 ml TSB with 0.25% glucose for 24 h at 37 °C. The biofilms were washed twice with 1× PBS, and treated with 0.05 μg/μl of CHAPk and CHAPk-SH3bk suspended in TSB with 0.25% glucose. Negative control blades were suspended in TSB with 0.25% glucose followed by incubation for 18 h at 37 °C. After treatment, the steel blades were washed with 1X PBS and kept for drying at 37 °C for 15 min. The biomass attached to the blades was stained with 0.1% w/v crystal violet for 15 min followed by washing with autoclaved water and solubilized in 33% glacial acetic acid. The OD of the final solution was measured at 570 nm.
Biofilm reduction activity on glass surface
The biofilm reduction ability of expressed chimeric endolysin CHAPk and CHAPk-SH3bk was observed against preformed biofilms on glass surfaces. The glass coverslips (Circular, 10 mm diameter) were sterilized by submerging them in 100% ethanol and followed by autoclaving. The coverslips were then placed in 12-well culture plates and incubated with 200 μl of MRSA S. aureus (ATCC® BAA-44™), prepared in 2 ml TSB with 0.25% glucose for 24 h and 48 h at 37 °C. The preformed biofilms were treated with 0.05 μg/μl of CHAPk and CHAPk-SH3bk suspended in TSB with 0.25% glucose after washing twice with 1X PBS. Control coverslips were suspended in TSB with 0.25% glucose followed by incubation for 18 h at 37 °C. The crystal violet assay was performed as described above and OD of the final solution was measured at 570 nm. The 24 h biofilm degradation was confirmed using 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI, D8417-1MG, Sigma) and propidium iodide (PI, 11195-10MG, SRL) live/dead staining by confocal laser scanning microscopy. The established biofilms were washed twice with 1X PBS and then stained with 300 μl of PI (500 nM prepared from 1 mg/ml of PI stock solution) for 15 min. Washed with 1X PBS thrice and stained with 300 μl of DAPI (10 μg/ml prepared from 1 mg/ml of DAPI stock solution) for 15 min. The stained coverslips were washed thrice with 1X PBS and mounted using 90% glycerol. The biofilms were analyzed at 100X followed by z-stack scanning using CLSM (Leica TCS SP8, AOBS-Acousto Optical Beam Splitter based, Germany). Three-dimensional projections of the biofilms were analyzed using ImageJ44. The biomass and maximum thickness of biofilms were analyzed using COMSTAT software45.
Biofilm formation inhibition assay
The assay was performed to study the ability of CHAPk, and CHAPk-SH3bk to inhibit biofilm formation of MRSA. The overnight culture of hospital-associated S. aureus (ATCC® BAA-44™) and four bovine-origin MRSA isolates (SA1, SA2, SA3, and SA4) was reinoculated for 3 h in TSB supplemented with 0.25% D- (+) -glucose. The culture was diluted to obtain OD600nm ~ 0.1 and 100 μl of diluted culture was added to a 96-well tissue culture plate. The 0.05 μg/ml of CHAPk and CHAPk-SH3bk were added to the experimental wells and incubated at 37 °C for 24 h. The negative control wells were added with 100 μl of diluted bacterial culture and 100 µl of TSB supplemented with 0.25% glucose. After 24 h incubation, the wells were washed with 1X PBS and stained with 0.1% w/v crystal violet for 15 min. The stain was aspirated out and washed with autoclaved water. The plate was air dried and biomass was solubilized in 33% glacial acetic acid. The OD of the final solution was measured at 570 nm. The inhibition of biofilm formation by chimeric endolysins CHAPk, and CHAPk-SH3bk was further observed by screening live/dead cells with DAPI/PI staining. The glass coverslips were incubated with S. aureus (ATCC® BAA-44™) bacterial culture (OD600nm ~ 0.1) and 0.05 μg/μl of CHAPk and CHAPk-SH3bk at 37 °C for 24 h. Following incubation, slides were washed with 1X PBS and stained with DAPI and PI as described above. The prepared slides were subjected to imaging at 100X and z-stack scanning using CLSM (Leica TCS SP8, AOBS-Acousto Optical Beam Splitter based, Germany).
Cytotoxicity activity
The cytotoxicity of CHAPk and CHAPk-SH3bk was evaluated on mammalian Madin-darby canine kidney (MDCK) cell lines, used as an epithelial model implementing MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide) assay as described earlier with slight modifications46. Briefly, the MDCK cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma, USA) with 10% Fetal Bovine Serum (ThermoFisher Scientific), 1% penicillin and streptomycin (Sigma, USA) in an incubator at 37 °C and 5% CO2. In a 96-well plate 8000 cells/well were seeded in 100 μl DMEM media and incubated at 37 °C, 5% CO2 for 24 h. Post 24 h CHAPk, and CHAPk-SH3bk were added at different concentrations (0.005 μg/μl, 0.05 μg/μl, 0.1 μg/μl, 0.15 μg/μl, 0.25 μg/μl, and 0.5 μg/μl) suspended in DMEM media to the wells and incubated at 37 °C, 5% CO2 for 24 h. The 100 μl of low salt buffer was used as buffer control, 0.02 μg/μl vancomycin was used as the positive control, and 100 μl of DMEM media was used for the negative control. After treatment media was aspirated and 100 μl of MTT (0.5 mg/ml stock solution) was added to wells and incubated at 37 °C in the dark for 4 h. After 4 h, the MTT solution was aspirated out, and formazan crystals were dissolved in 200 μl Dimethyl sulfoxide (DMSO), and OD was taken at 570 nm.
In-vivo evaluation of biofilm reduction ability in mice skin infection model
The in-vivo biofilm reduction assay was performed according to the approved guidelines from the Institutional Animal Ethics Committee (IAEC), Kirori Mal College, University of Delhi, New Delhi, India (Registration no. 1666/GO/Re/S/12/CPCSEA). The in-vivo experiments were performed as per the protocol no. KMC/IAEC/2024/02. The 6–8-week-old female Balb/c mice (body wt.: 20–25 g) were used for the non-surgical in-vivo experiment. All mice were kept for care and use in laboratoryas per the National Guidelines provided by the Committee for the Purpose of Control and Supervision of the Experiments on Animals (CPCSEA). For antibiofilm activity assessment of CHAPk and CHAPk-SH3bk, the MRSA murine skin infection model was prepared as earlier described previously with few modifications26. The mice were divided into four experimental groups (n = 3). The mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) and the dorsal surface of the mice was disinfected using 70% ethanol. The disinfected area was shaved to remove the hairs and create a dermal abrasion of 2 cm2 in the skin area. The overnight culture of MRSA S. aureus (ATCC® BAA-44™) was grown in TSB supplemented with 0.25% D- (+) -glucose. The bacterial inoculum was prepared by diluting overnight culture to procure an OD600nm of 1.0 and resuspended in 1X PBS. To attain a 24 h biofilm on mice skin, the shaved portion of the skin was sterilized with 70% ethanol and inoculated with 20 μl of the prepared bacterial suspension. The 24 h formed biofilms were treated with 0.5 μg/μl of CHAPk, 0.5 μg/μl of CHAPk-SH3bk, 0.1 μg/μl of Vancomycin, and 20 µl 1X PBS in CHAPk treated, CHAPk-SH3bk treated, positive control, and negative control groups, respectively for 20 h. Post-treatment the mice were anesthetized using mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), and experimental skin area was collected in 5 ml 1X PBS. The tissue was vortexed and properly scrapped to suspend all the bacterial cells in 1X PBS. The obtained culture was serially diluted and plated on the MSA agar plate supplemented with 20 μl oxacillin (1 mg/ml stock solution) for CFU counting. Skin samples from sacrificed mice were kept frozen at −80 °C until histopathological studies were carried out.
Ex-vivo evaluation of biofilm reduction ability using Hematoxylin and eosin (H&E) and Gram-staining
The ex-vivo skin samples of a 2 cm2 area were obtained from sacrificed mice. Skin samples were cleaned using a scalpel to remove underneath fat tissues to obtain skin with unvaried thickness. The skin samples were washed with 1X PBS, sterilized using 70% ethanol, and stored in 1X PBS. The overnight culture of MRSA S. aureus (ATCC® BAA-44™) was prepared in TSB supplemented with 0.25% D- (+) -glucose and diluted to obtain culture with OD600nm = 1.0. The culture was washed and resuspended in 1X PBS. The skin tissues were placed in 12-well plates and inoculated with 200 μl MRSA S. aureus (ATCC® BAA-44™) suspended in 2 ml 1X PBS for 24 h at 37 °C for biofilm formation. Post 24 h of infection, the tissue was treated with 0.05 μg/μl of CHAPk, and CHAPk-SH3bk suspended in 1X PBS. The negative control group was treated with 1X PBS. Post-treatment, the treated skin tissues and untreated skin samples were processed for paraffin block preparation. The skin tissue samples were fixed in 10% neutral-buffered formalin (NBF) overnight at room temperature. The formalin-fixed skin samples were embedded in paraffin, and processed for sectioning. The 5-micron thick skin sections were proceeded for H&E-staining and Gram-staining as described in the previous publications47,48. The H&E and Gram-stained slides were imaged with bright field microscopy (Cilika BT-P-2021, India).
Ex-vivo evaluation of biofilm reduction ability using Immunohistochemistry (IHC)
Immunohistochemistry was performed on the sectioned skin samples to detect the presence of HA-MRSA in infected skin samples after the treatment of CHAPk, and CHAPk-SH3bk. Following deparaffinization and rehydration, slides were incubated in sodium citrate buffer (pH = 6) at 95–100 °C for 15 min for antigen retrieval. The slides were washed twice with distilled water followed by washing with 1X TBS (Tris-buffered saline). The slides were blocked with 1% BSA (Bovine Serum Albumin) prepared in 1X TBS with 0.025% Triton-X100 for 1 h at room temperature in a humidified chamber. The slides were then incubated overnight in primary antibody Staphylococcus aureus Polyclonal Antibody (Invitrogen, PA1-7246) prepared in 0.025% Triton-X100 TBS with 1% BSA in 1:100 dilution at 4 °C. Successively, slides were washed twice in 1X TBS with 0.025% Triton-X100 and incubated with Goat anti-Rabbit IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (Invitrogen, A11008) in dilution 1:500 prepared in 0.025% Triton-X100 TBS with 1% BSA for 1 h in dark at room temperature. The slides were washed thrice with 1X TBS with 0.025% Triton-X100 and mounted using SlowFade™ Gold Antifade Mountant with DAPI (Invitrogen, S36939). The prepared slides were imaged at 20X using confocal laser scanning microscopy (CLSM, Leica TCS SP8, AOBS-Acousto Optical Beam Splitter based, Germany).
Ex-vivo evaluation of biofilm reduction ability using confocal laser scanning microscopy (CLSM) and scanning electron microscope (SEM)
The ex-vivo skin samples of 2 cm2 square area and after sacrificing mice. Skin samples were scrubbed to remove underneath fat tissues to obtain skin with unvaried thickness. The skin samples were washed with 1X PBS followed by sterilization using 70% ethanol and UV treatment. The overnight culture of MRSA S. aureus (ATCC® BAA-44™) was diluted to obtain culture with OD600nm = 1.0 and suspended in 1X PBS. The skin tissues were placed in 12-well plates and inoculated with 200 μl MRSA S. aureus (ATCC® BAA-44™) suspended in 2 ml 1X PBS for 48 h at 37 °C for biofilm formation. Post-biofilm formation, the tissue was treated with 0.05 μg/μl of CHAPk, and CHAPk-SH3bk suspended in 1X PBS. The negative control group was treated with 1X PBS. Post-treatment, the skin tissues were prepared for CLSM analysis using DAPI/PI staining as described above. For SEM imaging skin samples were prepared as previously described32. Briefly, the skin tissues were fixed overnight in 2.5% glutaraldehyde solution at 4 °C, followed by ethanol dehydration. The skin samples were dried by the quorum critical point dryer, coated with chromium, and imaged using FESEM (TESCAN CLARA, TESCAN, Czech Republic).
Statistical analysis
All experiments were performed in triplicate. Statistical analysis and graphs were generated using GraphPad Prism 8.0 (GraphPad Software, USA). Results are shown as mean ± standard deviation of the mean. Statistical significance was analyzed using one-way ANOVA and two-way ANOVA with Dunnett’s multiple and Tukey’s multiple comparisons tests. P-values of <0.05 were considered statistically significant.
Data availability
The sequencing data generated for this manuscript are available in the National Center for Biotechnology Information (NCBI) GenBank under accession numbers OR709902 and OR709903.
References
Blaskovich, M. A. T. The fight against antimicrobial resistance is confounded by a global increase in antibiotic usage. ACS Infect. Dis. 4, 868–870 (2018).
World Health Organization, WHO releases report on state of development of antibacterials (2024).
De Oliveira, D. M. P. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33, 10–1128 (2020).
Howden, B. P. et al. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 21, 380–395 (2023).
Chambers, H. F. & DeLeo, F. R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641 (2009).
Lee, A. S. et al. Methicillin-resistant Staphylococcus aureus. Nat. Publ. Gr. 4, 1–23 (2018).
Bhavana, J. & Rama, N. K. Study of HA-MRSA and CA-MRSA isolated from clinical cases in a tertiary care hospital. Indian J. Public Health Res. Dev. 8, 106–111 (2017).
Vestergaard, M., Frees, D. & Ingmer, H. Antibiotic resistance and the MRSA problem. Microbiol. Spectr. 7 (2019).
Wielders, C. L. C., Fluit, A. C., Brisse, S., Verhoef, J. & Schmitz, F. J. mecA gene is widely disseminated in Staphylococcus aureus population. J. Clin. Microbiol. 40, 3970 (2002).
Cascioferro, S. et al. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 16, 65–80 (2021).
Cuny, C., Wieler, L. H. & Witte, W. Livestock-associated MRSA: the impact on humans. Antibiotics 4, 521–543 (2015).
Craft, K. M., Nguyen, J. M., Berg, L. J. & Townsend, S. D. Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm 10, 1231–1241 (2019).
Assefa, M. & Amare, A. Biofilm-associated multi-drug resistance in hospital-acquired infections: a review. Infect. Drug Resist. 15, 5061–5068 (2022).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Divakar, S., Lama, M. & Asad, U. K. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 8, 76 (2019).
Loessner, M. J. Bacteriophage endolysins—current state of research and applications. Curr. Opin. Microbiol. 8, 480–487 (2005).
Liu, H. et al. Therapeutic potential of bacteriophage endolysins for infections caused by Gram-positive bacteria. J. Biomed. Sci. 30, 29 (2023).
Ho, M. K. Y., Zhang, P., Chen, X., Xia, J. & Leung, S. S. Y. Bacteriophage endolysins against gram-positive bacteria, an overview on the clinical development and recent advances on the delivery and formulation strategies. Crit. Rev. Microbiol. 48, 303–326 (2022).
Lu, Y. et al. Phage endolysin LysP108 showed promising antibacterial potential against methicillin-resistant Staphylococcus aureus. Front. Cell. Infect. Microbiol. 11, 1–12 (2021).
Son, B., Kong, M., Lee, Y. & Ryu, S. Development of a novel chimeric endolysin, Lys109 with enhanced lytic activity against Staphylococcus aureus. Front. Microbiol. 11, 1–12 (2021).
Cha, Y., Son, B. & Ryu, S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol 84, 103245 (2019).
Liu, H. et al. LysSYL: a broad-spectrum phage endolysin targeting Staphylococcus species and eradicating S. aureus biofilms. Microb. Cell Fact. 23, 1–19 (2024).
Schuch, R. et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 209, 1469–1478 (2014).
Jun, S. Y. et al. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 61, 10–1128 (2017).
Totté, J. E. E., van Doorn, M. B. & Pasmans, S. G. M. A. Successful treatment of chronic staphylococcus aureus- related dermatoses with the topical endolysin Staphefekt SA.100: a report of 3 cases. Case Rep. Dermatol. 9, 19–25 (2017).
Eichenseher, F. et al. Linker-improved chimeric endolysin selectively kills Staphylococcus aureus in vitro, on reconstituted human epidermis, and in a murine model of skin infection. Antimicrob. Agents Chemother. 66, e02273–21 (2022).
O’Flaherty, S. et al. Genome of staphylococcal phage K: a new lineage of myoviridae infecting Gram-positive bacteria with a low G+C content. J. Bacteriol. 186, 2862–2871 (2004).
O’Flaherty, S., Coffey, A., Meaney, W., Fitzgerald, G. F. & Ross, R. P. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 187, 7161–7164 (2005).
Becker, S. C. et al. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 294, 52–60 (2009).
Horgan, M. et al. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 75, 872–874 (2009).
Kashani, H. H., Fahimi, H., Goli, Y. D. & Moniri, R. A novel chimeric endolysin with antibacterial activity against methicillin-resistant Staphylococcus aureus. Front. Cell. Infect. Microbiol. 7, 1–12 (2017).
Behera, M. et al. Expression and characterization of novel chimeric endolysin CHAPk-SH3bk against biofilm-forming methicillin-resistant Staphylococcus aureus. Int. J. Biol. Macromol. 254, 127969 (2024).
Laux, C., Peschel, A. & Krismer, B. Staphylococcus aureus colonization of the human nose and interaction with other microbiome members. Microbiol. Spectr. 7, 10–1128 (2019).
Moormeier, D. E. & Bayles, K. W. Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 104, 365–376 (2017).
Fenton, M., Ross, R. P., Mcauliffe, O., O’Mahony, J. & Coffey, A. Characterization of the staphylococcal bacteriophage lysin CHAP(K). J. Appl. Microbiol. 111, 1025–1035 (2011).
Filatova, L. Y., Becker, S. C., Donovan, D. M., Gladilin, A. K. & Klyachko, N. L. LysK, the enzyme lysing Staphylococcus aureus cells: specific kinetic features and approaches towards stabilization. Biochimie 92, 507–513 (2010).
Sanz-Gaitero, M., Keary, R., Garcia-Doval, C., Coffey, A. & Van Raaij, M. J. Crystal structure of the lytic CHAPKdomain of the endolysin LysK from Staphylococcus aureus bacteriophage K. Virol. J. 11, 1–10 (2014).
Gerstmans, H. et al. A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci. Adv. 6, eaaz1136 (2020).
Fenton, M. et al. The truncated phage lysin CHAPk eliminates staphylococcus aureus in the nares of mice. Bioeng. Bugs 1, 404–407 (2010).
Fenton, M. et al. Bacteriophage-derived peptidase CHAPK eliminates and prevents staphylococcal biofilms. Int. J. Microbiol. 1, 625341 (2013).
Arroyo-Moreno, S., Begley, M., Dembicka, K. & Coffey, A. Engineering of the chapk staphylococcal phage endolysin to enhance antibacterial activity against stationary-phase cells. Antibiotics 10, 1–14 (2021).
Roshan, M. et al. Comparative Immunology, Microbiology and Infectious Diseases Virulence and enterotoxin gene profile of methicillin-resistant Staphylococcus aureus isolates from bovine mastitis. Comp. Immunol. Microbiol. Infect. Dis. 80, 101724 (2022).
Patel, M. H., Lu, S. Y., Liu, S. & Skory, C. D. Novel endolysin LysMP for control of Limosilactobacillus fermentum contamination in small-scale corn mash fermentation. Biotechnol. Biofuels Bioprod. 16, 1–16 (2023).
Abràmoff, M. D., Hospitals, I., Magalhães, P. J. & Abràmoff, M. Image processing with ImageJ. Biophotonics Int 11, 36–42 (2004).
Heydorn, A. et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146, 2395–2407 (2000).
George, S. E. et al. Biochemical characterization and evaluation of cytotoxicity of antistaphylococcal chimeric protein P128. BMC Res. Notes 5, 280 (2012).
Cardiff, R. D., Miller, C. H. & Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 6, 655–658 (2014).
Brown, R. C. & Hopps, H. C. Staining of bacteria in tissue sections: a reliable Gram stain method. Am. J. Clin. Pathol. 60, 234–240 (1973).
Acknowledgements
We thank Principal, Hindu College, University of Delhi, for providing infrastructural facilities. We acknowledge the Council of Scientific and Industrial Research, India (CSIR) for financial support in the form of research fellowship to Manisha Behera (08/555(0001)/2019-EMR-I). Authors thank Confocal laser scanning microscopy and field emission scanning electron microscopy facility at Central Instrumentation Facility (CIF), University of Delhi South Campus, Delhi, India for their help and support. This study received no funding.
Author information
Authors and Affiliations
Contributions
M.B.: Conceptualization, Data curation, Formal analysis, Methodology, Investigation, Visualization, Writing—original draft. P.S.: Investigation, Writing—review and editing. A.K.V.: Resources. S.D.: Supervision, Project administration. S.M.G.: Project administration, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Behera, M., Singh, P., Verma, A.K. et al. Activity of GS-linked chimeric endolysin CHAPk-SH3bk against methicillin-resistant Staphylococcus aureus biofilms: an in-vitro, ex-vivo and in-vivo study. npj Biofilms Microbiomes 11, 94 (2025). https://doi.org/10.1038/s41522-025-00728-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-025-00728-4