Abstract
Spaceflight-Associated Neuro-Ocular Syndrome (SANS) affects astronaut vision, causing refraction and acuity changes during and after long-duration missions. As space agencies plan for extended exploration, real-time SANS detection is crucial. A systematic review of PubMed and EMBASE yielded 72 relevant studies out of 196 screened. Current measurement techniques, including invasive and noninvasive intracranial pressure (ICP) assessments and novel technologies, have limitations in quantifying SANS changes and lack remote monitoring capability. Emerging tools such as telemetric ICP monitoring, optical coherence tomography (OCT), and ultrasound show promise but require further validation. Given the constraints of invasive techniques in space, non-invasive technologies should be tested in terrestrial analogs before spaceflight implementation. The precise SANS etiology will determine optimal monitoring approaches, whether ICP-focused, ocular-based, or a combination of both. No single technology can independently track SANS progression, emphasizing the need for multiparametric integration and post hoc analysis to enhance in-flight monitoring and mitigation strategies.
Similar content being viewed by others
Introduction
Health concerns associated with long-duration spaceflight (LDSF) include bone and muscle degradation, cephalad fluid shifts, changes in cardiac morphology, post-flight orthostatic intolerance, immunological suppression, and psychological challenges1,2,3,4,5,6,7. Countermeasures have been developed for many of these challenges, including rigorous exercise schedules for astronauts on board the International Space Station (ISS), routine psychological screening, and pre-flight quarantining to avoid crew exposure to unnecessary pathogens prior to orbital flight5,6,8. However, one area of focus for which effective countermeasures have not yet been developed is that of Spaceflight-Associated Neuro-Ocular Syndrome (SANS). SANS is widely theorized to be due to abnormal fluid shifts in the brain leading to in- and post-flight visual changes in astronauts. The key clinical and imaging findings of SANS were first described by Mader et al. (2011) who characterized a population of astronauts after LDSF on the ISS with the clinical findings of optic disc edema, globe flattening (GF), choroidal and retinal folds, retinal nerve fiber layer thickening on optical coherence tomography and hyperopic shifts resulting in decreased near vision. About half of them also had clinical findings of isolated retinal nerve fiber layer infarcts, known as cotton wool spots. Lumbar puncture demonstrated elevated opening pressures in two astronauts following return to Earth and both distance and near visual acuity changes were documented, however, such changes were found to resolve over time (months to years)9. In another case, Mader et al. report globe flattening and hyperopic shift in refraction persistent for over 7 years after long duration space flight, suggesting the possibility of scleral remodeling and long-term anatomic changes10. Such permanent anatomic changes stress the need to anticipate long-term nonreversible visual changes that might impact future space missions beyond low-Earth orbit as well as permanent habitation on other planets.While many articles in recent years have proposed and tested possible etiologies of SANS, this review focuses on new and existing technologies to monitor SANS in astronauts, an area which has not been as thoroughly explored, and that is of critical importance. Without accurate monitoring, we cannot detect, study, or treat SANS effectively.
Although impact to the ISS missions cannot be claimed, the deficits caused by SANS already affect ISS operations: many astronauts experience hyperopia during LDSF, with documentation of use of hypermetropic corrective glasses to maintain functional visual acuity. The full clinical trajectory of SANS is still unclear due to a lack of long-duration data and small sample size. Given the limited number of people with SANS, it is not yet known whether this condition plateaus over time with new homeostatic set points or continues to worsen indefinitely. It is also unclear what factors determine the severity of SANS. These factors are not only relevant for the future of ISS missions, but also exploration-class missions as humans look to travel further from Earth. Missions to Mars will vary in duration, with a round trip suggested to take at least 245 days using on-orbit staging, there exists a distinct possibility that astronauts could be affected by SANS symptoms prior to their arrival on Mars.
Fortunately, alternate imaging modalities have facilitated further understanding of the underlying pathophysiology of SANS. Optical coherence tomography (OCT) imaging, both on ISS and post-space flight has shown choroidal expansion thought to at least partially account for the hyperopic shift and choroidal folds11. Similarly, OCT-Angiography (OCT-A) and enhanced depth imaging may be promising tools in quantitatively characterizing the effect of head-down tilt and transient microgravity on choroidal expansion and choroidal drainage12,13,14. OCT-Anterior Segment and ultrasound biomicroscopy have been proposed as tools to monitor the front of the eye for SANS related changes15.
Given the potential negative mission impacts of SANS, early detection and accurate in-flight monitoring is desired. If treatment can be developed, precise monitoring will also be needed to assess treatment response. Diagnosing SANS with an ophthalmologist-performed eye exam in a terrestrial clinic will not be possible in LDSF, and new methods are needed. In this literature review, we evaluate current technology available for monitoring SANS during ascent, in-flight, descent, and postflight. By additionally reviewing terrestrial methods and focusing on those that could be easily translatable to the spaceflight environment, we hope to shine light on the potential for multiparametric assessments to better characterize the life cycle of SANS at an individual level from preflight baseline to post flight resolution or plateau.
Results
Invasive ICP measurement
While the pathophysiology of SANS is still debated, monitoring technologies have historically focused on ICP. In conditions with elevated ICP such as traumatic brain injury, ICP is often measured invasively with intraventricular monitoring, through a burr hole in the skull16. This method provides benefits of accurate intraventricular monitoring, access to administer therapeutic agents, and the potential for CSF drainage for symptom relief. However, this methodology is invasive, with significant potential complications of hemorrhage and infection17,18,19. In addition, kinks, bubbles, clots, and tissue debris may detract from accuracy of ICP measurements and require repeat surgery20,21.
There are also telemetric ICP monitors which allow wireless measurement with the same accuracy as wired ICP monitors. There are two main systems, Neurovent p-Tel and Miethke Sensor Reservoir, both of which may be read by external hand-held equipment with slightly different system placements22. Telemetric ICP monitors benefit from both high accuracy with low drift (demonstrated to retain accuracy with low drift of 2.5 mmHg over a median 241 day implantation period), and safety and efficacy in situ beyond 3 months and up to 5 years of placement23,24,25. They are increasingly utilized in IIH with the only adverse event reported currently as local skin infection after placement26. Telemetric ICP monitors have demonstrated utility in a variety of settings, both in diagnostic decision making (potential efficacy of neurosurgical shunt placement) and in accurate monitoring with clear quantifiable correlation with ICP for up to 5 years22.
While highly accurate, invasive methods of measurement of ICP are unlikely to be suitable for use in spaceflight. Invasive procedures in microgravity have not yet been performed in humans, and in-dwelling catheters pose risk of infection27. Therefore, recent developments in non-invasive ICP monitoring technologies may be more applicable. The ideal non-invasive monitoring technology are easy availability, low cost, accuracy, simplicity, convenience, and minimal risk. In the space environment, especially on Mars missions, the procedure will need to be performed independent of real-time ground support due to a lack of synchronous communication. Currently, visual health on board ISS is tracked with visual acuity, Amsler grid, and self-reported survey, although the case has been made to expand these tools using wearable headsets28,29.
Non-invasive assessment – ultrasound
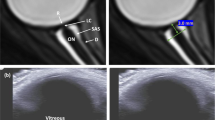
Ultrasound (US) measurement of optic nerve sheath diameter (ONSD) is a promising indirect measure of ICP and a correlate for fluid shifts potentially contributing to SANS. This technique is based on the anatomic flow of CSF fluid from the intracranial cavity to the subarachnoid space between the optic nerve and sheath surrounding the optic nerve30. This measurement is obtained by aligning the linear transducer probe (13–7.5 MHz) over a closed eyelid or directly over the globe with the optic nerve sheath appearing as a hypodense area behind the globe of the eye where the posterior pole of the eye ends (3 mm depth). ONSD has been shown to correlate with elevated ICP (ONSD > 5 mm associated with intracranial hypertension)31,32. However, while it is recognized as a screening tool for detection of moderate ICP changes, it cannot compete with the sensitivity and specificity of invasive monitoring devices, with less reliability for smaller or even moderate changes in ICP17. A paper by Kramer et al. studied MRI scans of 27 astronauts taken 100 days post flight, finding a mean ONSD of 6.2 mm33. Terrestrially, it has been found that ONSD > 5.82 mm showed a 90% probability of underlying intracranial hypertension34. ONSD measurement with ultrasound has the advantage of a short investigation time, is easily reproducible, with capabilities already in-flight. Intra- and inter-observer variability can be minimized with technique training31. In addition, regardless of ICP correlation, ultrasound ONSD measurement may demonstrate increased CSF volume in the optic nerve sheath, a key clinical feature of SANS35. Ultrasound ONSD may also objectively document possible asymmetry in optic nerve sheath pressure. It may also help elucidate the possibility of localized changes occurring at the level of the intraorbital optic nerve (with or without a rise in ICP), which may lead to a type of optic nerve compartment syndrome9. However, it is important to note that the methodology for studying ONSD has varied with older studies reporting absolute values derived from various patient populations, which may or may not be applicable to the present or future of SANS management. Therefore, ONSD analysis may benefit from interpretation as a trend rather than absolute value as ONSD has not been demonstrated to have predictive power in predicting ICP in this or a closely similar cohort.
Fundoscopy is a cornerstone of ophthalmic evaluation and allows for the identification of some signs of SANS. Optic disc swelling, seen as a blurring of the boundary of the optic disc on fundoscopy, is known to be a sign of increased ICP. However, the magnitude of optic disc swelling, as apparent on clinical examination or on two-dimensional imaging does not always correlate with ICP; it is well known that the optic disc swelling may persist for 6 or more weeks once the ICP settles. In addition, optic disc swelling can be milder in SANS than terrestrial cases of increased ICP, and a blinded study of ophthalmologists reviewing SANS fundoscopy images from ISS demonstrated insufficiency of fundoscopy, as well as the Frisen scale used to grade opic disc swelling, to consistently recognize subtle changes in early SANS36. Optic nerve sheath diameter can detect relatively increased or decreased intracranial pressure (ICP) but cannot quantify changes in ICP as can be done with OCT37,38,39. Additionally, ultrasound measures of optic nerve sheath diameter have been found to be user dependent unlike OCT imaging, which is objective and rapid.
Transcranial Doppler Ultrasonography (TDU) uses ultrasound to measure velocity of bloodflow in the middle cerebral artery. In neurocritical care patients, a significant correlation (r = 0.938) has been established between pulsatility index and ICP, however it becomes less precise as ICP increases over 30 mmHg40. Further research has shown excellent specificity of TDU for ruling in elevated ICP (96.3%) but further work needs to be done before TDU can be used to reliably quantify ICP41. These factors may limit its practical application in spaceflight, as ICP in astronauts during LDSF is theorized to be elevated over 30 mmHg. TDU has been used to detect changes in cerebral blood flow velocity in space albeit with unclear correlations to SANS42. Ultrasound acoustoelasticity of the brain is also on the horizon to predict ICP, and potentially fluid shifts contributing to SANS, however, as of now has only been validated in experimental models43. If validated in clinical models, this would prove beneficial for continuous monitoring of ICP, with the hope for more longitudinal correlates for SANS. Ultrasound has the added utility of being an excellent diagnostic tool for the detection of other space-flight associated health conditions such as internal jugular vein thrombosis which is also associated with increased ICP44,45,46,47,48.
Similarly, venous ophthalmodynamometry (measurement of the central retinal vein pressure) may be promising as a correlate for SANS. It has been primarily studied a surrogate of ICP for static measurements, however, this method is limited in its ability to allow for continuous monitoring49. This method has a likelihood of predicting raised ICP of 84.2% patients and 92.8% of patients with normal central retinal vein pressure have normal ICP, further supporting its potential50. That said, it still falls below the sensitivity and specificity of invasive ICP monitoring techniques, and does not clearly translate to quantifiable ICP changes. However, as a measure for venous pressure, it may represent a correlate for progression to SANS.
Many other SANS monitoring techniques derived from ICP have been proposed, for example measurement of tympanic membrane displacement, reflecting stapedius muscle dysfunction in the setting of elevated cochlear hydrostatic pressure51. This has been demonstrated to be a quality screening tool utilized in initial assessment and continued monitoring of patients with increased ICP, however, it may be considered more of a binary commentary on whether or not there is elevated ICP rather than specific quantification of reliable ICP values51. Similarly, distortion-product oto-acoustic emissions have been tested as a non-invasive ICP monitor specifically to apply to SANS, given the continuity of CSF with the perilymphatic space. Such studies have been promising, demonstrating correlation with invasive methods of ICP monitoring52,53,54,55,56.
Tissue resonance analysis (TRA), a novel ultrasound technique leveraging differential tissue specific ultrasound resonance of the brain to digitally obtain an echopulsogram is another promising method for ICP measurement which has not yet been studied in SANS57. Tonometry (measurement of intraocular pressure (IOP)) has also been studied as a correlate of ICP, however, the literature demonstrates that it is not a reliable method of non-invasive ICP monitoring as it only moderately correlates among severely elevated ICPs making it less helpful as a diagnostic tool in more nuanced settings58. Given tonometry’s availability on ISS, it may prove helpful in gathering additional complementary measurements in relation to SANS.
Similarly, clinical neurology-based techniques, such as electroencephalogram (EEG) measurements, have also been explored as a method of predicting ICP on the basis of changes in the cerebral metabolic rate of oxygen secondary to dysfunctional intracranial compliance59. This idea is particularly intriguing as neurophysiology changes have been shown to precede clinical changes, so in cases of unclear onset of ICP elevation in relation to functional and anatomic changes (as in SANS), this might be a particularly applicable tool59,60. However, there has not yet been a reliable correlation between EEG and ICP demonstrated in the literature, nor has there been a specific correlation established with the clinical features of SANS.
Near infra-red spectroscopy (NIRS) is a novel technology focused on the unique absorption of light near infrared spectrums in the presence of differing concentrations of oxygen and deoxyhemoglobin. It has been particularly studied in cases of head trauma, where it has demonstrated detectable changes in cerebral blood flow, blood volume, brain tissue oxygenation and ICP, which means it could also be studied for use in SANS28,60,61.
Pupillometry has also provided an interesting area of exploration, primarily validated and tested against ICP62. Pupil shape and response has long been associated with alterations in ICP and concern for imminent brain herniation, however, only more recently has this effect been quantified. In particular, Taylor et al. developed a pupillometer which has been able to measure velocity and extent of pupillary constriction, associating pupillary constriction velocity as being particularly sensitive to high ICP and 10% decrease in pupil size to be associated with ICP (>20 mmHg)63,64. Follow up studies demonstrated an inverse association between ICP and pupillary reactivity, however, they have not established a direct correlation to actual ICP values yet65. Commercial off the shelf extended reality headsets have been shown to be an accurate way to obtain detailed pupillometry data without bulky medical equipment which may be unsuited for spaceflight66. Similarly, visual evoked responses (VER) have been associated with variation in ICP, and an alteration in the latency of the N2 wave (normally found at 70 ms and considered to be representative of cortical activity) is associated with intracranial pressure elevation as well67. However, similar to pupillometry, the extent of this relationship has not been clearly delineated, and has not been explored as a correlate to SANS.
Radiologic techniques have also been considered as methods of detecting elevated ICP, fluid shifts, and anatomical changes associated with SANS. Computerized Tomography (CT) may be differentiated on the basis of loss of gray and white matter differentiation, midline shift, and basal cistern and ventricular enhancement, however, CT has not been demonstrated to be sensitive enough at this time68. Novel ICP methods in magnetic resonance imaging (MRI) measure the pulsatile differences in intracranial volume and pressure to predict a mean ICP. Surprisingly, while MRI of astronauts commonly show cerebral perivascular space expansion post-flight, analog studies in head-down bedrest have suggested that this is protective against SANS and it may be those with fewer MRI findings who are susceptible to SANS69. While brain imaging is helpful in mapping anatomy and measuring the optic nerve sheath, it is also subject to technique variation and does not capture dynamic changes70.
Non-invasive assessment – OCT
All of these described methods show promise in being able to identify changes in ICP, but are limited in utility as most have not been successfully used to study SANS. The most promising method for indirect assessment is OCT imaging of the optic nerve (specifically nerve head central thickness), as it has been demonstrated to correlate with quantifiable changes in ICP and anatomic parameters that demonstrate fluid shifts in the eye39. For instance, choroidal folds (Fig. 1) have been demonstrated in a higher proportion of eyes of astronauts after LDSF with changes in retinal nerve fiber layer thickness and total retinal thickness similar to that seen in the milder spectrum of terrestrial idiopathic intracranial hypertension (IIH)13,71,72. OCT measures have been demonstrated to correlate well with severity of papilledema according to Frisén papilledema grading13,39,73,74. A more complex association between OCT parameters, papilledema grade, and measured ICP has been demonstrated, and recent studies have demonstrated that OCT may not only have utility in monitoring papilledema, but also in noninvasively prognosticating ICP levels in IIH39,75,76,77. For example, OCT measurements of central thickness as measured on the optic nerve head volume scan have shown that changes in optic nerve head volume central thickness prognosticates changes in intracranial pressure at 12 months and 24 months39. Beyond its unique ability to pick up changes in ICP, terrestrial and in-flight ISS OCT has proved integral in identifying anatomic changes around the optic nerve head (total retinal thickness surrounding the optic nerve head, changes in Bruch’s membrane opening, choroidal thickness and of course choroidal folds)13,33.
Given ocular OCT’s unique ability to quantify anatomic and ICP changes, OCT may be one of the most important modalities in space flight13. In addition, recent advancements have led to more precise choroid tissue images and according to a study by Macias et al., it has been found that there is significant peripapillary choroid thickening during spaceflight78. OCT can also record 10 s videos which detect the presence or absence of spontaneous venous pulsation (SVP). Expert observers grading SVP on the infrared videos combined with a multiple linear regression model confirmed a statistically significant association between SVP and ICP, as measured with telemetric devices79.
Non-ICP related assessment and novel imaging techniques
Monitoring ICP directly or indirectly may be only relevant if ICP plays a role in the underlying cause of SANS. The ability to monitor the medical signs of SANS progression is vital for understanding the deployment of countermeasures. At the fore is utilizing OCT to monitor the features of SANS. In a recent review of SANS and novel techniques, OCT imaging featured prominently due to its ability to provide high quality measures of the optic nerve head, macula and choroid complex, vessels, and lymphatics in a rapid, non-invasive, safe, reliable and reproducible manner13. In particular, OCT may demonstrate SANS associated signs, such as nerve fiber layer thickening, and correlates well with fundoscopy findings without the need for slit-lamp exam13. As fundoscopy has been shown to have limitations in diagnosing early optic disc edema in SANS, OCT is considered a superior modality36. OCT-A technologies provide insight into specific vascular changes occurring during SANS. Furthermore, lymphatics may be assessed with OCT to measure and quantify paravascular spaces thought to be a continuous system between the eye and brain80. Neural networks trained to identify SANS from OCT images was able to successfully detect SANS without operator input in 84% of cases81. Helpfully, both fundoscopy and OCT capabilities are currently available on the ISS, and images are already being sent back to Earth to be analyzed, such as in a recent case report of an astronaut with severe SANS, where in-flight OCT was a primary means of tracking disease development and resolution through optic nerve and macular assessment82.
With the COVID pandemic-related push toward tele-ophthalmology and remote imaging tools in the ophthalmology space, there are many powerful remote handheld imaging devices on the horizon83. Multiple companies including Paxos Scope, D-Eye, Tesseract Health, Zeiss, Eyefficient, Volk, Nidek, Topcon and more have been developing hand-held retinal imaging devices with capacities to obtain images of the fundus, retina, with some even developing methods for improved vascular imaging84. These lightweight, handheld retinal or fundus cameras do not typically require the 20 min of patient dilation time and offer quick and easy dissemination of retinal images through the web from remote eye locations. They are typically rechargeable with battery power and have built in image quality analysis to ensure quality photos. With brief training sessions, exams may be performed quickly without the need for formally trained technologists. Such devices can also leverage machine learning and artificial intelligence (AI) based technologies to allow for automated image processing and analysis which would enable close in-flight monitoring81,85,86,87.
On Earth, even smartphone ophthalmic imaging has become popular, particularly in less-advantaged regions due to the easy portability, ubiquity, low cost, and diagnostic efficacy compared to traditional fundus images88. D-Eye, Peek Retina, Paxos Scope, Fundus on phone, and iExaminer exemplify such technologies with easy smartphone attachments that may mount to Android or iPhone, obtain non-mydriatic fundus images and offer 6 degree fields of view in undilated pupils, capable of delineating the optic nerve and posterior pole84. These platforms also allow for easy transmission of images and AI-based detection of certain biomarkers. While exciting, these imaging techniques and platforms require further validation in humans, and SANS as a disease entity has not yet been tested in these automated handheld imaging devices to determine whether these technologies could truly delineate its pathologies and isolate its retinal and choroidal biomarkers. Furthermore, it is important to consider the limits of 2-d retinal photography which may well-characterized papilledema but may not convey the small quantifiable changes in retinal fiber layer appearance. This however could be distinguished by the fast improving 3-d OCT technologies.
Discussion
While the discussed technologies may be able to evaluate the constellation of signs and symptoms associated with SANS, many of them are not suitable for practical use given the limitations of the spaceflight environment. Tests carried out in space are expensive and require extensive planning, therefore using terrestrial analogs to ascertain suitability prior to launch is paramount.
There is not a single analog which investigates every aspect of a potential methodology, and therefore methodologies should be validated across multiple analogs to ensure their efficacy in LDSF. For example, invasive ICP monitoring methodologies incur a risk of infection, as well as risks posed during surgical insertion89. In addition, testing such invasive ICP and LP measuring technologies poses an additional challenge as these procedures carry inherent risks of complications such as infection, bleeding, or nerve damage, which may be exacerbated by microgravity. Beyond ethical concerns, the translational validity of findings from animals to humans adds complexity and practical challenges, such as performing these invasive procedures in a microgravity setting, further limit their feasibility. While surgery has been performed in mouse models in microgravity, on-orbit human surgery is yet to become a reality, making invasive models of SANS assessment infeasible in the spaceflight environment90. Alternative non-invasive methods may offer safer and more practical solutions for studying and treating elevated ICP and SANS in space.
Much of the literature previously published on SANS hypothesize an associated increase in ICP during spaceflight. This therefore requires any potential ICP monitoring device to remain accurate, even when measuring vastly outside of a normal physiological range. It is therefore paramount during device/method validation that the correlation with gold standard monitoring remains constant even at such higher non-physiologic values. There are still gaps in literature and current understanding about the correlation between any extracranial findings and ICP. Discussion surrounding novel, non-ICP methodologies begs the question about true correlation between these surrogates and SANS changes. Furthermore, many technologies are validated against ICP, however, monitoring in spaceflight may reveal other correlates more sensitive and specific to SANS pathology.
An additional challenge in the monitoring and management of SANS during long-duration space missions is the impact of distance on communication delays. For missions beyond low Earth orbit, such as those to Mars, the signal delay ranges from 3 to 22 min one-way, depending on the relative positions of Earth and Mars. This latency makes real-time assessments and interventions challenging, particularly for conditions that require rapid evaluation, such as changes in intracranial pressure or neuro-ocular health. Furthermore, the potential for complete signal loss during periods of solar conjunction further underscores the limitations of relying on Earth-based support. To address these challenges, future advancements in SANS monitoring must prioritize the development of autonomous, reliable, and non-invasive diagnostic technologies. These systems should be capable of continuously assessing and managing SANS-related parameters without requiring immediate ground-based intervention.
Journeys further from Earth’s surface will incur greater launch costs for materials. This will increase a potential permanent habitat’s need for self-sufficiency beyond being sustained by resupply launches from Earth, as is the status quo aboard the ISS. This high cost associated with launch weight will lead to carefully selected items being launched, including medical supplies. It is therefore paramount that the relevant and necessary medical agents and equipment needed to sustain a habitat are chosen carefully prior to launch. Exacting knowledge on the etiology of SANS, and how to assess and address it, will inform key selection medical payload on future voyages. Further distances traveled also incur a greater time delay in communications; this drives a requirement for closed-loop analysis of results without relying on real-time communication with Earth. Devices and methodologies for SANS care will necessarily include autonomous measurement, analysis and clinical decision support (CDS) components. These can be tested in isolated environments on Earth, which don’t have access to real-time communications. Spaceflight professionals could use the discussed methodologies upon astronauts’ return to Earth, for example investigating ICP or an appropriate surrogate following a hard Soyuz landing to better understand the effects that re-entry can have on SANS. The hand-held devices discussed in this paper may provide immediate information on astronauts prior to air transport to Houston for further post-flight investigations. Any feasible device may have low inter- and intra-user variability. This is also the case with standard terrestrial medical monitoring; any user-dependent method introduces a greater potential for error and possibly decreases standards of care. With limited crews on board the ISS and in future Lunar and Martian missions, it may be possible to train multiple crew members in the techniques discussed to validate methodologies.
The technologies discussed in this review will provide valuable insight into both prevention and treatment of SANS. Early detection and intervention may prevent the changes seen in SANS from ever developing, and with continuous in-flight monitoring, indications for preventative treatment could be seen before clinical symptoms arise is met, preserving astronauts’ eyesight completely. Russian cosmonauts have been known to use lower body negative pressure devices and venoconstrictive thigh cuffs
to attempt to mitigate fluid shifts; the methodologies discussed here would allow dynamic ocular and ICP data to be collected to fully describe the abrupt pressure shifts associated with intermittent use of lower body negative pressure devices, as well as any potential benefit on SANS.
Future research lies in the effect of partial gravity (such as Lunar, 0.16 g or Martian, 0.3 g) on the landscape of SANS. Countermeasures for other microgravity related degenerative conditions have been found to be ineffective in partial gravity, while the deleterious nature of the conditions persists58. An example of this is musculoskeletal breakdown due to gravitational unloading; the SkinSuit, devised by researchers at the European Space Agency in collaboration with King’s College London, was found to simulate loading of 0.4 g when worn in microgravity. However, when this was worn in a simulated 0.16 g, the SkinSuit did not generate any additional loading91. This highlights the fundamental difference in conditions between micro- and partial gravity, and the need for additional investigation into the symptoms of SANS in these conditions. Should the etiology of SANS be found to include the fluid shifts associated with microgravity, its clinical course may well be influenced by partial gravity, further increasing the need for in-situ monitoring on the surface of other celestial bodies. It is assumed that partial gravity will be beneficial in mitigating the effects of SANS, but the magnitude of this impact thus far remains unknown.
Because the true cause of SANS remains unknown, monitoring must be multimodal. Monitoring of ICP and its surrogates (including MRI findings) has long been standard practice for space medicine professionals, however newer methodologies focus on the ophthalmic aspects of the condition. This may allow mission planners in the future to rely on more lightweight portable monitoring devices which allow for closed-loop monitoring of conditions, rather than relying on methods such as MRI which are not available in-flight. Future in-orbit studies can enhance our knowledge of the trajectory of SANS, potentially informing its future treatment. These methodologies, once validated, might also be used to determine long-term sequelae of SANS during post-flight follow-up.
SANS assessment will become more common and important with the advent of commercial space tourism; less rigorous medical selection for commercial spaceflight participants (CSPs) compared to professional astronauts mean a greater incidence of medical comorbidities and risk factors for SANS changes. The knowledge of SANS changes in CSP populations needs to be expanded in order to adequately educate CSPs prior to flight so that they might give informed consent to fly. In addition, while this review focused on diagnostics, future research will consider the challenges of deploying treatments, including exacerbation of increased calcium clearance, kidney stones and cognitive disturbances secondary to acetazolamide treatment for the treatment of ICP.
Many of the methodologies discussed in this paper are applicable to spaceflight, however none are currently fully sufficient to monitor all aspects of SANS. Many current SANS monitoring technologies are validated as correlates for ICP but may not capture as-of-yet unrecognized clinical features of SANS or correlations to other non-ocular pathologies. We believe to be able to assess the progression of this disease from preflight baseline to postflight resolution and plateau, multiparametric methodology with inflight closed loop capabilities will be required to generate actionable insights28. In future, exploration-class missions will require remote monitoring of SANS to introduce interposing countermeasures. We suggest an increased focus on post hoc analysis, AI driven imaging analysis, and synergistic post hoc integration of OCT, OCTA, IOP, and ultrasound. By improving our ability to combine the data from imaging technologies we already have, we believe there will be a greater potential for these monitoring methodologies to be used to introduce these countermeasures before astronauts begin to experience symptoms, effectively acting before the condition has reached a clinical horizon. Intervening early at the sub-clinical stage has the potential to improve clinical outcomes and prevent mission impacts due to SANS-related changes. Even when treatments for SANS emerge, early and accurate monitoring will be required to know when and how to deploy it.
Methods
Three databases, PubMed, EMBASE, and Google Scholar, were searched using the following search terms:
Spaceflight, astronaut, microgravity, technology, neuro-ocular, spaceflight associated neuro-ocular syndrome, and SANS.
Papers published on or before August 6th, 2024, were included. The search returned 196 results. Results were imported in Mendeley (London, UK) citation manager. Abstracts which described methods of monitoring SANS were included. Papers in analog environments were included if monitoring ICP, IOP, or the optic nerve was a focus. Papers not written in English, conference abstracts, or those which did not comment on detection or measurement or assessment of SANS were excluded. Notably, the search returned many reviews on SANS which focused on etiology but not detection. Of the 196 papers reviewed, 72 met inclusion criteria. Additional references from outside the SANS literature were selected individually to provide more background information about the technologies discussed.
Data availability
No datasets were generated or analyzed during the current study.
References
Stavnichuk, M., Mikolajewicz, N., Corlett, T., Morris, M. & Komarova, S. V. A systematic review and meta-analysis of bone loss in space travelers. npj Microgravity 2020 6(1 6), 1–9 (2020).
Fitts, R. H. et al. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 588, 3567–3592 (2010).
Perhonen, M. A. et al. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 91, 645–653 (2001).
Wieling, W., Halliwill, J. R. & Karemaker, J. M. Orthostatic intolerance after space flight. J. Physiol. 538, 1 (2002).
Crucian, B. E. et al. Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Front. Immunol. 9, 1437 (2018).
Deming, C. A. & Vasterling, J. J. Workplace social support and behavioral health prior to long-duration spaceflight. Aerosp. Med. Hum. Perform. 88, 565–573 (2017).
Buckey, J. C., Lan, M., Phillips, S. D., Archambault-Leger, V. & Fellows, A. M. A theory for why the spaceflight-associated neuro-ocular syndrome develops. J. Appl. Physiol. 132, 1201–1203 (2022).
Petersen, N. et al. Exercise in space: the European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem. Physiol. Med. 5, 1–13 (2016).
Mader, T. H. et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 118, 2058–2069 (2011).
Mader, T. H. et al. Persistent globe flattening in astronauts following long-duration spaceflight. Neuroophthalmology 45, 29–35 (2021).
Lee, A. G., Mader, T. H., Gibson, C. R., Brunstetter, T. J. & Tarver, W. J. Space flight-associated neuro-ocular syndrome (SANS). Eye32, 1164–1167 (2018).
Wostyn, P., Killer, H. E. & De Deyn, P. P. Why a one-way ticket to mars may result in a one-way directional glymphatic flow to the eye. J. Neuroophthalmol. 37, 462–463 (2017).
Lee, A. G. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity 6, 7 (2020).
Mader, T. H. et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J. Neuroophthalmol. 37, 133–139 (2017).
Soares, B. et al. Ultrasound biomicroscopy as a novel, potential modality to evaluate anterior segment ophthalmic structures during spaceflight: an analysis of current technology. Diagnostics 14, 639 (2024).
Smith, M. Monitoring intracranial pressure in traumatic brain injury. Anesthesia Analgesia 106, 240 (2008).
Raboel, P. H., Bartek, J., Andresen, M., Bellander, B. M. & Romner, B. Intracranial pressure monitoring: invasive versus non-invasive methods-a review. Crit. Care Res Pr. 2012, 950393 (2012).
Binz, D. D., Toussaint, L. G. & Friedman, J. A. Hemorrhagic complications of ventriculostomy placement: a meta-analysis. Neurocrit Care 10, 253–256 (2009).
Gardner, P. A., Engh, J., Atteberry, D. & Moossy, J. J. Hemorrhage rates after external ventricular drain placement. J. Neurosurg. 110, 1021–1025 (2009).
Hoefnagel, D., Dammers, R., Ter Laak-Poort, M. P. & Avezaat, C. J. J. Risk factors for infections related to external ventricular drainage. Acta Neurochir. (Wien.) 150, 209–214 (2008).
Dasic, D., Hanna, S. J., Bojanic, S. & Kerr, R. S. C. External ventricular drain infection: the effect of a strict protocol on infection rates and a review of the literature. Br. J. Neurosurg. 20, 296–300 (2006).
Mitchell, J. L., Mollan, S. P., Vijay, V. & Sinclair, A. J. Novel advances in monitoring and therapeutic approaches in idiopathic intracranial hypertension. Curr. Opin. Neurol. 32, 422–431 (2019).
Antes, S., Tschan, C. A., Heckelmann, M., Breuskin, D. & Oertel, J. Telemetric intracranial pressure monitoring with the raumedic neurovent P-tel. World Neurosurg. 91, 133–148 (2016).
Norager, N. H., Lilja-Cyron, A., Bjarkam, C. R., Duus, S. & Juhler, M. Telemetry in intracranial pressure monitoring: sensor survival and drift. Acta Neurochir.160, 2137–2144 (2018).
Antes, S., et al. Clinical and radiological findings in long-term intracranial pressure monitoring. Acta Neurochir. (Wien.) 156, 1009–1019 (2014).
Mitchell, J. L., Mollan, S. P., Tsermoulas, G. & Sinclair, A. J. Telemetric monitoring in idiopathic intracranial hypertension demonstrates intracranial pressure in a case with sight-threatening disease. Acta Neurochir.163, 725–731 (2021).
Nag, D. S., Sahu, S., Swain, A. & Kant, S. Intracranial pressure monitoring: gold standard and recent innovations. World J. Clin. Cases 7, 1535–1553 (2019).
Ong, J. et al. Neuro-ophthalmic imaging and visual assessment technology for spaceflight associated neuro-ocular syndrome (SANS). Surv. Ophthalmol. 67, 1443–1466 (2022).
Sampige, R. et al. XR-SANS: a multi-modal framework for analyzing visual changes with extended reality (XR) in Spaceflight Associated Neuro-Ocular Syndrome (SANS). Eye38, 2680–2685 (2024).
Hayreh, S. S. Pathogenesis of oedema of the optic disc (papilloedema). A preliminary report. Br. J. Ophthalmol. 48, 522–543 (1964).
Ballantyne, S. A., O’Neill, G., Hamilton, R. & Hollman, A. S. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur. J. Ultrasound 15, 145–149 (2002).
Kimberly, H. H., Shah, S., Marill, K. & Noble, V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad. Emerg. Med. 15, 201–204 (2008).
Kramer, L. A., Sargsyan, A. E., Hasan, K. M., Polk, J. D. & Hamilton, D. R. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology 263, 819–827 (2012).
Geeraerts, T. et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit. Care 12, R114 (2008).
Paez, M., Mudie, L. I. & Subramanian, P. S. Spaceflight associated neuro-ocular syndrome (SANS): a systematic review and future directions. Eye Brain 12, 105–117 (2020).
Valencia, W. E. et al. Evaluation of optic disc edema in long-duration spaceflight crewmembers using retinal photography. J. Neuro Ophthalmol. 43, 364–369 (2023).
Watanabe, A., Kinouchi, H., Horikoshi, T., Uchida, M. & Ishigame, K. Effect of intracranial pressure on the diameter of the optic nerve sheath. J. Neurosurg. 109, 255–258 (2008).
Hansen, H. C. & Helmke, K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J. Neurosurg. 87, 34–40 (1997).
Vijay, V. et al. Using optical coherence tomography as a surrogate of measurements of intracranial pressure in idiopathic intracranial hypertension. JAMA Ophthalmol. 138, 1264–1271 (2020).
Bellner, J. et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg. Neurol. 62, 45–51 (2004).
Wakerley, B. R. et al. Usefulness of transcranial Doppler-derived cerebral hemodynamic parameters in the noninvasive assessment of intracranial pressure. J. Neuroimaging 25, 111–116 (2015).
Bateman, G. A. & Bateman, A. R. A perspective on spaceflight associated neuro-ocular syndrome causation secondary to elevated venous sinus pressure. npj Microgravity 8, 3 (2022).
Wu, J., He, W., Chen, W.-M. & Zhu, L. Research on simulation and experiment of noninvasive intracranial pressure monitoring based on acoustoelasticity effects. Med Devices6, 123–131 (2013).
Pavela, J. et al. Surveillance for jugular venous thrombosis in astronauts. Vasc. Med. 27, 365–372 (2022).
Auñón-Chancellor, S. M., Pattarini, J. M., Moll, S. & Sargsyan, A. Venous Thrombosis during Spaceflight. N. Engl. J. Med. 382, 89–90 (2020).
Ridha, M. A. et al. Magnetic resonance imaging findings of elevated intracranial pressure in cerebral venous thrombosis versus idiopathic intracranial hypertension with transverse sinus stenosis. Neuro Ophthalmol. 37, 1–6 (2013).
Levasseur, S. et al. Venous thromboembolism in exploration class human spaceflight. Aerosp. Med. Hum. Perform. 95, 45–53 (2024).
Schuchardt, F. et al. Risk factors for the development of secondary intracranial hypertension in acute cerebral venous thrombosis. Neuroradiology 65, 463–477 (2023).
Firsching, R., Schütze, M., Motschmann, M. & Behrens-Baumann, W. Venous opthalmodynamometry: a noninvasive method for assessment of intracranial pressure. J. Neurosurg. 93, 33–36 (2000).
Firsching, R. et al. Noninvasive assessment of intracranial pressure with venous ophthalmodynamometry. Clinical article. J. Neurosurg. 115, 371–374 (2011).
Marchbanks, R. J. Measurement of tympanic membrane displacement arising from aural cardiovascular activity, swallowing, and intra-aural muscle reflex. Acta Otolaryngol. 98, 119–129 (1984).
Voss, S. E. et al. Posture systematically alters ear-canal reflectance and DPOAE properties. Hear Res 263, 43–51 (2010).
Bershad, E. M. et al. Intracranial pressure modulates distortion product otoacoustic emissions: a proof-of-principle study. Neurosurgery 75, 445–454 (2014).
Büki, B. et al. Otoacoustic emissions: a new tool for monitoring intracranial pressure changes through stapes displacements. Hear Res 94, 125–139 (1996).
Williams, M., Voss, S., Horton, N., Malm, J. & Eklund, A. Comparison of invasive ICP measurements to Distortion Product Otoacoustic Emissions (DPOAE) in adults during infusion testing for INPH. Fluids Barriers CNS 12, O60 (2015).
Williams, M. A., Malm, J., Eklund, A., Horton, N. J. & Voss, S. E. Distortion product otoacoustic emissions and intracranial pressure during CSF infusion testing. Aerosp. Med Hum. Perform. 87, 844–851 (2016).
Michaeli, D. Ultrasound apparatus and method for tissue resonance analysis. J. Acoust. Soc. Am. 112, 14 (2002).
Lashutka, M. K., Chandra, A., Murray, H. N., Phillips, G. S. & Hiestand, B. C. The relationship of intraocular pressure to intracranial pressure. Ann. Emerg. Med. 43, 585–591 (2004).
Lescot, T. et al. The relationship of intracranial pressure Lundberg waves to electroencephalograph fluctuations in patients with severe head trauma. Acta Neurochir.147, 125–129 (2005).
Ghosh, A., Elwell, C. & Smith, M. Review article: cerebral near-infrared spectroscopy in adults: a work in progress. Anesth. Analg. 115, 1373–1383 (2012).
Kirkpatrick, P. J., Smielewski, P., Czosnyka, M., Menon, D. K. & Pickard, J. D. Near-infrared spectroscopy use in patients with head injury. J. Neurosurg. 83, 963–970 (1995).
Marshall, L. F., Barba, D., Toole, B. M. & Bowers, S. A. The oval pupil: clinical significance and relationship to intracranial hypertension. J. Neurosurg. 58, 566–568 (1983).
Fountas, K. N. et al. Clinical implications of quantitative infrared pupillometry in neurosurgical patients. Neurocrit Care 5, 55–60 (2006).
Taylor, W. R. et al. Quantitative pupillometry, a new technology: normative data and preliminary observations in patients with acute head injury. Tech. note J. Neurosurg. 98, 205–213 (2003).
Chen, J. W. et al. Pupillary reactivity as an early indicator of increased intracranial pressure: the introduction of the Neurological Pupil index. Surg. Neurol. Int 2, 82 (2011).
Sarker, P. et al. Extended reality quantification of pupil reactivity as a non-invasive assessment for the pathogenesis of spaceflight associated neuro-ocular syndrome: a technology validation study for astronaut health. Life Sci. Space Res. 38, 79–86 (2023).
York, D., Legan, M., Benner, S. & Watts, C. Further studies with a noninvasive method of intracranial pressure estimation. Neurosurgery 14, 456–461 (1984).
Rosenberg, J. B., Shiloh, A. L., Savel, R. H. & Eisen, L. A. Non-invasive methods of estimating intracranial pressure. Neurocrit Care 15, 599–608 (2011).
Richmond, S. B. et al. Dynamic changes in perivascular space morphology predict signs of spaceflight-associated neuro-ocular syndrome in bed rest. npj Microgravity 10, 24 (2024).
Alperin, N., Mazda, M., Lichtor, T. & Lee, S. From cerebrospinal fluid pulsation to noninvasive intracranial compliance and pressure measured by MRI flow studies. CMIR 2, 117–129 (2006).
Patel, N., Pass, A., Mason, S., Gibson, C. R. & Otto, C. Optical coherence tomography analysis of the optic nerve head and surrounding structures in long-duration international space station astronauts. JAMA Ophthalmol. 136, 193–200 (2018).
Sibony, P. A. et al. Ocular deformations in spaceflight-associated neuro-ocular syndrome and idiopathic intracranial hypertension. Investig. Ophthalmo. Vis. Sci. 64, 32 (2023).
Scott, C. J., Kardon, R. H., Lee, A. G., Frisén, L. & Wall, M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch. Ophthalmol. 128, 705–711 (2010).
Frisén, L. Swelling of the optic nerve head: a staging scheme. J. Neurol. Neurosurg. Psychiatry 45, 13–18 (1982).
Patel, M. D., Malhotra, K., Shirazi, Z. & Moss, H. E. Methods for quantifying optic disc volume and peripapillary deflection volume using radial optical coherence tomography scans and association with intracranial pressure. Front Neurol. 10, 798 (2019).
Albrecht, P. et al. Optical coherence tomography for the diagnosis and monitoring of idiopathic intracranial hypertension. J. Neurol. 264, 1370–1380 (2017).
Kaufhold, F. et al. Optic nerve head quantification in idiopathic intracranial hypertension by spectral domain OCT. PLoS One 7, e36965 (2012).
Macias, B. R. et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol. 138, 553–559 (2020).
D’Antona, L. et al. Association of intracranial pressure and spontaneous retinal venous pulsation. JAMA Neurol. 76, 1502–1505 (2019).
Denniston, A. K., Keane, P. A., Aojula, A., Sinclair, A. J. & Mollan, S. P. The ocular glymphatic system and idiopathic intracranial hypertension: author response to ‘hypodense holes and the ocular glymphatic system’. Invest. Ophthalmol. Vis. Sci. 58, 1134–1136 (2017).
Kamran, S. A. et al. SANS-CNN: an automated machine learning technique for spaceflight associated neuro-ocular syndrome with astronaut imaging data. npj Microgravity 10, 40 (2024).
Brunstetter, T. J. et al. Severe spaceflight-associated neuro-ocular syndrome in an astronaut with 2 predisposing factors. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2024.2385 (2024).
Jin, K. et al. Telemedicine screening of retinal diseases with a handheld portable non-mydriatic fundus camera. BMC Ophthalmol. 17, 89 (2017).
Leonard, C. Retinal Imaging on the go. Rev. Ophthalmol. https://www.reviewofophthalmology.com/article/retinal-imaging-on-the-go (2020).
Ong, J. et al. Artificial Intelligence Frameworks to Detect and Investigate the Pathophysiology of Spaceflight Associated Neuro-Ocular Syndrome (SANS). Brain Sci. 13, 1148 (2023).
Kamran, S. A. et al. FA4SANS-GAN: a novel machine learning generative adversarial network to further understand ophthalmic changes in spaceflight associated neuro-ocular syndrome (SANS). Ophthalmol. Sci. 4, 100493 (2024).
Rajalakshmi, R., Subashini, R., Anjana, R. M. & Mohan, V. Automated diabetic retinopathy detection in smartphone-based fundus photography using artificial intelligence. Eye 32, 1138–1144 (2018).
Adam, M. K. et al. Quality and diagnostic utility of mydriatic smartphone photography: the smartphone ophthalmoscopy reliability trial. Ophthalmic Surg. Lasers Imaging Retin. 46, 631–637 (2015).
Chi, H., Chang, K.-Y., Chang, H.-C., Chiu, N.-C. & Huang, F.-Y. Infections associated with indwelling ventriculostomy catheters in a teaching hospital. Int J. Infect. Dis. 14, e216–219 (2010).
Drudi, L., Ball, C. G., Kirkpatrick, A. W., Saary, J. & Grenon, S. M. Surgery in space: where are we at now?. Acta Astronaut 79, 61–66 (2012).
Carvil, P. A., Attias, J., Evetts, S. N., Waldie, J. M. & Green, D. A. The effect of the gravity loading countermeasure skinsuit upon movement and strength. J. Strength Cond. Res. 31, 154–161 (2017).
Acknowledgements
For support of this work we would like to acknowledge the UCSF Department of Orthopaedic Surgery, the UC Space Health Program, and the Translational Research Institute of Space Health through NASA Cooperative Agreement NNX16AO69A.
Author information
Authors and Affiliations
Contributions
E.M., L.C., and B.J. wrote the main manuscript, J.R. helped develop Table 1. A.S., S.M, L.P., J.P., A.J.S. reviewed the manuscript and provided figures and insights about direction of the manuscript and reviewed the main manuscript. A.J.S. served as the senior author who helped with conception of the study, organization of the main manuscript, and review of the main manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The images are non-identifiable and each person has consented to IIH:Life study which has been approved by the NHS National Research Ethics Committee (14/LO/1208).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meer, E.A., Church, L.E., Johnson, B.A. et al. Non invasive monitoring for spaceflight associated neuro ocular syndrome: responding to a need for In flight methodologies. npj Microgravity 11, 61 (2025). https://doi.org/10.1038/s41526-025-00502-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41526-025-00502-y