Abstract
As a stress hormone existing in the human body, cortisol can reflect the psychological stress and health status in daily life, and is a potential biomarker of the body’s stress response. To effectively collect sweat and accurately identify the target, this paper reports a flexible wearable cortisol detection device with outstanding reliability and sensitivity. Molecular imprinted polymer (MIP) ensures cortisol specificity. And carbon nanotubes (CNT) on electrodes increase sensitivity, expanding the detection range to 10−3 to 104 nM, with sensitivity at 189.2 nA/lg(nM). In addition, porous chitosan hydrogel (PCSH) collects sweat effectively, its adhesive properties and 80% swelling rate offer a low-cost alternative to microfluidics. Flexible printed circuit board (FPCB) and serpentine electrode (SE) ensure device durability. This non-invasive, highly sensitive device offers a novel method for mental stress monitoring and clinical diagnosis, advancing human physiological state monitoring.
Similar content being viewed by others
Introduction
Our bodies need to feel stress to adapt to the changing environment. However, prolonged psychological stress can lead to various mental disorders, including depression, anxiety, and post-traumatic stress disorder1,2,3. Excessive stress can also profoundly impact an individual’s health and may result in long-term immune dysfunction. Traditional methods for measuring psychological stress include questionnaires and observation, but these methods are time-consuming and complex, such as the Copenhagen Psychosocial Questionnaire and Anxiety and Depression Scale4,5. In addition, patients may choose to retain private information due to the risk of privacy invasion, leading to misdiagnosis6,7,8. In recent years, cortisol levels have been considered as markers related to psychological stress. With the development of biosensor technology, cortisol sensors have gradually developed9,10,11,12,13,14,15. ELISA is currently the most commonly used method for detecting cortisol levels, but it has disadvantages such as high cost and short storage time16,17,18,19. Therefore, this study proposed a portable psychological stress sensor based on MIP, and improved its sensitivity in application by CNT deposition technology. At the same time, PCSH technology was used to complete the collection of sweat. Therefore, this research proposed a portable mental pressure sensor based on MIP, and used CNT deposition technology to improve the sensitivity of the sensor in the application.
Cortisol, a hormone released as part of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress in humans, is a mature biomarker for assessing stress levels (Fig. 1A)14,20. Specifically, the greater psychological stress leads to the higher cortisol concentration in the body. However, cortisol has a normal range, which provides a framework for assessing abnormal cortisol levels. In plasma, cortisol is 140–630 nM at 8 a.m. and 80–410 nM at 4 p.m. Therefore, it is possible to establish normal range of cortisol concentration for different times throughout a 24 h day21. The analysis of cortisol concentration during these periods can be used to assess psychological stress levels.
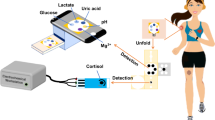
A Illustration of the induction of cortisol secretion by an acute physical stimulus synthesis of the MIP layer for cortisol sensing. B Surface structure of the three-electrode carbon electrode, C deposition of CNT on the printed electrode surface with increased surface attachment duty cycle, D electrochemical deposition of PB, cortisol, and Py on the printed carbon electrode. E The trapped cortisol template is eluted from the polymerized PPy, and the corresponding MIP recognition layer after cortisol elution, in which a cortisol-specific cavity is formed in the electrode; Touch-based palm-based sweat cortisol acquisition sensor, and F the sensor mainly consists of a three-electrode system, SE printed on SEBS substrate, and FPCB. G Illustration of the sensing mechanism in which cortisol diffused from accumulated palm sweat through the hydrogel to the MIP electrode. H Schematic diagram of the chemical structure and chemical formula of MIP.
MIP involves cortisol with pyrrole (Py) to create a highly specific cavity structure, simulating the interaction between natural receptors and ligands13. In contrast to other sensor technologies, the fabrication process of MIP sensors usually does not require complicated synthetic steps or expensive materials, which has the potential for a wide range of applications. MIP is typically prepared by polymerizing Py with cortisol (Fig. 1D), followed by removing the embedded cortisol from Polypyrrole (PPy), leaving behind an imprint structure with specific interactions for cortisol (Fig. 1E). The selectivity of MIP is determined by the interactions between cortisol and the Py (Fig. 1H). The imprint structure enables highly selective recognition of the target molecule. In the electric polymerization, Prussian blue (PB) was introduced in the MIP network as a “built-in” electrochemical signal probe. In Fig. 1E, PB is represented by a blue round ball. The selective binding of cortisol in the imprinted lumen leads to the blocking of the electron transfer pathway during PB oxidation, which results in a decrease in PB oxidation current (Supplementary Figs. 1, 2). The reduced electric current can be used as a signal for the increasing sweat cortisol concentration.
Sweat is one of the most information-rich bodily fluids and holds significance in various medical fields22,23,24. Traditional methods for measuring cortisol levels involve blood and saliva sampling, which presents a risk of biological contamination25,26. Fortunately, cortisol molecules are also present in sweat, so collection of sweat can avoid the risk of biological contamination (Fig. 1A)10. However, the cortisol content in sweat is much lower than that in blood, so to achieve accurate detection of cortisol concentration in sweat, it is necessary to design a highly sensitive electrochemical sensor. The electron transport rate and specific surface area of the electrodes of sweat electrochemical devices are very important to achieve high sensitivity and precision detection of the target, which directly affects the detection resolution and biochemical signal conversion efficiency. To improve the efficiency of cortisol detection in sweat, we deposited CNT on the substrate of MIP to improve the specific surface area (Fig. 1C).
It is an inevitable trend in the development of flexible sweat electrochemical sensors to design a fast and quantitative analysis system that closely integrates sweat collection and electrochemical sensors. The palm of the hand is one of the areas in the human body where sweat is the most abundant due to the high density of sweat glands. Factors such as exposure to a hot environment, anxiety, intense physical activity, emotional excitement, or certain medical conditions often contribute to an increase in sweat production on the palms13. The collection of sweat is a big challenge for sweat sensors. Currently, non-invasive sweat collection devices are mostly microfluidic chips27,28,29,30. The collection of sweat samples through microchannels is affected by time factors, limiting the detection of changes in human sweat composition over time. To address this issue, an approach utilizing PCSH (Supplementary Fig. 3), with an 80% expansion rate (Supplementary Fig. 4), is used for sweat collection and as the medium for chemical monitoring in electrochemical analysis. Collecting sweat by PCSH is more convenient than obtaining blood, urine, or tissue fluid samples, and sweat has minimal biological contamination effects. After absorbing sweat from the skin surface, the PCSH undergoes swelling, enabling sweat to reach the surface of MIP for cortisol recognition (Fig. 1G). Due to the specific recognition capability of the MIP polymer for template molecules, it interacts only with cortisol present in the sweat sample. We assessed the reactivity of MIP polymers with sweat samples, using this as the basis for determining cortisol concentration in sweat. This technique exploits the specific recognition ability of the MIP polymer for cortisol so that it interacts only with the cortisol present in the sweat sample, thereby enabling the measurement of cortisol. Compared with traditional testing methods, this technology is simpler and can quickly monitor the changes in cortisol concentration in humans.
The integrated mobile platform based on MIP is capable of accurate and real-time monitoring of cortisol levels in palm sweat. Smart wearable devices combine modern information technology with sensor technology31. Using wearable devices for monitoring psychological stress plays a crucial role in public healthcare: such devices continuously remind users of their mental state, takes effective measures and promotes a healthier lifestyle by helping prevent diseases resulting from the accumulation of psychological stress32. However, research on biosensors is still in its early stages, facing issues like bulky device sizes, inaccurate detection, and poor portability. Further development and research are required before widespread application in the detection of cortisol concentrations2,9,27. In this context, we utilized the FPCB and the SE (Supplementary Fig. 5) to miniaturize the wearable cortisol device, demonstrating the feasibility of incorporating this circuit into a wearable cortisol device (Fig. 1F).
Results
MIP function test of sedimentary CNT
Potassium ferricyanide and ferric chloride are commonly used as reagents for electrochemical reactions in cyclic voltammetry (CV) experiments. They can be used together with pyrrole for electrochemical deposition to form PPy films or coatings, which are useful electroactive materials13. In the process of PPy deposition, the pyrrole molecules were first reduced to pyrrole anions by applying a negative potential, and then the pyrrole anions were oxidized to PPy at a positive potential and immobilized on the electrode surface. Potassium ferricyanide is often used as an electrolyte in this process to provide ionic conductivity. Ferric chloride is commonly used as a catalyst, which acts as a catalyst in the reduction and oxidation of pyrrole molecules and promotes the growth of PPy. At the reducing potential, the pyrrole molecule accepts electrons and forms a pyrrole anion. At the oxidation potential, pyrrole anions lose electrons and oxidize to PPy. By repeatedly performing cyclic voltammetry scans during the CV experiments, the reduction and oxidation reactions of pyrrole can be controlled, thus gradually deposing PPy films on the electrode surface.
In the preparation process of MIP, cortisol acts as a template molecule bound to Py monomers free in solution, and with the electro-polymerization of Py, cortisol is encapsulated by PPy (Fig. 2B). Subsequently, PPy undergoes further oxidation to disrupt part of the binding bond between cortisol and PPy, resulting in cortisol detachment and formation of cortisol imprinting sites, a process known as “elution” (Fig. 2C). Py as MIP functional monomers and cortisol interact with each other through hydrogen bonding, ionic interaction, van der Waals force and other functional groups13,30. By controlling peroxidation, cortisol can be eluted from the electrode to form MIP, preserving weak cortisol interactions and providing the possibility of further cortisol sensing. The polymerization reaction results in the formation of a specific cavity structure of the functional Py with cortisol. Cortisol molecules were removed from MIP by oxidative elution through CV scanning in PBS solution. In this way, an MIP with template-specific recognition capability was formed. Images of the bare electrode, the PPy deposited electrode, and the electrode after MIP elution were taken under scanning electron microscopy (SEM) (Fig. 2A–C). The SEM showed that the MIP with high selectivity was successfully synthesized, and its surface morphology and pore structure matched the template molecules (Fig. 2B).
In a three-electrode system (Supplementary Fig. 7), between the working electrode (WE), reference electrode (RE), and counter electrode (CE), the current transmission path were independent of each other, and each has its different function (Fig. 2D). The electrochemical reaction occurring at the WE causes current flow, the RE is used to provide a stable potential reference, whereas the CE provides a low resistance path to ensure good current transmission. This configuration makes controlling and measuring current and potential changes in electrochemical experiments possible, thus obtaining information on the kinetics and analysis of electrochemical reactions. The peak value of redox measured by cyclic voltammetry before and after MIP recognition was nearly 19.241 μA smaller after the combination of cortisol than before cortisol binding (Fig. 2F). This suggested that the cortisol molecules were successfully captured by MIP and reduced the redox current of the system.
In order to solve the difficulty of identifying cortisol in sweat, a method of increasing the specific surface area of the electrode with CNT deposition structure was proposed, and at the same time, the high integration of the collection unit and the sensing unit was realized, which provided strong technical support for sweat sensing. CNT coverage of electrode has many important roles, including higher conductivity, increase of specific surface area, promotion of interaction with the analyte and improvement of stability and durability. This makes CNT an essential functional component in electrode materials33. CNT deposits can provide a high specific surface area, which means that they can provide more area for the deposition of MIP and PB, thereby contributing to more reaction sites that can be exposed to the reaction environment. By increasing the surface area, more opportunities can be provided for the target molecule to interact with the reaction site, thereby improving the sensitivity. At the same time, CNT are also excellent conductive materials, and they can effectively promote the electron transport between MIP and PB. They have high chemical stability and mechanical strength which provides a protective layer, preventing corrosion or damage on the surface of the electrode.
In the Randles–Sevcik equation, the peak current can be expressed as follows:
Where F is the Faraday constant, n is the number of electrons transferred during the redox process, A is the surface area of the electrode, C is the concentration of ions involved in the reaction, D is the diffusion coefficient, v is the scan rate set by cyclic voltammetry, R is the gas constant, and T is the temperature. In the same \({{Fe}}^{2+}/{{Fe}}^{3+}\) system reaction environment, Eq. (1) can be regarded as proportional to the peak current \({I}_{p}\) and the electrode surface area A:
Where \({C}_{n}\) is the constant of proportionality. In the modified electrode, CNT is doped, which increases the specific surface area of the electrode and thus improves the detection accuracy of cortisol concentration. The surface area of the bare electrode is denoted as \({A}_{b}\), while the surface area of the CNT electrode is denoted as \({A}_{{CNT}}\), therefore, the area ratio of the two can be expressed as:
Where, \({I}_{{pb}}\,\approx\, 35\) μA and \({I}_{{pCNT}}\,\approx\, 70\) μA, this indicates that the specific surface area of the CNT electrode is twice that of the bare electrode. At the same time, the current values for the recognition of the cortisol concentration upper and lower limits by the bare electrode are 16–700 nA (Fig. 3B), while the corresponding currents for the CNT-deposited electrode are 270–1963 nA (Fig. 3C). By comparing the data, it can also be concluded that the CNT-deposited electrode has a higher sensitivity for the recognition of cortisol. The higher surface area allows for more reactive sites. The use of CNT deposition on the electrode successfully increased the sensitivity of the electrode, providing favorable conditions for the detection of even lower concentrations of cortisol.
A Different concentration under NIP. B Verification of MIP function. C MIP layer gradient with the solution itself as the conductive layer (D) and the fitting curve. E MIP concentration gradient of PCSH conductive layer (F) and the fitting curve. G Repeated experiments in the PCSH-mediated layer proved the superiority of PCSH. H Interference experiments with impurities hardly affect the concentration identification of MIP. I The stability test was carried out after storage at different times.
To verify the specific recognition function of MIP for cortisol, we conducted the following experiments and established a blank control group (NIP). NIP is a PPy substance that does not have a cortisol recognition function, so it cannot detect the presence of cortisol (Fig. 3A). The preparation process of NIP is similar to that of MIP, but the difference is that the polymerization solution of NIP does not contain cortisol molecules, so there are no binding sites for cortisol in NIP. In the experiment, we selected three different concentrations of cortisol (1, 10, and 100 nM) and PBS buffer for the NIP blank group experiment. In the experimental data of Fig. 3A, the current value measured by NIP is between 900 and 1050 nA. Similarly, we also tested MIP and used 10−3 and 103 nM cortisol solutions for validation. The membrane formed by MIP showed a specific recognition function for the cavity, which can convert the biological signal cortisol concentration into an electrical signal, and different concentrations of cortisol correspond to different electrical signal curves. The currents corresponding to the cortisol concentrations of 10−3 and 103 nM are 732 and 103 nA, respectively (Fig. 3B). In summary, the experimental results show that MIP has the recognition function for cortisol and can convert different concentrations of cortisol into electrical signals that are negatively correlated with the concentration. Through these validation experiments, the successful application of MIP technology effectively identified the presence of cortisol.
Gradient experiments with PCSH
The preparation of PCSH is typically carried out under mild conditions. PCSH is a semi-transparent sheet-like solid structure that exhibits a certain level of stickiness and elasticity after being saturated with water, existing in a state between liquid and solid. This characteristic allows it to store moisture from sweat internally, replacing traditional microfluidic channel techniques. After absorption by PCSH, cortisol in sweat equilibrates with sweat outside the PCSH, resulting in a steady state of cortisol concentration in sweat and cortisol concentration in PCSH (Supplementary Fig. 8). By doping with CNT as conductive materials, PCSH can form loop currents under applied voltage conditions, providing a stable conductive medium channel for chronoamperometry. Additionally, during the manufacturing process, the evaporation of glycerol leads to the formation of a porous structure inside the PCSH, giving it porosity, which facilitates the penetration of cortisol to reach the electrode recognition layer while blocking large molecular impurities on the surface.
In the sensor electrode gradient experiment, two sets of experiments were performed to examine the application of PCSH (Supplementary Fig. 9). In the first group, without PCSH, 100 μL of different concentrations of cortisol solution was directly added to the electrode surface for ammeter time method measurement (Fig. 3c). In the second group, 100 μL of different concentrations of cortisol solution was drip-added to PCSH covering the electrode surface for ammeter time method measurement (Fig. 3E). The log of the cortisol concentration and the magnitude of the electrical signal were used to plot the correlation:
Where \({I}_{L}\) is the current magnitude, \({C}_{L}\) is the cortisol concentration, a = 1117.84 ± 14.05, b = −272.05 ± 6.25, coefficient of judgment R2 = 0.997 (Fig. 3d).
PCSH was overlaid on the electrode for testing. An appropriate concentration of cortisol solution was added by dripping, and the test began after PCSH water absorption reached saturation, and the correlation curve between cortisol concentration and electrical signal size was obtained (Fig. 3E, F). To verify the same stability after covering PCSH, we tested the concentration of 100 nM cortisol five times, and the electrical signal was stably distributed around 550 nA (Fig. 3G). So far, it was proved that the addition of PCSH still had the same stability. With the size of the cortisol concentration logarithm and electric signals (Fig. 3E) can be mapped both cover the PCSH correlation curve:
Where \({I}_{P}\) is the current magnitude, \({C}_{P}\) is the cortisol concentration, a = 904.05 ± 12.70, b = −189.20 ± 4.89, coefficient of judgment R2 = 0.995 (Fig. 3F).
The composition of sweat is complex, so it is necessary to explore whether other substances have an effect on MIP, or if PCSH can filter macromolecules, and ultimately whether MIP can identify small molecules and allow passing through PCSH. Interference experiments were performed with urea, uric acid, glucose, ascorbic acid, and lactic acid at concentrations of 20 mM, 20 μM, 100 μM, 100 μM, and 5 mM, respectively, to compare the electrical signal with a cortisol concentration of 100 nM. These concentrations were chosen to reach the levels of these compounds in human sweat. The current of these interference solutions was around 1100 nA compared to the current of the blank group PBS solution. These results indicate that the interference of these small molecules is within an acceptable range and has little effect on the detection of cortisol concentration (Fig. 3H). Then we tested the stability of the electrode over time, in which the electrodes were refrigerated at 5 °C for 4 weeks and tested weekly. As time accumulated, the current of the electrode decreased by about 5 nA per week using the time amperometry method in 102 nM solution, but the overall decrease was negligible. MIP electrode is more stable (Fig. 3I). The research established a correlation curve between cortisol concentration and current magnitude, providing reliable data support for the integration of wearable devices.
Clinical test
During the circadian cycle, cortisol levels typically peak in the morning and then gradually decrease until they reach their nadir in the evening (Fig. 4A). In addition, cortisol is a stress hormone whose levels can change under acute physical stimuli10. Acute physical stimulation includes vigorous exercise, physical labor, physical training, sports competition, etc. These stimuli are often accompanied by physiological changes such as increases in physical activity, heart rate, and metabolism. As stimulated by acute physical activities, cortisol levels tend to show a temporary increase (Fig. 4B)34.
A The variation of cortisol concentration in normal subjects during the day. B Cortisol levels increase significantly after exercise. C The electrodes were tested after the fingers were incubated for 30 s. D–I Five volunteers were selected to measure cortisol levels in their sweat between 7 a.m. and 7 p.m., and two volunteers were asked to observe the effect of exercise on cortisol levels after exercise.
During exercise or the cold pressor test (CPT), cortisol is released by the human body to regulate the body’s metabolism and energy expenditure, as shown in Figs. 4H, 5C, the cortisol content in sweat rises to about 250 nM in response to external stimuli. Cortisol returned to normal level by 2 h after exercise stimulation. The CPT was faster and returned to the normal level 20 min after the CPT was removed. The increase in cortisol induced by such stimulation is transient, and in general, cortisol levels gradually return to baseline levels after the end of the stimulus. In practice, it is very useful to know how a person’s stress changes by detecting cortisol levels at different time points. For example, cortisol testing at different times, such as upon waking up in the morning, during work, and before going to bed at night, can help people better understand their stress levels and trends. In people with high work stress or experiencing major life changes, regular and long-term monitoring of cortisol levels can help to understand the impact of stress on physiological health and provide a scientific basis for intervention measures.
A total of five volunteers were selected, three of whom measured the changes in cortisol during different periods of the day (Supplementary Fig. 10b, d, f), and the other two measured the changes in cortisol after exercise (Supplementary Fig. 10c, e). Experiments involving sweat samples from human volunteers and human volunteers were approved by the University Institutional Review Board of Qingdao University, in which volunteers participated after informed consent. Three randomly selected volunteers were tested for cortisol concentration in fingertip sweat every 2 h from 7 a.m. to 7 p.m., and the mean cortisol concentration in sweat decreased from 350 to 30 nM. Based on the negative correlation between the current and cortisol, human cortisol content continues to drop from 7 a.m. to 7 p.m. At the same time, two additional volunteers were randomly selected to do rope skipping at 4 p.m. and 12 p.m., respectively. Cortisol levels measured 1 h after exercise were 180 nM greater than the average levels of other resting volunteers (Fig. 4D–I), indicating that the cortisol concentration increased after acute physical activities. In conclusion, cortisol concentration in human sweat continued to decrease from 7 a.m. to 7 p.m., with a significant increase in cortisol concentration during a specific period of strenuous skipping exercise (Fig. 4A, B).
Cold stimulation activates the sympathetic nervous system, leading to the release of hormones such as adrenaline and cortisol. The release of these hormones is aimed at facilitating the body’s adaptation to cold environments and maintaining thermal and physiological balance35. In the CPT, the researchers randomly selected three volunteers for an ice water stimulation experiment, to observe the stimulation effect on levels of cortisol in the sweat. At the beginning of the experiment, three volunteers held their left hands in the ice water mixture at 0 °C for 3 min. Ice water stimulation causes slight sweating on the right hand. Cortisol is released as the body reacts to the stimulus. The amount of cortisol secreted in the body can be assessed by measuring the cortisol concentration in the sweat of the right hand (Fig. 5A).
The nerve pulse transmission speed is relatively fast, which is a fast way of information transmission36. When the stimulus of ice water is felt, this stimulus transmits electrical signals to the brain through sensory neurons. Nerve impulses can be quickly passed between neurons, making the body respond immediately. However, the rate of hormonal regulation is relatively slow37. In the ice water stimulation experiment, the stimulation of ice water may trigger a stress response, leading to the release of cortisol. However, due to the relatively slow rate of hormonal regulation, variations of data in cortisol concentrations were not immediately observed. In order to observe the trend of cortisol concentration, we choose certain time intervals, such as sampling every 5 min. With the accumulation of time, it can be observed that the cortisol concentration gradually rises after stimulation and reaches a peak. After a sustained ice water stimulus, cortisol levels gradually decrease and return to normal levels as the body gradually adjusts to the stimulus. Before CPT, the cortisol concentration of the three volunteers was less than 50 nM. When the hand reached the ice water mixture, the cortisol level increased sharply in 10–15 min, and gradually returned to the pre-CPT level after 20 min (Fig. 5B–D). Therefore, the concentration of cortisol in sweat under ice water stimulation presented dynamic changes, rising followed by immediate declining.
Integration and experiment of a portable sensor
To meet personalized mental health needs, we designed a psychological stress wearable device consisting of FPCB and SE38. To better fit the device to the human body, the sensing electrodes are designed with SE, which can provide better scalability and flexibility. The screen-printing technique employed in the SE part was printed on Styrene-ethylene/butene-styrene copolymer (SEBS) via silver electrode mesh and carbon electrode template, respectively (Fig. 6A, B). The SE with recognition function was obtained by functionalizing the SE structure using CNT deposition and MIP techniques (Fig. 6E). SE protects the sensing device from deformation under stretching, water immersion, and wrinkling conditions that may occur in everyday life (Fig. 6C). Even the curled equipment does not affect normal work. In practice, when the device was stretched, no deformation occurred at 15% stretch, and no delamination was observed at 30% stretch. At 60% stretch, there was a small tear at the fluid edge of the paper. However, the sensor current could still conduct normally due to the mechanical isolation of the serpentine design (Supplementary Fig. 11).
The specific circuit of the flexible wearable sensor requires fewer components and the overall complexity of the circuit is not high, which facilitates the realization of the design concept of miniaturization and lightweight (Fig. 6D). ADuCM355 is a microcontroller chip launched by the Analog Devices Inc. It is a member of the ADuCM3xx product family, aimed to design low-power and high-performance biosensors for applications39. The ADuCM355 chip and its circuits were integrated into a 4.5 × 3.56 cm flexible circuit board for portable application (Supplementary Fig. 5). The Bluetooth 4.0 (HC - 08) module, with low-power consumption and real-time transmission, is convenient and mobile. The integrated circuit board adopts two power supply ideas: one is to have a 5 V power supply to the circuit through the USB port, and the other is to connect the external power supply to the pin header to support the circuit. In the experiment, the power supply of the integrated circuit board was supported by a 3.7 V power supply output of 4.2 × 3.0 cm rechargeable polymer lithium battery (403040 model). For data acquisition, the AD conversion unit integrated into the ADuCM355 chip was used to apply an appropriate voltage to the WE pin on the sensor in order to collect the current flowing through the CE pin. Two ways of data transmission could be achieved through the UART serial communication of the ADuCM355 chip: one can be transmitted to the computer through the debugger for real-time display, and the other can be transmitted to the mobile phone through the Bluetooth module for real-time analysis (Supplementary Fig. 6).
We experimentally verified the feasibility of the detection method for flexible wearable sensors. To evaluate the effect of the SEBS substrate on the sensor performance, we performed an experimental comparison. First, in the PET substrate, the redox peaks of CV were 41.1 and 21.8 μA before and after cortisol binding (Fig. 2F). Next, the redox peaks of CV were 47.0 and 8.6 μA before and after cortisol binding to the PET substrate (Fig. 6E). The comparative experimental data showed that CV did not change much and differed before and after cortisol binding, indicating that SEBS substrate could still play an excellent detection performance. The experimental results show that the method has high sensitivity and specificity, and can effectively detect the content of cortisol in sweat. At the same time, sample processing is simple, and the detection process is fast and convenient (Fig. 6F–H). The precision of flexible sweat electrochemical sensors can not only effectively improve the reliability of personalized medical sensors, but also comply with the national demand for active precision medical equipment, especially in the fields of disease diagnosis, health monitoring, and drug detection, which has important scientific research and application value.
Discussion
In this paper, we aim to make an efficient and accurate wearable device for psychological stress by addressing the limitations of traditional cortisol measurement methods, such as tedious processing and low-pass quantification. The results show that the method has high stability, sensitivity, and specificity, and the sample processing is simple. The PCSH and MIP used in the experiments showed excellent performance for cortisol concentration detection. This paper has designed and developed a sweat cortisol concentration detection based on MIP integrated mobile platform. This device can noninvasively monitor and continuously transmit physiological indexes related to health in the human body to the user device. The advantage of the device is that it incorporated MIP technology, providing a novel method for monitoring human body physiologically.
The innovations of this study are mainly reflected in the following aspects: (i) MIP is used to selectively identify cortisol molecules, which is different from ELISA principle, has greatly improved stability and is easier to store; (ii) the deposition of CNT improves the electrical and mechanical properties of the electrode, and the recognition sensitivity of MIP is greatly improved; (iii) Unlike microfluidic conduit technology, the PCSH method collects sweat and serves as a conductive medium, effectively filtering impurities in sweat and stabilizing current analysis. (iv) The use of integrated small wearable devices, unique SE construction, and FPCB meets the needs of everyday life.
Touch-based cortisol sensing has great potential for non-invasive and real-time monitoring of cortisol levels, with potential to be applied in stress management, health monitoring, and medical diagnosis. Continued research and development focusing on improving sensor performance and solving problems in practical implementation are needed for further advance the field.
Methods
Materials
Hydrogel: Chitosan powder (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), glycerol (purchased from Shanghai Aladdin Biochemical Technology Co., LTD.), acetic acid (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), CNT (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.); MIP: Hydrochloric acid (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), potassium ferricyanide (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), potassium chloride (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), Prussian blue (purchased from Shanghai McLean Biochemical Technology Co, LTD.), Cortisol (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.), pyrrole (purchased From Shanghai Aladdin Biochemical Technology Co., LTD.), PBS solution (purchased from Shanghai Aladdin Biochemical Technology Co., LTD.); SEBS: Styrene-ethylene/butene-styrene copolymer (purchased from Taiwan Synthetic Rubber Co., LTD.), toluene (purchased from Merck Group, Germany); Electrode: carbon paste (purchased from Yantai Hennuo New Material Co, LTD.), silver chloride (purchased from Shanghai McLean Biochemical Technology Co, LTD.), PET substrate (purchased from Shanghai Kadeer Chemical Technology Co, LTD.), insulation paste (purchased from Shanghai McLean Biochemical Technology Co, LTD.); Electrode cleaning: sulfuric acid (purchased from Shanghai Aladdin Biochemical Technology Co, LTD.); Cortisol solution: cortisol stock solution and PBS solution diluted to the desired concentration; Experimental instruments :Mtrohm Autolab(purchased from Metrohm), DHG-9145A blast dryer, SC-MS-II desktop magnetic mixer, desktop homogenizer (KW-4BC) (purchased from Beijing Cedcais Electronics Co, LTD.), desktop baking machine (SC-H-I) (purchased from Beijing CedCAis Electronics Co, LTD.).
Preparation of sensing platform
Before printing, the PET substrate was rinsed with deionized water to clean the surface, and dried with nitrogen. The first step was to print the WE. The material of the WE was formed by mixing carbon paste, and dried at 130 °C for 30 min post-printing. The second step was to print the RE. The material was Ag/AgCl paste (Ag and AgCl in a ratio of 3:2), and was dried at 130 °C for 30 min post-printing. The third step was to print the insulating layer, by fixing the insulating ink onto the electrode layer, and leaving the working area of the RE and the WE outside. The insulation layer was solidified at 100 °C for 30 min. Thus, an ordinary screen-printing electrode was created (Supplementary Fig. 7).
Preparation of the porous CS hydrogel
First, chitosan powder and 1% acetic acid were mixed at a mass ratio of 1:30, and then glycerol containing 7% chitosan mass and 5 uL of CNT were added. The mixture was then thoroughly mixed at 60 °C in a magnetic stirrer to 500 RPM at 60 °C for 5 h to completely mix to form colloidal precursors. The mixing process of small bubbles was removed by resting for 24 h. Then 5 mL of the gel was taken and spin-coated to a thick and uniform hydrogel film at 500 r/min for 30 s on a circular glass material with a diameter of 8 cm. Excess water in the gel was removed by heating at 70 °C for 2 h. It was allowed to stand for 24 h until the gel cooled and oxidized to form PCSH. The gel film was partitioned to the desired size and covered the electrode working area (Supplementary Fig. 3).
Synthesis of the MIP
First, the MIP solution is produced using 0.02 M pyrrole, 5 mM ferric chloride, 5 mM potassium ferricyanide, 6 mM cortisol, 1.6 mM Prussian blue, and 0.1 M hydrochloric acid. After the electrodes have undergone ten cycles of washes in 0.5 M sulfuric acid solution (−1 to +1 V voltage range and scan rate 50 mV/s). About 150 uL MIP solution (−1 to +1 V voltage range and scan rate 50 mV/s) were applied to the electrodes drop wise for ten cycles aggregation. After the electrochemical polymerization, the electrodes were washed twice with deionized water to remove the remaining compounds. The embedded cortisol molecules were extracted from the MIP matrix by peroxidation of MIP with CV 20 cycles (50 mV/s) in PBS to produce complementary cavities.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
References
Ramón-Arbués, E. et al. The prevalence of depression, anxiety and stress and their associated factors in college students. Int. J. Environ. Res. Public Health 17, 15 (2020).
Yang, L. F. et al. The effects of psychological stress on depression. Curr. Neuropharmacol. 13, 494–504 (2015).
Kontoangelos, K., Economou, M. & Papageorgiou, C. Mental health effects of COVID-19 pandemia: a review of clinical and psychological traits. Psychiatry Investig. 17, 491–505 (2020).
Giannakakis, G. et al. Review on psychological stress detection using biosignals. IEEE Trans. Affect. Comput. 13, 440–460 (2022).
Szakonyi, B., Vassányi, I., Schumacher, E. & Kósa, I. Efficient methods for acute stress detection using heart rate variability data from ambient assisted living sensors. Biomed. Eng. Online 20, 73 (2021).
Colin, C., Prince, V., Bensoussan, J. L. & Picot, M. C. Music therapy for health workers to reduce stress, mental workload and anxiety: a systematic review. J. Public Health 45, E532–E541 (2023).
Shah, S. M. A., Mohammad, D., Qureshi, M. F. H., Abbas, M. Z. & Aleem, S. Prevalence, psychological responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Ment. Health J. 57, 101–110 (2021).
Myin‐Germeys, I. et al. Experience sampling methodology in mental health research: new insights and technical developments. World Psychiatry 17, 123–132 (2018).
An, J. E. et al. Wearable cortisol aptasensor for simple and rapid real-time monitoring. ACS Sens. 7, 99–108 (2022).
Russell, G. & Lightman, S. The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019).
Torrente-Rodríguez, R. M. et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2, 921–937 (2020).
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, 15 (2022).
Tang, W. et al. Touch‐based stressless cortisol sensing. Adv. Mater. 33, 2008465 (2021).
An, J. E. et al. Wearable cortisol aptasensor for simple and rapid real-time monitoring. ACS Sens. 7, 99–108 (2022).
Tian, G. et al. Oriented antibody-assembled metal–organic frameworks for persistent wearable sweat cortisol detection. Analyt. Chem. 95, 13250–13257 (2023).
Leitão, C. et al. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 29, e00587 (2021).
Villa, J. E. L. et al. SERS-based immunoassay for monitoring cortisol-related disorders. Biosens Bioelectron 165, 112418 (2020).
Saiyudthong, S., Suwannarat, P., Trongwongsa, T. & Srisurapanon, S. J. S. Comparison between ECL and ELISA for the detection of salivary cortisol and determination of the relationship between cortisol in saliva and serum measured by ECL. Sci. Asia 36, 169–171 (2010).
Zhang, Z. et al. Highly sensitive in-situ growth gold dendrite structure electrochemical sensor for early Alzheimer’s disease screening. Chem. Eng. J. https://doi.org/10.1016/j.cej.2024.151644 (2024).
Joseph, J. J. & Golden, S. H. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N.Y. Acad. Sci. 1391, 20–34 (2017).
Mohd Azmi, N. A. S. et al. Cortisol on circadian rhythm and its effect on cardiovascular system. Int. J. Environ. Res. Public Health 18, 676 (2021).
Robinson, S. & Robinson, A. H. Chemical composition of sweat. Physiol. Rev. 34, 202–220 (1954).
Xiao, J., Wang, J., Luo, Y., Xu, T. & Zhang, X. Wearable plasmonic sweat biosensor for acetaminophen drug monitoring. ACS Sens. 8, 1766–1773 (2023).
Xiao, J. et al. An electrochemical wearable sensor for levodopa quantification in sweat based on a metal–organic framework/graphene oxide composite with integrated enzymes. Sens Actuators B Chem. https://doi.org/10.1016/j.snb.2022.131586 (2022).
Imamovic, M. et al. Confounding effects of liquorice, hydrocortisone, and blood contamination on salivary cortisol but not cortisone. Endocr. Connect. https://doi.org/10.1530/ec-22-0324 (2023).
Qiu, Q. et al. The effects of forest therapy on the blood pressure and salivary cortisol levels of urban residents: a meta-analysis. Int. J. Environ. Res. Public Health 20, 458 (2022).
Shajari, S. et al. MicroSweat: a wearable microfluidic patch for noninvasive and reliable sweat collection enables human stress monitoring. Adv. Sci. 10, 16 (2023).
Baker, L. B. et al. Skin-interfaced microfluidic system with personalized sweating rate and sweat chloride analytics for sports science applications. Sci. Adv. 6, 12 (2020).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, 12 (2019).
Son, J. et al. Cactus‐spine‐inspired sweat‐collecting patch for fast and continuous monitoring of sweat. Adv. Mater. 33, 9 (2021).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 6, 1225–1235 (2022).
Iqbal, S. M. A., Mahgoub, I., Du, E., Leavitt, M. A. & Asghar, W. Advances in healthcare wearable devices. npj Flex. Electron. 5, 9 (2021).
Ma, P. C., Tang, B. Z. & Kim, J. K. J. C. Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composites. Carbon 46, 1497–1505 (2008).
Gonzalez-Bono, E., Salvador, A., Serrano, M. A. & Ricarte, J. Testosterone, cortisol, and mood in a sports team competition. Horm. Behav. 35, 55–62 (1999).
Srámek, P., Simecková, M., Janský, L., Savlíková, J. & Vybíral, S. Human physiological responses to immersion into water of different temperatures. Eur. J. Appl. Physiol. 81, 436–442 (2000).
Milenkovic, N., Wetzel, C., Moshourab, R. & Lewin, G. R. Speed and temperature dependences of mechanotransduction in afferent fibers recorded from the mouse saphenous nerve. J. Neurophysiol. 100, 2771–2783 (2008).
Luttbeg, B., Beaty, L. E., Ambardar, M. & Grindstaff, J. L. Mathematical modeling reveals how the speed of endocrine regulation should affect baseline and stress-induced glucocorticoid levels. Horm. Behav. 136, 105059 (2021).
Fu, X., Al-Jumaily, A. M., Ramos, M., Meshkinzar, A. & Huang, X. Stretchable and sensitive sensor based on carbon nanotubes/polymer composite with serpentine shapes via molding technique. J. Biomater. Sci. Polymer Ed. 30, 1227–1241 (2019).
Bembnowicz, P., Brom–Verheyden, G., Boonen, T., Philips, N. Water quality sensors-from transducer technology to environmental application. In IEEE Transactions on Instrumentation and Measurement (IEEE, 2023).
Acknowledgements
This research work was financially supported by the Natural Science Foundation of Shandong Province (No. ZR2022QF120), Qingdao Postdoctoral Fund (No. QDBSH20230101005), and Shandong Province Youth Innovation and Technology Support Plan for Higher Education Institutions (No. 2023KJ362).
Author information
Authors and Affiliations
Contributions
C.W. (Data curation: Equal; Formal analysis: Equal; Investigation: Lead; Methodology: Equal; Software: Equal; Validation: Equal; Visualization: Equal; Writing—original draft: Lead; Writing—review & editing: Equal) Z.W. (Formal analysis: Equal; Investigation: Equal; Methodology: Equal; Software: Lead; Validation: Equal; Visualization: Equal; Writing—original draft: Supporting; Writing—review & editing: Equal) W.W. (Formal analysis: Equal; Software: Equal; Validation: Equal; Visualization: Equal; Writing—review & editing: Equal) Z.Z. (Data curation: Equal; Investigation: Equal; Methodology: Equal; Validation: Equal; Visualization: Equal) A.A.L. (Data curation: Equal; Formal analysis: Equal; Investigation: Supporting; Validation: Equal; Visualization: Supporting; Writing—review & editing: Equal) G.H. (Writing—review & editing: Equal) X.L. (Writing—review & editing: Equal) S.S.G. (Writing—review & editing: Equal) L.Z. (Project administration: Equal; Resources: Supporting; Supervision: Equal; Validation: Equal; Visualization: Supporting; Writing—review & editing: Equal) H.K. (Conceptualization: Lead; Funding acquisition: Lead; Project administration: Equal; Resources: Equal; Supervision: Equal; Validation: Equal; Visualization: Equal; Writing—review & editing: Equal).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
The study was approved by the Medical College of Qingdao University (QDU-HEC-2022144). Written informed consents were obtained from each of the involved individuals. All experiments were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Wang, Z., Wei, W. et al. High-precision flexible sweat self-collection sensor for mental stress evaluation. npj Flex Electron 8, 47 (2024). https://doi.org/10.1038/s41528-024-00333-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-024-00333-z
This article is cited by
-
A quantitative, multimodal wearable bioelectronic device for comprehensive stress assessment and sub-classification
Nature Communications (2026)
-
Flexible and sensitive pressure sensor with enhanced breathability for advanced wearable health monitoring
npj Flexible Electronics (2025)
-
Fiber-based organic electrochemical transistors for re-shaping bioelectronics: integration on and in textiles
npj Flexible Electronics (2025)
-
Laser-induced graphene-based, flexible, and all-inorganic carbon thin-film transistor for non-invasive monitoring of cortisol
Microchimica Acta (2025)