Abstract
Parabacteroides, a promising candidate for the next generation of probiotics, plays a significant role in various diseases. In this review, we provide a comprehensive examination of the biological properties of Parabacteroides, summarize its effects on host health and potential associations with diseases, and discuss unresolved scientific questions regarding this genus. Our aim is to offer novel insights for the development of targeted gut microbiota interventions and their translational applications.
Similar content being viewed by others
Introduction
The gut microbiota is recognized as a crucial environmental factor that influences the regulation of host health. It significantly impacts host immunity, digestion and absorption, drug metabolism, hormone secretion, and nerve signal transmission1. A substantial body of research has demonstrated a correlation between the composition and interactions of gut microbes and the onset and progression of various diseases. For instance, specific functional bacterial genera, such as Akkermansia muciniphila (A. muciniphila), have been quantitatively characterized using multi-omics techniques, including metagenomics and metabolomics. Changes in their abundance are closely associated with metabolic diseases related to obesity, type 2 diabetes, neurodegenerative disorders, and even cancer2,3,4,5. However, the specific molecular mechanisms underlying their interactions with the host remain to be fully elucidated. Therefore, further research into the precise functions of specific microorganisms in disease processes and in maintaining healthy homeostasis is of utmost importance.
The Parabacteroides genus is increasingly gaining attention as a candidate for the next generation of beneficial microbes (Fig. 1). The bacterial strain Parabacteroides distasonis ATCC 8503, initially classified within the Bacteroides genus, was first isolated from clinical specimens in 19336,7. Subsequently, through phylogenetic analysis of 16S rRNA gene sequences and comparative genomic characterization, it was reclassified into the Parabacteroides genus in 2006. Parabacteroides strain ATCC 8503 is a Gram-negative, non-anaerobic, non-spore-forming, and non-motile bacterium that is found in the gastrointestinal tract of humans and animals8,9. To date, the Parabacteroides genus comprises 27 species (https://lpsn.dsmz.de/search?word=parabacteroides), with the majority of studies focusing on four species: P. distasonis, P. goldsteinii, P. johnsonii, and P. merdae. Among these, P. distasonis serves as the reference strain for the Parabacteroides genus. Following the successful completion of the first whole-genome sequencing of this species in 2007, P. distasonis strain ATCC 8503 has been established as the standard comparator for all species within the Parabacteroides genus. This strain plays a crucial role in facilitating taxonomic classification, phylogenetic analysis, and functional genomics studies10.
Existing research has demonstrated that Parabacteroides spp. are implicated in various pathological mechanisms. P. distasonis ATCC 8503 enhances glycitein (GLY) production through β-glucosidase activity, thereby alleviating sirtuin 5-dependent rheumatoid arthritis11. Furthermore, recent studies indicate that P. goldsteinii GDMCC 1.2815 can secrete outer membrane vesicles (OMVs) that translocate to articular regions, directly suppressing neutrophil extracellular trap (NET) formation via a caveolin-1 (Cav-1)-dependent mechanism, thereby mitigating the progression of rheumatoid arthritis12. Meanwhile, oral administration of P. distasonis ATCC BAA-1295 can significantly improve the degree of injury in rheumatoid arthritis and reduce the expression of joint inflammatory cytokines. Specifically, bile acid, a metabolite of P. distasonis ATCC BAA-1295, inhibits RORγt-mediated Th17 cell differentiation and promotes the polarization of M2 macrophages, thereby altering the Th17/Treg cell balance and subsequently exerting a synergistic protective effect13. It is noteworthy that some other metabolites of the Parabacteroides genus also contribute to alleviating the disease processes in the host. For example, the protective metabolite apigenin produced by P. goldsteinii JCM 13446 directly to the potential sites on Fgr (M341 and D404), preventing its inhibitory role on Vav1 phosphorylation, and consequently promoting the activation of Cdc42/Rac1, Arp2/3 expression, and subsequent actin reorganization, which enhances the phagocytosis of bacteria by macrophages14. Lipopolysaccharide (LPS), as an active component derived from P. goldsteinii MTS01 and also an antagonist of the toll-like receptor 4 (TLR4) signaling pathway, plays a crucial role in ameliorating chronic obstructive pulmonary disease (COPD)15. Additionally, P. distasonis ATCC 8503 improves testicular dysfunction by increasing microbially derived polyamines that upregulate heat shock protein 7016. The γ-linolenic acid derived from P. distasonis ATCC 8503 activates hepatic peroxisome proliferator-activated receptor α (PPARα) signaling, thereby alleviating metabolic dysfunction-associated steatotic liver disease (MASLD)17. P. distasonis DSM 20701 ameliorates alcohol-related liver disease (ALD) by enhancing its metabolite propionic acid to activate major urinary protein 1 (MUP1), which suppresses endoplasmic reticulum stress18. Furthermore, P. distasonis ATCC 8503 can alleviate heparin-exacerbated acute pancreatitis through the production of acetic acid and the reduction of neutrophil infiltration19. In conclusion, the significant role of Parabacteroides in host metabolic function and the immune system, as a key member of the gut microbiome, cannot be overlooked. Understanding the complexity of its relationship with health will help lay the foundation for the development of novel therapies for various diseases.
Characterization of Parabacteroides
Parabacteroides and Bacteroides were confirmed to have diverged from a common ancestor by complete 16S rRNA sequencing8. According to the NCBI database, the taxonomic lineages of Parabacteroides are: Bacteria; Bacteroidetes; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides (Table 1). Individual P. distasonis ATCC 8503 cells are 0.8–1.6 × 1.2–12 μm in size, and their colonies on Eggerth-Gagnon (EG) agar plates are 1–2 mm in diameter, gray to off-white, rounded, intact, slightly raised, and smooth. P. distasonis ATCC 8503 acts as a glycolytic bacterium capable of metabolizing carbohydrates such as mannose and raffinose for energy production, and can be grown on a culture medium containing 20% bile8.
Among the sequenced human gut-associated Parabacteroides species, P. distasonis has the smallest genome and the smallest repertoire of genes that are members of the environmental sensing and gene regulation GO categories. Additionally, P. distasonis has the smallest number of genes associated with carbon source degradation. Furthermore, P. distasonis lacks several hemicellulases, pectinases, and other polysaccharides that facilitate the breakdown of non-plant carbohydrates20. Intriguingly, the HLB5 protein from P. johnsonii DSM 18315, despite lacking cellulolytic activity and any conserved domains characteristic of carbohydrate-active enzymes (CAZymes), retains the ability to degrade cellulosic biomass21. Notably, compared to other gut Bacteroides species, the proteome of P. distasonis is enriched with two classes of carbohydrate-processing enzymes. The first class consists of glycoside hydrolase family 13 (GH13), classified under the CAZymes database, which are predominantly associated with α-amylase activity and mediate the hydrolytic metabolism of starch and related carbohydrates. The second class includes glycoside hydrolase family 73 (GH73), which encodes N-acetylhexosaminidases with broad substrate specificity. These enzymes not only act on host-derived glycans but also target bacterial cell wall components, playing pivotal roles in host-microbe interactions and the homeostatic regulation of bacterial cell wall dynamics. In addition, P. distasonis possesses a large number of lateral transfer genes, which may contribute to its successful colonization of the distal gut20. Although P. distasonis lacks polysaccharide degradation genes, its unique surface layer (S-layer) of this bacterium is composed of glycoproteins (this S-layer is absent in most Bacteroides species). This distinctive single-molecule surface layer can decompose complex polysaccharides that human enzymes cannot break down and may help mask the organism, making it resemble the surrounding environment more closely. This adaptation may enable P. distasonis to evade triggering strong immune responses from the host, providing a possible evolutionary explanation for its unique S-layer22. Furthermore, P. distasonis ATCC 8503 can secrete a C1B-like cysteine protease, which exhibits continuous diaminopeptidase activity and maintains a high degree of specificity for N-terminal glycine residues. This enzymatic activity offers a survival advantage to P. distasonis by inactivating various antimicrobial peptides present in the host gut23. Hildebrand et al. found that P. distasonis showed a dominant presence in the gut microbial community following antibiotic treatment. This bacterium can proliferate rapidly in the gut, becoming the second most abundant symbiotic bacterium, with a relative abundance of up to 95%. These short-term, non-invasive, conditioned, monodominant taxa may play a crucial role in ecological succession and contribute to the restoration and reconstruction of complex ecosystems. Additionally, they could serve as potential probiotic candidates for future research24.
To further elucidate the putative pathogenicity of P. distasonis and the dual nature of probiotics, Bank et al. developed a virulence (LPS/bacterial wall) “ rfbA O-antigen-synthesis gene” using DNA chip technology. They also proposed a PCR-based restriction fragment length polymorphism method. Cluster analysis revealed that P. distasonis could be categorized into four lineages, designated as rfbA-Types I, II, III, and IV. Notably, most pathogenic P. distasonis strains were identified as single-copy rfbA-Type I. This classification may aid in the differentiation of P. distasonis strains and provide a foundation for future research on functional pathogens and probiotics25.
Insight into specific diseases
The intestinal microbiota is involved in various physiological processes such as energy intake, immune system maturation, hormone secretion, etc. Parabacteroides is an important component of intestinal microbiota, and its changes can be significantly correlated with various physiological disorders such as obesity, diabetes, neurological diseases, inflammatory bowel diseases, and tumors (Fig. 2).
a Obesity: P. goldsteinii could alleviate obesity by increasing the thermogenesis of adipose tissue. P. distasonis significantly alters the bile acid profile and elevates the level of succinate in the gut, thereby facilitating lipid metabolism and glucose metabolism. b Diabetes mellitus: P. distasonis augments the production of its metabolites, namely indoleacrylic acid, nicotinic acid, and TDUAC, thereby activating the AhR signaling pathway, intestinal GPR109a, and regulating glucose tolerance and insulin sensitivity, thereby ameliorating diabetes and its complications. c Depression: The specific mechanism by which Parabacteroides affects the occurrence and development of depression is highly complex, and it might exert its role through metabolites such as GABA and IAld. d Neurological disorders: Parabacteroides can affect a variety of neurological diseases, including Alzheimer’s disease, Parkinson’s disease, epilepsy, autism spectrum disorder, amyotrophic lateral sclerosis, and multiple sclerosis. e Gut barrier and intestinal inflammation: Parabacteroides is capable of promoting intestinal self-repair and ameliorating intestinal inflammation by reducing the production of pro-inflammatory cytokines, down-regulating the NF-κB pathway, facilitating Wnt signaling, and increasing the mRNA level of tight junction proteins. f Tumor: Parabacteroides could inhibit tumor growth by enhancing anti-tumor immune responses, particularly the anti-tumor immunity represented by ICIs. (FBP fructose-1,6-bisphosphate, FBPase fructose-1,6-bisphosphatase, F6P fructose-6-phosphate, Glu6P glucose-6-phosphate, IGN intestinal gluconeogenesis, CA cholic acid, LCA lithocholic acid, FGF15 fibroblast growth factor 15, CDCA chenodeoxycholic acid, UDCA ursodesoxycholic acid, NLRP3 NOD-like receptor, thermal protein domain associated protein 3).
Parabacteroides & metabolic disorders
Parabacteroides & obesity
Obesity results from a prolonged imbalance between energy intake and energy expenditure, primarily characterized by fat accumulation26. Research has demonstrated that gut microbiota plays a significant role in the pathogenesis of obesity. Gut microbiota not only directly affects food digestion, absorption, and metabolism but also influences obesity by regulating appetite, the intestinal microenvironment, bile acid metabolism, hormone secretion, and the immune system27.
Current studies have demonstrated that the relative abundance of the Parabacteroides genus in the gut is significantly reduced in overweight/obese patients28. Furthermore, the abundance of the Parabacteroides genus is inversely correlated with various measures, including body weight (such as waist circumference and BMI) and blood lipids (including low-density lipoprotein, triglycerides, and total cholesterol)29. Furthermore, recent studies have demonstrated that the abundance of the Parabacteroides genus significantly increases following bariatric surgery, which may be closely associated with reductions in blood glucose levels30. Additionally, after a 10% weight loss in obese patients, the abundance of P. distasonis can increase markedly. It is suggested that Parabacteroides may have the potential to predict obesity-related metabolic abnormalities31. Intriguingly, during obesity, dysregulation of dopaminergically regulated hedonic food intake resulted in mice exhibiting a delayed preference for palatable diets (high-fat, high-sucrose, HFHS) and a decreased pleasure from eating compared to their lean counterparts. Notably, the abundance of the Parabacteroides genus is strongly positively correlated with HFHS intake, suggesting that this genus may actively modulate the hedonic components of food intake and could play a role in hedonic eating through the gut-brain axis32.
Emerging evidence demonstrates that theabrownin ameliorates obesity and MASLD by modulating the serotonin signaling pathway through gut microbiota-dependent mechanisms. Notably, the Parabacteroides genus has been identified as one of the functionally pivotal taxa that exhibit differential enrichment in the gut microbial community following the intervention with theabrownin33. Similarly, Panax notoginseng saponins (PNS) alleviate obesity by markedly elevating the abundance of P. distasonis and A. muciniphila in the gut microbiota, thereby activating the leptin-AMPK/STAT3 signaling pathway, enhancing thermogenesis in brown adipose tissue (BAT), and promoting the biogenesis of beige adipocytes34. Wu et al. demonstrated that the abundance of P. goldsteinii was reduced in mice fed a high-fat diet (HFD). Oral administration of P. goldsteinii ATCC BAA-1180 reduced adipocyte hypertrophy and crown-like structure (CLS) lesions in the adipose tissue of HFD-fed mice. Additionally, it significantly increases UCP1 mRNA and protein expression in BAT and inguinal white adipose tissue (iWAT), indicating its potential to combat obesity by inducing thermogenesis. Furthermore, P. goldsteinii ATCC BAA-1180 alleviated the progression of MASLD and metabolic dysfunction-associated steatohepatitis (MASH) in HFD-fed mice, normalizing the expression of hepatic genes involved in lipid transport, lipogenesis, and β-oxidation35. Similarly, oral treatment with P. distasonis CGMCC 1.30169 reduced weight gain and improved obesity-related dysfunction in ob/ob mice and HFD-induced metabolic syndrome mice. Mechanically, P. distasonis CGMCC 1.30169 therapy significantly alters the bile acid profile, resulting in an increase in secondary bile acids that activate the intestinal farnesoid X receptor (FXR) pathway. This activation subsequently influences lipid metabolism. Furthermore, P. distasonis CGMCC 1.30169 therapy significantly elevates the level of succinic acid in the gut, which acts as an agonist for a key enzyme in the gluconeogenic pathway, thereby enhancing glucose homeostasis and reducing food intake36. Recent research conducted by Qiao et al. has shown that the porA gene expressed in P. merdae, which was isolated from human feces, enhances the degradation of branched-chain amino acid (BCAA) degradation, thereby suppressing the mTOR/NF-κB pathway and activating AMPK, ultimately reducing obesity-associated atherosclerosis37. These findings suggest that Parabacteroides holds significant potential for anti-obesity treatment.
Parabacteroides & diabetes mellitus
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disease characterized by pancreatic β-cell dysfunction and peripheral insulin resistance (IR), resulting in impaired glucose metabolism and chronic low-grade inflammation38. Studies have demonstrated that gut microbiota can be implicated in the development of T2DM by reducing glucose tolerance and exacerbating IR39. Parabacteroides is one such microorganism that may influence disease progression by regulating glycolipid metabolism. Wang et al. found that Shouhuitongbian (SHTB) treatment effectively improves IR and reduces blood glucose levels in mice. This effect is primarily attributed to the enrichment of the genera Parabacteroides and Akkermansia, as well as the enhancement of short-chain fatty acid (SCFAs) production and the catabolism of branched-chain fatty acids40. The strains P. distasonis AS93 and P. distasonis PF-BaE11 can induce the secretion of glucagon-like peptide-1 (GLP-1) in vitro. GLP-1 is a multifaceted endocrine gut peptide that plays several metabolic roles, particularly in the regulation of glucose homeostasis41,42. In contrast, the serine protease DPP4 from P. merdae DSM 19495 can rapidly hydrolyze GLP-1, leading to glucose metabolism disorders in T2DM43. Recent studies have indicated that the efficacy of very low caloric restriction on T2DM can be partially attributed to the role of P. distasonis in enhancing glucose and lipid metabolism44. Furthermore, Liu et al. demonstrated that P. distasonis ATCC 8503 can stimulate the production of its metabolite indoleacrylic acid, which activates the aryl hydrocarbon receptor (AhR) signaling pathway and enhances the expression of IL-22, ultimately contributing to the treatment of T2DM45.
IR is a key characteristic of patients with T2DM. Recent studies have shown that the abundance of P. distasonis in T2DM patients is negatively correlated with the deterioration of IR. As an important bioactive molecule, niacin (NA) derived from P. distasonis NSP007 (GDMCC 61888) can enhance intestinal barrier function by activating intestinal G protein-coupled receptor 109a (GPR109a), thereby improving IR46. Furthermore, a study on the anti-obesity effects of Hirsutellasinensis mycelium by Wu et al. demonstrated the crucial role of P. goldsteinii. The oral administration of P. goldsteinii ATCC BAA-1180 to obese mice not only prevented weight gain but also improved intestinal integrity and reduced IR35. Diabetic peripheral neuropathy (DPN) is the most common complication of T2DM. Recent studies have demonstrated that P. goldsteinii and P. distasonis levels are significantly decreased in the stool of DPN patients. Parabacteroides can convert bile acids and increase the secondary bile acid taurine deoxycholic acid (TUDCA) content. Subsequently, TUDCA can regulate glucose tolerance and insulin sensitivity, enhance cell viability and migration by targeting NLRP3, and inhibit Schwann cell pyroptosis induced by high glucose levels, thereby mitigating DPN47.
Unexpectedly, contrary to T2DM, a recent study titled “The Environmental Determinants of Type 1 Diabetes in the Young” (TEDDY), which collected over 12,000 stool samples from 903 children at risk for T1DM in four countries, revealed that the Parabacteroides genus is most significantly associated with T1DM48. Matos et al. demonstrated that the P. distasonis PtF D14MH1 strain isolated from fecal samples of children with T1DM exhibited a higher culture recovery rate and showed adherence to intestinal epithelial cells, and was highly aggressive49. Furthermore, the P. distasonis D13 strain can accelerate the onset of disease in mouse models of T1DM due to the fact that the specific peptide hprt4-18 encoded in the genome of the P. distasonis D13 strain acts as a mimic of the major insulin epitope B9-23. This bacterial peptide, in conjunction with the insulin peptide, has the capacity to stimulate a bidirectional cross-reactive immune response, thereby initiating an autoimmune response to T1DM50. To date, existing research on Parabacteroides in relation to diabetes remains limited, inconclusive, and at times contradictory. However, this presents a promising opportunity for further exploration of the role of Parabacteroides in diabetes.
Parabacteroides & depression and neurological disorders
Parabacteroides & depression
Depression is a complex neuropsychiatric disorder determined by a variety of factors, including genetic, psychological, and environmental components51. Over the past twelve years, the gut microbiota dysbiosis has been extensively documented in patients with depression as well as in animal models, and several probiotics have shown promise in alleviating behavioral symptoms52,53. An increasing body of research has also focused on revealing how gut microbes act on neural activities and circuits that regulate behavior. In particular, bidirectional communication between the brain and gut, also known as the brain-gut axis, plays a key role in the pathogenesis of various central nervous disorders54.
Accumulating studies have demonstrated that the diversity and richness of the gut microbiome are significantly altered in patients with depression, and that the abundance of Parabacteroides is significantly up-regulated at the genus level55. For instance, Deng et al. demonstrated that ferulic acid and feruloylated oligosaccharides could alleviate anxiety and depressive symptoms in mice by reducing the abundance of the Parabacteroides genus in the gut and enhancing tryptophan biosynthesis56. Furthermore, Gomez-Nguyen et al. revealed that SAMP1 mice with ileitis exhibited spontaneous depression-like behaviors, which were strongly correlated with an increased abundance of the Parabacteroides genus. Notably, the oral administration of P. distasonis ATCC 8503 significantly mitigated these depression-like phenotypes in SAMP1 mice57. Interestingly, the abundance of the Parabacteroides genus was reduced in cases of chronic restraint stress-induced depression, yet it was inversely associated with depressive behavior58.
G protein-coupled receptor-35 (GPR35), an orphan receptor primarily expressed in the intestinal epithelium, serves as a novel regulator of intestinal symbiosis. This receptor may modulate host physiology and pathology through intestinal microbial signaling59. Recent research has indicated that GPR35 deficiency can lead to alterations to the intestinal microecological dysregulation, which in turn could affect indole—a microbial metabolite involved in the tryptophan metabolism pathway. These metabolites could potentially influence depression-like behavior in mice through the gut-brain axis. It is notable that P. distasonis is significantly enriched in Gpr35−/− mice, and oral administration of P. distasonis ATCC 8503 induces depression-like behavior in mice, accompanied by an increase in serum indole-3-lactate (ILA) levels and a significant decrease in indole-3-formaldehyde (IAld). This suggests that the imbalance of IAld and ILA induced by P. distasonis ATCC 8503 may collectively contribute to depression-like behavior in Gpr35−/− mice. Notably, IAld treatment effectively alleviates most core symptoms of depression in USM mice, while oral ILA induces a series of depression-like behaviors. Mechanically, these two bacterial metabolites can counterregulate the density of dendritic spines and synaptic transmission in the nucleus accumbens. More importantly, the researchers found that P. distasonis ATCC 8503 was similarly significantly enriched, while serum concentrations of IAld and its precursor indole-3-acetamide (IAM) were significantly reduced in depressed patients60. The above studies reveal an interrelationship among host genetics, Parabacteroides, and metabolite-mediated depression-like behavior, suggesting that targeting Parabacteroides could serve as a potential diagnostic and therapeutic approach for depression.
Parabacteroides & neurological disorders
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social interaction, cognitive inflexibility, repetitive/restrictive sensorimotor behavior, etc.61. Studies have shown that the microbiome characteristics differ significantly between individuals with ASD and typically developing controls, with a reduced relative abundance of Bacteroides and Parabacteroides, among other bacteria, in children with ASD62. Furthermore, the abundance of Bacteroides and Parabacteroides is generally decreased in germ-free mice that harbor the human ASD microbiome and exhibit ASD-like behaviors; both are correlated with reduced repetitive behaviors and increased social interactions63. Lin et al. found that P. goldsteinii could improve autism-related behaviors induced by maternal immune activation (MIA). By mechanism, P. goldsteinii MTS01 enhanced neuropeptide-associated signaling in the gut while suppressing aberrant cellular proliferation and inflammatory responses. Furthermore, this strain augmented ribosomal/mitochondrial and antioxidant activities in the hippocampus, concomitant with attenuated glutamate receptor signaling64.
Epilepsy, one of the most prevalent severe brain disorders, is characterized by a persistent tendency to spontaneous epilepsy and has many neurobiological, cognitive, and psychosocial consequences65. Studies have demonstrated that the natural steroid saponin diosgenin can inhibit the progression of epilepsy to some extent, partly by reversing the reduced abundance of Bacteroides and Parabacteroides in mouse models of epilepsy66. In addition, Olson et al. found that Parabacteroides mediated the anti-epileptic effect of the ketogenic diet (KD). In terms of mechanism, KD increased the relative abundance of A. muciniphila and Parabacteroides spp. in the gut, and the two bacteria acted synergically to inhibit γ-glutamylation, reduce levels of peripheral ketogenic amino acids, and increase the hippocampal gamma-aminobutyric acid (GABA)/glutamate ratio, thereby preventing seizures67. Intriguingly, Parabacteroides has been identified as a producer of GABA, a major inhibitory neurotransmitter that plays a crucial role in regulating brain states and cognitive functions, including learning and memory, sensory processing, circadian rhythms, and alertness68. Moreover, tonic GABA currents are essential for normal motor function, and their dysregulation has been associated with motor symptoms in Parkinson’s and Huntington’s diseases69. These studies suggest that Parabacteroides may play a significant role in neurological disorders through its metabolism of GABA; however, the specific mechanisms warrant further investigation.
Neurodegenerative diseases (NDs) are a heterogeneous group of complex disorders characterized by neuronal loss and progressive degeneration in various regions of the nervous system. This group primarily includes Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS)70. Recent studies have demonstrated that significant alterations in intestinal flora are associated with NDs, which may influence the progression of these diseases. Among the various bacterial genus, Parabacteroides is notably prevalent71,72,73. Taking MS as an example, a chronic inflammatory autoimmune disease of the central nervous system, the abundance of P. distasonis in patients with relapsing-remitting MS is significantly reduced compared with healthy controls74. In addition, Cekanaviciute et al. demonstrated that the abundance of P. distasonis was markedly diminished in MS patients, and peripheral blood mononuclear cells (PBMCs) from these patients exhibited an impaired ability to differentiate or expand the CD25⁺FoxP3⁺ Treg population. In vitro experiments have shown that P. distasonis ATCC 8503 can stimulate the expression of IL-10 in human CD4⁺CD25⁺ T cells and promote the generation of IL-10⁺FoxP3⁺ Tregs75. Similarly, P. distasonis has also been implicated in inducing Treg differentiation in germ-free mice, suggesting that P. distasonis may play a crucial role in alleviating the disease process of MS76. In conclusion, further investigation of the gut microbiota-gut-brain axis is essential to fully exploit its potential, thereby promoting the development of effective therapeutic interventions to ameliorate NDs.
Parabacteroides & gut barrier and intestinal inflammation
The gut barrier is a complex and dynamic assembly of physical and chemical structures that monitor the environment and protect the host from microbial invasion and harmful stimuli. It is ideally positioned to receive and convey signals from gut microbes, serving as a portal for communication between the gut microbiota and the host. Dysfunction of the gut barrier, resulting from alterations in the composition of the gut microbiome, may underlie various diseases. For instance, chronic drug use can disrupt gut microbiota, potentially leading to damage of the intestinal barrier77,78. Recent studies have shown that P. goldsteinii JCM 13446 can mitigate aspirin-induced intestinal damage. Mechanistically, P. goldsteinii JCM 13446 can enhance the production of bile acids, specifically ursodeoxycholic acid (UDCA) and its precursor 7-keto-LCA, through the hdhA gene. These bile acids can act as FXR antagonists to promote Wnt signaling, thereby facilitating the maintenance of the integrity of intestinal recess cells during the process of intestinal self-repair79. Lai et al. found that P. goldsteinii MTS01 can enhance colonic integrity by reducing the production of pro-inflammatory cytokines and upregulating the expression of genes involved in ribosome biogenesis and mitochondrial activity15. Recent studies have demonstrated that outer membrane protein A (OmpA) from P. goldsteinii strain ASF519 activates gut lymphocytes to produce IL-22. Subsequently, IL-22 interacts with its receptor, which is predominantly expressed on intestinal epithelial cells (IECs), to promote epithelial proliferation, mucus secretion, and the release of antimicrobial peptides80. In addition, P. johnsonii and P. distasonis isolated from the feces of healthy donors can inhibit E. coli-induced inflammatory responses by down-regulating the NF-κB pathway. Notably, P. distasonis can significantly improve mRNA levels of the tight junction protein Occludin and can completely or partially restore the tight junction barrier disruption induced by the pro-inflammatory cytokine IL-1β, demonstrating its potential to modulate epithelial barrier function81.
Inflammatory bowel disease (IBD) is a group of gastrointestinal inflammatory disorders characterized by complex pathogenesis and an unclear etiology. It is widely accepted that the onset of IBD results from the interplay of environmental and genetic factors. Key contributors to the development of IBD include defects in the intestinal epithelial barrier, dysregulation of immune responses, and disturbances in intestinal microecology82. Recent studies have demonstrated that synergistic interactions between the commensal bacterial strains A. muciniphila DMS 22959 and P. distasonis DSM 29491 can alleviate acute and chronic colitis by enhancing the integrity of the epithelial barrier and promoting group 3 innate lymphoid cells (ILC3s) in the colonic mucosa83. Notably, A. muciniphila and P. distasonis may also serve as prognostic markers for recurrence in patients with ulcerative colitis84. Furthermore, specific strains of P. distasonis (AS93, PF-BaE5, PF-BaE11) exert a protective effect in TNBS-induced acute colitis mouse models by enhancing the epithelial barrier and inducing the expression of the anti-inflammatory cytokine IL-10. Among these strains, IL-10 was sufficient to attenuate the pro-inflammatory response of Th1 and Th17 by reducing DC-derived IL-12 and IL-23. In particular, the P. distasonis strain PF-BaE5 and PF-BaE11 can induce immature regulatory DCs, thereby inducing Tregs85. In addition, Kverka et al. found that oral administration of P. distasonis membranous component (mPd) was effective in inhibiting acute colitis induced by dextran sulfate sodium salt (DSS). Mechanistically, mPd could promote an increase in specific serum antibody levels and the number of Tregs. Moreover, oral mPd can inhibit macrophage production of TNF-α and stabilize the gut microbiota in vitro86. Interestingly, in ulcerative colitis, P. merdae ATCC 43184 similarly ameliorates intestinal tissue damage by maintaining a balance between anti-inflammatory and pro-inflammatory cytokine levels. Besides, P. merdae ATCC 43184 may also relieve ulcerative colitis by regulating the levels of SCFAs, including acetic, propionic, and isovaleric acids87. It is worth mentioning that P. goldsteinii ATCC BAA-1180 can significantly relieve the typical symptoms of colitis and inhibit the activation of the inflammatory response associated with the PI3K-Akt pathway in mice exposed to DSS88.
In conclusion, the studies discussed above underscore the ability of Parabacteroides to enhance intestinal barrier integrity and its potential anti-inflammatory effects at various levels. These findings offer new insights into how Parabacteroides can positively influence gut homeostasis and also open up new avenues for using microbiome-derived strategies to address gut inflammation.
Parabacteroides & tumor
Intestinal microecology is a crucial environmental factor contributing to tumorigenesis and progression, which can affect tumor development through multiple pathways, including regulating immune response, inflammation, metabolism, genotoxicity, and virulence effects, as well as inducing cell cycle arrest89. Recent studies have revealed that HFD and cigarette smoke can disrupt the balance of intestinal flora, thereby promoting the development of colorectal cancer (CRC). Similarly, depletion of P. distasonis was observed in both studies90,91. Furthermore, Gu et al. found that the abundance of bacteria associated with CRC development was elevated in the feces of TGF-β signaling deficient mice with CRC, while the abundance of P. distasonis was significantly reduced92. Fascinatingly, the abundance of the Parabacteroides genus increased significantly markedly following the resection of colorectal adenomas93. More persuasively, in studies regarding the anti-tumor activity of O. sinensis, Auricularia delicate and berberine through regulating intestinal flora and related metabolites, Parabacteroides seems to present a trend in increasing relative abundance94,95,96,97. Notably, the Parabacteroides genus may modulate tumor progression as a component of the intratumoral microbiota98. For example, Cui et al. reported a significantly reduced relative abundance of the Parabacteroides genus in hepatoblastoma tissues compared to adjacent non-neoplastic tissues in pediatric patients99. These findings imply that Parabacteroides may play a crucial role in the development of tumors, which should not be disregarded.
Further research has indicated that the Parabacteroides genus can modulate tumor progression through multiple mechanisms. In vitro co-culture experiments have demonstrated that P. goldsteinii can inhibit the growth of CRC cells100. Additionally, mPd can induce apoptosis in CRC cells by impeding the TLR4-activated MyD88 and Akt signaling pathways101. Furthermore, P. distasonis isolated from human fecal samples could directly stimulate the AHR pathway in CRC cells through the production of its metabolite p-hydroxyphenylacetic acid, which inhibits the expression of the canonical oncogene VEGFA, thereby exerting an inhibitory effect on CRC metastasis102. In the context of the tumor immune microenvironment, Liang et al. found that OMVs derived from P. distasonis ATCC 8503 can enhance the anti-tumor immune response and inhibit CRC growth by upregulating the expression of the chemokine CXCL10, which promotes the infiltration of intratumoral CD8⁺ T cells103. Interestingly, the strain P. goldsteinii kh35, isolated from human feces, can improve anastomotic healing after CRC surgery by exerting local anti-inflammatory effects through the inhibition of the MyD88/NF-κB signaling pathway104. Ecologically, Fusobacterium nucleatum could promote the progression of KRAS p.G12D-mutant CRC by binding to DHX15, an RNA helicase family protein expressed in CRC cells. However, this effect can be mitigated by P. distasonis ATCC 8503105.
It is noteworthy that Parabacteroides also plays a vital role in anti-tumor immunotherapy. Studies have indicated that anti-PD-L1 treatment generally augments the abundance of P. goldsteinii in the gut of both male and female mice compared to CRC controls106. Clinically, the abundance of P. distasonis is also higher in responders to anti-PD-1 treatment for non-small cell lung cancer (NSCLC) than in non-responders107. Recent studies have demonstrated that P. distasonis, when combined with immune checkpoint inhibitors (ICIs), can significantly delay the progression of bladder cancer, increase the intratumoral infiltration of CD4+ T cells and CD8+ T cells, and upregulate pathways associated with the anti-tumor immune response108. Additionally, 11 related strains (including P. distasonis, P. gordonii, and P. johnsonii) have been isolated from healthy human feces, working synergistically to induce interferon-γ-producing CD8+ T cells and enhance ICI-mediated anti-tumor immunity109. Emerging research has found that P. distasonis significantly enhances the immune response to anti-PD-1 therapy in NSCLC mouse models by inducing tumor-associated macrophages (TAMs) to secrete CXCL9 and boosting CD8⁺ T cell activity110. In summary, Parabacteroides holds great potential for application in anti-tumor immunotherapy. A comprehensive understanding of the interactions between Parabacteroides and tumors may provide significant insights for the development of microbiome-based anti-tumor therapies and drug combinations, as well as promising prospects for future clinical translation (Table 2).
Conclusions and pending issues
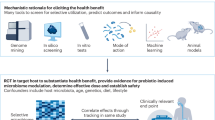
Although Parabacteroides exhibits a range of potentially beneficial effects on host health (Fig. 3), there are significant scientific questions that need to be comprehensively addressed that will be critical to the ultimate application of Parabacteroids-based therapeutic strategies in the treatment of clinical diseases (Fig. 4).
Firstly, the precise mechanism of action of Parabacteroides should be exhaustively contemplated. The variation of the relative abundance of Parabacteroides in diverse diseases and its correlation with the occurrence and development of diseases have been documented in existing literature111,112,113,114,115. Nevertheless, the specific causal mechanisms underlying these associations—namely, how Parabacteroides contributes to or responds to particular pathophysiological changes and influences disease progression—remain to be elucidated. Especially, the abundance of the Parabacteroides genus and its associations with diseases may be influenced by various factors, including dietary patterns and gender. For example, Liu et al. found that the abundance of the Parabacteroides genus may be gender-dependent in obese patients and that specific dietary habits may also influence its abundance116. Similarly, Wang et al. reported a higher abundance of the Parabacteroides genus in the gut of healthy adult males117. Notably, recent studies have further revealed stronger female-specific associations between its abundance and obesity118, which may be attributed to estrogen-mediated regulation of gut microbiota119. Therefore, comprehensive investigations in large-scale populations, particularly in prospective cohorts, are essential to define the precise role of Parabacteroides in specific disease contexts and enforce its causal mechanisms, which will provide critical foundations for developing targeted clinical strategies that modulate Parabacteroides and related gut microbiota for disease-specific interventions.
Secondly, there is a need for a systematic investigation into the safety of Parabacteroides. Parabacteroides have been observed to exert dual effects in certain diseases. For instance, P. distasonis F1-28 has been shown to possess anti-ulcerative colitis properties in DSS-induced mice120, while P. distasonis ATCC 8503 has been demonstrated to potentiate DSS-induced colitis and may predispose mice to IBD121. This might be due to the fact that disparate strains of the same species may exhibit considerable discrepancies in metabolic profiles, gene expression, and functional activity. In particular, significant functional heterogeneity in disease development has been observed among different Parabacteroides strains. For instance, P. distasonis AS93 and P. distasonis PF-BaE11 strains have been demonstrated to induce GLP-1 secretion in vitro41, whereas the serine protease DPP4 derived from P. merdae DSM 19495 T has been shown to rapidly hydrolyze GLP-1, resulting in impaired glucose metabolism in T2DM43. It is imperative to acknowledge that Parabacteroides does not universally confer protective effects across all diseases56,60. In order to ensure the safe application of Parabacteroides strains, rigorous strain-screening frameworks must be established in preclinical studies. Furthermore, given that Parabacteroides primarily exerts biological effects through active metabolites, membrane components, and secreted OMVs, the development of novel postbiotic formulations (e.g., purified bacterial proteins or signature metabolites) may mitigate risks associated with live bacterial transplantation. It is noteworthy that intermittent fasting (IF) interventions in obese populations have been shown to induce significant enrichment of P. distasonis, with its relative abundance exhibiting an inverse correlation with obesity and atherosclerotic cardiovascular disease (ASCVD)-related clinical markers (e.g., BMI, lipid profiles)122. Moreover, a significant increase in the abundance of the Parabacteroides genus has been observed in patients with T2DM following treatment with Chinese Medical Nutrition Therapy (CMNT)123. Taken together, these results indicate that modulating Parabacteroides through specific dietary approaches or by utilizing natural compounds may offer a promising intervention strategy for the management of the disease, informed by gut microbiome dynamics.
Thirdly, there are complex relationships between different bacteria in the gut microbiota. A considerable number of studies have shown that gut bacteria can collaborate to decelerate the course of disease. For example, recent studies have demonstrated that Lactobacillus johnsonii works in collaboration with Clostridium sporogenes to generate IPA, thereby modulating T cell dry-enhancement of tumor immunotherapy124. It is important to note that the function of Parabacteroides might be dependent on other gut microbes. It has been demonstrated that A. maciniphila is capable of metabolizing KD components to support the growth of P. merdae ATCC 43184. This interaction inhibits the γ-glutamylation of P. merdae ATCC 43184, thereby facilitating the growth of A. maciniphila to exert an anti-epileptic role67. In addition, Lai et al. discerned that P. goldsteinii MTS01 could modify the composition of intestinal microbiota and increase the number of beneficial bacteria, as well as markedly inhibit the action of Helicobacter pylori virulence factor, thereby alleviating gastritis125. These findings indicate that the interactions between different bacteria in different gut microbiota cannot be disregarded and require further exploration in subsequent studies before practical application, which is important for the development of new clinical therapeutic strategies.
Finally, the application of Parabacteroides in therapeutic strategies deserves further exploration. Although mouse models have long been an important foundation for biological research, there are still certain problems and limitations in their use. The structure and function of the mouse gut, as well as the anatomical sites where some nutrients are metabolized, are different from those of humans. As a result, there are differences in gut microbial composition and function between mice and humans126. The intricate nature of host-microbiome interactions renders the replication of the relationship between humans and their gut microbiome, which may be a challenging endeavor. Furthermore, in clinical practice, the situation is considerably more intricate, with a multitude of factors, including disparate disease stages and pharmacological interventions, influencing the equilibrium of the gut microbiome. Meanwhile, the standardized strategy for Parabacteroides is yet to be established, which includes the selection of its specific components and metabolites, dosage, time of administration, route of administration, survival, and other factors. Moreover, the protocol for its combination with other clinical interventions is still to be perfected. Additionally, the potential effects of a specific microbe are contingent upon a multitude of variables, extending beyond the bacteria themselves to encompass factors such as dietary patterns, age, genotype, and host immune status127,128. Therefore, how to better promote its clinical application from the perspective of gut microbes remains to be systematically and deeply explored.
In the future, with the rapid advancement of next-generation sequencing technology, spatial transcriptomics, non-targeted metabolomics, and other technologies, the integration of multi-level and multi-dimensional knowledge theories in microbiology, genetics, immunology, molecular pathology, and epidemiology will eventually facilitate targeted microbial therapy to become a component of future personalized disease treatment.
Data availability
The authors declare no competing interests.
References
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Liu, C. et al. Gut commensal Christensenella minuta modulates host metabolism via acylated secondary bile acids. Nat. Microbiol. 9, 434–450 (2024).
Zhang, Y. et al. Akkermansia muciniphila supplementation in patients with overweight/obese type 2 diabetes: efficacy depends on its baseline levels in the gut. Cell Metab. 37, 592–605.e6 (2025).
Ahmed, S. et al. In vitro characterization of gut microbiota-derived bacterial strains with neuroprotective properties. Front. Cell Neurosci. 13, 402 (2019).
Zhao, X. et al. Akkermansia muciniphila: a potential target and pending issues for oncotherapy. Pharm. Res. 196, 106916 (2023).
Eggerth, A. H. & Gagnon, B. H. The bacteroides of human feces. J. Bacteriol. 25, 389–413 (1933).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Sakamoto, M. & Benno, Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. Int. J. Syst. Evol. Microbiol. 56, 1599–1605 (2006).
Nakano, V. et al. Adherence and invasion of Bacteroidales isolated from the human intestinal tract. Clin. Microbiol. Infect. 14, 955–963 (2008).
Ezeji, J. C. et al. Parabacteroides distasonis: intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 13, 1922241 (2021).
Ma, F. et al. Dietary-timing-induced gut microbiota diurnal oscillations modulate inflammatory rhythms in rheumatoid arthritis. Cell Metab. 36, 2367–2382.e5 (2024).
Ye, H. et al. Guizhi Shaoyao Zhimu decoction inhibits neutrophil extracellular traps formation to relieve rheumatoid arthritis via gut microbial outer membrane vesicles. Phytomedicine 136, 156254 (2025).
Sun, H. et al. Gut commensal Parabacteroides distasonis alleviates inflammatory arthritis. Gut 72, 1664–1677 (2023).
Gu, P. et al. Aging-induced alternation in the gut microbiota impairs host antibacterial defense. Adv. Sci. 12, e2411008 (2025).
Lai, H. C. et al. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 71, 309–321 (2022).
Zhao, Q. et al. Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 9, 224 (2021).
Kuang, J. et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis. Cell Metab. 35, 1752–1766.e8 (2023).
Zhang, H. et al. MUP1 mediates urolithin A alleviation of chronic alcohol-related liver disease via gut-microbiota-liver axis. Gut Microbes 16, 2367342 (2024).
Lei, Y. et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome 9, 115 (2021).
Xu, J. et al. Evolution of symbiotic bacteria in the distal human intestine. PLoS Biol. 5, e156 (2007).
Chang, C. et al. A 2.08 Å resolution structure of HLB5, a novel cellulase from the anaerobic gut bacterium Parabacteroides johnsonii DSM 18315. Protein Sci. 28, 794–799 (2019).
Fletcher, C. M., Coyne, M. J., Bentley, D. L., Villa, O. F. & Comstock, L. E. Phase-variable expression of a family of glycoproteins imparts a dynamic surface to a symbiont in its human intestinal ecosystem. Proc. Natl. Acad. Sci. USA 104, 2413–2418 (2007).
Xu, J. et al. A commensal dipeptidyl aminopeptidase with specificity for N-terminal glycine degrades human-produced antimicrobial peptides in vitro. ACS Chem. Biol. 13, 2513–2521 (2018).
Hildebrand, F. et al. Antibiotics-induced monodominance of a novel gut bacterial order. Gut 68, 1781–1790 (2019).
Bank, N. C., Singh, V. & Rodriguez-Palacios, A. Classification of Parabacteroides distasonis and other Bacteroidetes using O- antigen virulence gene: RfbA-Typing and hypothesis for pathogenic vs. probiotic strain differentiation. Gut Microbes 14, 1997293 (2022).
Van Hul, M. & Cani, P. D. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat. Rev. Endocrinol. 19, 258–271 (2023).
McAllister, E. J. et al. Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 49, 868–913 (2009).
Palmas, V. et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 11, 5532 (2021).
Zeng, Q. et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 9, 13424 (2019).
Anhê, F. F. et al. Human gut microbiota after bariatric surgery alters intestinal morphology and glucose absorption in mice independently of obesity. Gut 72, 460–471 (2023).
Alili, R. et al. Characterization of the gut microbiota in individuals with overweight or obesity during a real-world weight loss dietary program: a focus on the Bacteroides 2 enterotype. Biomedicines 10, 16 (2021).
de Wouters d’Oplinter, A. et al. Gut microbes participate in food preference alterations during obesity. Gut Microbes 13, 1959242 (2021).
Li, H. Y. et al. Theabrownin inhibits obesity and non-alcoholic fatty liver disease in mice via serotonin-related signaling pathways and gut-liver axis. J. Adv. Res. 52, 59–72 (2023).
Xu, Y. et al. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 10, 11302–11323 (2020).
Wu, T. R. et al. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 68, 248–262 (2019).
Wang, K. et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5 (2019).
Qiao, S. et al. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nat. Metab. 4, 1271–1286 (2022).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
Yang, G. et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 117, 154712 (2021).
Wang, T. et al. Antidiabetic action of the Chinese formula Shouhuitongbian and the underlying mechanism associated with alteration of gut microbiota. Phytomedicine 129, 155575 (2024).
Cuffaro, B. et al. Characterization of Two Parabacteroides distasonis candidate strains as new live biotherapeutics against obesity. Cells 12, 1260 (2023).
Müller, T. D. et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019).
Olivares, M. et al. Gut microbiota DPP4-like enzymes are increased in type-2 diabetes and contribute to incretin inactivation. Genome Biol. 25, 174 (2024).
Gong, T. et al. Gut microbiota and metabolites exhibit different profiles after very-low-caloric restriction in patients with type 2 diabetes. Front. Endocrinol. 14, 1289571 (2024).
Liu, D. et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 21, 90 (2023).
Sun, Y. et al. Parabacteroides distasonis ameliorates insulin resistance via activation of intestinal GPR109a. Nat. Commun. 14, 7740 (2023).
Wang, Q. et al. Tauroursodeoxycholic acid protects Schwann cells from high glucose-induced cytotoxicity by targeting NLRP3 to regulate cell migration and pyroptosis. Biotechnol. Appl. Biochem. 71, 28–37 (2024).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588 (2018).
Matos, J. et al. Insights from Bacteroides species in children with type 1 diabetes. Microorganisms 9, 1436 (2021).
Girdhar, K. et al. A gut microbial peptide and molecular mimicry in the pathogenesis of type 1 diabetes. Proc. Natl Acad. Sci. USA 119, e2120028119 (2022).
Marwaha, S. et al. Novel and emerging treatments for major depression. Lancet 401, 141–153 (2023).
Simpson, C. A. et al. The gut microbiota in anxiety and depression - a systematic review. Clin. Psychol. Rev. 83, 101943 (2021).
Liu, L. et al. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 90, 104527 (2023).
Cryan, J. F. et al. The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013 (2019).
Nikolova, V. L. et al. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry 78, 1343–1354 (2021).
Deng, L. et al. Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 162, 111887 (2022).
Gomez-Nguyen, A. et al. Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav. Immun. 98, 245–250 (2021).
Yang, H. L. et al. Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation. Inflammation 44, 2448–2462 (2021).
Zheng, X., Cai, X. & Hao, H. Emerging targetome and signalome landscape of gut microbial metabolites. Cell Metab. 34, 35–58 (2022).
Cheng, L. et al. A Gpr35-tuned gut microbe-brain metabolic axis regulates depressive-like behavior. Cell Host Microbe 32, 227–243.e6 (2024).
Hirota, T. & King, B. H. Autism spectrum disorder: a review. JAMA 329, 157–168 (2023).
Xu, M., Xu, X., Li, J. & Li, F. Association between gut microbiota and autism spectrum disorder: a systematic review and meta-analysis. Front Psychiatry 10, 473 (2019).
Sharon, G. et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17 (2019).
Lin, T. L. et al. Amelioration of maternal immune activation-induced autism relevant behaviors by gut commensal Parabacteroides goldsteinii. Int. J. Mol. Sci. 23, 13070 (2022).
Goldberg, E. M. & Coulter, D. A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 14, 337–349 (2013).
Li, X. et al. Gut microbiota modification by diosgenin mediates antiepileptic effects in a mouse model of epilepsy. J. Neurochem. 168, 3982–4000 (2024).
Olson, C. A. et al. The gut microbiota mediates the anti-seizure effects of the Ketogenic diet. Cell 174, 497 (2018).
Strandwitz, P. et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403 (2019).
Koh, W., Kwak, H., Cheong, E. & Lee, C. J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 24, 523–539 (2023).
Fang, P., Kazmi, S. A., Jameson, K. G. & Hsiao, E. Y. The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 28, 201–222 (2020).
Blacher, E. et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480 (2019).
Ning, M. et al. Causal associations between gut microbiota, gut microbiota-derived metabolites, and Alzheimer’s disease: a multivariable Mendelian randomization study. J. Alzheimers Dis. 100, 229–237 (2024).
Lin, C. H. et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 16, 129 (2019).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016).
Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl Acad. Sci. USA 114, 10713–10718 (2017).
Faith, J. J., Ahern, P. P., Ridaura, V. K., Cheng, J. & Gordon, J. I. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci. Transl. Med 6, 220ra11 (2014).
Aburto, M. R. & Cryan, J. F. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 21, 222–247 (2024).
Becattini, S., Taur, Y. & Pamer, E. G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478 (2016).
Li, T. et al. A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage. Cell Host Microbe 32, 191–208.e9 (2024).
Wang, Y., Ngo, V. L., Zou, J. & Gewirtz, A. T. Commensal bacterial outer membrane protein A induces interleukin-22 production. Cell Rep. 43, 114292 (2024).
Pan, M., Barua, N. & Ip, M. Mucin-degrading gut commensals isolated from healthy faecal donor suppress intestinal epithelial inflammation and regulate tight junction barrier function. Front. Immunol. 13, 1021094 (2022).
Schirmer, M., Garner, A., Vlamakis, H. & Xavier, R. J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 17, 497–511 (2019).
Gaifem, J. et al. Akkermansia muciniphila and Parabacteroides distasonis synergistically protect from colitis by promoting ILC3 in the gut. mBio. 15, e0007824 (2024).
Mendes-Frias, A. et al. Akkermansia muciniphila and Parabacteroides distasonis as prognostic markers for relapse in ulcerative colitis patients. Front. Cell Infect. Microbiol. 14, 1367998 (2024).
Cuffaro, B. et al. In vitro characterization of gut microbiota-derived commensal strains: selection of parabacteroides distasonis strains alleviating TNBS-induced colitis in mice. Cells 9, 2104 (2020).
Kverka, M. et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 163, 250–259 (2011).
Zhu, J. et al. Integrative analysis with microbial modelling and machine learning uncovers potential alleviators for ulcerative colitis. Gut Microbes 16, 2336877 (2024).
Li, Z. et al. Parabacteroides goldsteinii enriched by Pericarpium Citri Reticulatae ‘Chachiensis’ polysaccharides improves colitis via the inhibition of lipopolysaccharide-involved PI3K-Akt signaling pathway. Int. J. Biol. Macromol. 277, 133726 (2024).
Liu, J. et al. Intestinal microbiota: a bridge between intermittent fasting and tumors. Biomed. Pharmacother. 167, 115484 (2023).
Yang, J. et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology 162, 135–149.e2 (2022).
Bai, X. et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 71, 2439–2450 (2022).
Gu, S. et al. Mutated CEACAMs disrupt transforming growth factor beta signaling and alter the intestinal microbiome to promote colorectal carcinogenesis. Gastroenterology 158, 238–252 (2020).
Yu, S. Y., Xie, Y. H., Qiu, Y. W., Chen, Y. X. & Fang, J. Y. Moderate alteration to gut microbiota brought by colorectal adenoma resection. J. Gastroenterol. Hepatol. 34, 1758–1765 (2019).
Ji, Y. et al. Comparison of effects on colitis-associated tumorigenesis and gut microbiota in mice between Ophiocordyceps sinensis and Cordyceps militaris. Phytomedicine 90, 153653 (2021).
Li, L. et al. Intestinal microbiota and metabolomics reveal the role of auricularia delicate in regulating colitis-associated colorectal cancer. Nutrients 15, 5011 (2023).
Chen, H. et al. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int J. Biol. Sci. 19, 2097–2113 (2023).
Ruiz-Malagón, A. J. et al. Tigecycline reduces tumorigenesis in colorectal cancer via inhibition of cell proliferation and modulation of immune response. Biomed. Pharmacother. 163, 114760 (2023).
Sheng, Q. S. et al. Comparison of gut microbiome in human colorectal cancer in paired tumor and adjacent normal tissues. Onco Targets Ther. 13, 635–646 (2020).
Cui, J. et al. Existence and distribution of the microbiome in tumour tissues of children with hepatoblastoma. Heliyon 10, e39547 (2024).
Wang, L. et al. Male-biased gut microbiome and metabolites aggravate colorectal cancer development. Adv. Sci. 10, e2206238 (2023).
Koh, G. Y. et al. Parabacteroides distasonis attenuates toll-like receptor 4 signaling and Akt activation and blocks colon tumor formation in high-fat diet-fed azoxymethane-treated mice. Int. J. Cancer 143, 1797–1805 (2018).
Yu, J. et al. Interleukin-10 deficiency suppresses colorectal cancer metastasis by enriching gut Parabacteroides distasonis. J. Adv. Res. https://doi.org/10.1016/j.jare.2024.11.024.
Liang, R. et al. Parabacteroides distasonis-derived outer membrane vesicles enhance antitumor immunity against colon tumors by modulating CXCL10 and CD8+ T cells. Drug Des. Dev. Ther. 18, 1833–1853 (2024).
Hajjar, R. et al. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut 72, 1143–1154 (2023).
Zhu, H. et al. Fusobacterium nucleatum promotes tumor progression in KRAS p.G12D-mutant colorectal cancer by binding to DHX15. Nat. Commun. 15, 1688 (2024).
Song, C. H. et al. Anti-PD-L1 antibody and/or 17β-estradiol treatment induces changes in the gut microbiome in MC38 colon tumor model. Cancer Res. Treat. 55, 894–909 (2023).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2022).
Wang, B. et al. Gut microbiota Parabacteroides distasonis enchances the efficacy of immunotherapy for bladder cancer by activating anti-tumor immune responses. BMC Microbiol 24, 237 (2024).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Fan, Z., Yi, Z., Li, S. & He, J. Parabacteroides distasonis promotes CXCL9 secretion of tumor-associated macrophages and enhances CD8T cell activity to trigger anti-tumor immunity against anti-PD-1 treatment in non-small cell lung cancer mice. BMC Biotechnol. 25, 30 (2025).
Alderete, T. L. et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes 13, 1961203 (2021).
Gallardo-Becerra, L. et al. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Micro Cell Fact. 19, 61 (2020).
Moreno-Arrones, O. M. et al. Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J. Eur. Acad. Dermatol. Venereol. 34, 400–405 (2020).
Huang, R. et al. Metagenome-wide association study of the alterations in the intestinal microbiome composition of ankylosing spondylitis patients and the effect of traditional and herbal treatment. J. Med. Microbiol. 69, 797–805 (2020).
Shapiro, J. et al. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J. Dermatol. 46, 595–603 (2019).
Liu, Y. et al. Interplay between dietary intake, gut microbiota, and metabolic profile in obese adolescents: Sex-dependent differential patterns. Clin. Nutr. 41, 2706–2719 (2022).
Wang, J. J. et al. Sex differences in colonization of gut microbiota from a man with short-term vegetarian and inulin-supplemented diet in germ-free mice. Sci. Rep. 6, 36137 (2016).
Zhang, F. et al. Sex- and age-dependent associations between parabacteroides and obesity: evidence from two population cohort. Microorganisms 11, 2087 (2023).
Zeibich, L. et al. Surgical menopause and estrogen therapy modulate the gut microbiota, obesity markers, and spatial memory in rats. Front. Cell. Infect. Microbiol. 11, 702628 (2021).
Ma, M. et al. Polysaccharide from edible alga Enteromorpha clathrata improves ulcerative colitis in association with increased abundance of Parabacteroides spp. in the gut microbiota of dextran sulfate sodium-fed mice. Mar. Drugs 20, 764 (2022).
Dziarski, R., Park, S. Y., Kashyap, D. R., Dowd, S. E. & Gupta, D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and bacteroides eggerthii enhance and alistipes finegoldii attenuates colitis in mice. PLoS ONE 11, e0146162 (2016).
Hu, X. et al. Intermittent fasting modulates the intestinal microbiota and improves obesity and host energy metabolism. NPJ Biofilms Microbiomes 9, 19 (2023).
Luo, W. et al. A Chinese medical nutrition therapy diet accompanied by intermittent energy restriction alleviates type 2 diabetes by enhancing pancreatic islet function and regulating gut microbiota composition. Food Res. Int. 161, 111744 (2022).
Jia, D. et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 187, 1651–1665.e21 (2024).
Lai, C. H. et al. Gut commensal Parabacteroides goldsteinii MTS01 alters gut microbiota composition and reduces cholesterol to mitigate helicobacter pylori-induced pathogenesis. Front. Immunol. 13, 916848 (2022).
Hugenholtz, F. & de Vos, W. M. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol. Life Sci. 75, 149–160 (2018).
Li, Z. et al. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 17, 33 (2024).
Zmora, N., Suez, J. & Elinav, E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 16, 35–56 (2019).
Song, Y. et al. “Bacteroides goldsteinii sp. nov.” isolated from clinical specimens of human intestinal origin. J. Clin. Microbiol. 43, 4522–4527 (2005).
Sakamoto, M., Kitahara, M. & Benno, Y. Parabacteroides johnsonii sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 57, 293–296 (2007).
Johnson, J. L., Moore, W. E. C. & Moore, L. V. H. Bacteroides caccae sp. nov., Bacteroides merdae sp. nov., and Bacteroides stercoris sp. nov. Isolated from Human Feces. Int. J. Syst. Evol. Microbiol. 36, 499–501 (1986).
Liao, J. et al. Docosahexaenoic acid promotes Cd excretion by restoring the abundance of parabacteroides in Cd-Exposed mice. Molecules 28, 4217 (2023).
Zhao, Q. et al. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat. Commun. 14, 1829 (2023).
Wei, W. Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid. Nat. Microbiol. 8, 1534–1548 (2023).
Chen, X. et al. Pregnancy-induced changes to the gut microbiota drive macrophage pyroptosis and exacerbate septic inflammation. Immunity 56, 336–352.e9 (2023).
Jiraskova Zakostelska, Z. et al. Lysate of Parabacteroides distasonis prevents severe forms of experimental autoimmune encephalomyelitis by modulating the priming of T cell response. Front. Immunol. 15, 1475126 (2024).
Gervason, S. et al. Antihyperalgesic properties of gut microbiota: Parabacteroides distasonis as a new probiotic strategy to alleviate chronic abdominal pain. Pain 165, e39–e54 (2024).
Sepulveda, M. et al. Coordinated elimination of bacterial taxa optimally attenuates alloimmunity and prolongs allograft survival. Am. J. Transpl. 24, 1573–1582 (2024).
Zhu, X. X. et al. Gut microbiome and metabolites mediate the benefits of caloric restriction in mice after acute kidney injury. Redox Biol. 77, 103373 (2024).
Wei, H. et al. Gut commensal Parabacteroides distasonis exerts neuroprotective effects in acute ischemic stroke with hyperuricemia via regulating gut microbiota-gut-brain axis. J. Transl. Med. 22, 999 (2024).
Su, D. et al. Gut commensal bacteria Parabacteroides goldsteinii-derived outer membrane vesicles suppress skin inflammation in psoriasis. J. Control. Release 377, 127–145 (2025).
Acknowledgements
We sincerely thank to the valuable suggestions provided by Professor Sidong Xiong (Soochow University). This work was supported by the National Natural Science Foundation of China (Grant No. 82160503, 32160178, 82272812), the Project of the Guizhou Provincial Department of Science and Technology (Grant No. QKHZC-2020-4Y156, QKH-JC-2018-1428, QKHJC-ZK-2022-624), the collaborative Innovation Center of Chinese Ministry of Education (2020-39), and the Project of the Department of Science and Technology of Zunyi (Grant No. ZKH-HZ-2021-193).
Author information
Authors and Affiliations
Contributions
Original draft preparation: J.L. Manuscript review and editing: J.L., H.Q., J.Z., N.S., C.C., Z.H., and X.Z. Supervision and conceptualization: J.Z. and Y.Z. Supervision and funding acquisition: L.X. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, J., Qiu, H., Zhao, J. et al. Parabacteroides as a promising target for disease intervention: current stage and pending issues. npj Biofilms Microbiomes 11, 137 (2025). https://doi.org/10.1038/s41522-025-00772-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-025-00772-0