Abstract
Fusobacterium functions as both commensal and pathogen, linking the oral–gut axis to diverse diseases, including cancer. Evidence shows it modulates microbial balance, promotes dysbiosis, and contributes to carcinogenesis by driving inflammation, proliferation, invasion, and immune evasion. This review integrates ecological, molecular, and clinical insights, highlighting its roles in oral and systemic disease and discussing therapeutic potential, underscoring Fusobacterium’s dualistic nature and implications for microbiome-targeted interventions.
Similar content being viewed by others
Introduction
Fusobacterium occupies a distinctive niche in microbial pathogenesis, balancing between benign commensalism and opportunistic pathogenicity. This dual identity grants it a disproportionate influence within the human microbiome, shaping both local and systemic disease processes, from chronic infections to malignancies1,2. Compared with other oral microbes, Fusobacterium exhibits unique ecological and functional characteristics, notably its capacity to tolerate and persist under both aerobic and anaerobic conditions and its role as a “bridging organism” that links early and late microbial colonizers3,4. As commensal members of the human and animal microbiota, Fusobacterium species primarily inhabit mucosal surfaces, with the oral cavity and colon serving as major reservoirs. Among anaerobic Gram-negative bacteria, Fusobacterium stands out for its prevalence, intricate host interactions, and involvement in a wide spectrum of diseases. Its clinical importance is further underscored by associations with conditions ranging from periodontal diseases to colorectal cancer, implicating diverse and complex pathogenic mechanisms that merit in-depth investigation5,6,7.
Historically, research focused on its role in oral health, where Fusobacterium was recognized as a key architect of dental biofilms, contributing to periodontal disease and shaping oral microbial ecology8. Over the past few decades, however, interest has expanded markedly due to its strong association with colorectal cancer. The recognition of Fusobacterium as a potential oncogenic microbe represents a pivotal shift in understanding its role beyond the oral cavity. Initial studies documented its enrichment in colorectal tumors and its ability to promote tumor progression via immune modulation and chronic inflammation. Evidence now suggests that Fusobacterium could traverse anatomical boundaries, contributing to the pathogenesis of pancreatic, esophageal, and gastric cancers9. These findings raise the possibility that its presence may not be merely correlative but potentially causal in oncogenesis.
Mechanistic studies have identified key molecular pathways linking Fusobacterium to tumor biology. A landmark discovery was the binding of the Fusobacterium adhesin FadA to E-cadherin, a critical epithelial adhesion protein10,11,12,13. This interaction activates β-catenin signaling, driving cell proliferation, survival, and metastatic potential12,14,15. Such signaling perturbations not only promote tumor growth but also enhance invasiveness, positioning Fusobacterium as an active facilitator of malignant transformation.
Equally notable is Fusobacterium’s remarkable adaptability to diverse environments, ranging from oxygen-rich to strictly anaerobic niches, and its capacity to shift from a harmless commensal to a pathogen under dysbiotic or immunocompromised conditions16,17,18. This adaptability, coupled with its frequent association with aberrant tissue proliferation in multiple cancers, has earned it the designation of an “oncobacterium”19,20. Its dual nature presents both a challenge and an opportunity: understanding when and how Fusobacterium transitions from symbiont to pathogen may yield critical insights into microbe–host dynamics in health and disease.
In this review, we synthesize current knowledge on Fusobacterium’s ecological functions, its pathogenic mechanisms in infectious diseases and cancer, and its potential as a therapeutic target. By integrating ecological, molecular, and clinical perspectives, we aim to illuminate the multifaceted roles of Fusobacterium and outline avenues for innovative research and intervention strategies.
Characteristics and significance
Overview of Fusobacterium
Fusobacterium is an anaerobic, Gram-negative bacterial genus that exhibits diverse morphologies, ranging from spherical to rod-shaped forms, and is predominantly found in the human oral cavity and gastrointestinal tract16,21,22,23. Unlike most oral bacteria, Fusobacterium possesses a remarkable ability to maintain anaerobic metabolism under microaerophilic conditions, enabling it to persist in varied microbial communities. While typically commensal, certain species, most notably Fusobacterium nucleatum (F. nucleatum) and Fusobacterium necrophorum (F. necrophorum), are well-recognized pathogens capable of causing systemic infections across various age groups. Their abundance, measured in colony-forming units (CFU) from oral and stool samples, varies over the human lifespan, suggesting dynamic shifts in colonization patterns24,25. Distinct phenotypic traits, such as hemolytic activity and specific metabolic profiles observed in vitro, further aid in differentiating species and strains within the genus26,27.
Importantly, Fusobacterium exhibits a context-dependent duality: functioning as a benign commensal under normal physiological conditions but transitioning to a pathogen in dysbiotic or immunocompromised states. This switch underscores its relevance in both maintaining mucosal homeostasis and contributing to diverse disease processes when microbial balance is disrupted.
Ecological interactions and diversity
Fusobacterium occupies a central ecological position in the oral and gastrointestinal microbiomes, acting as a bridging organism that facilitates co-aggregation between early colonizers (e.g., Streptococcus spp.) and late-stage anaerobes (e.g., Porphyromonas spp.). This bridging role promotes the hierarchical assembly and structural stability of biofilms. The process is mediated by specific molecular interactions, including FadA adhesins that bind both host tissues and neighboring bacteria, and virulence factors such as Fap2 that enhance co-aggregation and biofilm integrity.
Taxonomically, the genus comprises at least nine phylogenetic lineages and over 30 distinct species, as revealed by genomic sequencing28, marking a shift from earlier classifications based solely on metabolic and fermentative traits29. Ecologically, Fusobacterium shares close relationships with genera such as Prevotella and Porphyromonas, occupying overlapping niches and contributing to host cell invasion2,4. Its capacity to persist alongside oxygen-sensitive microbes even in oxygen-rich environments highlights its importance in maintaining microbial homeostasis under fluctuating oxygen conditions8,16.
Beyond its ecological contributions, Fusobacterium is implicated in a wide range of systemic conditions, particularly those affecting the oral and gastrointestinal tracts30,31,32,33. Its metabolic versatility, fermenting carbohydrates and proteins to produce short-chain fatty acids such as butyrate and acetate, affects host–microbe interactions in both healthy and dysbiotic states. Virulence factors, including leukotoxins and lipopolysaccharides (LPS), further enable its transition from commensal to pathogen, particularly in contexts of tissue damage or immune suppression8,34.
Within the genus, Fusobacterium nucleatum subsp. animalis has emerged as a clinically significant subspecies, especially in gastrointestinal diseases such as colorectal cancer and inflammatory disorders28,35,36. Recent studies indicate that this subspecies may play a prominent role in systemic infections and immune modulation. For example, it can bind to Siglec-7 receptors on natural killer (NK) cells, thereby suppressing NK cell-mediated cytotoxicity against cancer cells37. In addition, tandem mass spectrometry has been applied to detect F. nucleatum subspecies in the saliva from pre-colorectal cancer patients, underscoring the potential of subspecies-level detection as a biomarker in cancer-associated microbial communities38. Collectively, these findings position F. nucleatum subsp. animalis as a key focus for future research aimed at elucidating its ecological functions and pathogenic potential.
Role of Fusobacterium species in oral diseases
Fusobacterium species are pivotal contributors to the pathogenesis of oral diseases, with their diverse virulence factors and complex interactions within microbial communities driving processes ranging from plaque formation and gingivitis to invasive infections. Among these, F. nucleatum, a prominent bacterium in human dental plaque, plays a crucial role in plaque formation and is implicated in conditions such as gingivitis39,40. Recent research highlights its ability to influence microbial community structure through adhesive interactions, forming microbial aggregates critical for plaque stability and pathogenicity. This capacity stems from its remarkable ability to adhere to a broad spectrum of Gram-positive and Gram-negative microorganisms within plaque, including Porphyromonas gingivalis (P. gingivalis). Strongly linked to periodontitis, F. nucleatum is also frequently associated with invasive infections affecting the head and neck, thorax, lungs, liver, and abdomen. Its adhesive capabilities facilitate interactions with viruses and bacteria, promoting viral attachment to host tissue cells, thereby enhancing invasion and modulating host immune responses41,42,43,44.
In addition to F. nucleatum, F. necrophorum and other Fusobacterium species play significant roles in oral diseases. For example, F. necrophorum is frequently associated with severe forms of periodontal disease, particularly in immunocompromised patients45,46,47,48. These species contribute to pathogenic biofilm formation, and both F. nucleatum and F. necrophorum are implicated in the development of gingivitis, periodontitis, and more invasive oral infections.
Fusobacterium in oral squamous cell carcinoma
Recent studies have identified an enrichment of Fusobacterium in oral squamous cell carcinoma (OSCC) tissues, as shown in Table 141,49,50,51,52. The proportion of anaerobic bacteria is higher in OSCC tumors, whereas aerobic bacteria predominate in normal controls. These differences are primarily determined through sequencing methods, which, despite their high-throughput advantages, may be affected by technical biases such as DNA extraction efficiency or sequencing depth. This microbial shift highlights anaerobic bacteria as a potential reservoir on the OSCC surface, possibly contributing to cellular dysregulation. The distribution of Fusobacterium within OSCC tissues may differ greatly from that in healthy oral mucosa53. Notably, while several studies confirm the bacterium’s enrichment in OSCC, others report an absence of detectable Fusobacterium in cancer samples53 (Table 1).
This clinical association has prompted numerous in vitro studies exploring mechanisms by which Fusobacterium may mediate oral cancer progression50,54,55,56,57. F. nucleatum exhibits strong adherence to and invasion of human gingival epithelial cells (HGEC)58 (Table 1). It has also been shown to activate the NF-κB pathway, disrupt epithelial adhesion, and promote epithelial-mesenchymal transition (EMT), key steps in tumor progression and metastasis. Invasion appears to depend on specific bacterial components, as spontaneous mutants lacking invasive capabilities fail to penetrate HGEC59,60. Lectins, carbohydrate-binding proteins involved in cell–cell interactions, such as Galectin-9, are implicated in F. nucleatum-mediated adhesion and immune modulation in OSCC61 (Table 1).
Emerging evidence suggests that Fusobacterium does not act alone. Co-pathogens such as P. gingivalis and Candida albicans (C. albicans) have been observed to co-aggregate with Fusobacterium in biofilm communities. This co-localization enhances microbial colonization, sustains chronic inflammation, and may further promote oncogenic signaling in epithelial cells. Specifically, P. gingivalis could augment EMT via ZEB1-mediated signaling, while C. albicans may contribute to local acetaldehyde production, a known carcinogen. Furthermore, several studies link Fusobacterium presence to patient survival outcomes and clinicopathological parameters in oral cancer62,63,64. Notably, the detection of Fusobacterium correlates with clinicopathological parameters, including tumor size and stage65. This relationship suggests that Fusobacterium may influence disease progression and clinical prognosis, underscoring its importance in cancer biology.
Fusobacterium’s role also extends to interactions with human papillomavirus (HPV), particularly in head and neck cancers, suggesting that it can modulate immune responses and foster an inflammatory microenvironment that may exacerbate HPV-mediated carcinogenesis66. However, it is important to note that the interaction between Fusobacterium and HPV may vary among oral cancer subtypes. Some studies suggest mutual exclusivity between the two in certain head and neck cancers, indicating a complex interplay in cancer progression67
Multiple molecular pathways are activated by Fusobacterium in OSCC cells. Notably, the NF-κB pathway is markedly upregulated in its presence, promoting cancer cell survival, proliferation, and invasiveness68. Additionally, Fusobacterium can disrupt E-cadherin-mediated cell adhesion, facilitating EMT and metastasis69,70. These molecular interactions underscore the bacterium’s significant role in tumor progression and metastasis71.
A critical aspect of Fusobacterium infection is its induction of cytokine secretion. Studies have demonstrated that it stimulates the production of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α in oral cancer cells, contributing to a pro-tumorigenic environment72. This cytokine release can promote tumor growth, invasion, and resistance to apoptosis, highlighting the potential for targeting these inflammatory pathways as a therapeutic strategy.
Fusobacterium as a periodontal pathogen
Toxic metabolites produced by Fusobacterium species can contribute to periodontal pathology by inhibiting or killing periodontal cells, such as fibroblasts73,74,75,76 (Table 1). Furthermore, Fusobacterium’s ability to evade immune responses through sulfide production and to modulate local inflammation underscores its role in chronic periodontal diseases77. For instance, sulfides generated by F. nucleatum may help the bacterium evade host immune defenses78. Butyric acid, along with propionic acid and ammonium ions produced by F. nucleatum, inhibits the proliferation of human gingival fibroblasts, further impairing periodontal tissue health79. Fusobacterium can also penetrate the gingival epithelium and is frequently found in plaque associated with periodontal disease, underscoring its prevalence and pathogenic potential80.
Beyond toxic metabolite production, Fusobacterium employs various molecular strategies to sustain infection within periodontal tissues81,82,83. Adhesins such as FadA facilitate bacterial adherence to and invasion of host epithelial cells, enabling deeper tissue colonization13,84. Furthermore, Fusobacterium modulates host immune responses by secreting enzymes and factors that degrade host tissues and immune components, thereby promoting bacterial survival and persistence. These toxins, while not directly cytotoxic, significantly impair fibroblast proliferation, delaying wound healing and contributing to chronic inflammation85,86,87. This interplay between bacterial virulence factors and host immune modulation highlights Fusobacterium’s pivotal role in the pathogenesis of periodontal disease, including gingivitis88.
Role of Fusobacterium in gut cancers and diseases
The genus Fusobacterium exhibits a multifaceted role in gastrointestinal pathologies, spanning neoplastic and non-neoplastic diseases. Accumulating evidence implicates Fusobacterium, particularly F. nucleatum, not only in colorectal carcinogenesis but also in other gastrointestinal malignancies and inflammatory conditions, underscoring its systemic pathogenic potential.
Impact of Fusobacterium on colorectal cancer
The Fusobacterium detected in colorectal cancer tissues is genetically identical to strains from the oral cavity, suggesting microbial translocation via hematogenous or enteric routes89,90. Whole-genome sequencing studies support this oral-gut axis, positioning Fusobacterium as a mediator of distal carcinogenesis. Clinically, meta-analyses demonstrate significantly higher Fusobacterium DNA detection rates in colorectal tumor tissues compared to adjacent healthy tissues or controls, with a less pronounced but still significant enrichment observed in precancerous polyps, particularly those with high-grade dysplasia91. Fecal microbiome analyses reveal elevated F. nucleatum abundance in colorectal cancer patients, with pooled odds ratios indicating stronger associations in colorectal cancer than in healthy controls or polyp-bearing individuals23,92,93. Beyond colorectal cancer, F. nucleatum has been detected in pancreatic, esophageal, and gastric cancers, suggesting broad oncogenic relevance94,95.
CpG islands are regions of DNA rich in cytosine and guanine dinucleotides, often located near gene promoters. In cancer, hypermethylation of these regions, frequently catalyzed by DNA methyltransferases (DNMTs), can silence tumor suppressor genes, contributing to tumorigenesis. The CpG Island Methylator Phenotype (CIMP) and Microsatellite Instability (MSI) are hallmark epigenetic and genetic alterations, respectively, in colorectal and gastric cancers, both of which have been linked to Fusobacterium presence. Specifically, studies demonstrate that Fusobacterium infection enhances DNMT-mediated CpG island methylation, which silences tumor suppressor genes such as MLH1 and CDKN2A, thereby driving cancer progression. Moreover, Fusobacterium triggers MSI through inflammatory responses that impair DNA mismatch repair proteins, further promoting genetic mutations. Mechanistic studies also show that F. nucleatum interacts with host cells via its FadA adhesin protein and E-cadherin, activating the β-catenin signaling pathway to promote tumor cell proliferation and invasion13,96. Additionally, F. nucleatum can inhibit the function of NK cells, diminishing immune surveillance and creating favorable conditions for tumor metastasis97,98.
Moreover, multiple studies associate intratumoral Fusobacterium with poorer survival outcomes in colorectal cancer99,100. Fusobacterium presence correlates with adverse clinicopathological features, such as larger tumor size, increased depth of invasion, poor differentiation, lymph node or distant metastasis, and advanced tumor stages7,101,102,103,104,105.
In summary, genomic analyses of the colorectal cancer microbiome consistently show significant enrichment of Fusobacterium species, particularly strains closely related to F. nucleatum, F. mortiferum, and F. necrophorum. These species have been identified in various clinical settings and are increasingly recognized for their potential role in human health and disease. This enrichment is corroborated by molecular analyses, such as fluorescence in situ hybridization (FISH), quantitative PCR, and sequencing-based methods, that identify Fusobacterium DNA within primary tumor tissues and colorectal metastases, highlighting its potential involvement in tumor progression and metastasis.
Association of Fusobacterium with other gastrointestinal cancers
Recent studies reveal that beyond its established role in colorectal cancer, Fusobacterium is intricately linked to a spectrum of other gastrointestinal tumors, notably showing high prevalence in esophageal, gastric, and pancreatic cancers (Table 1). Pan-cancer analyses demonstrate an increased abundance of Fusobacterium in both primary tumors and adjacent normal tissues across gastrointestinal cancers, contrasting with non-gastrointestinal malignancies. Specifically, a significant prevalence of Fusobacterium species in gastric cancer patients suggests a potential contributory role in the pathogenesis of this cancer106. The enrichment of F. nucleatum in esophageal cancer has been correlated with poor prognosis95,107,108; however, direct mechanistic evidence remains limited.
CCL20 (C-C motif chemokine ligand 20) is a chemokine critically involved in inflammatory responses by recruiting immune cells, especially dendritic cells and Th17 cells, into inflamed tissues and tumor microenvironments. This association may be driven by F. nucleatum’s ability to activate chemokines such as CCL20, intensifying inflammatory responses within the tumor microenvironment109 (Table 1). Such an inflammatory milieu fosters a favorable environment for tumor progression.
Additionally, METTL3 (Methyltransferase-like 3) is an enzyme involved in m6A methylation, which plays a significant role in regulating RNA stability and splicing. Enhanced METTL3 activity has been associated with increased tumor metastasis and progression. Specifically, F. nucleatum infection has been shown to enhance METTL3-mediated m6A methylation, promoting metastasis in esophageal squamous cell carcinoma (ESCC)110 (Table 1).
Furthermore, NOD1 (nucleotide-binding oligomerization domain-containing protein 1) is an intracellular pattern-recognition receptor (PRR) that senses bacterial components, activating downstream signaling pathways involving the adaptor protein RIPK2 (receptor-interacting serine/threonine-protein kinase 2). F. nucleatum further invades ESCC cells, activating the NF-κB pathway via the NOD1/RIPK2 signaling axis, thereby facilitating tumor progression111 (Table 1). Collectively, these findings suggest that F. nucleatum contributes to cancer advancement through diverse mechanisms, including chemokine activation, epigenetic modifications, and inflammatory signaling.
In gastric cancer, the enrichment of bacterial taxa including Fusobacterium, Lactobacillus, and Veillonella underscores the significance of microbiome composition alterations in tumorigenesis112 (Table 1). Notably, F. nucleatum and other Fusobacterium species have emerged as potential diagnostic biomarkers for gastric cancer113,114. High expression of the Gal-GalNAc antigen, a carbohydrate structure serving as a binding target for specific bacterial adhesins, in gastric and esophageal cancers may facilitate increased adherence and enrichment of F. nucleatum within the tumor microenvironment, thereby influencing disease progression115.
In pancreatic cancer, while Fusobacterium spp. have been detected in pancreatic tumor tissues (Table 1), other studies have not consistently confirmed its involvement116. However, the presence of Fusobacterium within pancreatic ductal adenocarcinoma (PDAC) tumors correlates with a poorer prognosis in PDAC patients117. This observation suggests that Fusobacterium may serve as a prognostic biomarker for PDAC, indicating potentially variable roles of Fusobacterium across gastrointestinal cancers, possibly mediated by distinct mechanisms95.
In summary, the extensive presence of Fusobacterium across gastrointestinal tumors, particularly its dominance in the colorectal cancer microenvironment, highlights the complex and multifaceted relationship between F. nucleatum and gastrointestinal cancers. However, the evidence linking Fusobacterium to non-colorectal gastrointestinal cancers, such as pancreatic and esophageal cancer, remains largely correlative, and further mechanistic studies are needed to clarify its causal role in these contexts.
Association of Fusobacterium with other gastrointestinal diseases
The relationship between Fusobacterium and inflammatory bowel disease (IBD) is particularly noteworthy within the broader context of digestive system disorders. IBD, which includes ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic intestinal inflammation. Recent research has increasingly focused on the genus Fusobacterium, especially F. nucleatum, due to its association with IBD118,119. Studies have demonstrated that both the frequency and abundance of F. nucleatum in the intestines of IBD patients are significantly elevated compared with healthy individuals, correlating closely with disease severity120.
F. nucleatum appears to drive UC progression by promoting a shift toward pro-inflammatory M1-type macrophages. Mechanistically, this occurs in part through activation of the AKT2 signaling pathway, an intracellular serine/threonine kinase pathway that regulates diverse cellular processes including proliferation, survival, metabolism, and inflammation. Targeting F. nucleatum or its AKT2-mediated inflammatory signaling could therefore represent a promising therapeutic approach for UC121. Furthermore, F. nucleatum exacerbates intestinal inflammation, epithelial barrier dysfunction, dysbiosis, and metabolic disruption, thereby worsening UC through its capacity to adhere to and invade host epithelial cells122.
The association between Fusobacterium and IBD underscores its broader role in digestive disease pathogenesis. Its high invasiveness and positive correlation with disease activity suggest that F. nucleatum may promote inflammation and disease progression by modulating host immune responses and destabilizing the intestinal microenvironment. Recent studies have further implicated Fusobacterium in functional gastrointestinal disorders, such as irritable bowel syndrome (IBS) and functional dyspepsia123,124,125. In these conditions, F. nucleatum can exacerbate symptoms by altering intestinal microbiota composition, disrupting the mucosal barrier, increasing intestinal permeability, and facilitating the release of inflammatory mediators126. Interactions between Fusobacterium and abnormalities in the enteric nervous system may also contribute to visceral hypersensitivity and motility disorders in IBS127. These findings suggest that rebalancing the intestinal microbiota, particularly by controlling Fusobacterium overgrowth, could offer a potential strategy for managing functional gastrointestinal diseases128,129.
Although direct studies on the association between Fusobacterium and liver diseases are limited, emerging research has begun to explore its potential impact130. In liver diseases, especially non-alcoholic fatty liver disease (NAFLD) and liver fibrosis, recent findings indicate a close link with gut microbiota dysbiosis131. Fusobacterium may exacerbate liver damage by directly influencing hepatocyte function and structure through its virulence factors132.
Fusobacterium species are also implicated in various gastrointestinal infections31. They have been isolated from intra-abdominal abscesses, liver abscesses, and colonic lesions. F. nucleatum, in particular, has been linked to colorectal cancer, where it promotes tumor growth and metastasis through interactions with host immune cells and the tumor-associated infectious microenvironment31.
In summary, the genus Fusobacterium demonstrates complex and multifaceted associations with a variety of non-tumoral digestive diseases. These connections enhance our understanding of the pathogenesis of these conditions and suggest potential avenues for novel therapeutic strategies.
Pathogenic mechanisms of Fusobacterium species in disease progression
Impact of epigenetic changes
Fusobacterium was once regarded as a passive resident in the gastrointestinal tract; however, it is now recognized that F. nucleatum infection can trigger specific tumor-associated molecular events in colorectal cancer, including the CpG island methylation phenotype, microsatellite instability, and mutations in the BRAF and TP53 genes100,133,134.
Epigenetic studies have shown that F. nucleatum can alter host cell DNA methylation and histone modification via various pathways. In particular, elevated CpG island methylation is associated with silencing of tumor suppressor genes. F. nucleatum has been linked to increased CpG island methylation, often mediated by DNA methyltransferases (DNMTs), enzymes that add methyl groups to DNA, thereby regulating gene expression2,100,133,135. This effect may be enhanced by F. nucleatum’s inhibition of molecules that compete with DNMTs, leading to amplified DNMT activity, further silencing tumor suppressor genes and promoting cancer progression.
Recent studies have also associated F. nucleatum enrichment in head and neck squamous cell carcinoma (HNSCC) with hypermethylation of tumor suppressor gene promoters. In addition, F. nucleatum can influence chromatin structure and regulate gene expression by modifying histones, resulting in transcriptional repression of key tumor suppressor genes23,136,137.
The epigenetic alterations induced by F. nucleatum extend to non-coding RNAs, particularly microRNAs. Several studies have shown that F. nucleatum can modulate host cell behavior by altering microRNA expression138,139, leading to enhanced inflammatory responses, increased cell proliferation, and reduced apoptosis. These intricate regulatory networks reveal how F. nucleatum reshapes the host epigenetic landscape, directly driving tumorigenesis and progression.
In conclusion, F. nucleatum plays a multifaceted role in colorectal cancer by inducing epigenetic changes. These alterations not only deepen our understanding of its contribution to cancer progression but also suggest potential therapeutic targets among these epigenetic markers. As research continues to elucidate F. nucleatum’s influence on the epigenetic landscape, new opportunities may arise for its integration into cancer prevention and treatment strategies.
Activation of cell proliferation
At its core, cancer is characterized by uncontrolled cell growth, and F. nucleatum influences cancer cell proliferation through interactions with host proteins. The FadA–E-cadherin–β-catenin pathway is also implicated in colorectal cancer proliferation140,141. In colorectal cancer, F. nucleatum enhances tumor cell proliferation in mouse xenografts by activating Toll-like receptor 4 (TLR4) signaling via MYD88, which subsequently triggers NF-κB activation and upregulates miR-21. This microRNA downregulates RASA1, a RAS GTPase that normally restrains cell proliferation and differentiation.
In addition, F. nucleatum promotes intestinal tumor growth by increasing cyclin D1 expression, a key cell cycle regulator73. In OSCC, both F. nucleatum and P. gingivalis significantly stimulate cell proliferation through upregulation of cyclin D1 and c-Myc142. Activation of TLR4 by F. nucleatum elevates IL-6 production, which in turn activates STAT3, a transcription factor that regulates cyclin D1 and c-Myc41. Moreover, F. nucleatum induces DNA damage and promotes proliferation in oral cancer cells by reducing the expression of p27, a cyclin-dependent kinase inhibitor, and by downregulating DNA repair proteins such as Ku70 and p53 56. These molecular interactions facilitate cancer cell proliferation, accelerate cell cycle progression, and impair DNA repair, contributing to tumor initiation and progression in multiple cancer types.
Induction of inflammation
The pro-inflammatory potential of F. nucleatum is well-documented, as it can stimulate the production of reactive oxygen species (ROS) and pro-inflammatory cytokines73. Chronic inflammation is a major driver of carcinogenesis, which may partly explain the strong association between periodontitis and an increased risk of OSCC143. Elevated F. nucleatum levels correlate with increased inflammatory cytokines in both colorectal cancer and OSCC142,144, fostering a tumor-promoting inflammatory microenvironment.
As mentioned before, F. nucleatum activates the TLR4-NF-κB signaling axis, leading to pro-inflammatory cytokine production in both oral and colorectal tissues145,146. This inflammatory cascade reinforces tumor-supportive conditions, underscoring F. nucleatum’s central role in cancer progression.
Anti-tumor immune response and immunotherapy
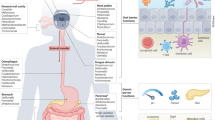
As illustrated in Fig. 1, F. nucleatum exerts profound effects on host immune cells. It impairs dendritic cell antigen-presenting capacity, thereby weakening anti-tumor immunity, and polarizes tumor-associated neutrophils (TANs) toward a pro-tumorigenic phenotype that supports cancer cell proliferation, invasion, and metastasis. While the bulk of evidence underscores Fusobacterium’s immunosuppressive and tumor-promoting actions, emerging studies suggest a more nuanced role—one that may also involve modulating anti-tumor immunity and shaping responses to immunotherapy in specific contexts147,148. Together with its effects on dendritic cells and TANs, F. nucleatum also shapes other immune compartments, including myeloid-derived suppressor cells, T cells, and NK cells, thereby reinforcing an immunosuppressive tumor microenvironment.
The schematic depicts epithelial cells in the upper layer, with blood vessels and tumor cells in the lower portion. F. nucleatum activates multiple signaling pathways that stimulate the production of inflammatory cytokines, leading to inflammation and DNA damage, thereby fostering tumor initiation, proliferation, recurrence, and metastasis. In addition, F. nucleatum modulates the activity of various immune cells, including tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), dendritic cells, T cells, natural killer (NK) cells, and myeloid-derived suppressor cells (MDSCs), ultimately influencing tumor growth, invasion, and dissemination. Solid lines indicate direct effects, whereas dashed lines represent indirect effects. This figure was created using MedPeer (medpeer.cn), and appropriate publication and licensing rights have been obtained.
In the ApcMin/+ mouse model, F. nucleatum has been shown to recruit bone marrow-derived suppressor cells, particularly myeloid-derived suppressor cells (MDSCs), to the tumor site. These MDSCs inhibit T cell proliferation and induce T cell apoptosis, fostering an immunosuppressive microenvironment101,144. This is supported by observations of a negative correlation between F. nucleatum abundance and CD3 and CD4 T cell infiltration in colorectal and breast cancer tissues. Additionally, a similar negative association exists between F. nucleatum load and markers of immune cells, such as B lymphocytes, CD4 T helper cells, M2 macrophages, and fibroblasts, in OSCC, underscoring F. nucleatum’s broad immunosuppressive influence149. F. nucleatum uses specific inhibitory proteins to impair T cell activation, arresting cells in the G1 phase of the cell cycle. The interaction between the Fusobacterium Fap2 adhesin and the TIGIT receptor on T cells and NK cells exemplifies an immune evasion strategy that protects both F. nucleatum and nearby tumor cells from immune-mediated destruction98,150. In addition to Fap2, other adhesins such as FadA and RadD play critical roles in F. nucleatum’s ability to adhere to and co-aggregate with host cells and other microbes, contributing to its pathogenic potential. Furthermore, the outer membrane proteins Fap2 and RadD induce lymphocyte cell death, and the polarization of M2 macrophages via the TLR4/IL-6/p-STAT3/c-MYC pathway reflects Fusobacterium’s multifaceted approach to immune suppression151,152.
In the context of tumor immunotherapy, F. nucleatum may modulate treatment effectiveness. Differences in colorectal cancer microsatellite instability (MSI) status show that high levels of F. nucleatum correlate with an enhanced response to PD-1 blockade therapy100,153. In mouse models of colorectal cancer, F. nucleatum not only amplifies the effects of PD-L1 inhibitors but also synergizes with these therapies to extend survival. As has been observed in OSCC, F. nucleatum can induce PD-L1 expression, potentially via the STING signaling pathway, which may contribute to resistance or response modulation in PD-L1 blockade therapy154,155,156. Furthermore, patient-derived organoid models have shown a positive association between F. nucleatum levels and response to PD-L1 blockade, highlighting the bacterium’s potential as a biomarker or adjunct in colorectal cancer immunotherapy. Beyond single-taxon effects such as F. nucleatum, system-level modulation of the gut ecosystem via fecal microbiota transplantation (FMT) can reshape responses to immune checkpoint inhibitors (ICIs). However, evidence remains mixed and context-dependent, underscoring the need for standardized, patient-tailored FMT–ICI protocols and rigorous multicenter randomized trials157. In parallel, analogous microbiota-transfer strategies along the oral–gut axis, such as transplanting defined oral microbial consortia to recalibrate dysbiosis, may likewise be explored to achieve disease-modifying benefits.
Cell migration and invasion
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases responsible for degrading extracellular matrix (ECM) components, and play a key role in pathological processes that involve excessive ECM degradation, such as tumor invasion and metastasis. Both P. gingivalis and F. nucleatum can stimulate MMP production through distinct mechanisms, promoting cancer cell invasion and metastasis158,159.
In OSCC, periodontal pathogens like P. gingivalis and F. nucleatum have been shown to induce the expression of MMPs, particularly MMP-1 and MMP-9160. In breast cancer models, co-culture of AT3 mouse breast carcinoma cells with F. nucleatum resulted in overexpression of MMP-9, implicating this bacterium in enhancing metastatic potential161,162.
F. nucleatum is also associated with the dysregulation of genes involved in the EMT, a process critical for cell migration and invasion. In colorectal cancer, elevated F. nucleatum levels correlate with decreased expression of the epithelial marker E-cadherin and increased expression of the mesenchymal marker N-cadherin163. Similarly, OSCC cell lines exposed to F. nucleatum exhibit downregulation of E-cadherin and upregulation of N-cadherin, vimentin, and Snail142. These changes signify an EMT-like process, essential for cancer cells to acquire invasive properties.
F. nucleatum has been shown to upregulate ZEB1, promoting a mesenchymal phenotype in oral cancer cells142. This mechanism mirrors bacterial modulation of EMT observed in Helicobacter pylori-infected gastric epithelial cells, suggesting a conserved role for bacterial influence on EMT in cancer progression.
Co-aggregation and oral carcinogenesis
F. nucleatum acts as a keystone species in both the oral and gastrointestinal microbiomes, often serving as a bridge for co-aggregation among various microbial species. This ability to facilitate multispecies biofilm formation largely stems from its outer membrane adhesin, Fap2, which mediates interactions with other bacteria, including both Gram-negative and Gram-positive species164.
In the oral cavity, F. nucleatum can significantly alter the local environment, affecting the colonization and pathogenic potential of other microbes. For example, in the presence of P. gingivalis, a key periodontal pathogen, F. nucleatum is consistently observed, suggesting that its colonization precedes, and is necessary for, that of P. gingivalis. Through the fermentation of glutamate and aspartate, F. nucleatum produces ammonia, which neutralizes acidic metabolic byproducts like lactic acid, creating a more favorable environment for P. gingivalis and promoting a synergistic relationship within the biofilm165.
The co-aggregation of F. nucleatum with P. gingivalis has been implicated in both periodontal disease progression and tumor development. F. nucleatum facilitates multispecies assemblies through outer membrane adhesins such as Fap2 and RadD. It promotes the colonization of P. gingivalis and C. albicans by neutralizing local acidity via ammonia production, thereby creating a favorable environment for microbial synergy. Together, they may contribute to tumorigenesis by inducing chronic inflammation and stimulating OSCC cell proliferation in vitro142. Additionally, F. nucleatum modulates the immune response, potentially suppressing host defenses and facilitating the persistence of other pathogens.
Interactions between Fusobacterium species and Candida albicans (C. albicans), an opportunistic pathogenic yeast found in the gastrointestinal tract and oral cavity, further illustrate the role of this co-aggregation in disease progression. One proposed mechanism involves Candida-derived ethanol dehydrogenase converting ethanol into acetaldehyde, a recognized carcinogen in the oral environment. Co-aggregation between Fusobacterium species and Candida enhances colonization166, and elevated levels of F. nucleatum have been observed in Candida-colonized oral leukoplakia lesions. This co-localization may increase epithelial exposure to acetaldehyde and contribute to early-stage malignant transformation, although this remains to be clinically validated. Further studies are warranted to confirm the role of Fusobacterium-Candida synergy in the pathogenesis of OSCC.
In conclusion, the complex network of interactions between Fusobacterium and other microbes within the oral and gastrointestinal microbiomes plays a pivotal role in shaping its impact on health and disease. These interactions drive chronic inflammation, modulate immune responses, and contribute to genotoxic stress, collectively promoting carcinogenesis and other pathological conditions167.
Association with other related diseases and cancers
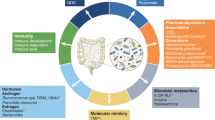
Fusobacterium-related diseases span a range of conditions, reflecting the bacterium’s broad impact on human health (Fig. 2). Since its initial association with infections in 1936, particularly jugular vein septic thrombophlebitis, Fusobacterium has been increasingly implicated in various health conditions. The rising incidence of Fusobacterium infections is likely due to improved detection methods and changes in antibiotic use168. These infections, especially severe in pediatric cases, frequently affect the head and neck, with acute otitis media being a prominent manifestation169,170,171,172,173. Rapid progression of these infections can lead to serious complications, including bacteremia and osteomyelitis174, underscoring the need for early detection and treatment.
The left side highlights major diseases linked to microbiota in the oral cavity and pharynx, including periodontitis, endodontic infections, gingivitis, tonsillitis, and head and neck cancers. The central human figure indicates bodily systems potentially impacted by microbiota-related conditions, including the gastrointestinal tract, respiratory system, and urogenital tract. In the gastrointestinal tract, specific conditions such as appendicitis, inflammatory bowel diseases, and colorectal cancer are shown. The right side lists additional diseases associated with microbiota, such as endocarditis, atherosclerosis, respiratory tract infections, brain abscess, liver abscess, and osteomyelitis. In females, microbiota imbalances may also be linked to adverse pregnancy outcomes, including preterm labor, stillbirth, and chorioamnionitis. The color coding represents the strength of evidence linking these diseases to microbiota: red for moderate evidence, orange for some evidence, and green for associative evidence. This figure was created using MedPeer (medpeer.cn), and appropriate publication and licensing rights have been obtained.
Lemierre’s syndrome, a severe form of septic thrombophlebitis affecting the internal jugular vein, exemplifies the significant morbidity associated with Fusobacterium infections, particularly those involving F. necrophorum175,176,177. The bacterium’s role in systemic infections, such as bacteremia in individuals with malignancies or immunosuppression, highlights its potential for high mortality rates and necessitates vigilant monitoring and comprehensive management178. Fusobacterium-related endocarditis, though rare, poses a serious risk to individuals with preexisting heart conditions179.
Recent large-scale analyses of the global burden and inequalities associated with COVID-19 underscore its far-reaching health impacts beyond acute infection, raising questions about potential effects on the human microbiome180,181. Indeed, emerging evidence suggests that the COVID-19 pandemic may influence the oral and gut microbiome, with some research reporting shifts in Fusobacterium abundance; however, the clinical relevance of this observation remains to be clarified182. Some preliminary studies have reported increased Fusobacterium levels in individuals with gout, but whether this reflects a causal relationship or secondary microbial shift remains unclear183,184. This expanding association between Fusobacterium and various health conditions reinforces the need for advanced research and targeted therapeutic strategies. However, these associations remain speculative and should be interpreted with caution. Further longitudinal and mechanistic studies are needed to validate any potential causative links.
Beyond its well-documented connection to colorectal and oral cancers, Fusobacterium has been implicated in other malignancies, including breast and pancreatic cancers. Studies have detected Fusobacterium in breast cancer tissues, suggesting a potential role in cancer progression, and have associated its presence with poorer prognostic outcomes101,185,186,187. F. nucleatum has also been detected in liver abscesses and in advanced liver cancer, suggesting it may contribute to liver cancer progression and could serve as a therapeutic target188.
The association between Fusobacterium and diverse cancer types has become a focal point of research, with recent studies linking F. nucleatum to breast cancer through Fap2-mediated adhesion to elevated levels of the Gal-GalNAc antigen in breast tissues161. F. nucleatum-derived extracellular vesicles stimulate breast cancer proliferation and metastasis, potentially through TLR4 activation186. Moreover, F. nucleatum may promote breast cancer cell proliferation and treatment resistance by stimulating autophagy and facilitating immune evasion through immune suppression189. In liver cancer, F. nucleatum has been detected in liver abscesses and in patients with advanced disease190. Although the exact mechanisms remain unclear, they may involve pathways similar to those observed in breast cancer, including enhanced tumor growth and metastasis.
Research into Fusobacterium’s role in cancer also examines its interactions within the hypoxic tumor microenvironment191. Hypoxia, common in solid tumors, affects tumor cell behavior and their response to bacterial infections191,192. Understanding how F. nucleatum interacts with hypoxic tumor cells could uncover new insights into its role in cancer progression and suggest potential therapeutic approaches.
In summary, the growing body of research on Fusobacterium and its involvement in cancers beyond the oral and gastrointestinal tracts underscores its potential as a therapeutic target. Novel therapies, such as F. nucleatum-mimicking nanovehicles, which utilize F. nucleatum’s surface characteristics to target specific cancer cells, show promise in improving the treatment of cancers193,194. These nanovehicles can be engineered to deliver therapeutic agents directly to Fusobacterium-enriched tumor sites, enhancing the specificity and efficacy of cancer treatments. As research in this area advances, the intricate relationship between Fusobacterium and cancer is likely to reveal additional intervention opportunities.
In conclusion, expanding research on Fusobacterium, particularly F. nucleatum, underscores its multifaceted role beyond the oral cavity, linking it to a wide spectrum of systemic diseases, from inflammatory bowel disease to colorectal, pancreatic, and esophageal cancers. Acting both as a commensal organism and a potent pathogen, Fusobacterium serves as a microbial bridge within complex communities, facilitating co-aggregation, shaping ecosystem dynamics, and influencing disease pathogenesis through inflammation, cell proliferation, immune modulation, and tumor microenvironment remodeling. Its detection in malignancies beyond the colorectum suggests a broader oncogenic potential that warrants deeper investigation into its molecular mechanisms and host interactions. Clinically, its involvement in systemic infections such as Lemierre’s syndrome, bacteremia, and cervicofacial and gastrointestinal infections further highlights its significance. As the understanding of Fusobacterium-driven tumorigenesis advances, emerging therapeutic strategies, such as nanovehicle-based targeted drug delivery and novel antibiotics directed at specific strains, offer promising avenues to enhance treatment efficacy, minimize side effects, and improve patient outcomes. These innovations, coupled with continued elucidation of Fusobacterium’s pathogenic mechanisms, could transform both the prevention and management of its associated diseases.
Data availability
No datasets were generated or analysed during the current study.
References
Hofer, U. Fusobacterium orchestrates oral biofilms. Nat. Rev. Microbiol. 20, 576 (2022).
Brennan, C. A. & Garrett, W. S. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166 (2019).
Bradshaw, D. J., Marsh, P. D., Watson, G. K. & Allison, C. Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration. Infect. Immun. 66, 4729–4732 (1998).
Kolenbrander, P. E. Multispecies communities: interspecies interactions influence growth on saliva as sole nutritional source. Int J. Oral. Sci. 3, 49–54 (2011).
Karahashi, Y. et al. Fusobacterium nucleatum putatively affects the alveoli by disrupting the alveolar epithelial cell tight junction, enlarging the alveolar space, and increasing paracellular permeability. Biochem. Biophys. Res Commun. 682, 216–222 (2023).
Michikawa, C. et al. Fusobacterium is enriched in oral cancer and promotes induction of programmed death-ligand 1 (PD-L1). Neoplasia 31, 100813 (2022).
Bullman, S. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017).
Sakanaka, A. et al. Fusobacterium nucleatum metabolically integrates commensals and pathogens in oral biofilms. mSystems 7, e0017022 (2022).
Mitsuhashi, K. et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 6, 7209–7220 (2015).
Li, D., Li, Z., Wang, L., Zhang, Y. & Ning, S. Oral inoculation of Fusobacterium nucleatum exacerbates ulcerative colitis via the secretion of virulence adhesin FadA. Virulence 15, 2399217 (2024).
Guo, P. et al. FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 39, 202 (2020).
Jiang, Y. et al. Banxia Xiexin Decoction delays colitis-to-cancer transition by inhibiting E-cadherin/β-catenin pathway via Fusobacterium nucleatum FadA. J. Ethnopharmacol. 328, 117932 (2024).
Xu, M. et al. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J. Biol. Chem. 282, 25000–25009 (2007).
Li, B. et al. Fusobacterium nucleatum induces oxaliplatin resistance by inhibiting ferroptosis through E-cadherin/β-catenin/GPX4 axis in colorectal cancer. Free Radic. Biol. Med. 220, 125–138 (2024).
Zhou, Z. et al. Vanillin Derivatives Reverse Fusobacterium nucleatum-Induced Proliferation and Migration of Colorectal Cancer Through E-Cadherin/β-Catenin Pathway. Front. Pharm. 13, 841918 (2022).
Choi, E., Murray, B. & Choi, S. Biofilm and Cancer: Interactions and Future Directions for Cancer Therapy. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms241612836 (2023).
Han, Y. W. Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147 (2015).
Furuichi, M. et al. Commensal consortia decolonize Enterobacteriaceae via ecological control. Nature 633, 878–886 (2024).
Duong, M. T., Qin, Y., You, S. H. & Min, J. J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp. Mol. Med. 51, 1–15 (2019).
Wang, Q. et al. Fusobacterium nucleatum promotes colorectal cancer through neogenesis of tumor stem cells. J. Clin. Invest. 135, https://doi.org/10.1172/jci181595 (2025).
Irfan, M., Delgado, R. Z. R. & Frias-Lopez, J. The oral microbiome and cancer. Front. Immunol. 11, 591088 (2020).
Matthes de Freitas Pontes, K. et al. Clinical study of the biofilm of implant-supported complete dentures in healthy patients. Gerodontology 39, 148–160 (2022).
Wang, N. & Fang, J. Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol 31, 159–172 (2023).
Idrissi Janati, A. et al. Investigation of Fusobacterium Nucleatum in saliva and colorectal mucosa: a pilot study. Sci. Rep. 12, 5622 (2022).
Hida, Y., Nishida, T., Taniguchi, C. & Sakakibara, H. Association between swallowing function and oral bacterial flora in independent community-dwelling elderly. Aging Clin. Exp. Res. 33, 157–163 (2021).
Amoako, K. K., Goto, Y. & Shinjo, T. Studies on the factors affecting the hemolytic activity of Fusobacterium necrophorum. Vet. Microbiol 41, 11–18 (1994).
Strauss, J., White, A., Ambrose, C., McDonald, J. & Allen-Vercoe, E. Phenotypic and genotypic analyses of clinical Fusobacterium nucleatum and Fusobacterium periodonticum isolates from the human gut. Anaerobe 14, 301–309 (2008).
Zepeda-Rivera, M. A., Dewhirst, F. E., Bullman, S. & Johnston, C. D. Addressing controversy in Fusobacterium nomenclature: what exactly does “F. nucleatum” refer to?. Gut Microbes 17, 2514797 (2025).
Michels, N. et al. Human microbiome and metabolic health: An overview of systematic reviews. Obes. Rev. 23, e13409 (2022).
Curtis, M. A., Diaz, P. I. & Van Dyke, T. E. The role of the microbiota in periodontal disease. Periodontol 2000 83, 14–25 (2020).
Dougherty, M. W. & Jobin, C. Intestinal bacteria and colorectal cancer: etiology and treatment. Gut Microbes 15, 2185028 (2023).
Yamazaki, K. & Kamada, N. Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal. Immunol. https://doi.org/10.1016/j.mucimm.2023.11.006 (2023).
Gao, L. et al. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 9, 488–500 (2018).
Narayanan, S. et al. Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes. Infect. Immun. 70, 4609–4620 (2002).
Galaski, J. et al. Fusobacterium nucleatum subsp. nucleatum RadD binds Siglec-7 and inhibits NK cell-mediated cancer cell killing. iScience 27, 110157 (2024).
Robinson, A. V. & Allen-Vercoe, E. Strain specificity in fusobacterial co-aggregation with colorectal cancer-relevant species. Anaerobe 82, 102758 (2023).
Lamprinaki, D. et al. Siglec-7 Mediates Immunomodulation by Colorectal Cancer-Associated Fusobacterium nucleatum ssp. animalis. Front Immunol. 12, 744184 (2021).
Morsi, H. et al. Detection of Fusobacterium nucleatum subspecies in the saliva of pre-colorectal cancer patients, using tandem mass spectrometry. Arch. Oral. Biol. 134, 105337 (2022).
Chen, Y. et al. More Than Just a Periodontal Pathogen -the Research Progress on Fusobacterium nucleatum. Front. Cell Infect. Microbiol 12, 815318 (2022).
Liu, P. et al. Detection of fusobacterium nucleatum and fadA adhesin gene in patients with orthodontic gingivitis and non-orthodontic periodontal inflammation. PLoS One 9, e85280 (2014).
Stasiewicz, M. & Karpinski, T. M. The oral microbiota and its role in carcinogenesis. Semin. Cancer Biol. 86, 633–642 (2022).
Louis-Jean, S. F., Agrawal, N. & Bisht, S. Fusobacterium nucleatum Pyogenic Liver Abscess and the Role of Bacterial Virulence and Gut Microbiota Dysbiosis. Cureus 15, e34548 (2023).
Wu, C. Y., Yu, Z. Y., Hsu, Y. C. & Hung, S. L. Enhancing production of herpes simplex virus type 1 in oral epithelial cells by co-infection with Aggregatibacter actinomycetemcomitans. J. Formos. Med. Assoc. 121, 1841–1849 (2022).
Engevik, M. A. et al. Fusobacteriumnucleatum Adheres to Clostridioides difficile via the RadD Adhesin to Enhance Biofilm Formation in Intestinal Mucus. Gastroenterology 160, 1301–1314.e1308 (2021).
Malheiros Vde, J. & Avila-Campos, M. J. Detection of pathogens from periodontal lesions. Rev. Saude Publica 38, 723–728 (2004).
Ogugua, C. Bilateral lemierre syndrome secondary to periodontitis: a case report and review of the literature. J. Bronchol. Inter. Pulmonol. 16, 115–120 (2009).
de Sousa, E. L. et al. Bacteriological study of root canals associated with periapical abscesses. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radio. Endod. 96, 332–339 (2003).
Roberts, G. L. Fusobacterial infections: an underestimated threat. Br. J. Biomed. Sci. 57, 156–162 (2000).
Li, R. et al. Role of oral microbiome in oral oncogenesis, tumor progression, and metastasis. Mol. Oral. Microbiol. 38, 9–22 (2023).
Yan, K. et al. Microbial changes associated with oral cavity cancer progression. Otolaryngol. Head. Neck Surg. 168, 1443–1452 (2023).
Su, S. C. et al. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 42, 127–135 (2021).
Li, Z. et al. Alterations of the oral microbiota profiles in Chinese patient with oral cancer. Front Cell Infect. Microbiol. 11, 780067 (2021).
Gopinath, D. et al. Culture-independent studies on bacterial dysbiosis in oral and oropharyngeal squamous cell carcinoma: A systematic review. Crit. Rev. Oncol. Hematol. 139, 31–40 (2019).
Yao, Y., Shen, X., Zhou, M. & Tang, B. Periodontal pathogens promote oral squamous cell carcinoma by regulating ATR and NLRP3 Inflammasome. Front. Oncol. 11, 722797 (2021).
Meng, Q. et al. Fusobacterium nucleatum secretes amyloid-like FadA to enhance pathogenicity. EMBO Rep. 22, e52891 (2021).
Geng, F., Zhang, Y., Lu, Z., Zhang, S. & Pan, Y. Fusobacterium nucleatum Caused DNA damage and promoted cell proliferation by the Ku70/p53 Pathway in Oral Cancer Cells. DNA Cell Biol. 39, 144–151 (2020).
Zhang, S. et al. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 287, 4032–4047 (2020).
Li, Y. et al. Coinfection with Fusobacterium nucleatum can enhance the attachment and invasion of Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans to human gingival epithelial cells. Arch. Oral. Biol. 60, 1387–1393 (2015).
Yang, R. et al. The regulatory effect of coaggregation between Fusobacterium nucleatum and Streptococcus gordonii on the Synergistic Virulence to Human Gingival Epithelial Cells. Front. Cell Infect. Microbiol 12, 879423 (2022).
Han, Y. W. et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect. Immun. 68, 3140–3146 (2000).
Muñoz-Grez, C. P. et al. Host-microbe computational proteomic landscape in oral cancer revealed key functional and metabolic pathways between Fusobacterium nucleatum and cancer progression. Int. J. Oral. Sci. 17, 1 (2025).
Li, Z. et al. The significant clinical correlation of the intratumor oral microbiome in oral squamous cell carcinoma based on tissue-derived sequencing. Front. Physiol. 13, 1089539 (2022).
Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Chiscuzzu, F. et al. Current evidence on the relation between microbiota and oral cancer-the role of Fusobacterium nucleatum-A Narrative Review. Cancers 17, https://doi.org/10.3390/cancers17020171 (2025).
Liu, Y., Li, Z., Qi, Y., Wen, X. & Zhang, L. Metagenomic analysis reveals a changing microbiome associated with the depth of invasion of oral squamous cell carcinoma. Front. Microbiol 13, 795777 (2022).
Rui, M. et al. The baseline oral microbiota predicts the response of locally advanced oral squamous cell carcinoma patients to induction chemotherapy: A prospective longitudinal study. Radiother. Oncol. 164, 83–91 (2021).
Desai, S. et al. Fusobacterium nucleatum is associated with inflammation and poor survival in early-stage HPV-negative tongue cancer. NAR Cancer 4, zcac006 (2022).
Chen, Z. et al. The intersection between oral microbiota, host gene methylation and patient outcomes in head and neck squamous cell carcinoma. Cancers) 12, https://doi.org/10.3390/cancers12113425 (2020).
Fan, C. C. et al. Expression of E-cadherin, Twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J. Cancer Res Clin. Oncol. 139, 1735–1744 (2013).
Hakim, S. G. et al. Prognostic impact of the loss of E-cadherin and de novo expression of N-cadherin at the invasive front of primary and recurrent oral squamous cell carcinoma. Front. Oncol. 13, 1151879 (2023).
Gopinath, D., Li, Z., Mohammed, M. M. & Panda, S. Role of oral microbes in epithelial-mesenchymal transition in cancer progression. Mol. Oral Microbiol., e70001, https://doi.org/10.1111/omi.70001 (2025).
Neuzillet, C. et al. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci. Rep. 11, 7870 (2021).
Kang, W. et al. Fusobacterium nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-kappaB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell Longev. 2019, 1681972 (2019).
Kang, W., Ji, X., Zhang, X., Tang, D. & Feng, Q. Persistent Exposure to Fusobacterium nucleatum Triggers Chemokine/Cytokine Release and Inhibits the Proliferation and Osteogenic Differentiation Capabilities of Human Gingiva-Derived Mesenchymal Stem Cells. Front. Cell Infect. Microbiol 9, 429 (2019).
Despins, C. A. et al. Modulation of the Host Cell Transcriptome and Epigenome by Fusobacterium nucleatum. mBio 12, e0206221 (2021).
Wang, Y. et al. Study of the inflammatory activating process in the early stage of Fusobacterium nucleatum infected PDLSCs. Int. J. Oral. Sci. 15, 8 (2023).
Torresyap, G., Haffajee, A. D., Uzel, N. G. & Socransky, S. S. Relationship between periodontal pocket sulfide levels and subgingival species. J. Clin. Periodontol. 30, 1003–1010 (2003).
Yoshida, A. et al. Hydrogen sulfide production from cysteine and homocysteine by periodontal and oral bacteria. J. Periodontol. 80, 1845–1851 (2009).
Sun, J. et al. Fusobacterium nucleatum dysregulates inflammatory cytokines and NLRP3 inflammasomes in oral cells. Oral Dis. https://doi.org/10.1111/odi.14899 (2024).
Xiao, X. et al. A qualitative and quantitative analysis of the human gingival crevicular fluid proteome and metaproteome. Proteomics 21, e2000321 (2021).
Nogueira, A. V. B. et al. Effect of Bacterial Infection on Ghrelin Receptor Regulation in Periodontal Cells and Tissues. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms23063039 (2022).
Liu, H., Liu, Y., Fan, W. & Fan, B. Fusobacterium nucleatum triggers proinflammatory cell death via Z-DNA binding protein 1 in apical periodontitis. Cell Commun. Signal 20, 196 (2022).
De Andrade, K. Q., Almeida-da-Silva, C. L. C., Ojcius, D. M. & Coutinho-Silva, R. Differential involvement of the canonical and noncanonical inflammasomes in the immune response against infection by the periodontal bacteria Porphyromonas gingivalis and Fusobacterium nucleatum. Curr. Res Micro Sci. 2, 100023 (2021).
Zhang, Z. et al. Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of Fusobacterium nucleatum into oral epithelial cells by downregulating FadA and FomA. J. Periodontol. 93, 515–525 (2022).
Wilson, M. Biological activities of lipopolysaccharides from oral bacteria and their relevance to the pathogenesis of chronic periodontitis. Sci. Prog. 78, 19–34 (1995).
Bapat, R. A. et al. Antimicrobial FiteBac® K21 promotes antimicrobial Potency and wound healing. Heliyon 9, e19282 (2023).
Bhattacharya, R. et al. Effect of bacteria on the wound healing behavior of oral epithelial cells. PLoS One 9, e89475 (2014).
Di Spirito, F. et al. Human Herpesviruses, bacteria, and fungi in gingivitis and periodontitis pediatric subjects: a systematic review. Children 12, https://doi.org/10.3390/children12010039 (2024).
Zepeda-Rivera, M. et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 628, 424–432 (2024).
Flemer, B. et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67, 1454–1463 (2018).
Janati, A. I., Karp, I., Laprise, C., Sabri, H. & Emami, E. Detection of Fusobaterium nucleatum in feces and colorectal mucosa as a risk factor for colorectal cancer: a systematic review and meta-analysis. Syst. Rev. 9, 276 (2020).
He, Z., Tian, W., Wei, Q. & Xu, J. Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front. Immunol. 13, 968649 (2022).
Idrissi Janati, A., Karp, I., Sabri, H. & Emami, E. Is a fusobacterium nucleatum infection in the colon a risk factor for colorectal cancer?: a systematic review and meta-analysis protocol. Syst. Rev. 8, 114 (2019).
Baima, G., Ribaldone, D. G., Romano, F., Aimetti, M. & Romandini, M. The Gum-Gut Axis: Periodontitis and the risk of gastrointestinal cancers. Cancers 15, https://doi.org/10.3390/cancers15184594 (2023).
Ye, C. et al. Fusobacterium nucleatum in tumors: from tumorigenesis to tumor metastasis and tumor resistance. Cancer Biol. Ther. 25, 2306676 (2024).
Chen, Y. et al. Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 Expression. Theranostics 10, 323–339 (2020).
Han, J. et al. Gut microbiome: decision-makers in the microenvironment of colorectal cancer. Front. Cell Infect. Microbiol. 13, 1299977 (2023).
Gur, C. et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355 (2015).
Gao, Z. et al. Heterogeneity of intratumoral microbiota within the tumor microenvironment and relationship to tumor development. Med Res. 1, 32–61 (2025).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
Castellarin, M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012).
Lu, X. et al. Long non-coding RNA EVADR induced by Fusobacterium nucleatum infection promotes colorectal cancer metastasis. Cell Rep. 40, 111127 (2022).
Martin-Gallausiaux, C. et al. Fusobacterium nucleatum promotes inflammatory and anti-apoptotic responses in colorectal cancer cells via ADP-heptose release and ALPK1/TIFA axis activation. Gut Microbes 16, 2295384 (2024).
Chen, S. et al. Fusobacterium nucleatum reduces METTL3-mediated m(6)A modification and contributes to colorectal cancer metastasis. Nat. Commun. 13, 1248 (2022).
Hsieh, Y. Y. et al. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci. Rep. 8, 158 (2018).
Song, X., Greiner-Tollersrud, O. K. & Zhou, H. Oral microbiota variation: a risk factor for development and poor prognosis of esophageal cancer. Dig. Dis. Sci. 67, 3543–3556 (2022).
Muszyński, D. et al. Esophageal cancer and bacterial part of gut microbiota - A multidisciplinary point of view. Front. Cell Infect. Microbiol. 12, 1057668 (2022).
Yamamura, K. et al. Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res 22, 5574–5581 (2016).
Guo, S. et al. Intracellular Fusobacterium nucleatum infection increases METTL3-mediated m6A methylation to promote the metastasis of esophageal squamous cell carcinoma. J. Adv. Res. https://doi.org/10.1016/j.jare.2023.08.014 (2023).
Nomoto, D. et al. Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression via the NOD1/RIPK2/NF-kappaB pathway. Cancer Lett. 530, 59–67 (2022).
Castaño-Rodríguez, N., Goh, K. L., Fock, K. M., Mitchell, H. M. & Kaakoush, N. O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 7, 15957 (2017).
Yang, J., Zhou, X., Liu, X., Ling, Z. & Ji, F. Role of the gastric microbiome in gastric cancer: from carcinogenesis to treatment. Front Microbiol 12, 641322 (2021).
Kaźmierczak-Siedlecka, K., Daca, A., Roviello, G., Catalano, M. & Połom, K. Interdisciplinary insights into the link between gut microbiome and gastric carcinogenesis-what is currently known?. Gastric Cancer 25, 1–10 (2022).
Abed, J. et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 20, 215–225 (2016).
Stasiewicz, M., Kwaśniewski, M. & Karpiński, T. M. Microbial associations with pancreatic cancer: a new frontier in biomarkers. Cancers 13, https://doi.org/10.3390/cancers13153784 (2021).
Hayashi, M. et al. Intratumor Fusobacterium nucleatum promotes the progression of pancreatic cancer via the CXCL1-CXCR2 axis. Cancer Sci. 114, 3666–3678 (2023).
Karpinski, T. M., Ozarowski, M. & Stasiewicz, M. Carcinogenic microbiota and its role in colorectal cancer development. Semin Cancer Biol. 86, 420–430 (2022).
Fan, Z. et al. Fusobacterium nucleatum and its associated systemic diseases: epidemiologic studies and possible mechanisms. J. Oral. Microbiol 15, 2145729 (2023).
Glassner, K. L., Abraham, B. P. & Quigley, E. M. M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 145, 16–27 (2020).
Liu, L. et al. Fusobacterium nucleatum aggravates the progression of Colitis by regulating M1 Macrophage Polarization via AKT2 Pathway. Front Immunol. 10, 1324 (2019).
Lin, S. et al. Fusobacterium nucleatum aggravates ulcerative colitis through promoting gut microbiota dysbiosis and dysmetabolism. J. Periodontol. 94, 405–418 (2023).
Quaglio, A. E. V., Grillo, T. G., De Oliveira, E. C. S., Di Stasi, L. C. & Sassaki, L. Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 28, 4053–4060 (2022).
Cui, X. et al. Fecal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease patients with irritable bowel syndrome-type symptoms. BMC Gastroenterol. 21, 433 (2021).
Villanueva-Millan, M. J. et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am. J. Gastroenterol. 117, 2055–2066 (2022).
Gu, X. et al. Fusobacterium nucleatum causes microbial dysbiosis and exacerbates visceral hypersensitivity in a colonization-independent manner. Front Microbiol 11, 1281 (2020).
Yang, J. et al. Involvement of mucosal flora and enterochromaffin cells of the caecum and descending colon in diarrhoea-predominant irritable bowel syndrome. BMC Microbiol. 21, 316 (2021).
Simon, E., Călinoiu, L. F., Mitrea, L. & Vodnar, D. C. Probiotics, Prebiotics, and synbiotics: implications and beneficial effects against irritable Bowel Syndrome. Nutrients 13, https://doi.org/10.3390/nu13062112 (2021).
Di Rosa, C. et al. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 15, https://doi.org/10.3390/nu15071647 (2023).
Chung, M. et al. Comparisons of oral, intestinal, and pancreatic bacterial microbiomes in patients with pancreatic cancer and other gastrointestinal diseases. J. Oral. Microbiol 13, 1887680 (2021).
Su, X. et al. Composition of gut microbiota and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Obes. Rev. 25, e13646 (2024).
Kuraji, R. et al. Nisin lantibiotic prevents NAFLD liver steatosis and mitochondrial oxidative stress following periodontal disease by abrogating oral, gut and liver dysbiosis. NPJ Biofilms Microbiomes 10, 3 (2024).
Tahara, T. et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 74, 1311–1318 (2014).
Nishihara, R. et al. Abstract PR01: Fusobacterium nucleatum and mutational landscape of colorectal cancer in whole-exome sequencing analysis. Cancer Res. 77, PR01–PR01 (2017).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Kong, C. et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 72, 1129–1142 (2023).
Zhang, J. W. et al. Fusobacterium nucleatum promotes esophageal squamous cell carcinoma progression and chemoresistance by enhancing the secretion of chemotherapy-induced senescence-associated secretory phenotype via activation of DNA damage response pathway. Gut Microbes 15, 2197836 (2023).
Wei, S. et al. Exosomal-miR-129-2-3p derived from Fusobacterium nucleatum-infected intestinal epithelial cells promotes experimental colitis through regulating TIMELESS-mediated cellular senescence pathway. Gut Microbes 15, 2240035 (2023).
Tang, B. et al. MicroRNA-31 induced by Fusobacterium nucleatum infection promotes colorectal cancer tumorigenesis. iScience 26, 106770 (2023).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 20, https://doi.org/10.15252/embr.201847638 (2019).
Li, X. et al. Fusobacterium nucleatum promotes the progression of colorectal cancer through Cdk5-activated Wnt/beta-Catenin Signaling. Front Microbiol 11, 545251 (2020).
Pignatelli, P., Nuccio, F., Piattelli, A. & Curia, M. C. The role of Fusobacterium nucleatum in Oral and Colorectal Carcinogenesis. Microorganisms 11, https://doi.org/10.3390/microorganisms11092358 (2023).
Engevik, M. A. et al. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation. mBio 12, https://doi.org/10.1128/mBio.02706-20 (2021).
Wu, J., Li, Q. & Fu, X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 12, 846–851 (2019).
Hu, L. et al. Fusobacterium nucleatum facilitates M2 macrophage polarization and colorectal carcinoma progression by activating TLR4/NF-kappaB/S100A9 Cascade. Front Immunol. 12, 658681 (2021).
Koike, R. et al. Heat-Killed Fusobacterium nucleatum triggers varying heme-related inflammatory and stress responses depending on primary human respiratory epithelial cell type. Molecules 25, https://doi.org/10.3390/molecules25173839 (2020).
Lin, A. et al. Microbiota boost immunotherapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors. BMC Med. 23, 341 (2025).
Situ, Y. et al. The metabolic dialogue between intratumoural microbes and cancer: implications for immunotherapy. EBioMedicine 115, 105708 (2025).
Liang, M. et al. Fusobacterium nucleatum induces MDSCs enrichment via activation the NLRP3 inflammosome in ESCC cells, leading to cisplatin resistance. Ann. Med. 54, 989–1003 (2022).
Gur, C. et al. Fusobacterium nucleatum supresses anti-tumor immunity by activating CEACAM1. Oncoimmunology 8, e1581531 (2019).
Chen, T. et al. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 67, 1635–1646 (2018).
Hu, L. et al. Fusobacterium nucleatum Facilitates M2 macrophage polarization and colorectal carcinoma progression by activating TLR4/NF-κB/S100A9 Cascade. Front Immunol. 12, 658681 (2021).
Gao, Y. et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target Ther. 6, 398 (2021).
Li, Y. et al. Intracellular Fusobacterium nucleatum infection attenuates antitumor immunity in esophageal squamous cell carcinoma. Nat. Commun. 14, 5788 (2023).
Jiang, S. S. et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797 e789 (2023).
Ugai, T. et al. Inverse relationship between Fusobacterium nucleatum amount and tumor CD274 (PD-L1) expression in colorectal carcinoma. Clin. Transl. Immunol. 12, e1453 (2023).
Lin, A. et al. From chaos to order: optimizing fecal microbiota transplantation for enhanced immune checkpoint inhibitors efficacy. Gut Microbes 17, 2452277 (2025).
Ding, Y. et al. Release and activation of human neutrophil matrix metallo- and serine proteinases during phagocytosis of Fusobacterium nucleatum, Porphyromonas gingivalis and Treponema denticola. J. Clin. Periodontol. 24, 237–248 (1997).
Tipton, D. A., Babu, J. P. & Dabbous, M. Effects of cranberry components on human aggressive periodontitis gingival fibroblasts. J. Periodontal Res. 48, 433–442 (2013).
Sztukowska, M. N. et al. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol 18, 844–858 (2016).
Parhi, L. et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 11, 3259 (2020).
Guo, X., Yu, K. & Huang, R. The ways Fusobacterium nucleatum translocate to breast tissue and contribute to breast cancer development. Mol. Oral. Microbiol 39, 1–11 (2024).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Ali Mohammed, M. M., Pettersen, V. K., Nerland, A. H., Wiker, H. G. & Bakken, V. Label-free quantitative proteomic analysis of the oral bacteria Fusobacterium nucleatum and Porphyromonas gingivalis to identify protein features relevant in biofilm formation. Anaerobe 72, 102449 (2021).
Yamaguchi-Kuroda, Y., Kikuchi, Y., Kokubu, E. & Ishihara, K. Porphyromonas gingivalis diffusible signaling molecules enhance Fusobacterium nucleatum biofilm formation via gene expression modulation. J. Oral. Microbiol. 15, 2165001 (2023).
Thurnheer, T., Karygianni, L., Flury, M. & Belibasakis, G. N. Fusobacterium Species and Subspecies Differentially Affect the Composition and Architecture of Supra- and Subgingival Biofilms Models. Front Microbiol 10, 1716 (2019).
Li, G. et al. Breaking boundaries: chronic diseases and the frontiers of immune microenvironments. Med. Res. 1, 62–102 (2025).
Li, Z., Wang, Q., Huang, X., Wu, Y. & Shan, D. Microbiome’s role in musculoskeletal health through the gut-bone axis insights. Gut Microbes 16, 2410478 (2024).
Arane, K. & Goldman, R. D. Fusobacterium infections in children. Can. Fam. Phys. 62, 813–814 (2016).
Stergiopoulou, T. & Walsh, T. J. Fusobacterium necrophorum otitis and mastoiditis in infants and young toddlers. Eur. J. Clin. Microbiol Infect. Dis. 35, 735–740 (2016).
Brook, I. Fusobacterial infections in children. J. Infect. 28, 155–165 (1994).
Haraldsson, G., Holbrook, W. P. & Könönen, E. Clonal similarity of salivary and nasopharyngeal Fusobacterium nucleatum in infants with acute otitis media experience. J. Med. Microbiol. 53, 161–165 (2004).
Giridharan, W. et al. Complicated otitis media caused by Fusobacterium necrophorum. J. Laryngol. Otol. 118, 50–53 (2004).
Pandey, S. et al. Septic thrombophlebitis of portal and splenic vein secondary to Fusobacterium nucleatum: A case report of an abdominal variant of lemierre syndrome. Medicine 102, e35622 (2023).
Nygren, D., Elf, J., Torisson, G. & Holm, K. Jugular Vein Thrombosis and Anticoagulation Therapy in Lemierre’s Syndrome-A Post Hoc Observational and Population-Based Study of 82 Patients. Open Forum Infect. Dis. 8, ofaa585 (2021).
Lee, W. S., Jean, S. S., Chen, F. L., Hsieh, S. M. & Hsueh, P. R. Lemierre’s syndrome: A forgotten and re-emerging infection. J. Microbiol. Immunol. Infect. 53, 513–517 (2020).
Riordan, T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre’s syndrome. Clin. Microbiol. Rev. 20, 622–659 (2007).
Kajihara, T. et al. Distribution, Trends, and Antimicrobial Susceptibility of Bacteroides, Clostridium, Fusobacterium, and Prevotella Species Causing Bacteremia in Japan During 2011-2020: A Retrospective Observational Study Based on National Surveillance Data. Open Forum Infect. Dis. 10, ofad334 (2023).
Hatz, C. R., Cremona, M., Liu, C. C., Schmidlin, P. R. & Conen, A. Antibiotic prophylaxis with amoxicillin to prevent infective endocarditis in periodontitis patients reconsidered: a narrative review. Swiss Med. Wkly 151, w30078 (2021).
Shan, D. et al. Identifying Potential Vulnerability to Long COVID Through Global-to-Local Inequalities in Years Lived With Disability Attributed to COVID-19, 2020–2021, Across 920 Locations. Med. Res. (2025).
Shan, D. et al. Global, Regional, National, and Local Burden of COVID-19 With Inequality Analysis Across 920 Locations, 2020–2021. Med. Res. https://doi.org/10.1002/mdr2.70013 (2025).
Wolff, L. et al. COVID-19-Associated Fusobacterium nucleatum Bacteremia, Belgium. Emerg. Infect. Dis. 27, 975–977 (2021).
Chu, Y. et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 7, 66 (2021).
Cao, C. et al. Association of gut microbiota and biochemical features in a chinese population with renal uric acid stone. Front Pharm. 13, 888883 (2022).
Yusuf, E., Wybo, I. & Piérard, D. Case series of patients with Fusobacterium nucleatum bacteremia with emphasis on the presence of cancer. Anaerobe 39, 1–3 (2016).
Li, G. et al. Fusobacterium nucleatum-derived small extracellular vesicles facilitate tumor growth and metastasis via TLR4 in breast cancer. BMC Cancer 23, 473 (2023).
Hieken, T. J. et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 6, 30751 (2016).
Shigefuku, R. et al. Fusobacterium nucleatum detected simultaneously in a pyogenic liver abscess and advanced sigmoid colon cancer. Anaerobe 48, 144–146 (2017).
Van der Merwe, M., Van Niekerk, G., Botha, A. & Engelbrecht, A. M. The onco-immunological implications of Fusobacterium nucleatum in breast cancer. Immunol. Lett. 232, 60–66 (2021).
Soni, K., Fricker, Z. & Long, M. T. Pyogenic liver abscesses from Fusobacterium nucleatum in an immunocompetent host. Clin. Res Hepatol. Gastroenterol. 44, e59–e60 (2020).
Udayasuryan, B. et al. Fusobacterium nucleatum infection modulates the transcriptome and epigenome of HCT116 colorectal cancer cells in an oxygen-dependent manner. Commun. Biol. 7, 551 (2024).
Shukla, S. D. et al. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology 25, 53–63 (2020).
Geng, S. et al. Biomimetic Nanovehicle-Enabled Targeted Depletion of Intratumoral Fusobacterium nucleatum Synergizes with PD-L1 Blockade against Breast Cancer. ACS Nano 18, 8971–8987 (2024).
Chen, L. et al. Antibacterial Fusobacterium nucleatum-Mimicking Nanomedicine to Selectively Eliminate Tumor-Colonized Bacteria and Enhance Immunotherapy Against Colorectal Cancer. Adv. Mater. 35, e2306281 (2023).
Kamarajan, P. et al. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 16, e1008881 (2020).
Acknowledgements
The authors received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Additionally, the authors acknowledge the use of MedPeer (medpeer.cn) for creating Figures 1 and 2. Appropriate publication and licensing rights have been obtained (License Nos.: 8cf274jvhh979kgt21753945877 and 22f326f6ea05ak3dqy1755176713).
Author information
Authors and Affiliations
Contributions
Z.R.L., P.L., and D.S. were responsible for research conception and design. Z.R.L., J.A.L., J.L., Z.K.Z., D.G., and P.L. were responsible for manuscript drafting. Z.R.L., X.F.H., and Q.W. were responsible for figure preparation and visualization. Z.R.L., J.A.L., Q.W., and D.S. were responsible for critical revision and final approval of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Liu, J., Li, J. et al. Fusobacterium in the microbiome: from health to disease across the oral–gut axis and beyond. npj Biofilms Microbiomes 11, 200 (2025). https://doi.org/10.1038/s41522-025-00838-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-025-00838-z