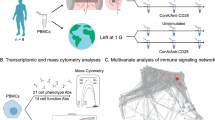
Abstract
Given NASA’s plans for manned lunar and Mars missions, it is critical to assess the risk of splenic immune dysregulation by using ground-based models of simulated microgravity (SMG) and/or chronic irradiation (CIR). To address this, C57BL/6 J mice of both sexes exposed to SMG and/or CIR for 29 days and alterations in immune cell distribution, function and phenotype were assessed. SMG and/or CIR altered a greater variety of immune cells in both lymphoid and myeloid lineages in female mice than in male mice; the function of splenic CD4 + T cells, CD8 + T cells, and CD19 + B cells altered in a sex-specific manner; and the distribution of different immune cells altered based on animal sex. These findings indicate that SMG and/or CIR alter the splenic immune cell distribution, phenotype and function in a sex-specific manner, underscoring the need for tailored strategies to mitigate health risks for crew members on long-term deep-space missions.
Similar content being viewed by others
Introduction
Spaceflights such as short-duration (a few days to weeks) missions within low Earth orbit (altitude of 1200 miles) adversely affect the function of the immune system1,2,3. Specifically, the NASA Human Research Program recognized that 2 major factors contributing to immune dysfunction during and after space missions are concurrent uninterrupted exposure to low-dose and low-dose-rate ionizing radiation (chronic irradiation, CIR) with high-energy heavy particles (HZE) and weightlessness (microgravity). Notably, space radiation is distinct from terrestrial radiation (such as X-rays and γ-rays); space radiation generates dense ionization events along its track, resulting in highly complex DNA damage that is difficult to repair, thus exerting greater cell-killing efficacy than terrestrial radiation. The HZE radiation is generated from galactic cosmic rays, with occasional short-term exposure from solar energetic particles during solar flares and coronal mass ejections4. However, the effects of HZE radiation during long-term missions beyond low Earth orbit such as cis-lunar and Mars missions are unknown.
The radiation dose measurement data obtained from the Mars Science Laboratory spacecraft, containing the Curiosity rover, estimated that crew members will receive ~1.84 mSv/day from both galactic cosmic rays and solar energetic particles5, resulting in a cumulative dose of ~2 Sv or ~2 Gy during a 3-year Mars exploration mission5,6. However, the actual radiation dose absorbed by crew members will vary depending on habitat shielding, solar activity, and mission duration; the dose could range between 0.5 and 2 Gy over 3 years6, resulting in a significantly higher dose than background radiation on Earth and exceeding the astronaut career exposure limit of 1 Sv adopted by several space agencies. Importantly, no ground-based facility is available to exactly mimic space radiation, particularly chronic exposure to HZE radiation.
Before the end of the next decade, NASA is planning for long-term (months to years) deep-space missions (traveling beyond the Earth-Moon Lagrange points), such as to asteroids (at least 3.1 million miles from Earth) and Mars (34.6 million miles from Earth), with an aim to extend human existence across the solar system. During these long-term deep-space missions, as compared to short-term missions within the low Earth orbit, crew members would receive a higher radiation dose and experience microgravity for an extended period of time, which could further increase the risk of immune dysfunction. Because it is not practical to conduct deep-space experiments with animals or humans to assess immune risk before the proposed missions, ground-based models capable of subjecting animals to CIR and simulated microgravity (SMG) would provide crucial insights into the immune risks associated with deep-space missions.
There are no animal or human data on immune dysfunction in the secondary lymphoid organs, such as the spleen, following deep-space missions. Additionally, we cannot collect spleen samples from crew members to assess immune dysfunction. Splenic immune dysfunction can adversely affect the immune response and thus overall health during and after deep-space missions. Therefore, we decided to predict the risk of splenic immune dysfunction after concurrent exposure to CIR and SMG using a pre-clinical mouse model.
Ground-based models testing a single stressor demonstrated that SMG altered splenic cytokine expression7, suppressed immune function8, and modified dendric cell activation and maturation9; while CIR altered splenic immune cell phenotype and function10, T cell signaling response11, and cytokine expression patterns12. These findings clearly indicate that exposure to a single stressor, specifically SMG or CIR, has the potential to impact the splenic immune system; however, there are limited biological data on the effects of concurrent exposure to SMG and CIR on the splenic immune system. We previously reported that concurrent exposure of C57BL/6 J male mice to SMG and CIR for 30 days altered the thymic immune cell phenotype, but did not alter splenic immune cell phenotype13. Additionally, concurrent exposure adversely affected body weight, food consumption, and water intake; and enhanced plasma levels of catecholamines (a stress marker)13.
Interestingly, short-duration spaceflight experiments with rodents in low Earth orbit indicated that space travel decreased spleen mass and altered cancer-related gene expression in the spleen14, differentially altered splenic transcription factors15, decreased the percentage of selectin-positive splenocytes16, decreased IFNγ and increased IL2 production by splenocytes following stimulation17, and altered homeostatic gene expression in the spleen18. These findings suggest that short-duration space missions within low Earth orbit alter the splenic immune phenotype and function. However, the effects of long-term deep-space missions on the splenic immune system, in humans or rodents, are unknown.
We addressed the issue with a ground-based model capable of subjecting mice to SMG and CIR simultaneously (at a dose rate relevant to space radiation). We used the NASA-approved SMG model for hind-limb unloading in rodents and a 137Cs gamma source to deliver CIR. While the radiation quality of HZE particles is significantly different than that of γ-rays, there is no ground-based research facility capable of mimicking the dose rate and radiation quality encountered in space. Although the NASA Space Radiation Laboratory at Brookhaven National Laboratory can deliver a mixture of galactic cosmic radiation, there are limitations. For example, the radiation is delivered in a single acute exposure or in multiple fractions, not in an uninterrupted chronic exposure as in space. Thus, our ground-based model will aid in the understanding of the health risks associated with concurrent, uninterrupted exposure to SMG and/or CIR.
In the current study, we exposed 12- to 16-week-old male and female C57BL/6 J mice to SMG and/or CIR for 29 days (equivalent to the time required to complete a Mars mission, where 1 month of age in mice is equivalent to 3 years of age in humans). We observed sex-specific changes in splenic immune cell phenotype and function after exposure to SMG and/or CIR. These findings suggest that long-term deep-space missions may negatively affect the splenic immune system, and appropriate, sex-specific measures are required to protect the health of crew members.
Results
Subsequent trainings for hind-limb unloading prevents body weight loss
The analysis of body weight changes in mice across the training sessions revealed notable findings. Prior to the first training session, the estimated mean body weight of mice was 18.13 g (95% CI: 17.71 to 18.56). Following the first training session (HLU for 24 h), a significant reduction in body weight was observed, with an estimated mean decrease of 0.556 g (95%CI: –0.90 to –0.21, p = 0.024). In contrast, following the second training session (HLU for 48 h after a rest of 24 h from the first training), mice did not lose body weight, rather slightly gain body weight with an estimated mean of 0.020 g (95%CI: -0.08 to 0.11, p = 0.69), when compared to the body weight before starting the second training. Similarly, following the third training session (HLU for 72 h after a rest of 72 h from the second training), mice did not lose body weight, rather slightly gained body weight with an estimated mean of 0.07 g (95%CI: 0.0007 to 0.14, p = 0.07), when compared to the body weight before starting the third training. These findings suggest that the training sessions helped mice to better adapt to the SMG condition and prevented SMG-induced body weight loss (Fig. 1).
Mice were subjected to 3 training sessions for adaptation to the SMG condition. Body weights were recorded before and after each training session. During the first training (T1) mice were subjected to HLU for 24 h. After a rest of 24 h following T1, mice were subjected to a second training (T2) for 48 h. After a rest of 72 h following T2, mice were subjected to a third training (T3) for 72 h. Body weight represents Mean ± SE. *, p < 0.05.
SMG and/or CIR alter the splenic lymphoid cell population in a sex-specific manner
Immune phenotyping with flow cytometry revealed that exposure to SMG and/or CIR for 29 days did not significantly alter the percent of CD4 + T cells, CD8 + T cells, regulatory T cells (T-reg), or NKT cells in female or male mice (Fig. 2). However, SMG significantly decreased the percent of B cells in female mice (95% CI: 4.40 to 22.03; p = 0.005), but not in male mice (Fig. 2). In addition, exposure to SMG + CIR significantly decreased the percent of NK cells in female mice (95% CI: 0.15 to 2.37; p = 0.03), while CIR significantly increased the percent of NK cell in male mice (95% CI: 0.79 to 0.05; p = 0.03) (Fig. 2). These findings clearly indicate that some splenic lymphoid components are more sensitive to space hazards in female mice than in male mice.
The percentage of (A) CD4 + T cells, (B) CD8 + T cells, (C) T-reg cells, (D) NKT cells, (E) NK cells, and (F) B cells in female C57BL/6 J mice after exposure to no stressor (sham; n = 8), simulated microgravity (SMG; n = 8), chronic irradiation (CIR; n = 8), and SMG + CIR (n = 8) for 29 days. The percentage of (G) CD4 + T cells, (H) CD8 + T cells, (I) T-reg cells, (J) NKT cells, (K) NK cells, and (L) B cells in male C57BL/6 J mice after exposure to no stressor (sham; n = 8), SMG (n = 8), CIR (n = 8), and SMG + CIR (n = 8) for 29 days. Data represent Mean ± SE. *, p < 0.05; **, p < 0.01.
SMG and/or CIR alter the splenic myeloid cell population in a sex-specific manner
Immune phenotyping with flow cytometry revealed alterations in various components of the myeloid cell population, mostly in a sex-specific manner. SMG + CIR significantly decreased the percent of macrophages in female mice (95% CI: 0.24 to 0.97; p = 0.004), while SMG alone significantly increased the percent of macrophages in male mice (95% CI: 4.81 to 0.43; p = 0.02) (Fig. 3). Then we looked at alterations in the percent of splenic migratory leukocytes, specifically monocytes and neutrophils. SMG significantly increased the percent of monocytes in female mice (95% CI: 0.98 to 0.17; p = 0.007), but not in male mice (Fig. 3). SMG significantly increased the percent of neutrophils in female mice (95% CI: 6.82 to 1.45; p = 0.007) and in male mice (95% CI: 1.93 to 0.008; p = 0.048). SMG + CIR increased the percent of neutrophils in female mice (95% CI: 6.76 to 0.51; p = 0.03) (Fig. 3). Finally, we determined space factors-induced alterations in the percent of different subtypes of the major antigen presenting cells, the DCs. SMG + CIR significantly increased the percent of cDC1 in female mice (95% CI: 0.1 to 0.01; p = 0.016) (Fig. 3). CIR significantly decreased the percentage of cDC2 in male mice (95% CI: 0.05 to 0.24; p = 0.004). However, no significant changes were observed in the percent of pDC under any conditions in both female or male mice (Fig. 3).
The percentage of (A) macrophages, (B) monocytes, (C) neutrophils, (D) cDC1 cells, (E) cDC2 cells, and (F) pDC cells in female C57BL/6 J mice after exposure to no stressor (sham; n = 8), simulated microgravity (SMG; n = 8), chronic irradiation (CIR; n = 8), and SMG + CIR (n = 8) for 29 days. The percentage of (G) macrophages, (H) monocytes, (I) neutrophils, (J) cDC1 cells, (K) cDC2 cells, and (L) pDC cells in male C57BL/6 J mice after exposure to no stressor (sham; n = 8), SMG (n = 8), CIR (n = 8), and SMG + CIR (n = 8) for 29 days. Data represent Mean ± SE. *, p < 0.05; **, p < 0.01.
SMG and/or CIR alter the splenic CD4 + T cell function in a sex-specific manner
Isolated CD4 + T cells were stimulated with anti-CD3/anti-CD28 magnetic beads for 24 h, and cell culture medium was collected and assayed for TNFα, IFNγ, and IL2. We observed that SMG + CIR significantly decreased TNFα secretion by stimulated CD4 + T cells from female mice (95% CI: 0.13 to 9.34; p = 0.04), but not male mice (Fig. 4). However, SMG and/or CIR did not affect the level of IFNγ or IL2, regardless of animal sex (Fig. 4).
CD4 + T cells were isolated from spleen with magnetic beads and purity assessment was validated with flow cytometry. Dot plots showing (A) all cells isolated from spleen with a CD4 + T cell isolation kit were acquired by FACS, (B) the proportion of CD3 + T cells was identified for downstream analysis, and (C) within the CD3 + T cell population, the true proportion of CD4 + T cells were detected based on CD4 positivity and CD8 negativity. Red box indicates negligible contamination of CD8 + T cells. Functional changes were determined by measuring the level of secreted cytokines in the media by stimulated CD4 + T cells. Splenic CD4 + T cells were isolated after exposure to SMG and/or CIR for 29 days followed by stimulation with anti-CD3/anti-CD28 magnetic beads for 24 h. The level of (D) TNFα, (E) IFNγ, and (F) IL2 in female mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). The level of (G) TNFα, (H) IFNγ, and (I) IL2 in male mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). Data represent Mean ± SE. *, p < 0.05.
SMG and/or CIR alter the splenic CD8 + T cell function in a sex-specific manner
Isolated CD8 + T cells were stimulated with anti-CD3/anti-CD28 magnetic beads for 24 h, and the cell culture medium was collected and assayed for TNFα, IFNγ, and IL2. CIR significantly increased TNFα secretion by stimulated CD8 + T cells from female mice (95% CI: 10.93 to 1.19; p = 0.016), but CIR significantly decreased TNFα secretion by stimulated CD8 + T cells from male mice (95% CI: 0.90 to 12.02; p = 0.03) (Fig. 5). SMG significantly increased IFNγ secretion by stimulated CD8 + T cells from female mice (95% CI: 15.75 to 4.63; p = 0.001), but not male mice (Fig. 5), and SMG increased IL2 secretion by stimulated CD8 + T cells from female mice (95% CI: 8.37 to 2.57; p = 0.001), but not male mice (Fig. 5).
CD8 + T cells were isolated from spleen with magnetic beads and purity assessment was performed with flow cytometry. Dot plots showing (A) all cells isolated from spleen with a CD8 + T cell isolation kit were acquired by FACS, (B) the proportion of CD3 + T cells was identified for downstream analysis, and (C) within the CD3 + T cell population, the true proportion of CD8 + T cells were detected based on CD8 positivity and CD4 negativity. Red box indicates negligible contamination of CD4 + T cells. Functional changes were determined by measuring the level of secreted cytokines in the media by stimulated CD8 + T cells. Splenic CD8 + T cells were isolated after exposure to SMG and/or CIR for 29 days followed by stimulation with anti-CD3/anti-CD28 magnetic beads for 24 h. The level of (D) TNFα, (E) IFNγ, and (F) IL2 in female mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). The level of (G) TNFα, (H) IFNγ, and (I) IL2 in male mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). Data represent Mean ± SE. *, p < 0.05; **, p < 0.01.
SMG and/or CIR alter the splenic B cell function in a sex-specific manner
Isolated CD19 + B cells were stimulated with LPS for 24 h, and the cell culture medium was collected and assayed for IgA and IgM. CD19 + B cell-mediated IgA secretion following stimulation was significantly decreased after exposure to CIR (95% CI: 0.27 to 4.18; p = 0.03) and SMG + CIR (95% CI: 0.68 to 4.54; p = 0.015) in female mice (Fig. 6). While CD19 + B cell-mediated IgA secretion following stimulation was significantly increased after exposure to CIR (95% CI: 14.16 to 4.08; p = 0.004) and SMG + CIR (95% CI: 7.74 to 3.04; p = 0.001) in male mice (Fig. 6). In addition, CD19 + B cell-mediated IgM secretion following stimulation was significantly increased after exposure to CIR (95% CI: 263.82 to 90.36; p = 0.002) and SMG + CIR (95% CI: 172.37 to 81.23; p = 0.0002) in male mice, but not in female mice (Fig. 6). These data clearly exhibit sex-specific effects on the B cell response to antigen.
B cells were isolated from spleen with magnetic beads and purity assessment was performed with flow cytometry. Dot plots showing (A) all cells isolated from spleen with B cell isolation beads were acquired by FACS for downstream analysis, (B) the proportion of CD3- cells ensuring no T cell contamination, and (C) true proportion of B cells as validated with B220 positivity by FACS. Functional changes were determined by measuring the level of secreted immunoglobins in the media by stimulated CD19 + B cells. Splenic CD19 + B cells were isolated after exposure to SMG and/or CIR for 29 days followed by stimulation with LPS for 24 h. The level of (D) IgA and (E) IgM in female mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). The level of (F) IgA and (G) IgM in male mice after exposure to no stressor (sham; n = 8); SMG (n = 8), CIR (n = 8) and SMG + CIR (n = 8). Data represent Mean ± SE. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
SMG and/or CIR alter the splenic CD4+ and CD8 + T cell immunoreactivity area in a sex-specific manner
Immunohistochemistry performed on spleen tissue demonstrated that immunoreactive area for CD4 + T cells was significantly decreased after exposure to SMG (95% CI: 0.36 to 1.12; p = 0.0005) and SMG + CIR (95% CI: 0.65 to 0.83; SE = 0.19; p = 0.024) in female mice; but not in male mice (Fig. 7). In addition, SMG + CIR increased the CD8 + T cells immunoreactive area in female mice (95% CI: 10.59 to 0.41; p = 0.035), but not in male mice (Fig. 8).
Representative photomicrographs showing CD4 + T cell immunoreactive areas in the spleen tissue collected from C57BL/6 J mice after exposure to (A) no stressor (sham), (B) SMG, (C) CIR; and (D) SMG + CIR. The percent area positive for CD4 + T cell immunoreactivity in the spleen collected from (E) female C57BL/6 J mice exposed to stressor (sham; n = 8); SMG (n = 7); CIR (n = 7) and SMG + CIR (n = 7). The percent area positive for CD4 + T cell immunoreactivity in the spleen collected from (F) male C57BL/6 J mice exposed to stressor (sham; n = 8); SMG (n = 8); CIR (n = 8) and SMG + CIR (n = 7). Data represent Mean ± SE. *, p < 0.05; ***, p < 0.001.
Representative photomicrographs showing CD8 + T cell immunoreactive areas in the spleen tissue collected from C57BL/6 J mice after exposure to (A) no stressor (sham), (B) SMG, (C) CIR; and (D) SMG + CIR. The percent area positive for CD8 + T cell immunoreactivity in the spleen collected from (E) female C57BL/6 J mice exposed to stressor (sham; n = 8); SMG (n = 7); CIR (n = 8) and SMG + CIR (n = 8). The percent area positive for CD8 + T cell immunoreactivity in the spleen collected from (F) male C57BL/6 J mice exposed to stressor (sham; n = 8); SMG (n = 8); CIR (n = 8) and SMG + CIR (n = 8). Data represent Mean ± SE. *, p < 0.05.
However, no changes were observed in the immunoreactivity of CD68+ macrophages in the splenic red pulp and white pulp regions (Supplementary Fig. 3).
Discussion
The risk of immune dysregulation after deep-space missions (beyond low Earth orbit) is one of the major concerns of NASA. Although no animal or human data are available on splenic immune dysregulation after long-term space missions beyond low Earth orbit, immune dysregulation was reported during and after short-duration missions to low Earth orbit2. Altered immune function may modify host–microorganism interactions, wound healing ability, and allergic reactions. The current study demonstrated that SMG and/or CIR alters the immune phenotype of splenic lymphoid- and myeloid-lineage cells, in a sex-specific manner.
We observed that exposure to SMG and/or CIR for 29 days did not change the percent of splenic CD4 + , CD8 + , T-reg, or NKT cells of the lymphoid lineage in male or female C57BL/6 J mice. Our previous study also demonstrated no change in the percent of splenic CD4+ or T-reg cells in C57BL/6 J male mice after 30 days of SMG and/or CIR, corroborating our current findings; however, the CIR dose in the previous study was substantially lower (0.5 Gy over the period of 30 days) than in the current study, and only male mice were used13. Similarly, no change was observed in the percent of splenic CD4+ or CD8 + T cells in rats following 10 days of SMG19 or 14 days on the SLS-2 mission20. These findings indicate that space hazards exert no significant phenotypic change in certain types of splenic immune cells (e.g., CD4 + , CD8 + , T-reg, and NKT) of the lymphoid lineage.
However, we found that SMG and SMG + CIR decreased the percent of splenic B cells and NK cells of the lymphoid lineage, respectively, in female mice, but not in males, suggesting a sex-specific effect. Notably, a 41% decrease in splenic B cells was observed in C57BL/6 mice after exposure to 1 month onboard the BION-M1 satellite followed by 1 week of recovery21, suggesting that spaceflight compromises B cell lymphopoiesis. As B cells exert antimicrobial immunity and NK cells are a crucial component of innate immunity, a decrease in the proportion of these cells may adversely affect B and NK cell-mediated immunity.
With respect to the myeloid lineage, SMG and/or CIR altered a greater variety of cells in female mice than male mice. SMG increased the percent of macrophages in male mice and SMG + CIR decreased it in females. A similar decrease in splenic macrophages was observed in rats that underwent SMG for 10 days19. This SMG-mediated decrease in the macrophage percentage could be due to suppression of macrophage development. Indeed, a previous study demonstrated that SMG suppressed macrophage development and differentiation via the RAS/ERK/NFκB axis22. Because macrophages are responsible for phagocytosis, alterations in the macrophage percentage could affect phagocytic activity. In addition, SMG increased the percent of monocytes and neutrophils in female mice, but not in males. Although relevant reports on splenic monocytes and neutrophils are limited, previous studies reported that SMG23 and spaceflight24 increased the percentage of monocytes in rat bone marrow and in peripheral blood of crew members25, respectively. Moreover, spaceflight increased neutrophils in the peripheral blood of rats20 and of crew members with a median mission duration of 162 days25, suggesting that space factors may promote the generation of monocytes and neutrophils. Notably, increased percentages of monocytes and neutrophils are associated with chronic inflammation.
Few studies have directly assessed immune function during and after spaceflight. In the NASA Twins Study, the response to flu vaccination did not differ during and after spaceflight compared to ground. However, several cytokine levels were altered during spaceflight and remained altered for months following return to Earth, suggesting there were changes in immune response26. Immune cells exert functional activity by secreting cytokines upon stimulation. Antigenic stimulation can gradually differentiate CD4+ and CD8 + T cells into central memory T cells and terminal effector cells, which secrete TNFα, IFNγ, and/or IL2. TNFα and IFNγ are major regulators of inflammatory responses, while IL2 is a potent inducer of CD4 + T cells, CD8 + T cells, and NK cells. The current study demonstrated that SMG + CIR suppressed TNFα secretion by stimulated CD4+ cells only in female mice, suggesting that space factors compromise the CD4 + T cell response in a sex-specific manner. However, SMG and/or CIR did not change IFNγ or IL2 secretion, irrespective of sex, when CD4 + T cells were stimulated with a specific antigen. A previous study also demonstrated that SMG did not alter IL2 production when rat splenic lymphocytes were stimulated27. Additionally, a spaceflight experiment demonstrated no change in IFNγ or IL2 secretion when mouse splenocytes were isolated after 13 days on the STS-135 mission and stimulated with mitogen17. However, CD4 + T cells isolated from the peripheral blood of crew members after spaceflight produced less INFγ in response to mitogenic stimulation, compared to no spaceflight28. This apparent discrepancy in INFγ secretion by stimulated lymphocytes following spaceflight or ground-based simulation experiments could be due to differences in the duration of exposures, the source of CD4 + T cells, and the method of T cell stimulation.
With respect to CD8 + T cells, we observed that CIR increased TNFα secretion by stimulated CD8 + T cells in female mice, but TNFα secretion decreased in males under the same conditions, suggesting that this was a radiation-induced sex-specific effect in mice. Increased TNFα secretion was also observed when splenocytes obtained from C57BL/6 mice that spent 13 days on the STS-135 mission were stimulated with TLR agonists17. Similarly, TNFα increased in stimulated peripheral blood lymphocytes isolated from 12 male cosmonauts (median age: 42 years) after a long-term space mission (median mission duration: 162 days)25. However, TNFα secretion decreased in PMA+ionomycin-stimulated blood lymphocytes collected from 23 crew members (18 males and 5 females) after long-term space missions29. These findings indicate that TNFα secretion by splenic immune cells depends on the method of stimulation. In addition, our study revealed that SMG increased IFNγ and IL2 secretion by stimulated splenic CD8 + T cells from female mice, but not from males. A similar increase in IFNγ secretion was observed in stimulated splenocytes from C57BL/6NTac female mice that spent 13-days on the STS-118 mission30. However, the same study reported a decrease in IL2 secretion by stimulated splenocytes30, which contradicts our current findings. This discrepancy in IL2 secretion could be due to the use of splenocytes as opposed to CD8 + T cells, as in our study.
Splenic B cells regulate humoral immunity. When stimulated, B cells secrete immunoglobins such as IgM and IgA, which regulate host defense against infection. The current study revealed that CIR and SMG + CIR enhanced the secretion of IgM by stimulated B cells from male mice, but not female mice. A similar increase in salivary IgM level was observed in crew members subjected to the isolated environment of the Mars Desert Research Station31. Notably, IgM is markedly elevated in a number of autoimmune diseases32. In addition, we observed that CIR and SMG + CIR decreased IgA secretion by stimulated B cells from female mice, but increased IgA secretion by stimulated B cells from male mice, suggesting a sex-specific effect. An increase in IgA level was also observed in C57Bl/6NCrl male mice subjected to chronic unpredictable, mild psychosocial and environmental stressors (a ground-based analog of spaceflight) for 3 weeks33. Moreover, crew members subjected to the isolated environment of the Mars Desert Research Station also exhibited increased salivary IgA. These findings suggest that space hazards modify the B cell response to antigens.
Finally, we observed that SMG and SMG + CIR suppressed the immunoreactive area for CD4+ cells in female mice, suggesting SMG in combination with or without CIR adversely affects the survival and proliferation of CD4 + T cells. This adverse effects on CD4 + T cells could be due to altered function of DC cells, which play crucial role for survival and proliferation of CD4 + T cells. A previous study demonstrated that SMG-induced altered DC cell function limits CD4 + T cell survival and proliferation9. Additionally, we observed SMG + CIR increased the immunoreactive area for CD8+ cells in female mice.
We acknowledge the limitations of the current study, which include the quality of radiation used; lack of investigation into the mechanistic basis of sex-specific functional changes in immune cells; exclusion of the contribution of other space hazards (e.g., isolation and confinement, distance from Earth, and hostile environment); the inability to simulate the stress associated with spacecraft landing; and the fact that our SMG model is primarily a fluid-shift model. Lastly, we assessed spleen cell types in percentages, which may not necessarily reflect actual changes in cell count. Future studies require the use of counting beads to quantify different cell types during immune phenotyping. However, considering the cost effectiveness, experimental complexity, and impracticality of performing deep-space experiments on secondary lymphoid organs, the data generated from our ground-based models are critically important to move the field forward.
In conclusion, the current study with a pre-clinical mouse model demonstrated that SMG and/or CIR alter the splenic immune cell phenotype and function in a sex-specific manner; and alter the splenic immune cell distribution. Collectively, these data contribute to our understanding of the risk of immune dysfunction associated with long-term deep-space missions.
Materials and methods
Animal model and experimental groups
All animal experiments were conducted in strict accordance with the guidelines established for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol (No. 4139) was designed to ensure animal welfare and minimize pain and distress and was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas for Medical Sciences (UAMS). Throughout the study, all animals were housed in facilities that met the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and were provided with appropriate environmental enrichment, nutrition, and veterinary care.
C57BL/6 J female or male mice (12 to 16 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and acclimatized at the UAMS animal facility for 2 weeks before the experiments were performed. The mice were housed in conventional cages in a pathogen-free environment with controlled humidity, temperature, and a 12:12 h light–dark cycle, with free access to drinking water and standard chow.
Experiments were conducted separately for male and female mice. For each sex, 32 mice were randomly assigned to one of four groups (sham, SMG, CIR, and SMG + CIR), with 8 mice/group.
Method for simulated microgravity (SMG) and training schedule for SMG
The NASA-approved hind-limb unloading (HLU) model was used to simulate microgravity, as described in our previous publication13. The mice were briefly anesthetized (for 3-5 min) by isoflurane inhalation. To anesthetize the mice, we used 2–3% isoflurane with an oxygen (100%) flow rate of 1.5-2 L/min. Skin–Trac double-sided adhesive orthopedic foam (Urofoam-2, UROCARE, Pomona, CA) was attached to the dorsal and/or ventral sides of the tail without covering the tip/base or sides of the tail to spare the lateral tail veins. One end of an 8-10–cm bead chain swivel was attached to the foam and then wrapped with cotton gauze to allow air flow; the gauze was then draped with self-adherent tape. The other end was passed through a “split key ring.” The hindlimbs were suspended by passing the key ring onto a metal rod placed horizontally on top of the cage, allowing the animal to move freely. The entire procedure took 6-10 min to complete.
Prior to the experiment, mice were subjected to a 18-day training period to adapt them to individual housing and the SMG condition, with the aim of assimilating them to these new conditions before performing the actual experiment, similar to training that crew members receive. All mice were placed individually in custom-made cages for 72 h. Then, mice in SMG and SMG + CIR groups were subjected to HLU for 24 h followed by rest (unsuspended) for 24 h. Then, mice were again subjected to 48 h of HLU followed by 72 h of rest (unsuspended). Then, mice were subjected to 72 h of HLU followed by a 120 h resting period before starting the experiment.
Chronic irradiation (CIR) method and dosimetry
We developed a CIR facility in the UAMS Winthrop P. Rockefeller Cancer Institute as described elsewhere13. Briefly, a Mark I Model 81 Category I panoramic irradiator (JL Shepherd and Associates, San Fernando, CA, USA) was commissioned in a basement laboratory. A lead attenuator was placed around the source to reduce the dose rate by twofold34. The chronic irradiator vault dose rate was characterized using an Exradin A5 ionization chamber and a Standard Imaging Max 4000 electrometer. Both instruments had NIST-traceable air kerma calibrations performed at the University of Wisconsin Accredited Dosimetry Calibration Laboratory. All requirements for an animal facility, including heating, ventilation, and air conditioning, were fulfilled. A time-controlled lighting system (12:12 h light–dark cycle) was used to ensure a uniform diurnal lighting cycle in the facility.
Single-housing cages were placed around the CIR source on 2-tiered plastic tables so that all animals received a uniform radiation dose. Each table contained 4 mouse cages on the upper tier. Two sets of cages were alternately used and changed so that one set was cleaned while the other set was used. The mice were checked daily, during which time the radiation source was lowered for no more than 30 min. Animals received a total of 1.74 Gy gamma radiation over 29 days at a dose rate of 0.0025 Gy/h.
Tissue harvest and euthanasia
To harvest tissue, the mice were anesthetized by isoflurane inhalation. The abdomen was sterilized with 70% ethanol before dissection. Whole spleens were collected in 2 ml PBS with 5% antibiotic-antimycotic (Gibco, Life Technologies). A 2–3–mm section of the anterior end of the spleen was collected for immunohistochemistry, and the rest of the spleen was used to isolate splenic immune cells. After tissue harvest, mice were deeply anesthetized in an isoflurane chamber, and the depth of anesthesia was assessed by testing the absence of the toe pinch reflex; if no response was observed, mice were euthanized by cervical dislocation.
Preparation of single splenocyte suspension
The spleen was placed in 3 ml of DMEM (Gibco, Life Technologies) supplemented with 10% FBS and 1% antibiotics. Splenocytes were collected by gently flushing the spleen using a 5 ml syringe with 23-gauge needle. To eliminate any clumps and debris, the cell suspension was finally filtered through a 70-µm cell strainer (BD Falcon, BD Biosciences), and cells were counted with an automated cell counter (Countless 3, Thermo Scientific). The single-cell suspension of splenocytes was placed on ice before further processing. Splenocytes were used for immunophenotyping and functional analysis.
Multicolor flow cytometry
Reagents: Fluorophore-labeled anti-mouse antibodies (Supplementary Table 1) were purchased from BD Biosciences (San Jose, CA, USA) and used for splenic immune cell phenotyping. Stain buffer containing FBS (Cat. No. 554656); brilliant staining buffer (Cat. No. 566385); and FACS lysing solution (Cat. No 349202) were also obtained from BD Biosciences. Fixable viability dye (eFluor 780, Cat. No. 65-0865-14) was purchased from ThermoFisher Scientific (Waltham, MA, USA).
Flow cytometry protocol: Cells (1 × 107 and 1.5 × 107) from splenic single-cell suspensions from individual mice were centrifuged at 400 g for 10 min at 4 oC. The supernatant was removed, and the cell pellets were resuspended in 110 μl of staining buffer containing 2% FBS. Cells were incubated on ice for 15 min for blocking. Two master mixes were prepared by adding conjugated antibodies to brilliant staining buffer to target immune cells of lymphoid and myeloid lineage separately. Each master mix was then aliquoted to the designated number of wells in a 96-well round-bottom plate, and 100 μl of each sample was divided equally into wells containing antibody cocktails to characterize the lymphoid or myeloid lineages. Cells were stained with conjugated antibodies for 30 min at room temperature in the dark. Following incubation, cells were washed twice with PBS by centrifugation and resuspended in 150 μl of FACS lysing solution for red blood cell lysis and fixation. Fixed cells were then transferred to 5-ml polystyrene round-bottom tubes before analysis with a BD LSRFortessa flow cytometer (BD Biosciences).
Gating strategy for immune cell subpopulations: We developed 2 gating strategies to identify distinctive lymphoid- and myeloid-lineage immune populations (Supplementary figs. 1, 2). Cell populations were defined as shown in Supplementary Table 2. Each antibody was used at an optimal concentration as determined with titration. Before starting experiments, 3 sets of compensation controls were used: single staining for single color compensation, fluorescent minus one (FMOs) for multicolor compensation, and cocktail control as the negative control. Flow Cytometry Standard files (FCS) were analyzed using FlowJo software (v10.10.0, BD Biosciences) as follows: single cells were identified, and aggregates were excluded using forward scatter area (FSC-A) vs. height (FSC-H) axes. We excluded dead cells by gating out eFluor 780-positive cells. In both lymphoid- and myeloid-lineage cells, live cells were analyzed by plotting CD45 vs. SSC-A axes to identify leukocytes as CD45+ cells. For the lymphoid lineage gating strategy, CD45+ cells were plotted on NK1.1 vs. CD49b to identify all natural killer (NK) cells, including natural killer T (NKT) cells. Double-positive (Nk1.1 + /CD49b + ) cells were then plotted on the CD3 axis to define NK cells (NK1.1 + /CD49b + /CD3-) from NKT cells (NK1.1 + /CD49b + /CD3 + ). Double-negative cells (NK1.1-/CD49b-) were plotted on the CD3 axis to identify T cells. To further identify T cell subsets, CD3-positive cells were plotted on CD4 and CD8 axes to gate CD4 + T cells (CD3 + / CD8-/CD4 + ) and CD8 + T cells (CD3 + /CD4-/CD8 + ). CD4 + T cells were plotted on CD25 and FoxP3 axes to identify regulatory T cells (CD3 + /CD8-/CD4 + /CD25 + ). CD3- cells (Nk1.1-/CD49b-/ CD3-) were plotted on CD19 and B220 (CD45R) axes to identify B cells (CD3-/CD19 + /B220 + ).
For the myeloid lineage gating strategy, CD45+ cells were plotted on the CD3 axis to exclude CD3 + T cells. CD3- cells were plotted on Ly6G vs. Ly6C axes or MHCII (I-A/I-E) vs. CD11b. Ly6G vs. Ly6C axes were used to identify neutrophils (Ly6G + /Ly6C + ) and monocytes (Ly6G-/Ly6Chigh). Double-negative cells (Ly6G-/Ly6C-) were then plotted on the F4/80 axis. F4/80+ cells were defined as macrophages (Ly6G-/Ly6C-/F4/80 + ). F4/80- cells were plotted on CD4 vs. CD11C axes to identify type 2 conventional dendritic cells (cDC2, CD4 + /CD11c + ). CD3- cells (CD45 + /CD3-) were also plotted on MHCII vs. CD11b axes. MHCII+ cells (MHCII + /CD11b-) were then plotted on B220 vs. Ly6C axes. The double-positive cells (B220 + /Ly6C + ) were identified as plasmacytoid dendritic cells (pDCs), while double-negative cells were plotted in the context of CD8 vs. CD11C to gate type 1 conventional dendritic cells (cDC1, CD11C + /CD8 + ). Each immune cell type was quantified as a proportion of CD45+ viable cells acquired with the flow cytometer.
Functional assay
Isolation and culture of CD4 + T cells, CD8 + T cells, and CD19 + B cells: EasySep™ Mouse kits (StemCell Technologies) were used to isolate CD4 + T cells (Cat #19852); CD8 + T cells (Cat #19853); and CD19 + B (Cat #18954). The purity of each preparation was further validated with flow cytometry. These isolation methods resulted in 80%, 97% and 96% purity of CD4 + T cells, CD8 + T cells, and CD19 + B cells, respectively. Isolated cells were counted with an automated cell counter (Countless 3, Thermo Scientific) and seeded into a 24-well plate at a density of 1.5 × 105 cells per well for CD4+ and CD8 + T cells and 1.0 × 106 cells per well for B cells, with 1 ml of complete DMEM medium. Cells were cultured for 24 h in a humidified CO2 incubator at 37 °C.
Stimulation of CD4 + T cells, CD8 + T cells, and CD19 + B cells and collection of medium: CD4+ and CD8 + T cells were stimulated with Dynabeads™ Mouse T-Activator CD3/CD28 (Gibco) for 24 h, while CD19 + B cells were stimulated with 100 ng/ml LPS (eBioscience) for 24 h. After stimulation, the medium was collected in a microcentrifuge tube and centrifuged at 1,000 g for 10 min at 4 °C; the supernatant was collected in a separate centrifuge tube and stored at –80 °C until further use.
ELISA: The concentration of cytokines in the medium was determined with commercially available enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (for measuring TNFα and IFNγ) and Invitrogen (for measuring IL2, IL10, IgA, and IgM). Absorbance at 450 nm was measured with a microplate reader (GloMax, Promega). Data were expressed as the mean concentration of duplicate readings per biological sample.
Immunohistochemistry
Tissue fixation and processing: Spleen samples were fixed in 10% neutral buffered formalin fixative (Tissue-Tek Clear FIX, Sakura) for 24 h at room temperature, embedded in paraffin blocks, sectioned at 4 µm thickness, and mounted on glass slides (Premium Superfrost, FisherBrand). The slides were allowed to dry overnight and stored at room temperature until further use.
Deparaffinization, processing, and staining: Spleen sections were deparaffinized, rehydrated, and subjected to heat-induced IHC Antigen Retrieval Solution (Invitrogen, Thermo Fisher Scientific) at low pH, as described elsewhere35,36. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide. Non-specific binding was blocked by incubating the slides for 1 h with 20% normal goat serum (Vector Laboratories) in BLOTTO. Sections were incubated with different monoclonal primary antibodies overnight at 4oC: anti-CD4 (Invitrogen MA5-44519, rabbit), anti-CD8 (Invitrogen MA5-44531, rabbit), anti-CD68 (Cell signaling 97778S, rabbit). After washing, goat anti-rabbit IgG HRP-conjugated secondary antibody (Invitrogen 31460) was applied, and samples were further incubated for 1 h at room temperature. To detect biotinylated targets, sections were incubated with Vectastain avidin–biotin complex (ABC KIT, Vector Laboratories). Finally, the slides were incubated for 3 min with DAB (Sigma-Aldrich, CAS-No. 868282-85-9) substrate for color development. To visualize nuclei, sections were counterstained with Hematoxylin 2 (7231, Epredia). Sections were then dehydrated and mounted using Permount mounting medium (Fisher Scientific), and a coverslip was placed on the slides.
Imaging and analysis: Slides were examined with an Olympus BX50 optical microscope (Olympus-Life Science) equipped with Image-Pro 10 image analysis software (Media Cybernetics). Digital images were captured at a magnification of 4× for quantifying immunoreactivity of splenic CD4+ and CD8 + T cells in white pulp regions. Images were captured under the same exposure conditions; then ImageJ (NIH) was used to quantify positively stained areas. To process, images were converted to binary 8-bit images, three specific areas were chosen at random, and a consistent thresholding was applied to count the immunopositively stained percentage area. A total of 8 biological replicates were evaluated for each treatment group. Images were captured at a magnification of 40× for quantifying CD68+ stain area in the red and white pulp, following the same method described above.
Statistical analysis: One-way Analysis of Variance (ANOVA) models were performed to estimate and compare the mean percentages of specific cells across the four groups. Levene’s tests were conducted to access the homogeneity of variances for one-way ANOVA models. Following a conservative approach, if a Levene’s test yielded a p-value less than 0.1, ANOVA assuming unequal variances was performed. Given the small sample size in experimental studies, we are more concerned about insufficient statistical power to detect true difference than minimizing Type I errors, thus post-hoc tests with p-value adjustments were not performed. The SAS Proc Mixed procedure, capable of accommodating both equal and unequal variance assumptions, were used to fit the data. Raw percentage values were analyzed, as visual inspections did not indicate notable deviations from the normality assumption required for ANOVA. All analyses were conducted separately for male and female mice due to the sex-specific nature of the experiments. For the analysis of mouse body weight changes across the three training sessions, a mixed effect model accounting for the correlation of the repeated measure within the same mice was applied.
Data availability
No datasets were generated or analysed during the current study.
References
Stowe, R. P., Mehta, S. K., Ferrando, A. A., Feeback, D. L. & Pierson, D. L. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat., space, Environ. Med. 72, 884–891 (2001).
Crucian, B. et al. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 33, 456–465 (2013).
Krieger, S. S. et al. Alterations in Saliva and Plasma Cytokine Concentrations During Long-Duration Spaceflight. Front. Immunol. 12, 725748 (2021).
Furukawa, S. et al. Space radiation biology for “Living in Space”. BioMed. Res. Int. 2020, 4703286 (2020).
Zeitlin, C. et al. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Sci. (N. Y., N. Y.) 340, 1080–1084 (2013).
Cucinotta, F. A. Review of NASA approach to space radiation risk assessments for Mars exploration. Health Phys. 108, 131–142 (2015).
Felix, K. et al. Altered cytokine expression in tissues of mice subjected to simulated microgravity. Mol. Cell. Biochem. 266, 79–85 (2004).
Aviles, H., Belay, T., Vance, M. & Sonnenfeld, G. Effects of space flight conditions on the function of the immune system and catecholamine production simulated in a rodent model of hindlimb unloading. Neuroimmunomodulation 12, 173–181 (2005).
Calcagno, G., Jeandel, J., Frippiat, J.-P. & Kaminski, S. Simulated microgravity disrupts nuclear factor ?B signaling and impairs murine dendritic cell phenotype and function. Int. J. Mol. Sci. 24, 1720 (2023).
Gridley, D. S. et al. Space-relevant radiation modifies cytokine profiles, signaling proteins and Foxp3+ T cells. Int. J. Radiat. Biol. 89, 26–35 (2013).
Rizvi, A., Pecaut, M. J., Slater, J. M., Subramaniam, S. & Gridley, D. S. Low-dose γ-rays modify CD4( + ) T cell signalling response to simulated solar particle event protons in a mouse model. Int. J. Radiat. Biol. 87, 24–35 (2011).
Rizvi, A., Pecaut, M. J. & Gridley, D. S. Low-dose gamma-rays and simulated solar particle event protons modify splenocyte gene and cytokine expression patterns. J. Radiat. Res. 52, 701–711 (2011).
Sadhukhan, R. et al. Simultaneous exposure to chronic irradiation and simulated microgravity differentially alters immune cell phenotype in mouse thymus and spleen. Life Sci. space Res. 28, 66–73 (2021).
Gridley, D. S. et al. Changes in mouse thymus and spleen after return from the STS-135 mission in space. PloS one 8, e75097 (2013).
Han, Y., Shi, S., Liu, S. & Gu, X. Effects of spaceflight on the spleen and thymus of mice: Gene pathway analysis and immune infiltration analysis. Math. Biosci. Eng. : MBE 20, 8531–8545 (2023).
Grove, D. S., Pishak, S. A. & Mastro, A. M. The effect of a 10-day space flight on the function, phenotype, and adhesion molecule expression of splenocytes and lymph node lymphocytes. Exp. cell Res. 219, 102–109 (1995).
Hwang, S.-A., Crucian, B., Sams, C. & Actor, J. K. Post-spaceflight (STS-135) mouse splenocytes demonstrate altered activation properties and surface molecule expression. PloS one 10, e0124380 (2015).
Horie, K. et al. Down-regulation of GATA1-dependent erythrocyte-related genes in the spleens of mice exposed to a space travel. Sci. Rep. 9, 7654 (2019).
Pecaut, M. J., Simske, S. J. & Fleshner, M. Spaceflight induces changes in splenocyte subpopulations: effectiveness of ground-based models. Am. J. Physiol. Regulatory Integr. Comp. Physiol. 279, R2072–R2078 (2000).
Ichiki, A. T. et al. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J. Leukoc. Biol. 60, 37–43 (1996).
Tascher, G. et al. Analysis of femurs from mice embarked on board BION-M1 biosatellite reveals a decrease in immune cell development, including B cells, after 1 wk of recovery on Earth. FASEB J. Publ. Federation Am. Societies Exp. Biol. 33, 3772–3783 (2019).
Shi, L. et al. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 18, 1489–1502 (2021).
Dai, S. et al. Effect of simulated microgravity conditions of hindlimb unloading on mice hematopoietic and mesenchymal stromal cells. Cell Biol. Int. 44, 2243–2252 (2020).
Meehan, R. T. et al. Alteration in human mononuclear leucocytes following space flight. Immunology 76, 491–497 (1992).
Buchheim, J.-I. et al. Stress related shift toward inflammaging in cosmonauts after long-duration space flight. Front. Physiol. 10, 85 (2019).
Garrett-Bakelman, F. E. et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science (New York, N.Y.) 364; https://doi.org/10.1126/science.aau8650 (2019).
Nash, P. V., Bour, B. A. & Mastro, A. M. Effect of hindlimb suspension simulation of microgravity on in vitro immunological responses. Exp. Cell Res. 195, 353–360 (1991).
Crucian, B. E., Cubbage, M. L. & Sams, C. F. Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. J. Interferon Cytokine Res. J. Int. Soc. Interferon Cytokine Res. 20, 547–556 (2000).
Crucian, B. et al. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 1, 15013 (2015).
Gridley, D. S. et al. Spaceflight effects on T lymphocyte distribution, function and gene expression. J. Appl. Physiol. (Bethesda, Md. : 1985) 106, 194–202 (2009).
Rai, B., Kaur, J. & Foing, B. H. Wound healing and mucosal immunity during short Mars analog environment mission: salivary biomarkers and its clinical implications. Eurasia J. Med. 44, 63–67 (2012).
Duarte-Rey, C., Bogdanos, D. P., Leung, P. S. C., Anaya, J.-M. & Gershwin, M. E. IgM predominance in autoimmune disease: Genetics and gender. Autoimmun. Rev. 11, A404–A412 (2012).
Gaignier, F. et al. A model of chronic exposure to unpredictable mild socio-environmental stressors replicates some spaceflight-induced immunological changes. Front. Physiol. 9, 514 (2018).
Papagiannis, P. et al. Radiation transmission data for radionuclides and materials relevant to brachytherapy facility shielding. Med. Phys. 35, 4898–4906 (2008).
Moon, Y., Park, G., Han, K., Kang, C.-S. & Lee, W. Mouse spleen tissue as a staining intensity reference for immunohistochemistry. Ann. Clin. Lab. Sci. 38, 215–220 (2008).
Rehg, J. E., Bush, D. & Ward, J. M. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol. Pathol. 40, 345–374 (2012).
Acknowledgements
This work was funded by a NASA-EPSCoR under grant number 80NSSC21M0323 (RP) and partially supported by a collaborative research grant from the National Institute of Allergy and Infectious Diseases (NIAID) under grant number 5U01AI170039 (RP); the National Institute of General Medical Sciences (NIGMS) under grant number P20 GM109005 (RP). We would like to thank Keith Kunugi and Autumn Rasmussen of the University of Wisconsin Department of Medical Physics for radiation dosimetry. Editorial assistance was provided by the Science Communication Group at the University of Arkansas for Medical Sciences.
Author information
Authors and Affiliations
Contributions
E.N.P. performed chronic irradiation and simulated microgravity experiments, collected tissues, processed tissues, performed functional assay and immunohistochemistry, compiled data, and wrote the material method section partly. B.N. performed flow cytometry analysis and wrote materials and methods for flow cytometry analysis. E.K.L., C.G., and H.C. participated in experiments and collected tissues. M.B. and I.K. contributed to design the study, dosimetry, and revise the manuscript. R.P. contributed to study design, performed and supervised research, analyzed results, wrote the manuscript, and critically revised the manuscript. R.D. contributed to statistical analysis and critically revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pineda, E.N., Nounamo, B., Du, R. et al. Sex-specific immune alterations in mice following long-term simulated microgravity and chronic irradiation. npj Microgravity 11, 24 (2025). https://doi.org/10.1038/s41526-025-00480-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-025-00480-1