Abstract
Palbociclib (PAL) combined with endocrine therapy (ET) is approved for hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2− ) advanced/metastatic breast cancer (MBC). However, there is limited data on the effectiveness of PAL + ET in patients with MBC and low socioeconomic status. This retrospective study of the Flatiron Health database compared overall survival (OS) and real-world progression-free survival (rwPFS) in patients with MBC living in disadvantaged neighborhoods who received either first-line PAL with an aromatase inhibitor (AI) or an AI alone. Of 723 patients, 394 received PAL + AI and 329 received AI alone. After stabilized inverse probability of treatment weighting, median OS was 57.1 months versus 38.2 months (hazard ratio, 0.70, P = 0.0053) and median rwPFS was 19.1 months versus 14.0 months (hazard ratio, 0.66, P = 0.0007) for PAL + AI versus AI alone, respectively. This real-world data analysis demonstrated that first-line PAL + AI versus AI alone was associated with survival benefit in patients with HR+/HER2− MBC living in disadvantaged neighborhoods. Trial registration number: NCT06495164.
Similar content being viewed by others
Introduction
Palbociclib, the first-in-class oral cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, was initially approved in the United States in combination with endocrine therapy (ET) for the treatment of hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced/metastatic breast cancer (MBC) in February 20151. Randomized controlled trials (RCTs) have demonstrated that first-line palbociclib in combination with AI versus placebo plus AI significantly prolonged progression-free survival (PFS, primary endpoint)2,3; overall survival (OS, secondary endpoint) was numerically prolonged across most subgroups, but did not reach statistical significance4.
A growing body of real-world evidence has demonstrated that palbociclib plus ET is more effective than ET alone in various populations of patients with HR+/HER2− MBC5,6,7,8,9,10. In a large (n = 2888), heterogeneous population in the United States, palbociclib plus ET was associated with a 30% reduction in risk of disease progression and a 24% reduction in risk of death relative to ET alone8. Real-world data sets have outcomes from all patients in routine clinical practice including patients who are often excluded from RCTs, and have been increasingly recognized as important complementary data sources that supplement evidence from RCTs11,12. Real-world evidence has demonstrated that patients aged ≥ 65 years and ≥ 75 years and those with metastases to the lungs and/or liver achieve a greater clinical benefit with palbociclib plus ET than with ET alone5,6,10. In African Americans with HR+/HER2− MBC, a population historically underrepresented in RCTs, relative risk reduction of progression (26%) and death (44%) were observed with palbociclib plus ET versus ET alone9. Despite this demonstration of palbociclib effectiveness in diverse patient subgroups, there is evidence that palbociclib and other CDK4/6 inhibitors have not been deployed as frequently as would be expected for a standard of care13,14.
There exist well-documented disparities in outcomes among racial and ethnic groups of patients with breast cancer15,16, including those with MBC17. A multitude of demographic, tumor, and other disease-related factors likely contribute to these inequalities, but social determinants of health (SDOH)18 have emerged as important predictors of breast cancer survival17,19,20,21. Using Surveillance, Epidemiology, and End Results (SEER) registry data, it was shown that patients residing in the most disadvantaged neighborhoods (bottom quintile) had a 43% greater risk of breast cancer-related death than those in the most advantaged neighborhoods (top quintile)19. Another SEER analysis was able to attribute approximately half of the excess risk for breast cancer-specific death experienced by non-Hispanic Black women with MBC aged < 65 years relative to their non-Hispanic White peers to socioeconomic factors17. Poorer outcomes among groups with unmet social needs may be linked to differential breast cancer management, as evidenced by longer time to treatment initiation21 and suboptimal treatment selection13.
Generating more equitable outcomes for patients with MBC in vulnerable populations such as those residing in low-income areas is a crucial public health objective. However, data regarding the use and effectiveness of CDK4/6 inhibitor/ET combination in patients with MBC living in disadvantaged neighborhoods are lacking; such findings may help to guide treatment decision-making by clinicians serving patient populations with SDOH-related barriers to care. Therefore, the current study aimed to compare OS and real-world PFS (rwPFS) with first-line palbociclib plus an AI versus an AI alone in patients with HR+/HER2− MBC living in disadvantaged neighborhoods in the United States.
Results
Patients
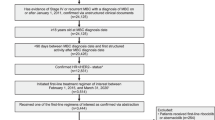
Of the 5087 patients with HR+/HER2− MBC, 1342 received palbociclib plus an AI and 1108 received an AI alone as first-line therapy. Of 1342 patients who received palbociclib plus an AI, 1231 (91.7%) had neighborhood socioeconomic status (SES) assessments and 394 (29.4%) met the criteria for living in disadvantaged neighborhoods. Of 1108 patients who received an AI alone, 992 (89.5%) had neighborhood SES assessments and 329 (29.7%) met the criteria for living in disadvantaged neighborhoods (Fig. 1). Median follow-up duration was 27.2 months for palbociclib plus an AI and 25.7 months for patients treated with an AI alone. Compared with the AI alone group, the palbociclib plus AI group was younger (median age 66.0 versus 69.0 years) and had higher proportions of de novo MBC (39.8% versus 24.0%) and visceral metastasis (32.0% versus 19.8%; Table 1). After stabilized inverse probability of treatment weighting (sIPTW) and 1:1 propensity score matching (PSM), baseline demographic and clinical characteristics were generally well balanced between the palbociclib plus AI and AI alone groups.
Overall survival
In the unadjusted analysis, patients from disadvantaged neighborhoods treated with palbociclib plus AI had significantly longer median OS than patients treated with AI alone (57.2 months [95% confidence interval (CI), 49.6–70.8] versus 37.7 months [95% CI, 28.7–44.8]; hazard ratio, 0.64 [95% CI, 0.52–0.80]; P < 0.0001; Fig. 2a). After sIPTW, palbociclib plus AI was associated with significantly longer OS versus treatment with AI alone (57.1 months [95% CI, 47.2–70.8] versus 38.2 months [95% CI, 29.6–48.0]; hazard ratio, 0.70 [95% CI, 0.55–0.90]; P = 0.0053; Fig. 2b). Similar results were seen in the PSM sensitivity analysis (57.1 months [95% CI, 41.2–72.9] versus 35.3 months [95% CI, 26.3–43.2]; hazard ratio, 0.65 [95% CI, 0.48–0.87]; P = 0.0035; Fig. 2c).
Real-world PFS and PFS2
In the unadjusted analysis, patients from disadvantaged neighborhoods who were treated with palbociclib plus AI had median rwPFS of 21.8 months (95% CI, 18.8–28.0) compared with patients treated with AI alone (13.5 months [95% CI, 11.2–19.3]; hazard ratio, 0.59 [95% CI, 0.48–0.73]; P < 0.0001; Fig. 3a). After sIPTW, median rwPFS was 19.1 months (95% CI, 15.8–24.2) in the palbociclib plus AI-treated group versus 14.0 months (95% CI, 10.7–19.7) in the AI-treated group (hazard ratio, 0.66 [95% CI, 0.52–0.84]; P = 0.0007; Fig. 3b). Median rwPFS after PSM was 21.0 months (95% CI, 16.5–27.4) in the palbociclib plus AI group versus 13.3 months (95% CI, 10.7–22.9) in the AI alone group (hazard ratio, 0.63 [95% CI, 0.47–0.84]; P = 0.0018; Fig. 3c).
Graphical representation of rwPFS in a the unadjusted population, b following sIPTW, and c following PSM. AI aromatase inhibitor, CI confidence interval, PAL palbociclib, PSM propensity score matching, rwPFS real-world progression-free survival, sIPTW stabilized inverse probability of treatment weighting.
Median rwPFS2 was significantly longer (36.9 months [95% CI, 31.1–44.1]) in the palbociclib plus AI group versus the AI alone group (21.1 months [95% CI, 15.4–23.4]; hazard ratio, 0.51 [95% CI, 0.42–0.63]; P < 0.0001; Fig. 4a). The results were similar after sIPTW (32.7 months [95% CI, 27.8–41.0] versus 22.5 months [95% CI, 16.9–27.3] in the palbociclib plus AI versus AI alone groups, respectively; hazard ratio, 0.62 [95% CI, 0.49–0.79]; P = 0.0001; Fig. 4b) and after PSM (30.5 months [95% CI, 23.2–40.7] versus 22.5 months [95% CI, 15.2–28.5], respectively; hazard ratio, 0.65 [95% CI, 0.49–0.85]; P = 0.0019; Fig. 4c).
Graphical representation of rwPFS 2 in a the unadjusted population, b following sIPTW, and c following PSM. AI aromatase inhibitor, CI confidence interval, PAL palbociclib, PSM propensity score matching, rwPFS2 real-world progression-free survival 2, sIPTW stabilized inverse probability of treatment weighting.
Subsequent treatment
In the unadjusted cohort, 177 of 394 patients (44.9%) treated with palbociclib plus AI received subsequent treatment: 77 (19.5%) received subsequent treatment with a CDK4/6 inhibitor; 37 (9.4%) received ET alone, 36 (9.1%) received chemotherapy, and 30 (7.6%) received another form of anticancer treatment (Table 2). Of the 235 of 329 patients (71.4%) in the AI alone group who received subsequent treatment, 120 (36.5%) received a CDK4/6 inhibitor, 80 (24.3%) received ET alone, 29 (8.8%) received chemotherapy, and 10 (3.0%) received other treatment (Table 2). Similar results were seen after the sIPTW and PSM analyses.
In the unadjusted analysis, median time to subsequent chemotherapy (TSC) was 43.7 months (95% CI, 37.4–54.1) in the patients treated with palbociclib plus AI versus 26.6 months (95% CI, 22.6–31.7) in the patients treated with AI alone (hazard ratio, 0.63 [95% CI, 0.52–0.77]; P < 0.0001; Fig. 5a). Similar results were seen after sIPTW (median TSC of 41.8 months versus 27.3 months respectively; hazard ratio, 0.68 [95% CI, 0.54–0.86]; P = 0.001; Fig. 5b) and after PSM (median TSC of 41.8 months versus 24.9 months, respectively; hazard ratio, 0.69 [95% CI, 0.53–0.90]; P = 0.0066; Fig. 5c).
Discussion
Using the Flatiron Health longitudinal database, our analysis identified patients with HR+/HER2– MBC who received palbociclib plus AI or AI alone as first-line treatment and who resided in disadvantaged neighborhoods. Compared to an AI alone, palbociclib plus AI was associated with significantly prolonged median OS, rwPFS, rwPFS2, and TSC in the unadjusted analysis and after the sIPTW and PSM analyses.
Inequality in oncologic survival outcomes among various patient populations is a persistent challenge for care providers and policy makers. Patients with low SES are underrepresented in RCTs for cancer treatments22, thus creating data gaps in this context. As such, real-world data are necessary to determine the effectiveness of therapies for this vulnerable population. This study’s findings for patients living in disadvantaged neighborhoods and treated with first-line palbociclib are consistent with the PFS benefits demonstrated in RCT populations2,3 and observed in real-world studies7,8. The significant OS improvement with first-line palbociclib shown in this study is also in alignment with previous real-world studies7,8.
Even though it is recognized as guideline-recommended standard of care for HR+/HER2− MBC, combination CDK4/6 inhibitor and AI therapy has been deployed inequitably in patients in disadvantaged neighborhoods13,14. In a single institution study (N = 211), there were lower rates of CDK4/6 inhibitor use in urban versus suburban sites and a trend toward lower use for patients residing in low-income neighborhoods14. A more recent analysis using SEER data (N = 752) found that patients in counties with lower median household income (≤ $45 K USD) had significantly lower rates of treatment initiation with CDK4/6 inhibitors than those in counties with high median household income (> $60 K USD)13. Furthermore, in counties with a high percentage of dual Medicare–Medicaid coverage, a common proxy for social risk, the rate of treatment with CDK4/6 inhibitors was lower than in counties with a higher percentage of Medicare-only insurance13. Given the better clinical outcomes observed in our study with palbociclib plus an AI versus an AI alone, we speculate that the survival disparities for patients with HR+/HER2− MBC residing in areas with low SES may be driven in part by suboptimal administration of CDK4/6 inhibitors.
A variety of factors may contribute to underutilization of CDK4/6 inhibitors in patients living in disadvantaged neighborhoods. SDOH-related factors (e.g., lack of transportation, stable housing, and caregiver assistance) have been identified as key contributors to disparities in cancer management21. An observational study using the Flatiron Health database found that patients’ source of health insurance (Medicaid versus commercial provider) was associated with lower use of and reduced time on CDK4/6 inhibitors23. Financial toxicity associated with drug cost, treatment monitoring, and side-effect management may discourage patients and care providers from selecting optimal treatments24,25. Even after optimal treatment selection, financial stressors may negatively affect quality of life26 and contribute to medical non-compliance and/or poor treatment adherence27,28. CDK4/6 inhibitors may be insufficiently covered by insurance and there are time, cost, and transportation challenges inherent in cardiac, hematologic, and gastrointestinal monitoring, which is crucial for guideline-compliant care29. Cancer treatment decision-making is a complex process often affected by resource limitations30, but the evidence of improved clinical outcomes provided in this study may help more patients from disadvantaged neighborhoods to receive standard of care for MBC.
African American patients with cancer, a group that is disproportionally affected by SDOH16, have been shown to experience a greater risk of breast cancer-related death31. It has also been shown that Black/African American patients receive CDK4/6 inhibitors at a lower rate than White patients23. Indeed, population-level survival improvements since the introduction of CDK4/6 inhibitors have been driven by non-Hispanic White women without similar improvements for non-Hispanic Black and Hispanic women32. Real-world evidence suggests that African American patients with HR + /HER2 − MBC receive a significant survival benefit from palbociclib plus an AI compared with an AI alone9, so treatment disparities may represent opportunities to improve survival.
This study has some limitations. Because this was a retrospective observational study, it can only infer associations between treatments and outcomes, but not causality. Retrospective database analyses are also limited by the quality of the data captured and may be subject to incomplete, missing, or inaccurate data. Disease progression was not evaluated according to a predefined schedule, nor were standardized clinical trial assessments used, such as the Response Evaluation Criteria in Solid Tumors. As a result, rwPFS and rwPFS2 data relied on the treating physician’s interpretation of pathology reports and scan results. Additionally, treatments were not randomly assigned; instead, they were selected for each patient based on the treating physician’s clinical judgment and experience, which may have introduced selection bias. sIPTW and PSM were used to balance patient characteristics between treatment groups, but the effect of unmeasured potential confounders could not be adjusted for in these analyses. Our findings here inform only the comparison between palbociclib plus an AI versus an AI alone and do not assess comparisons among other CDK4/6 inhibitors in combinations with ET; future studies are necessary to assess the relative effectiveness of various CDK4/6 inhibitors in patients living in disadvantaged neighborhoods. Finally, the Flatiron Health database is limited to practices in the network in the United States, and hence these results may not be generalizable outside of this network. Nevertheless, our study fills an important gap in knowledge regarding palbociclib effectiveness in a vulnerable population in which there has been limited research and provides important evidence for stakeholders in the MBC treatment decision-making process.
In conclusion, this real-world database analysis demonstrates that first-line treatment with palbociclib plus AI, when compared with an AI alone, was associated with extended OS, rwPFS, rwPFS2, and TSC in patients with HR+/HER2– MBC living in disadvantaged neighborhoods. These findings support first-line palbociclib with ET as an effective option in this patient population.
Methods
Study design and data source
This was a retrospective, observational study of patient data captured in the United States nationwide Flatiron Health electronic health records-derived deidentified Enhanced Datamart for MBC (EDM; N = 33,448 at the time of data cutoff, December 2022). The Flatiron Health database contains patient-level structured and unstructured data, originating from ~280 cancer clinics (~ 800 sites of care), representing > 3 million actively treated cancer patients in the United States. Patients in the EDM who had HR+/HER2− MBC, were aged ≥ 18 years, and who started first-line treatment between February 2015 and June 2022 were randomly sampled (n = 5500). Of these, 5087 patients had first-line treatment data manually confirmed via abstraction (Fig. 1).
Neighborhood disadvantage was measured with the Yost index33, a composite measure of neighborhood social and economic vulnerability that has been well validated in previous cancer studies19,34,35. The Yost Index is reported as a percentile, with a higher percentage indicating higher socioeconomic status. The score for each census tract is calculated using seven variables: median household income, median home value, median gross rent, percentage of individuals living below 150% of the poverty line, percentage of individuals considered working class, percentage of individuals who are unemployed, and education index19,35.
To calculate the area-level socioeconomic status (SES) Index linked to patients in the Flatiron network, Flatiron uses a 5-year estimate of the variables listed above from the Census Bureau’s American Community Survey (2015–2019), the largest household survey in the United States. Yost Index quintiles, relative to the entire United States, were used to categorize neighborhood SES, with quintile 1 (i.e., the 20th percentile or lower) representing the lowest and quintile 5 (the 80th percentile or higher) representing the highest33,35,36. Disadvantaged neighborhoods were defined as Yost Index quintiles 1–2.
Patients included in the analysis had a diagnosis of HR+/HER2− MBC, 18 years of age or older, initiated palbociclib plus an AI or an AI alone as first-line therapy between February 2015 and June 2022 (index period), and lived in disadvantaged neighborhoods (Yost Index quintiles 1–2; Fig. 1). Patients were retrospectively assessed from start of palbociclib plus an AI or AI alone to December 2022 (data cutoff), death, or last medical activity, whichever came first.
This retrospective database analysis was conducted in accordance with the Guidelines for Good Pharmacoepidemiology Practice, Good Practices for Outcomes Research issued by the International Society for Pharmacoeconomics and Outcomes Research, Good Practices for Real-world Data Studies of Treatment and/or Comparative Effectiveness, and in accordance with the Declaration of Helsinki. As this study was retrospective, non-interventional, and used anonymized data, it was exempt from institutional review board approval and included a waiver of informed consent.
Outcomes
The outcomes assessed were OS, rwPFS, rwPFS2, and TSC. OS was defined as the number of months from start of palbociclib plus AI or AI alone to death7,8. Date of death was a composite variable from three data sources: Social Security Death Index (SSDI), obituary data, and electronic health records-derived mortality data and has been previously validated against the National Death Index (NDI)21,37. rwPFS was defined as the number of months from the start of palbociclib plus AI or AI alone to death or disease progression, evaluated based on clinical assessment or radiographic scan/tissue biopsy7,8. rwPFS2 was defined as the number of months from the start of palbociclib plus AI or AI alone to disease progression on second-line therapy as determined by the treating physician, or death from any cause in either first-line or second-line, whichever occurred first38. TSC was defined as the length of time from the start of treatment to the beginning of subsequent chemotherapy or death from any cause, whichever came first38.
Statistical analysis
Baseline demographics and disease characteristics were summarized using descriptive statistics. Median survival times for time-to-event endpoints were calculated using the Kaplan–Meier method and displayed graphically. The weighted Cox proportional hazards model was used to compute hazard ratios and corresponding 95% CIs. sIPTW (primary analysis) was used to balance baseline patient demographics and clinical characteristics, and 1:1 PSM was performed as a sensitivity analysis. Both sIPTW and PSM were based on propensity scores calculated using a multivariable logistic regression model. The variables included in the model were age group, sex, race/ethnicity, practice type, disease stage at initial diagnosis, Eastern Cooperative Oncology Group performance status, bone disease, visceral disease, interval from initial breast cancer diagnosis to MBC diagnosis, and number of metastatic sites8. The balance in these baseline characteristics was assessed using a standardized differences approach, with values ≥ 0.1 indicating a non-negligible imbalance. No imputation for missing values was performed. All data analyses were executed using statistical software SAS version 9.4 or later.
Data availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Beaver, J. A. et al. FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin. Cancer Res. 21, 4760–4766 (2015).
Finn, R. S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 16, 25–35 (2015).
Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 375, 1925–1936 (2016).
Slamon, D. J. et al. Overall survival with palbociclib plus letrozole in advanced breast cancer. J. Clin. Oncol. 42, 994–1000 (2024).
Brufsky, A. et al. Palbociclib combined with an aromatase inhibitor in patients with breast cancer with lung or liver metastases in US clinical practice. Cancers15, 5268 (2023).
Brufsky, A. et al. Real-world treatment patterns and effectiveness of palbociclib plus an aromatase inhibitor in patients with metastatic breast cancer aged 75 years or older. Front. Oncol. 13, 1237751 (2023).
DeMichele, A. et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in US real-world clinical practice. Breast Cancer Res 23, 37 (2021).
Rugo, H. S. et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 8, 114 (2022).
Rugo, H. S. et al. Real-world effectiveness of palbociclib plus aromatase inhibitors in African American patients with metastatic breast cancer. Oncologist 28, 866–874 (2023).
Rugo, H. S. et al. Real-world comparative effectiveness of palbociclib plus letrozole versus letrozole in older patients with metastatic breast cancer. Breast 69, 375–381 (2023).
Harbeck, N. et al. Real-world effectiveness of CDK4/6i in first-line treatment of HR+/HER2- advanced/metastatic breast cancer: updated systematic review. Front. Oncol. 15, 1530391 (2025).
Wilson, B. E. & Booth, C. M. Real-world data: bridging the gap between clinical trials and practice. EClinicalMedicine 78, 102915 (2024).
Goyal, R. K. et al. Social determinants of health and other predictors in initiation of treatment with CDK4/6 inhibitors for HR+/HER2- metastatic breast cancer. Cancers16, 2168 (2024).
Lee, K. T. et al. Predictors of non-receipt of first-line CDK 4/6 inhibitors (CDK4/6i) among patients with metastatic breast cancer (MBC). J. Clin. Oncol. 39, Accessed August 20, 2024, https://doi.org/10.1200/JCO.2021.39.15_suppl.1016 (2021).
Albain, K. S. et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J. Natl. Cancer Inst. 113, 390–399 (2021).
Giaquinto, A. N. et al. Cancer statistics for African American/Black People 2022. CA Cancer J. Clin. 72, 202–229 (2022).
Ren, J. X., Gong, Y., Ling, H., Hu, X. & Shao, Z. M. Racial/ethnic differences in the outcomes of patients with metastatic breast cancer: contributions of demographic, socioeconomic, tumor and metastatic characteristics. Breast Cancer Res. Treat. 173, 225–237 (2019).
US Department of Health and Human Services. Healthy People 2030. https://health.gov/healthypeople/priority-areas/social-determinants-health/literature-summaries, Accessed October 08, 2024 (2024).
Goel, N., Hernandez, A. E. & Mazul, A. Neighborhood disadvantage and breast cancer-specific survival in the US. JAMA Netw. Open 7, e247336 (2024).
Unger, J. M. et al. Persistent disparity: socioeconomic deprivation and cancer outcomes in patients treated in clinical trials. J. Clin. Oncol. 39, 1339–1348 (2021).
Zhang, F. G. et al. Association between social determinants of health and cancer treatment delay in an urban population. JCO Oncol. Pract. 20, 1733–1743 (2024).
Donzo, M. W. et al. Effects of socioeconomic status on enrollment in clinical trials for cancer: A systematic review. Cancer Med. 13, e6905 (2024).
Sathe, C. et al. Social determinants of health and CDK4/6 inhibitor use and outcomes among patients with metastatic breast cancer. Breast Cancer Res. Treat. 200, 85–92 (2023).
Carrera, P. M., Kantarjian, H. M. & Blinder, V. S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 68, 153–165 (2018).
Williams, C. P. et al. Guideline discordance and patient cost responsibility in medicare beneficiaries with metastatic breast cancer. J. Natl. Compr. Canc. Netw. 17, 1221–1228 (2019).
Rosenzweig, M. et al. Financial toxicity among women with metastatic breast cancer. Oncol. Nurs. Forum 46, 83–91 (2019).
Conley, C. C. et al. Barriers and facilitators to taking CDK4/6 inhibitors among patients with metastatic breast cancer: a qualitative study. Breast Cancer Res. Treat. 192, 385–399 (2022).
Knight, T. G. et al. Financial toxicity in adults with cancer: adverse outcomes and noncompliance. J. Oncol. Pract. 14, e665–e673 (2018).
Lin, W., Zeng, Y., Weng, L., Yang, J. & Zhuang, W. Comparative analysis of adverse events associated with CDK4/6 inhibitors based on FDA’s adverse event reporting system: a case control pharmacovigilance study. BMC Pharmacol. Toxicol. 25, 47 (2024).
Dong, B., Lusen, R., Chick, E. & Kline, L. Shared decision-making on using a CDK4/6 inhibitor plus an aromatase inhibitor for HR+/HER2- metastatic breast cancer: a podcast. Oncol. Ther. 11, 411–418 (2023).
Torres, J. M., Sodipo, M. O., Hopkins, M. F., Chandler, P. D. & Warner, E. T. Racial differences in breast cancer survival between Black and White Women according to tumor subtype: a systematic review and meta-analysis. J. Clin. Oncol. 42, 3867–3879 (2024).
Alvarez, A., Bernal, A. M. & Anampa, J. Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res. Treat. 198, 75–88 (2023).
Yost, K., Perkins, C., Cohen, R., Morris, C. & Wright, W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12, 703–711 (2001).
Barber, L. E. et al. A Comparison of Three Area-Level Indices of Neighborhood Deprivation and Socioeconomic Status and their Applicability to Breast Cancer Mortality. J. Urban Health 101, 75–79 (2024).
Mani, K. et al. Association of socioeconomic status with worse overall survival in patients with bone and joint cancer. J. Am. Acad. Orthop. Surg. 32, e346–e355 (2024).
Ko, T. M. et al. Low neighborhood socioeconomic status is associated with poor outcomes in young adults with colorectal cancer. Surgery 176, 626–632 (2024).
Curtis, M. D. et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv. Res. 53, 4460–4476 (2018).
Rugo, H. S. et al. Real-world treatment patterns for palbociclib plus an aromatase inhibitor, or an aromatase inhibitor alone, for patients with metastatic breast cancer in the Flatiron Database. Int. J. Cancer 154, 701–711 (2024).
Acknowledgements
This study was sponsored by Pfizer Inc. Medical writing support was provided by Kevin Woolfrey, PhD, of Oxford PharmaGenesis (Newtown, PA, USA) and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
F.L., X.L, L.M., C.C., R.L., and H.S.R. contributed to the conception and study design. B.L. contributed to the study design, data analysis, and interpretation of the data. All authors contributed to drafting/revision of the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
F.L. reports consulting/advisory role for AstraZeneca, Eli Lilly, Pfizer, Merck, and Daiichi Sankyo; and institutional research funding from Eisai, AstraZeneca, Zentalis, Merck, CytomX, and Gilead Sciences. R.L. reports travel, accommodation, and expenses from Pfizer and institutional research funding from Genentech, AstraZeneca, Exact Sciences, Biotheranostics, and BeiGene. H.S.R. reports consulting or advisory role for Napo Pharmaceuticals, Scorpion Therapeutics, Blueprint Medicines, and Puma Biotechnology; research funding for OBI Pharma, Pfizer, Novartis, Lilly, Genentech, Merck, Daiichi Sankyo, Sermonix Pharmaceuticals, AstraZeneca, Gilead Sciences, Astellas Pharma, Pionyr, Taiho Oncology, and GlaxoSmithKline; travel, accommodations, expenses for Merck, AstraZeneca, and Gilead Sciences. X.L., B.L., L.McR., and C.C. are employees of, and stockholders in, Pfizer Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lynce, F., Liu, X., Li, B. et al. Real-world effectiveness of palbociclib plus an aromatase inhibitor in HR+/HER2− MBC patients living in disadvantaged neighborhoods. npj Breast Cancer 11, 75 (2025). https://doi.org/10.1038/s41523-025-00786-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-025-00786-z