Abstract
This study characterizes trends in contralateral breast cancer (CBC) and contralateral prophylactic mastectomy (CPM) in Ontario, Canada, by breast cancer risk level. Overall, there has been a decline in CBC rates and the concomitant rise in CPM rates after unilateral breast cancer diagnosis, largely driven by high-risk individuals. Considerations for breast cancer risk level are recommended in evaluations of CBC and CPM trends.
Similar content being viewed by others
Introduction
Among females diagnosed with a first primary breast cancer, the occurrence of a second primary contralateral breast cancer (CBC) is the most common malignancy, accounting for nearly 50% of all second cancers in this population1. As the 5-year breast cancer survival rate approaches 90% in Canada, a growing population of breast cancer survivors is at risk of CBC2. To our knowledge, very few studies have characterized CBC incidence trends in Canada, particularly within the last two decades3,4. In addition, individuals with a high lifetime risk of breast cancer (due to a genetic predisposition, strong family history, or other important risk factors)5 need to be considered when delineating trends. Thus, the study describes the annual incidence rates of CBC and CPM in Ontario from 2009 to 2016, while considering differences in trends by breast cancer risk level.
Among 64,779 eligible females diagnosed with primary breast cancer (invasive or in situ) in Ontario, 879 (1.36%) developed CBC and 720 (1.11%) underwent a CPM procedure during the follow-up period (Supplementary Table 1). Of those, 1560 (2.41%) qualified for high-risk breast cancer screening and were considered high-risk. The mean latency time between primary breast cancer diagnosis and contralateral breast cancer was 3.63 years (range 1.00–9.38 years).
CBC incidence was high among those with primary breast cancer diagnosed from age 20 to 29, 30–39, and 70–79 (incidence rate (IR): 0.44, 0.42 and 0.43 per 100 person-years; incidence proportion (IP): 1.46%, 1.48% and 1.49%, respectively), as presented in Table 1. CBC incidence was also high among individuals initially diagnosed with in situ tumors (IR: 0.55 per 100 person-years; IP: 1.86%), ER/PR/HER2 negative cancers (IR: 0.31–0.47 per 100 person-years; IP: 0.72%–1.47%), and lobular breast cancers (IR: 0.45 per 100 person-years; IP: 1.61%). The demographic characteristics demonstrated that CBC incidence was high for those in the lowest income quintile (IR: 0.41 per 100 person-years; IP: 1.48%) and those living in rural communities (IR: 0.48 per 100 person-years; IP: 1.40%) at first diagnosis. High-risk individuals had a higher incidence of CBC in comparison to those at population-level risk (IR: 0.99 vs. 0.37 per 100 person-years; IP: 2.95 vs. 1.32%).
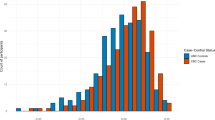
The age-adjusted annual CBC incidence rate by year of primary breast cancer diagnosis suggests a declining trend of CBC in Ontario from 2009 to 2016 (Fig. 1). The CBC incidence rate for breast cancers diagnosed in 2009 was 0.48 (95% CI 0.42, 0.55) CBC cases per 100 person-years which declined to 0.35 (95% CI 0.21, 0.59) cases per 100 person-years in 2016. Individuals at high-risk had higher annual incidence rates for CBC compared to those at population-level risk (Supplementary Fig. 1). Age-adjusted annual incidence rates for CPM show an overall trend of increasing rates (Fig. 1). The incidence rate of CPM for breast cancers diagnosed in 2009 was 0.12 (95% CI 0.10, 0.15) surgeries per 100 person-years, rising to 0.16 (95% CI 0.11, 0.24) CPM surgeries per 100 person-years in 2016. Individuals at high-risk had higher annual rates of CPM compared to those at population-level risk (Supplementary Fig. 2).
Poisson regression models were used to estimate the age-adjusted annual incidence rate of contralateral breast cancer (CBC) and contralateral prophylactic mastectomy (CPM) per 100 person-years and 95% confidence intervals (CI). Blue line represents the annual age-adjusted incidence rate of CBC, blue band represents the 95% CI. Green line represents the annual age-adjusted incidence rate of CPM, green band represents the 95% CI.
Compared to those at population-level risk, high-risk individuals had significantly higher cumulative risk of developing CBC in the study period (P < 0.0001) (Fig. 2). The risk of CBC continued to increase after the primary diagnosis among those at high-risk, however, the cumulative risk of CBC among those at population-level risk stabilized at 7.9 person-years. The cumulative risk of CBC among those at high-risk was 0.85% at 1 year, 4.41% at 5 years, and 10.15% at 10 years, while the cumulative risk for those at population-level risk was 0.42% at 1 year, 1.80% at 5 years, and 3.00% at 10 years. The cumulative incidence for CPM after the primary breast cancer diagnosis was significantly higher for those at high-risk compared to those at population-level risk (P < 0.0001). The cumulative risk for CPM among those at high-risk was 0.71% at 1 year and stabilized at 5.06% at 5 and 10 years, while those at population-level risk had a stable cumulative risk of CPM of 0.41% at 1 year, 1.16% at 5 years, and 1.29% at 10 years.
Kaplan–Meier cumulative risk curve for CBC and CPM stratified by high-risk and population-level risk. The cumulative risk for CBC is represented as the black dotted line for individuals at high-risk, blue solid line for individuals at population-level risk. The cumulative risk for CPM is represented as the gray dotted line for individuals at high-risk, green solid line for individuals at population-level risk. Logrank P = < 0.0001.
The current study findings are consistent with U.S. national data, demonstrating a reduction in CBC cases over the last few decades6,7 and a rise in contralateral prophylactic mastectomy (CPM) procedures among women diagnosed with unilateral breast cancer8. Recent declines in CBC rates are attributed to advances in treatment, including the use of adjuvant hormone therapy such as tamoxifen, aromatase inhibitors, taxane-containing drugs, and trastuzumab, preventing CBC in ER-positive patients7.
Although CPM is an effective option for reducing the risk of CBC, it does not affect long-term mortality outcomes in individuals diagnosed with unilateral breast cancer9. Our study, however, demonstrates that CPM surgeries are predominantly performed in high-risk individuals in Ontario, who are at increased risk of developing CBC. CPM is a cost-effective treatment that offers survival benefits to women who test positive for a pathogenic mutation, such as in BRCA1 or BRCA2, or to young women diagnosed with early-stage ER-negative breast cancer10,11,12. Although only a small proportion of the study population is represented by those who qualify for high-risk breast cancer screening in Ontario (2.41%), this population contributes greatly to the increasing CBC rates in Ontario. Our data demonstrates that the majority of those choosing to undergo CPM to prevent a future CBC diagnosis are individuals at high-risk, possibly warranting the rising rates of this procedure in Ontario.
Study limitations include missing or incomplete health administrative data, potential underestimation of the CPM rate due to missing laterality of some breast surgical procedures, and potential misclassification of CBC diagnoses or CPM procedures.
In conclusion, there has been an overall decline in CBC rates and the concomitant rise in CPM rates after unilateral breast cancer diagnosis in Ontario over the period of 2009 to 2016. These trends are largely driven by individuals who qualify for high-risk breast cancer screening. The majority of CPM procedures were in individuals at high-risk within 5 years of primary unilateral diagnosis, likely contributing to the reduced trend of CBC cases in Ontario. Future evaluations of CBC and CPM trends are recommended to be examined by breast cancer risk level.
Methods
Data sources
Females diagnosed with primary breast cancer in Ontario were identified through the Ontario Cancer Registry (OCR). The OCR is a population-based cancer registry that contains diagnostic information on incident cancers (excluding non-melanoma skin cancer) in Ontario residents13. To ensure the completeness of provincial cancer records, the OCR exchanges information with other Canadian provinces and territories if residents are diagnosed or treated outside of Ontario13. The OCR was reported to have a 95% completeness rate for incident breast cancers in the province14. The Ontario Breast Screening Program (OBSP) is a provincial, population-based breast cancer screening program for people aged 50–74 years. In 2011, the High Risk OBSP was established to screen individuals aged 30–69 years found to be at high risk with annual mammogram and MRI5. Individuals were further linked to the Canadian Institute of Health Information Discharge Abstract Database (CIHI-DAD) and Registered Persons Database (RPDB) to evaluate surgical and demographic data, respectively.
This study was approved by the Research Ethics Board at the University of Toronto (REB Protocol #: 00037488). The need for informed consent was waived, given that the data was provided by Ontario Health (Cancer Care Ontario), a prescribed entity under Ontario’s privacy legislation, which is authorized to collect and use personal health information without consent for the purpose of research. All research was performed in accordance with the Declaration of Helsinki.
Study population
The OCR database identified 98,826 females diagnosed with breast cancer (invasive or in situ) between January 01, 2009, and December 31, 2017. Individuals were excluded if their primary breast cancer diagnosis was classified as stage IV (n = 3791), had bilateral breast cancer at diagnosis (n = 1136), had insufficient or missing laterality information (n = 1478), had a previous cancer (n = 13,198), or were younger than 20 years of age or older than 85 years of age at diagnosis (n = 2774). To mitigate the possibility of undetected synchronous bilateral breast cancer, those who developed contralateral breast cancer within the first 12 months of their primary diagnosis were excluded from the analyses (n = 1262). As a result, follow-up accrual began 12 months after primary diagnosis, and anyone with less than 12 months of follow-up due to death or bilateral mastectomy was further excluded (n = 10,408). Therefore, the remaining 64,779 individuals had a primary unilateral breast cancer with known laterality and follow-up ≥12 months from 2009 to 2016. Out of these individuals, 879 CBC cases occurred over the duration of the follow-up, and a total of 720 had contralateral prophylactic mastectomies.
Case definitions and study measures
CBC was defined as having a tumor (invasive or in situ) diagnosed in the opposite breast ≥12 months following the detection of the first unilateral breast cancer. Any breast cancer diagnosed within 12 months of the first primary breast cancer was considered synchronous bilateral breast cancer and excluded from the analysis6. Histology and cancer type were based on International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes.
CPM was defined as having both breasts surgically removed among individuals diagnosed with unilateral breast cancer. Canadian Classification of Health Intervention (CCI) codes were used to determine the type of surgical intervention, including total mastectomy (surgical codes 1YM89, 1YM90, 1YM91, or 1YM92). Individuals who had at least two mastectomy codes recorded during the study period were considered to have received a CPM, due to missing surgical laterality indicators (n = 806).
Characteristics of the primary tumor such as age at diagnosis (years), date of diagnosis (date), laterality (left or right), hormone receptor status (estrogen receptor [ER], progesterone receptor [PR]), human epidermal growth factor receptor 2 (HER2) status, cancer stage using the American Joint Committee on Cancer (AJCC) staging system (0–IV), cancer type (in situ or invasive), and histology (ductal, lobular, or other) were available in the OCR. In situ breast cancers were designated to have non-invasive morphology behavior, while stage 0 disease breast cancers were designated as mixed types of malignant primary histology (i.e., Paget’s disease).
Individuals were additionally linked to the Registered Persons Database (RPDB) to obtain demographic data, including neighborhood income quintile and community size. Community size was categorized as urban (census metropolitan areas (CMA)) and rural (metropolitan influence zones outside of CMAs)15. Neighborhood income was quantified as pre-tax neighborhood income quintile from lowest to highest quintile of income16.
The breast cancer risk level was determined by the High Risk OBSP database. People are eligible to participate in the High Risk OBSP if they meet one of the following criteria: tested positive for a genetic mutation that increases their risk for breast cancer (e.g., BRCA1 or BRCA2), have a first-degree relative with a genetic mutation that increases their risk for breast cancer but are themselves untested, have a 25% or greater lifetime risk of breast cancer based on family history and other risk factors, and/or had chest radiation before the age of 30 at least 8 years ago17. This study considered differences in trends by identifying those who qualified for high-risk screening (i.e., high-risk) and those not in the high-risk screening program (i.e., population-level risk).
Statistical analysis
Characteristics of the first primary unilateral breast cancer and neighborhood demographics were summarized for the overall study population and by CBC diagnosis or CPM procedure during the follow-up. Mean and standard deviations were calculated for continuous characteristics (e.g., age), while frequencies and percentages were reported for categorical characteristics (e.g., neighborhood income quintile).
In the CBC analysis, person-years were accrued 12 months following the primary diagnosis until date of death, second breast cancer, CPM, or administrative censoring on December 31, 2016, whichever occurred first. In the CPM analysis, the calculation of person-years is accumulated immediately after the date of primary diagnosis until the date of death, second breast cancer, CPM, or administrative censoring on December 31, 2016. Thus, person-years only included individuals at risk for CBC or CPM in their respective analyses.
IR per 100 person-years and IP of CBC were calculated, stratified by the primary tumor characteristics and demographic data. Incidence rates were calculated by dividing the number of incident CBC cases by the total person-years, then multiplying by 100. Incidence proportions were calculated by dividing the number of incident CBC cases by the total population, then multiplying by 100%.
Poisson regression models were used to estimate the age-adjusted annual incidence rates and 95% confidence intervals (CI) of CBC per 100 person-years. Estimates were plotted by year of primary breast cancer diagnosis. Similarly, these methods were used to estimate the age-adjusted annual incidence rate and 95% CI of CPM per 100 person-years during the same period. Cumulative risks for CBC and CPM after the diagnosis of a primary unilateral breast cancer were presented using Kaplan-–Meier curves. Rates and risks for CBC and CPM were stratified by breast cancer risk level (high-risk or population-level risk). Analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Data availability
The data that support the findings of this study are available from Ontario Health (Cancer Care Ontario), a prescribed entity under Section 45 of the Personal Health Information Protection Act. Data sharing regulations prevent these data from being made available publicly due to the personal health information in the datasets. Data are, however, available from the authors upon reasonable request and with permission of Ontario Health (Cancer Care Ontario).
Code availability
The underlying code for this study is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author. Analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
References
Harvey, E. B. & Brinton, L. A. Second cancer following cancer of the breast in Connecticut, 1935-82. Natl Cancer Inst. Monogr. 68, 99–112 (1985).
Statistics Canada. Cancer survival statistics, 2020 update. https://www150.statcan.gc.ca/n1/daily-quotidien/201127/dq201127b-eng.htm (2020).
Burns, P. E. et al. Bilateral breast cancer in northern Alberta: risk factors and survival patterns. Can. Med. Assoc. J. 130, 881–886 (1984).
Chen, Y., Semenciw, R., Kliewer, E., Shi, Y. & Mao, Y. Incidence of second primary breast cancer among women with a first primary in Manitoba, Canada. Breast Cancer Res. Treat. 67, 35–40 (2001).
Cancer Care Ontario. Ontario Breast Cancer Screening Program (OBSP) Guidelines Summary. https://www.cancercareontario.ca/sites/ccocancercare/files/assets/OBSPGuidelinesSummary.pdf (2015).
Nichols, H. B., de González, A. B., Lacey, J. V., Rosenberg, P. S. & Anderson, W. F. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J. Clin. Oncol. 29, 1564–1569 (2011).
Ramin, C. et al. Risk of contralateral breast cancer according to first breast cancer characteristics among women in the USA, 1992–2016. Breast Cancer Res. 23, 1–10 (2021).
Wong, S. M. et al. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann. Surg. 265, 581–589 (2017).
Giannakeas, V., Lim, D. W. & Narod, S. A. Bilateral mastectomy and breast cancer mortality. JAMA Oncol. 10, 1228–1236 (2024).
Bedrosian, I., Hu, C. Y. & Chang, G. J. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. JNCI J. Natl Cancer Inst. 102, 401–409 (2010).
Davies, K. R. et al. Outcomes of contralateral prophylactic mastectomy in relation to familial history: a decision analysis (BRCR-D-16-00033). Breast Cancer Res. 18, 93 (2016).
Schrag, D., Kuntz, K. M., Garber, J. E. & Weeks, J. C. Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA 283, 617–624 (2000).
Cancer Care Ontario. Ontario Cancer Registry. https://www.cancercareontario.ca/en/cancer-care-ontario/programs/data-research/ontario-cancer-registry (2017).
Prodhan, S., King, M. J., De, P. & Gilbert, J. Health Services Data: The Ontario Cancer Registry (a Unique, Linked, and Automated Population-Based Registry). In Data and Measures in Health Services Research [Internet] (eds, Sobolev, B., Levy, A. & Goring, S.) 1–27 (Springer US, Boston, MA, 2016.
Statistics Canada. Census metropolitan influenced zones: detailed definition. 2011 [Accessed 2024 April 22]. Available from: https://www150.statcan.gc.ca/n1/pub/92-195-x/2011001/other-autre/miz-zim/def-eng.htm
Canadian Institute for Health Information. Measuring health inequalities: a toolkit — area-level equity stratifiers using PCCF and PCCF+. https://www.cihi.ca/sites/default/files/document/toolkit-area-level-measurement-pccf-en.pdf (2018).
Cancer Care Ontario. Breast cancer screening for women at high risk. https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/screening/breast-cancer-high-risk-women (2017).
Acknowledgements
Parts of this material are based on data and information provided by Ontario Health (Cancer Care Ontario) [and includes data received by Ontario Health (Cancer Care Ontario) from the Canadian Institute for Health Information (CIHI)]. The opinions, reviews, views, and conclusions reported in this publication are those of the authors and do not necessarily reflect those of Ontario Health (Cancer Care Ontario) [CIHI]. No endorsement by Ontario Health (Cancer Care Ontario) [CIHI] is intended or should be inferred. This study was funded by the Canadian Institutes of Health Research Catalyst Grant: 155465. The funder played no role in study design, data collection, analysis, and interpretation of data, or the writing of this manuscript. S.J.K. is supported by the Canadian Institutes of Health Research Canada Graduate Scholarship Doctoral Award and a Canadian Cancer Society Research Training Award – PhD. J.A. is supported by the Canadian Institutes of Health Research Vanier Canada Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
S.J.K. conceived and designed the work that led to the submission, conducted the data analysis, played an important role in interpreting the results, and drafted the manuscript. J.A. and R.A.G.C. acquired the data, played an important role in interpreting the results, and revised the manuscript. G.M.A. played an important role in interpreting the results and revised the manuscript. J.D.B. conceived and designed the work that led to the submission, played an important role in interpreting the results, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, S.J., Arneja, J., Christensen, R.A.G. et al. Trends in contralateral breast cancer and contralateral prophylactic mastectomy from 2009 to 2016. npj Breast Cancer 11, 55 (2025). https://doi.org/10.1038/s41523-025-00763-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-025-00763-6