Abstract
Neuro-ocular changes, such as globe flattening, optic disc edema or chorioretinal folds, are a major worry when considering astronaut health. These findings are now known as Spaceflight Associated Neuro-Ocular Syndrome. This systematic review aims to discuss the possible mechanisms involved in the pathogenesis of this syndrome. Contemplating the different reports regarding the impact of intracranial pressure (ICP), cardiovascular system, hypercapnia or glymphatic system, we hypothesize that a relationship exists between variations in ICP and SANS development. A literature search of five databases was conducted using the PICO model. Twenty studies were subsequently included, and two main theories discussed. The first suggests that cerebrospinal fluid (CSF) shifts can lead to a rise in ICP, while the second supports the importance of CSF compartmentalization, independently of ICP variation. These hypotheses are not mutually exclusive and environmental factors may also be essential for the development of this syndrome.
Similar content being viewed by others
Introduction
Long-duration spaceflight (LDSF) has a strong impact on the human body, exposing astronauts for extended periods of time to unique conditions, such as microgravity, radiation, and hypercapnia. This extreme environment leads to various health and physiologic alterations in astronauts during space missions. Following decades of human space exploration, it has been possible to identify and study several neuro-ocular findings in astronauts submitted to LDSF1. These ophthalmic changes are called Spaceflight Associated Neuro-Ocular Syndrome (SANS), a syndrome characterized by the development of different neuro-ocular alterations, such as hyperopic refractive error shifts, optic disc edema, choroidal and retinal folds and globe flattening after LDSF2.
SANS was first described in 2011, by Mader et al.2, with the study and presentation of different clinical and imaging findings in seven astronauts after LDSF, and soon became one of the most important astronaut health risks identified. Five of the seven participants presented with optic disc edema, which led to the presumption that elevated intracranial pressure (ICP) could be the cause of this syndrome. Moreover, the similarities with Idiopathic Intracranial Hypertension (IIH) symptoms, such as optic nerve sheath (ONS) expansion, stasis of axoplasmic flow and globe flattening, also contributed to this theory2. Consequently, this syndrome was initially termed Visual Impairment and Intracranial Pressure (VIIP) syndrome3.
However, it was noted over time that astronauts did not develop the typical symptoms of IIH, such as severe headaches, structural changes in the olfactory nerve, transient visual obscurations, or diplopia, and it remained unclear whether the optic disc edema could be considered papilledema4. In addition, measurement of post-LDSF ICP in astronauts has shown values considered to be only borderline high. In other words, the impact of ICP has not been significant enough to solely explain the symptoms described above4. The realization that the rise of intracranial pressure was not the only possible pathophysiology mechanism and with new theories strengthening the hypothesis of a multifactorial etiology, the syndrome was redefined as SANS5.
Although the SANS pathophysiology remains uncertain, several studies in the last decade have contributed to two main and non-mutually exclusive theories that try to explain the mechanism responsible for SANS4. The first considers the neuro-ocular findings to result from a rise in ICP from cerebrospinal fluid (CSF) shifts6. In normal terrestrial environment conditions, ICP depends on three main factors: CSF production volume, system resistance to CSF and venous pressure in the intracranial space, or equivalently, pressure in the superior longitudinal sinus7. Meanwhile, the second theory states that SANS can be explained by a compartmentalization of CSF in the optic nerve sheath2,8. On the one hand, this hypothesis suggests a fragile flow equilibrium may lead to locally elevated sheath pressures, regardless of a rise, or not, of ICP, or the optic nerve and globe being retracted posteriorly due to the brain shifting upward during LDSF, and may cause localized pressure elevation and optic nerve sheath expansion6. On the other hand, this optic nerve compartment syndrome could be explained by the existence of a glymphatic system, responsible for the exchange of CSF with the interstitial fluid4.
During LDSF, astronauts are exposed to completely different and extreme conditions that can strongly impact on ICP, brain structure and CSF hemodynamics, such as changes in cerebral blood flow and glymphatic drainage, hypercapnia, intense resistive exercises, and imbalances in daily circadian cycles and genetics, among other variables. Therefore, these theories of potential pathophysiological mechanisms prove that SANS may have a multifactorial pathogenesis, strongly affected by changes in ICP and CSF shifts.
The main objective of the present study was to systematically review the scientific literature addressing the mechanisms responsible for SANS, focusing on knowledge related to the possible impact that variations in intracranial pressure can have in the development of this syndrome. Accordingly, the PI(C)O question we want to answer is “Is the variation of ICP (I) in astronauts who undergo LDSF (P) related to the development of SANS (O)?”.
Results
Study selection
A total of 852 studies were identified after applying the keywords in several databases, of which 114 duplicates were removed before screening. The remaining 738 studies were screened independently by two reviewers, based on the title and abstract, and, following a subsequent discussion between reviewers, 240 results remained. After applying the eligibility criteria once again, a final discussion led to 20 studies being included in the review. Figure 1 summarizes this process.
Several studies met most of the inclusion criteria but were excluded for other reasons (referred to on the flow diagram as “inclusion criteria, but different purpose”). “Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution”6, “Effects of Spaceflight Stressors on Brain Volume, Microstructure, and Intracranial Fluid Distribution”8 and “Association of Structural Changes in the Brain and Retina After Long-Duration Spaceflight”9 were centered on describing how the brain structures change after spaceflight and not on SANS itself. The main focus of “Mechanical countermeasures to headward fluid shifts”10, “Effect of Nightly Lower Body Negative Pressure on Choroid Engorgement in a Model of Spaceflight-Associated Neuro-ocular Syndrome”11 and “Daily generation of a footward fluid shift attenuates ocular changes associated with head-down tilt (HDT) bed rest”12 focused more on the description of different countermeasures.
Study Characteristics
Table 1 summarizes all of the study characteristics (title, doi, authors, month and year of publication, country, journal and type of study design), organized by date of publication.
Risk of bias in studies
Considering the non-randomized studies, six of the eleven were considered to have a “Moderate” risk of confounding bias due to insufficient information regarding the control for any post-intervention variables that could have been affected by the intervention. No apparent source of bias was identified in the selection of study participants, classification of interventions, deviations from the intended interventions, or due to missing data in all the included studies. When talking about bias in the measurement of the outcome, six studies were also considered to have a “Moderate” risk, as the assessors were aware of the intervention received by study participants, and it is believed that outcome measures could be minimally influenced by knowledge of the intervention received. In relation to any bias in the selection of the reported result, Ombergen et al.13 and Mader et al.14 presented a “Moderate” and “Serious” risk, respectively. The former was considered as “Moderate” risk due to the existence of different subgroups, while the latter was considered to have a “Serious” risk of bias due to multiple outcome measurements within the outcome domain and different subgroups. Finally, the overall risk of bias was “Low” for all, except Mader et al.14, which was considered “Moderate” due to the combination of a “Moderate” risk of confounding bias and in outcome measurement, together with a “Serious” risk of bias in the selection of the reported result. This information is displayed in Figs. 2 and 3.
In relation to the randomized studies, two15,16 of the three were considered to have “Some concerns” about the randomization process due to insufficient information for the allocation sequence. All three studies were evaluated as having “Some concerns” in relation to the bias risk due to deviations from the intended interventions, since in these studies, the participants, carers and people delivering the intervention were aware of the interventions assigned to participants. It was concluded that all had a “Low” risk of bias due to missing outcomes. The study by Scott et al.15 was considered to have “Some concerns” and “High” risk of bias in terms of the measurement of outcome, and in the selection of the reported result, respectively. These conclusions were drawn from this study as, not only were the assessors aware of the intervention, there was a higher probability that outcomes could have been influenced by having this knowledge of the intervention received. Moreover, the numerical results being assessed were likely selected, based on the results from multiple eligible analyses of the data. Accordingly, Scott et al.15 was considered to have “Some concerns” in the overall risk, while the other studies were considered to have a “Low” overall risk. This information is displayed in Figs. 4 and 5.
All three of the systematic reviews included were considered to have a “Low” risk of bias when contemplating the eligibility criteria and identification and selection of studies. In regards to the data collection and study appraisal domain, the review by Elwy et al.5 was concluded to have an “Uncertain” risk of bias due to insufficient information about the criteria to assess bias risk or methodological study and to minimize the errors associated with its assessment. Furthermore, both Galdamez et al.17 and Paez et al.4 were considered to have “High” risk for this parameter as the risk of bias was not formally assessed, and its criteria not defined. Considering the synthesis and findings domain, Galdamez et al.17 and Elwy et al.5 both lacked description regarding following analysis and biases in primary studies, indicating an “Uncertain” risk of bias, whereas, Paez et al.4 was considered “High” risk due to insufficient details in the analysis while addressing the synthesis and biases. Finally, the systematic reviews by Galdamez et al.17 and Elwy et al.5 have a “Low” risk of bias, while Paez et al.4 has a “High” risk of bias. This information is displayed in Fig. 6.
Of the two cohort studies included, Wåhlin et al.18 was considered of “Good quality”, fulfilling eight of the nine criteria, while Rosenberg et al.19 was noted as “Fair Quality”, fulfilling six of the criteria but having a lack of information for the selection parameter. The only case report, Mader et al.1, was “Included” through satisfying all the criteria that formed part of the respective evaluation scale.
Results of syntheses
The research synthesis for the present review was conducted based on analysis of each included article and subsequent creation of Table 2, in which a description, main findings and significance of each study were summarized by the reviewers.
Discussion
Summary of evidence and interpretation
Visual Impairment and Intracranial Pressure (VIIP) was the first denomination of this syndrome, since it was thought that the permanent elevation of Intracranial pressure during spaceflight was the pathophysiological mechanism responsible for SANS. Mader et al.2, the first group to report this syndrome, in 2011, followed up seven astronauts who had experienced a LDSF. Several neuro-ocular changes, such as optic disc edema, choroidal folds and hyperopic shift, were described after 6 months of spaceflight, while a post-flight lumbar puncture also revealed five of the group to have increased ICP2. Therefore, it was hypothesized that, during spaceflight, there was a mild, but chronic elevation in ICP. This raised ICP was then transmitted to the globe via the optic nerve sheath4, becoming responsible for the ocular changes also seen in pathologies associated with elevated ICP on Earth, such as Idiopathic Intracranial Hypertension20. In this theory, it is believed that the microgravity environment leads to increased venous outflow resistance from the head, causing increased cerebral venous pressure and decreased CSF resorption, and, consequently, a rise of ICP4.
Iwasaki et al.21 sought to validate this hypothesis by noninvasively estimating ICP (nICP) in 11 astronauts, before and shortly after spaceflight, using transcranial doppler to measure blood flow. This study showed that supine nICP decreased or remained unchanged in 10 of the 11 participants after spaceflight and that it increased and decreased in the two astronauts who developed neuro-ocular changes. However, the investigators concluded that any change, such as structural ocular findings or elevation of mean cerebral blood velocity in the middle cerebral artery, occurred independently of the variations in nICP21.
In 2020, a longitudinal follow up conducted by the same Mader et al.2 group, identified persistent globe flattening in three of the seven astronauts from the 2011 report. Aside from this alteration, no other symptoms, such as transient visual obscurations, headaches, diplopia, pulsatile tinnitus or vision changes with eye movement, were reported by the astronauts during their space missions, as well as no complaint of any symptoms associated with increased ICP14.
Another report also related neuro-ocular changes with ICP through studying a subset of LDSF astronauts. It observed that only one subject with significant retinal thickening, diagnosed with Frisen grade 1 optic disc edema, also displayed the largest bilateral increases in ONS area, compared to the other participants, however, the increase was considered relatively small in comparison to clinical populations with increased ICP22.
Opportunities to conduct research with astronauts are limited, therefore, several researchers over the years have tried other ways to corroborate the hypothesis described above. Lawley et al.23 were the first to directly measure ICP in humans, during zero gravity (0 G) and prolonged simulated microgravity. These investigators placed a fluid-filled 25 g butterfly needle into the Ommaya reservoir inserted in 8 participants, following a single intravenous dose of cefazolin, and studied them in different conditions. During the 0 G phase of parabolic flight, ICP decreased, compared to the supine posture. A more significant variation in ICP was noted during a prolonged microgravity exposure countermeasure achieved through a -6° head-down tilt position, which mimics spaceflight only in terms of fluid pressure and flow dynamics5. In the first seconds, ICP suffered a slight increase in every participant. After 3 hours, the ICP returned to the supine value, reducing through the night and increasing again to baseline supine values by the end of 24 hours of HDT23.
Using -15° HDT, a report showed a mismatch between arterial inflow and venous outflow, with a phenotype consistent with venous congestion, suggesting that venous engorgement is associated with increased jugular venous pressure (JVP) and cerebral venous congestion15,19. These can then result in a rise in ICP that may be transferred to the ONS, leading to ONS expansion, stasis of axoplasmic flow and globe flattening3. Using the same kind of countermeasure, Sater et al.24 studied possible changes of the optic nerve and ONS, seen by MRI. No change in the optic nerve area was found, but there were important increases in optic nerve deviations and ONS area, suggesting that CSF pressures were elevated during HDT. Kermorgant et al.25 reported a small increase in ONS diameter and found a decrease in intraocular pressure (IOP)24 when using dry-immersion as a microgravity analog. Thus, a spectrum of results can be seen from the above-described studies that try to correlate ICP and SANS. In general, the results do not reinforce the theory that ICP is pathologically elevated in microgravity, indeed to the contrary, they suggest the possibility of other factors existing that are involved in the development of SANS.
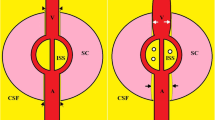
A second theory that tries to explain the development of SANS is the compartmentalization of CSF within the orbital subarachnoid space3, in which a one-way valve mechanism traps pressurized CSF within the optic nerve and ONS, without requiring elevated ICP4.This hypothesis is explained by several possible mechanisms, the first one being based on MRI findings showing an upward shift of the brain, with a retraction posteriorly of the optic nerve and globe4. Wåhlin et al.18 supported this theory through a study involving 22 astronauts that showed the optic nerve to be structurally altered after spaceflight. More precisely, they detected an anterior displacement of the optic nerve head (related to mission duration), superior displacement of the optic chiasm, and an overall increase in length of the optic nerve. However, they could not prove these alterations were caused by general white matter swelling, associated with CSF18.
Research by Ombergen et al.13 using MRI exams of a group of astronauts before and after LDSF found an increased ventricular CSF volume in supratentorial ventricular structures, with results revealing that the greater the lateral ventricular expansion, the more visual acuity was decreased postflight13. Findings from another report showed the development of significant peripapillary neural, retinal, and choroid tissue thickening in both eyes during early spaceflight, persisting throughout the mission26. Furthermore, the authors discuss the limitation of axoplasmic flow when CSF is chronically elevated, which can result in neuronal swelling of retinal nerve fibers. Additionally, the headward fluid shift could result in a chronic elevation of arterial or venous pressure at the optic nerve head, increasing local capillary filtration and, consequently, contributing to edema formation or direct compression of neural tissue17,26,27.
One of the astronauts accompanied by Mader et al.14 presented with postflight unilateral disc swelling, asymmetric globe flattening and ONS expansion. These findings suggest asymmetric CSF flow, volume and pressure changes within the ONS that worsened during LDSF, which supports the idea of asymmetric CSF flow along the ONS cul-de-sac and CSF compartmentalization. These investigators also reached the same conclusion earlier, in 2013, when one of the astronauts returning from LDSF presented with unilateral disc swelling and unilateral loss of spontaneous venous pulsations in the same eye1.
The second mechanism that tries to explain this optic nerve compartment syndrome is the existence of a glymphatic system, responsible for the exchange of CSF with interstitial fluid4. Wostyn et al.28 hypothesized that the glymphatic outflow from the eye into the optic nerve might be blocked under prolonged microgravity conditions, since elevation of ONS pressure due to a rise in ICP or sequestration of CSF within the orbital subarachnoid space, might lead to a reduction or reversal of the normal posteriorly directed trans-lamina pressure difference (TLPD). This results in fluid stasis within the prelaminar optic nerve head, and, consequently, various degrees of papillary edema can be seen without an increase in ICP29. Macias et al.26 noted that the lack of a retinal-blood barrier in the prelaminar region of the optic nerve head may be associated with greater extravasation of fluid due to the headward fluid shift in weightlessness, and may be a contributing factor to the retinal thickening quantified in this location26,28. It is therefore understandable that gravitational dependency for the functioning of the glymphatic system30 may exist.
In addition to the aforementioned theories and considering the variety of conditions affecting the spaceflight environment, many other factors for the development of SANS and variations in ICP have been proposed. Laurie et al.31 studied hypercapnia, one of the most important environmental conditions on the International Space Station (ISS), where values of CO2 can be 15-20 times higher than found on Earth16. The investigators compared the effects of 60 minutes acute exposure to a seated position breathing room air, -6° HDT breathing room air, and -6° HDT breathing 1% CO2 (HDT + CO2). A small increase in IOP was found after HDT, in comparison to seated, while the addition of CO2 to HDT resulted in a significant increase in IOP. A significant rise in nICP was seen in HDT, when compared to seated, however, no difference was found between HDT and HDT + CO2. No participant showed significant ophthalmic changes31.
Roberts et al. (2021a)30 developed a study with a similar countermeasure (-6°HDT + 0.5% CO2), but the 11 participants were exposed to the conditions for 30 days. No neurological symptoms of chronic hypercapnia were revealed, however, 5 subjects developed optic disc edema. These same participants also presented a greater decrease in perfusion, when compared to the rest of the group, progressively recovering towards the end of the exposure30. Comparing these two studies and knowing that CO2 has a strong impact on cerebrovascular modulation, we believe a time effect on the progression of neuro-ocular changes may exist, and CO2 may be an important environmental factor for the development of SANS.
Another risk factor associated with LDSF is the intense resistive and aerobic exercise that astronauts must practice 6 days per week, for at least two hours a day. Mekjavic et al.16, using HDT, sought to observe the acute effect of isometric exercise. Their findings showed that exercise caused a significant increase in mean arterial pressure (MAP), as well as an increase in IOP. Scott et al.15 used the same countermeasure, but with additional participant exposure to moderate-intensity aerobic, resistance, or high-intensity interval aerobic exercise. Interestingly, compared with HDT rest, they observed a decrease in IOP during HDT exercises. Fischman et al.32 exposed six volunteers to low level resistance exercise on the Advanced Resistive Exercise Device (ARED), and measured noninvasive ICP, assessing tympanic membrane displacement (Vm) in the inner ear following auditory stimulus. Knowing that Vm and ICP have an inverse relationship, a slight decrease in Vm in the supine position was observed, indicating a nonsignificant increase in ICP. The authors suggest that the use of ARED is unlikely to have a prominent impact on the development of SANS during spaceflight32. These divergent results highlight the current lack of understanding and need for more studies when discussing the effect of exercise on astronaut cerebro-ocular hemodynamics.
A further important topic to consider in relation to astronauts as individuals is genetics. As already mentioned, not all astronauts develop ophthalmic symptoms and neuro-ocular changes, which could suggest the possibility of existing predisposing factors that increase susceptibility. The one-carbon metabolic pathway and its link with single-nucleotide polymorphisms (SNPs) has been associated with genetic differences between astronauts and the development, or not, of neuro-ocular alterations, such as choroidal fold, optic disc edema and cotton-wool spots31. A study in which individuals bearing the GG allele of the MTRR 66 gene (associated with ocular alterations) were identified, grouped the participants according to their genetic polymorphisms (SNP+ and SNP-) and exposed them to HDT + CO2. The results revealed both groups to demonstrate a similar TLPD and no differences in cerebral blood flow and nICP were seen during HDT + CO231.
As previously stated, SANS is one of the biggest causes of concern for aerospace professionals, so further research to gain a better understanding of its development and consequences becomes very important. The present review systematically analyzed data about SANS and its development, reporting information on variations in ICP and how this relates to the other theories regarding SANS pathophysiology. Considering all the information discussed above, we believe that SANS has a multifactorial etiology, where ICP variation and its relationship with CSF shifts and compartmentalization, together with environmental factors have an important influence on the development of neuro-ocular changes.
We believe this work gives an interesting view on the development of this syndrome and provides a step forward to better understand it. We further recognize how essential it is to study astronauts and to find new ways of analyzing, in real-time, the alterations that occur during spaceflight.
Methods
This systematic review was structured according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist33.
Eligibility Criteria
Inclusion and exclusion criteria were defined before the study selection process, respecting the PICO method34. The inclusion criteria considered all clinical trials, cohort studies, case studies and systematic reviews that discussed SANS and its pathophysiology, established a relationship with ICP variation and/or CSF fluid shifts and/or studied neuro-ocular changes in the spaceflight environment or analog conditions and countermeasures. Furthermore, all studies were required to be in English and published in 2011 or after.
Studies that did not discuss SANS or the effect of microgravity and space environmental conditions on astronaut health were not considered. Animal studies, narrative reviews, background literature, letters, editorials, reports, thesis, and commentaries were also excluded from our review. Studies that met the inclusion criteria, but were considered by the reviewers to be unclear, insufficient, or did not add new data for the analysis were excluded.
Information sources and Search Strategy
The search was conducted in five databases, PubMed, Web of Science, Scopus, Google Scholar and Wiley Online Library. The search terms used were “Brain”, “Neuroocular”, “Astronaut”, “Spaceflight” and “Microgravity”. In all five databases, the search strategy was based on applying the keywords in the following way: “((brain) and (eye) or (neuro-ocular)) AND ((astronaut) or (spaceflight) or (microgravity))”. The search was conducted in August 2022.
The search returned 244 results in Pubmed, 149 in Web of Science, 121 in Scopus, 310 in Google Scholar and 28 in Wiley Online. The 852 results were then imported to RAYYAN35, a systematic review screening software and automation tool, to remove duplicates. The remaining 738 results were subsequently screened for inclusion by two reviewers.
Selection and data collection processes
Two reviewers (GR and EO) worked independently, applying, individually, the inclusion and exclusion criteria to the 738 results. An important factor in permitting this independent work was the blinding (each reviewer worked without access to the other reviewer’s work), made possible by using the Rayyan tool. Firstly, each reviewer applied the eligibility criteria based on the titles and abstracts of each result. If this information was not sufficient for decision-making, the study would have a full-text review. Coincidentally, each reviewer included 250 results and excluded 488. After screening and the final discussion between reviewers, 240 results were included and 498 excluded.
Following the screening process, the studies were again included or excluded based on the study type. Additionally, the two reviewers read all the studies and applied the eligibility criteria, excluding those articles that did not consider a relationship between the findings and SANS and/or ICP. Six studies met the inclusion criteria but were excluded based on different reasons. Therefore, after this final selection and discussion between the reviewers, 20 studies were included.
Data items
Population (P) data comprised of all astronauts who had completed a long-duration spaceflight and developed neuro-ocular symptoms, independently of gender, age or background.
Intervention (I) data comprised of the descriptions about ICP variations in the different conditions, such as spaceflight and countermeasures.
Comparison (C) data comprised of all astronauts who completed a long-duration spaceflight and did not develop neuro-ocular symptoms, independently of gender, age or background.
Outcomes (O) data comprised of the development of SANS and description of different neuro-ocular findings, after spaceflight or analogs.
Data on general information (title, DOI, authors, year, month and country), type of publication, and study characteristics (study design) were also extracted. Where data was missing, protocols and project overviews cited were consulted.
Study risk of bias assessment
Two independent reviewers (GR and EO) carried out a risk of bias assessment. The included research articles were divided according to study type.
The risk of bias of the eleven non-randomized studies2,13,14,21,22,23,24,26,30,31,36 was evaluated using the “ROBINS-I” tool, developed by the BMJ37. This tool evaluates the presence of bias due to confounding (D1), due to the selection of study participants (D2), in classification of interventions (D3), due to deviations from intended interventions (D4), due to missing data (D5), in measurement of outcomes (D6), and in the selection of the reported result (D7). Each parameter and the overall study were then rated using a scale comprising of “Low risk”, “Moderate risk”, “Serious risk”, and “Critical risk” of bias levels, as well as a “No information” level, for risk of bias assessment. The “Robvis” web-app was then used to create risk of bias plots for our study, to present the results of the risk of bias assessment38.
The risk of bias assessment for the three randomized trials15,16,25 was performed using the “RoB 2 tool”39. This instrument evaluates the presence of bias arising from the randomization process (D1), due to deviations from intended interventions (D2), due to missing outcome data (D3), in measurement of the outcome (D4) and in selection of the reported result (D5). Each parameter and the overall study were then evaluated according to a scale comprising of “Low”, “Some concerns” and “High” risk of bias levels. The “Robvis” web-app was then used to create risk of bias plots for our study, to present the results of the risk of bias assessment38.
The ”ROBIS” tool, developed by Bristol Medical School40, was used to assess the risk of bias of the three systematic reviews4,5,17. This tool evaluates the presence of Concerns regarding Study eligibility criteria, Identification and selection of studies, Data collection and study appraisal, and Synthesis and findings. Each parameter and the overall study were then evaluated in a scale with “Low”, “High” and “Uncertain” risk of bias levels.
The Newcastle-Ottawa Quality Assessment Form41 was used to evaluate the risk of bias of the two cohort studies18,19. This form evaluates the quality of Selection (Representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure and demonstration of the outcome of interest), Comparability (Comparability of Cohorts on the Basis of the Design or Analysis) and Outcome (Assessment of Outcome, duration of follow-up and Adequacy of Follow-up of Cohorts). After the evaluation of each parameter, the quality of the study is designated as “Good”, “Fair” or “Poor”.
The risk of bias of the only case study1 was evaluated using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports42. This tool evaluates the demographic characteristics, description of patient history, presentation of the current clinical condition, description of diagnostic/treatment procedures, description of post-intervention clinical condition, adverse effects, and takeaway lessons of the report. The end results in a decision to “Include”, “Exclude” or “Seek further information”.
Any disagreement between the two reviewers concerning risk of bias assessment was resolved through discussion. Consensus was reached on all articles included in the review.
Synthesis methods
The research synthesis for the present review was conducted based on analysis of each included article and subsequent creation of Table 2, in which the main findings and significance of each study were summarized by the reviewers.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
Code availability
No code was developed during the analysis and review of this manuscript.
References
Mader, T. H. et al. Optic Disc Edema in an Astronaut After Repeat Long-Duration Space Flight. J. Neuro-Ophthalmol. 33, 249–255 (2013).
Mader, T. H. et al. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight. Ophthalmology 118, 2058–2069 (2011).
Lee, A. G. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: A review and an update. Npj Microgravity 6, 7 (2020).
Martin Paez, Y., Mudie, L. I. & Subramanian, P. S. Spaceflight Associated Neuro-Ocular Syndrome (SANS): A Systematic Review and Future Directions. Eye Brain 12, 105–117 (2020).
Elwy, R. et al. Visual changes after space flight: Is it really caused by increased intracranial tension? A systematic review. Journal of Neurosurgical Sciences, 64. https://doi.org/10.23736/S0390-5616.20.04927-9 (2020).
Lee, J. K. et al. Spaceflight-Associated Brain White Matter Microstructural Changes and Intracranial Fluid Redistribution. JAMA Neurol. 76, 412 (2019).
Rodríguez-Boto, G., Rivero-Garvía, M., Gutiérrez-González, R. & Márquez-Rivas, J. Basic concepts about brain pathophysiology and intracranial pressure monitoring. Neurol.ía (Engl. Ed.) 30, 16–22 (2015).
Lee, J. K. et al. Effects of Spaceflight Stressors on Brain Volume, Microstructure, and Intracranial Fluid Distribution. Cerebral Cortex Communications, 2. https://doi.org/10.1093/texcom/tgab022 (2021).
Marshall-Goebel, K. et al. Association of Structural Changes in the Brain and Retina After Long-Duration Spaceflight. JAMA Ophthalmol. 139, 781 (2021).
Marshall-Goebel, K. et al. Mechanical countermeasures to headward fluid shifts. J. Appl. Physiol. 130, 1766–1777 (2021).
Hearon, C. M. et al. Effect of Nightly Lower Body Negative Pressure on Choroid Engorgement in a Model of Spaceflight-Associated Neuro-ocular Syndrome: A Randomized Crossover Trial. JAMA Ophthalmol. 140, 59–65 (2021).
Lawley, J. S. et al. Daily generation of a footward fluid shift attenuates ocular changes associated with head-down tilt bed rest. J. Appl. Physiol. 129, 1220–1231 (2020).
Van Ombergen, A. et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl Acad. Sci. 116, 10531–10536 (2019).
Mader, T. H. et al. Persistent Globe Flattening in Astronauts following Long-Duration Spaceflight. Neuro-Ophthalmol. 45, 29–35 (2020).
Scott, J. M. et al. Association of Exercise and Swimming Goggles With Modulation of Cerebro-ocular Hemodynamics and Pressures in a Model of Spaceflight-Associated Neuro-ocular Syndrome. JAMA Ophthalmol. 137, 652 (2019).
Mekjavić, I. B., Amoaku, W., Mlinar, T. & Mekjavić, P. J. Hypercapnia augments resistive exercise‐induced elevations in intraocular pressure in older individuals. Exp. Physiol. 105, 641–651 (2020).
Galdamez, L. A., Brunstetter, T. J., Lee, A. G. & Tarver, W. J. Origins of Cerebral Edema: Implications for Spaceflight-Associated Neuro-Ocular Syndrome. J. Neuro-Ophthalmol. 40, 84–91 (2020).
Wåhlin, A. et al. Optic Nerve Length before and after Spaceflight. Ophthalmology 128, 309–316 (2020).
Rosenberg, M. J. et al. Comparison of Dural Venous Sinus Volumes Before and After Flight in Astronauts With and Without Spaceflight-Associated Neuro-Ocular Syndrome. JAMA Netw. Open 4, e2131465 (2021).
Marshall-Goebel, K., Damani, R. & Bershad, E. M. Brain Physiological Response and Adaptation During Spaceflight. Neurosurgery 85, E815–E821 (2019).
Iwasaki, K. et al. Long‐duration spaceflight alters estimated intracranial pressure and cerebral blood velocity. J. Physiol. 599, 1067–1081 (2021).
Rohr, J. J. et al. Quantitative magnetic resonance image assessment of the optic nerve and surrounding sheath after spaceflight. Npj Microgravity 6, 30 (2020).
Lawley, J. S. et al. Effect of gravity and microgravity on intracranial pressure: Gravity on intracranial pressure. The. J. Physiol. 595, 2115–2127 (2017).
Sater, S. H. et al. MRI-based quantification of ophthalmic changes in healthy volunteers during acute 15° head-down tilt as an analogue to microgravity. J. Royal Soc. Interface, 18, rsif.2020.0920, 20200920. https://doi.org/10.1098/rsif.2020.0920 (2021).
Kermorgant, M. et al. Effects of Venoconstrictive Thigh Cuffs on Dry Immersion-Induced Ophthalmological Changes. Front. Physiol. 12, 692361 (2021).
Macias, B. R. et al. Association of Long-Duration Spaceflight With Anterior and Posterior Ocular Structure Changes in Astronauts and Their Recovery. JAMA Ophthalmol. 138, 553 (2020).
Starling, E. H. On the Absorption of Fluids from the Connective Tissue Spaces. J. Physiol. 19, 312–326 (1896).
Wostyn, P., Gibson, C. R. & Mader, T. H. The odyssey of the ocular and cerebrospinal fluids during a mission to Mars: The “ocular glymphatic system” under pressure. Eye 36, 686–691 (2022).
Ozelbaykal, B., Oğretmenoğlu, G. & Gedik, Ş. The Effects of Space Radiation and Microgravity on Ocular Structures. Turkish J. Ophthalmol. 52, 57–63 (2022).
Roberts, D. R. et al. Altered cerebral perfusion in response to chronic mild hypercapnia and head-down tilt Bed rest as an analog for Spaceflight. Neuroradiology 63, 1271–1281 (2021).
Laurie, S. S. et al. Effects of short‐term mild hypercapnia during head‐down tilt on intracranial pressure and ocular structures in healthy human subjects. Physiol. Rep. 5. https://doi.org/10.14814/phy2.13302 (2017).
Fischman, J. J., Cowen, R., Petersen, L., Healey, R. & Hargens, A. The Effects of Resistance Exercise on Intracranial Pressure. FASEB J. 32. https://doi.org/10.1096/fasebj.2018.32.1_supplement.587.8 (2018)
Page, M. J. et al. The PRISMA 2020 statement: an Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 372. https://doi.org/10.1136/bmj.n71 (2021).
LibGuides: Evidence Based Medicine: PICO. (n.d.). https://mcw.libguides.com/EBM/PICO.
Ouzzani, M., Hammady, H. M., Fedorowicz, Z. & Elmagarmid, A. K. Rayyan—a web and mobile app for systematic reviews. 5 https://doi.org/10.1186/s13643-016-0384-4 (2016).
Roberts, D. R. et al. Longitudinal change in ventricular volume is accelerated in astronauts undergoing long-duration spaceflight. Aging Brain 1, 100017 (2021).
Sterne, J. A. C. et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 355, i4919 (2016).
McGuinness, L. A. & Higgins, J. P. T. Risk‐of‐bias VISualization (robvis): An R package and Shiny web app for visualizing risk‐of‐bias assessments. Res. Syn. Methods 12. https://doi.org/10.1002/jrsm.1411 (2020).
Higgins, J. P. T. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ, 343. https://doi.org/10.1136/bmj.d5928 (2011).
Whiting, P. et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 69, 225–234 (2016).
Gierisch, J. M. et al. Newcastle-Ottawa Quality Assessment Form for Cohort Studies. Health Disparities in Quality Indicators of Healthcare Among Adults with Mental Illness. Appendix B (2014).
Moola, S. et al. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual (eds Aromataris, E. & Munn, Z.). Available from https://reviewersmanual.joannabriggs.org/ (The Joanna Briggs Institute, 2017).
Acknowledgements
This work forms part of the doctoral studies of Edson Santos Oliveira at Faculdade de Medicina (Universidade de Lisboa), carried out under the supervision of Prof. Thaís Russomano.
Author information
Authors and Affiliations
Contributions
Gabriela Alves Rodrigues, the main author, was responsible for the search and selection of studies and writing the article, under the supervision of Thais Russomano and Edson Santos Oliveira, who also contributed to the study inclusion process and article review. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rodrigues, G.A., Russomano, T. & Santos Oliveira, E. Understanding the relationship between intracranial pressure and spaceflight associated neuro-ocular syndrome (SANS): a systematic review. npj Microgravity 11, 22 (2025). https://doi.org/10.1038/s41526-025-00464-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41526-025-00464-1