Abstract
Underactive bladder (UAB) patients experience straining to void and typically cannot sense bladder fullness. Previous closed-loop bladder volume control systems are limited in neurogenic UAB patients and face infection risk due to wired connections. Here, we propose an intelligent bladder volume control system (IBCS) combining an implantable meshed magnetic soft robot (MMR) with a wearable magnetic field sensor. The MMR, tightly sutured to the bladder, compresses the bladder to facilitate urination under magnetic actuation, achieving a voiding efficiency of 94.8%. The wearable magnetic field sensor outside the abdomen achieves continuous and wireless monitoring of bladder volume with a 4.8% error in time. The MMR was validated on a UAB pig model, demonstrating a pressure increase of up to 33 cmH2O and voiding efficiency of over 83%. Our IBCS provides a biocompatible solution for wireless and continuous bladder volume management by integrating wearable sensors and magnetic robotics.
Similar content being viewed by others
Introduction
Underactive bladder (UAB) is a common type of bladder dysfunction, in which patients usually suffer from nerve or muscle damage, resulting in a loss of bladder volume sensitivity and a decrease in detrusor contraction strength. This condition prevents UAB patients from perceiving bladder fullness and from completely emptying the bladder within the normal voiding time. Chronic bladder dysfunction leads to a series of complications, including urinary tract infections, urinary retention, and renal impairment, which brings great damage to both the physical and mental health of patients1,2,3.
Current clinical treatments for UAB focus on bladder emptying, yet there is still a lack of effective and widely applicable methods to assist patients with involuntary urination. The micturition reflex involves numerous nerves and muscles, making it particularly challenging to help patients achieve voluntary urination4. The preferred treatment, intermittent catheterization (IC), helps patients empty the bladder but treats the symptoms rather than the root cause. Patients are unable to determine when to urinate but to regularly perform catheterization 4–6 times per day to avoid overfilling, which can easily lead to urinary tract infections and cause great discomfort for patients5,6. Neuromodulation surgeries, such as sacral nerve stimulation, use implanted electrodes to stimulate the sacral nerves to enhance the contraction of the detrusor, assisting certain UAB patients in bladder voiding. However, sacral nerve stimulation is not universally applicable, as patients with neurogenic UAB do not respond to neural stimulation. Additionally, sacral nerve stimulation does not help with bladder volume monitoring, leaving patients at risk of prolonged overfilling, which might lead to bladder and kidney damage7,8. In previous studies, researchers have utilized implantable soft robots to apply direct pressure on the bladder to assist all UAB patients with voiding. However, lacking sensing components, these soft robots cannot achieve bladder volume monitoring9,10,11. To address the issue of lacking sensing components, researchers have developed implantable sensing-actuation systems, where the sensors monitor bladder volume and the actuators perform bladder compression12,13,14. However, current sensing-actuation systems still face challenges in bladder pressure increase (ΔP), wireless monitoring, and passive power supply before they can be truly accepted for clinical treatment. To the best of our knowledge, there is currently no intelligent voiding system that combines wireless bladder volume monitoring with bladder voiding for practical human-size bladder volume control.
Here, we propose an intelligent bladder volume control system (IBCS) composed of an implantable meshed magnetic soft robot (MMR) and a wearable magnetic field sensor. The IBCS is a closed-loop, wireless system designed for continuous monitoring of bladder volume and initiation of bladder voiding when fullness is detected. The magnetic soft robot can increase bladder pressure by 24 to 37 cmH2O, which is within the typical range of pressure required for human urination15,16. The wearable magnetic field sensor achieves continuous monitoring of bladder volume with a sensitivity of 0.9 μT/ml during the storage phase. The IBCS detects bladder filling with a monitoring error of 4.8% and can elevate bladder pressure up to 36.7 cmH2O, achieving a voiding efficiency of 94.8% in in vitro experiments. IBCS increases the bladder pressure up to 33 cmH2O in a UAB pig, resulting in a voiding efficiency exceeding 83%. The IBCS provides a biocompatible, continuous, and wireless solution for controlling bladder volume, demonstrating strong potential for clinical applications.
Results
The intelligent bladder volume control system (IBCS)
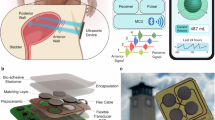
The IBCS consists of a mesh-structured magnetic soft robot (Fig. 1a) and a wearable flexible magnetic field sensor (Fig. 1b). Figure 1c illustrates the working principle of the system. The meshed magnetic soft robot, tightly sutured to the bladder, emits a variable magnetic flux density corresponding to bladder deformation as volume changes. The wearable flexible magnetic field sensor, adhered outside to the patient’s abdomen, measures bladder volume in real-time through a fitted linear relationship, with a sensitivity close to 0.9 μT/ml (Fig. 1d). When the magnetic field sensor monitors bladder filling, it alerts patients to approach an external magnetic field actuation which activates the meshed magnetic soft robot that directly compresses the bladder to facilitate rapid and complete urination.
a The meshed magnetic soft robot (MMR). b The wearable magnetic field sensor. c Schematic of the intelligent bladder volume control system (IBCS). d Fitted curve between magnetic flux density measured by the wearable sensor and actual bladder volume, along with the sensor’s sensitivity. e Comparison diagram of the hysteresis loops of the four groups of ferromagnetic particles.
Rational design of the meshed magnetic soft robot (MMR)
Our previous work on the magnetic soft robot bladder (MRB) demonstrated an ability to generate a high-pressure increase in the UAB pigs. However, without a sensing component, it was only able to assist with involuntary urination9. To achieve our envisioned closed-loop system with internal actuation and external sensing, simply adding an external sensor to the MRB is not sufficient. Since the sensor no longer directly contacts the bladder, it cannot directly receive physical changes that correspond to bladder volume fluctuations, such as bladder wall pressure or capacitance12,14. The challenge places new demands on our actuator design, not only has to be able to apply strong mechanical pressure to empty the bladder but also accurately relay the aforementioned volume-related changes to the sensor in real time. Additionally, the magnetically controlled soft robot should be lightweight enough to avoid causing a strong foreign body sensation. Finally, it needs to be well biocompatible.
Considering the above needs, inspired by the technology of hernia repair mesh17,18, we combine the mesh technology with magnetic soft robotics. The composite mesh serves as the load-bearing structure for ferromagnetic particles and adheres closely to the bladder. As the bladder fills, it changes shape and the corresponding changes in magnetic flux density reflect the changes in the bladder volume. We name this mesh-structured magnetic soft robot the “meshed magnetic soft robot (MMR)”. The mesh is made of thermoplastic polyurethane (TPU), with vertical and horizontal filaments printed at a height difference of 2 mm, resulting in a woven structure that combines good biocompatibility with strength and toughness19,20. The shape of the mesh is based on the filled state of Bama miniature pigs bladder21, with rectangles (60 mm × 30 mm) cut from the corners of a square (180 mm × 180 mm) to form a gripper-like structure that wraps tightly around the bladder. The composite mesh is printed with a combination of large (30 mm × 15 mm) and small (14 mm × 6.5 mm) TPU meshes, based on the forces applied to the bladder during compression by the detrusor muscle, to carry ferromagnetic particles. The ferromagnetic particles were prepared by mixing NdFeB magnetic powder and PDMS. The preparation of ferromagnetic particles is detailed in the Experimental Section and the Supplementary Fig. 1. NdFeB magnetic powder was chosen because it is currently the strongest hard magnetic material. After magnetization, it possesses an intrinsic magnetic field22. Under the excitation of the external driving magnetic field, it will be turned by the moment of the magnetic couple, thereby guiding the movement of the magnetic soft robot, which in this scenario is to contract and squeeze the bladder. We designed three magnetization methods for ferromagnetic particles (Supplementary Fig. 2). After comparing the urodynamic data of the prepared ferromagnetic particles, we chose magnetization along the positive Z-axis direction (Supplementary Fig. 3). Then we designed ferromagnetic particles with four different volume fractions of NdFeB magnetic powder, ranging from 30% to 45%. Their hysteresis loops (as shown in Fig. 1e) indicate that as the volume fraction of NdFeB magnetic powder increases, the remanent magnetization of the ferromagnetic particles also increase. This allows us to control the compression capability of the MMR by changing the volume fraction of NdFeB powder in the ferromagnetic particles, thereby customizing “artificial detrusor” for different UAB patients’ urodynamic conditions. For each volume fraction (30%, 35%, 40%, 45%), three different mesh combinations were designed, as shown in Fig. 2a. In total, we designed 12 groups of meshed magnetic soft robots.
a Illustration of three MMR patterns: pattern A, pattern B, and pattern C. Each pattern has four NdFeB volume fractions (30, 35, 40 and 45%). b The schematic of the in vitro setup. c Experimental results of the maximum pressure increase ΔP of 12 MMRs. d Pressure increases efficiency ΔP/W on striped structure9 and meshed structure artificial bladder developed in this work. e Voided volume and voiding efficiency of 12 MMRs.
The meshed magnetic soft robot has two working modes, urine storage and urine emptying (Supplementary Fig. 4). Under the urine storage mode, the meshed magnetic soft robot is tightly fitted to the outer surface of the bladder, and as the bladder fills up and deforms, the magnetic field signal generated will change accordingly. Under the urine emptying mode, when the bladder is full, the patient approaches or activates the external magnetic field excitation source (an N52 neodymium-iron-boron permanent magnet, as detailed in Supplementary Fig. 5), under the influence of the magnetic field, the MMR compresses the bladder towards the pelvic cavity, causing the intravesical pressure to rapidly increase and overcome the resistance at the urinary outlet, thereby emptying the bladder. After the urination is complete, the external magnetic field excitation source is withdrawn, and the system transitions into the next urine storage cycle, establishing a complete urination control loop, as shown in Fig. 2d.
In vitro test of MMR
Figure 2b shows the setup we built to explore the performance of MMR as an artificial detrusor. MMR is fixed to the surface of the silicone bladder by an adhesive. The shape and size of the silicone bladder are obtained through Computed Tomography (CT) of the human bladder (Supplementary Fig. 6) and are fabricated using Ecoflex00-30, which has similar physical properties (Poisson’s ratio, density, etc.) to human bladder tissue. The preparation of the silicone bladder is detailed in the Experimental Section. The constructed bladder model helps us simulate the physical properties of a real bladder in an in vitro environment.
Successful urination requires the detrusor to increase the pressure inside the bladder above a certain threshold, so we first test the ability of MMR to increase the pressure inside the bladder. In this paper, we conducted pressure augmentation tests on 12 groups of MMRs, including four volume fractions of NdFeB magnetic powder and three grid arrangements. ΔP is defined as the internal pressure difference of the filled bladder outlet measured by the pressure sensor before and after the external excitation of the NdFeB permanent magnet. The results are shown in Fig. 2c. The MMR in group C, with a 45% NdFeB magnetic powder volume fraction, exhibited the highest ΔP, reaching 37 cmH2O. Even the mesh that had the weakest ΔP (group A with a 30% NdFeB powder volume fraction) still produced an ΔP of 24 cmH2O. Normally, human intravesical pressure during urination ranges from 30 cmH2O to 40 cmH2O. The baseline intravesical pressure of UAB patients is above 10 cmH2O when the bladder is full. MMR can increase the pressure inside the bladder by more than 20 cmH2O, which can completely overcome the resistance of the urination outlet. It is worth noting that the material and arrangement of ferromagnetic particles play a critical role in increasing the pressure. NdFeB has a high coercive force and will generate an intrinsic magnetic field after magnetization. The intrinsic magnetic field interacts with the external excitation magnetic field source, and the generated moment of the magnetic couple drives the ferromagnetic particles to rotate, guiding the MMR to contract and compress the bladder. The ferromagnetic particles are arranged to simulate the force exerted on the bladder by the detrusor muscle. Large ferromagnetic particles are placed in the center of the MMR to apply a stronger squeezing force to the middle of the bladder. Small ferromagnetic particles are placed on both sides of the MMR to strengthen the combination and fixation of the MMR and the bladder surface. All meshes we designed meet the demand of artificial detrusor muscle in terms of compressing ability.
Furthermore, since the MMR needs to be implanted in the human body, excessive size and weight will cause a strong foreign body sensation, making lightweight design particularly important. We used the pressure increase value divided by the weight of the MMR, ΔP/W, as the standard to measure the degree of lightweight. Figure 2d shows the ΔP/W values of the 12 MMRs obtained from testing. Compared to the MRB with a striped structure in our previous work9, the MMR featuring a meshed structure has an average pressure increase efficiency that is more than twice that of the MRB, while maintaining a similar ability to increase bladder pressure, demonstrating the advantages of the MMR as an implantable artificial detrusor. This advantage arises from the combination of mesh technology with magnetic soft robotics in the MMR we developed. While maintaining a powerful driving force comparable to that of traditional magnetic robots, the introduction of a mesh structure makes it more lightweight.
Bladder voiding efficiency is also an important metric to measure the performance of MMR as an artificial detrusor. Urine retention exceeding 100 ml is prone to complications, and avoiding efficiency above 80% can ensure a lower risk of complications such as urinary tract infections. We slowly injected 400 ml of water into the silicone bladder applied an external driving magnetic field, and measured the amount of water discharged at the drain outlet with a beaker. The voiding test was performed once in each group of MMR, for a total of 12 tests. The voiding efficiency is defined as the ratio of the discharged volume to the injected volume (400 ml). Figure 2e shows the test data. Among the 12 MMR, the highest voiding efficiency reached 97.5%, the lowest voiding efficiency was 95%, and the average voiding efficiency was 96.6%. It is worth noting that compared with some existing artificial detrusors, MMR exhibits excellent bladder-voiding ability.
MMR has demonstrated excellent capabilities in three key performance metrics: pressure increase capacity, pressure increase efficiency, and voiding efficiency, making it a promising candidate as an implantable artificial detrusor muscle.
Rational design of the wearable magnetic field sensor
Supplementary Fig. 7 shows that as the bladder volume increases, the magnetic flux density emitted by the MMR increases accordingly, showing a strong positive correlation, which verifies the feasibility of our scheme of monitoring bladder volume by measuring the changing magnetic flux density. In addition, to determine the required working range of the wearable magnetic field sensor, we performed a three-dimensional magnetic field scan on the predetermined working area of the MMR with a volume fraction of 30% NdFeB and 45% NdFeB in group A. The obtained magnetic field cloud map is shown in Supplementary Fig. 8. The magnetic flux density is in the mT range, with a minimum of 0.5 mT and a maximum of 4.5 mT, which is within the working range of the TMR2901 magnetoresistive chip. In addition, the high sensitivity of the TMR2901 magnetoresistive chip enables it to respond to slight changes in bladder volume. Supplementary Fig. 9 are the design drawings of the four-channel magnetic field sensors. The morphological changes during bladder filling are relatively complex, thus the multi-channel design can avoid the impact of insignificant changes in magnetic field signals in some areas on monitoring accuracy. In addition, considering that the magnetic field sensor needs to fit tightly to the patient’s abdomen when in use and needs to withstand a certain degree of bending, we use a serpentine design to improve the sensor’s resistance to bending23,24. The magnetic field sensor is mainly encapsulated by PDMS, which is soft, comfortable, has good biocompatibility, and is suitable for prolonged wear by patients25,26. The fabrication process of the wearable magnetic field sensor is shown in Supplementary Fig. 10. Figure 3a shows the performance of the wearable magnetic field sensor under different mechanical deformations, including bending, twisting, and stretching, showing its adaptability. The serpentine design and PDMS material ensure that the wearable magnetic field sensor will not be physically damaged during deformation.
a The wearable magnetic field sensor undergoes various mechanical deformations, including bending, twisting, and stretching. b The schematic of the in vitro setup. c The magnetic flux density characteristics of the three working areas (0°, 30°, 60°) during bladder expansion. d The database of magnetic flux density and bladder volume for the most sensitive channel at each working angle. e The intravesical pressure changes induced by the IBCS during urination. f Comparison of bladder volume monitored by IBCS, actual injected volume, and voided volume.
In vitro test of the wearable magnetic field sensor and IBCS
The wearable magnetic field sensor was first demonstrated in the setup shown in Fig. 3b. Silicone bladder (similar in shape, size, density, and Poisson’s ratio to human bladders) simulate the physical properties of human bladders in an in vitro environment. The MMR is fixed to the bladder surface with Siloxy silicone adhesive. To explore the optimal working angle of the wearable magnetic field sensor, three working areas of 0°, 30°, and 60° were set up on the C-shaped column simulating human abdominal skin, and a wearable magnetic field sensor was placed respectively to monitor bladder volume. We injected 50 ml each time through the syringe at a speed of 5 ml/s, for a total of 400 ml. Figure 3c shows the real-time changes in the magnetic flux density of the four channels in the three working areas during the process of increasing bladder volume. When the injected water volume was below 150 ml, the magnetic flux density hardly varied with the bladder volume. After reaching 150 ml, the magnetic flux density increased significantly as the bladder volume increased. When the wearable magnetic field sensor operates at a 0° working angle, the magnetic flux density measured by the M1 channel is higher than that of the other channels, indicating the highest response to the same change in bladder volume. When the angle increases to 30°, the M2 channel becomes the most sensitive, and as it reaches 60°, the M4 channel shows a sensitivity that greatly exceeds the other channels. This variation arises from changes in the relative positioning of the four TMR2901 chips and the MMR at different angles, with certain chips moving closer to regions of higher magnetic flux sensitivity, while others shift away. Variations in the most sensitive channel at different angles demonstrate the necessity of a multi-channel design for the wearable magnetic field sensor. Within the 0° to 60° working angle range, there is always a channel that shows the highest sensitivity to changes in magnetic flux density. The data from this channel can represent the magnetic flux density monitored by the wearable sensor at that angle, enabling sensitive detection of bladder volume changes within the 0° to 60° working angle range. Assuming that the bladder is filled with a volume of 400 ml, the wearable magnetic field sensor can monitor 50% to 100% of the bladder’s filling. The positive correlation between bladder volume and magnetic flux density can be attributed to the expansion of the bladder surface as the volume increases, leading to the mechanical deformation or unfolding of the MMR. This deformation alters the spatial distribution of the ferromagnetic particles, resulting in some particles moving closer to the wearable magnetic field sensor, thereby increasing the local magnetic flux density. This increase in local magnetic field induction intensity becomes more significant as bladder volume increases.
The wearable magnetic field sensor was used to repeatedly monitor real-time changes in magnetic flux density in real-time across five repeated infusions in three working angles, establishing a database related to magnetic flux density and bladder volume. At each of the three working angles, the channel with the highest sensitivity to changes in magnetic flux density at the same volume was selected to represent the measurement value of the wearable magnetic field sensor. The data is shown in Fig. 3d. We fitted the data collected at the M1 channel in the 0° working area, revealing a linear relationship between magnetic flux density and bladder volume as follows: V = 0.1474 + 1091.64 × B, where V is the bladder volume and B is the magnetic flux density monitored by the sensor. The Pearson correlation coefficient between V and B reaches 0.99669, indicating that there is a very strong positive linear relationship between the bladder volume and the magnetic flux density measured by the wearable magnetic field sensor, verifying the accuracy and reliability of calculating bladder volume through the measured magnetic flux density27. The fitting results for other working areas are similar, as detailed in Supplementary Fig. 11.
The working performance of MMR and wearable magnetic field sensor when working separately has been verified. To achieve our ultimate design goal: of intelligent control of bladder capacity, we need to further verify whether the system can completely: monitor bladder filling to alert the patient, help the patient empty the bladder when he approaches the external magnetic field source, and initiate the next cycle of urine storage and voiding once the patient moves away from the magnetic field source.
Fig. 3b shows the setup used in the experiment. The syringe evenly injected water into the silicone bladder. When the magnetic flux density measured by the wearable magnetic field sensor is close to 231.4μT (the magnetic flux density corresponding to the volume of 400 ml obtained by the fitting formula V = 0.1474 + 1091.64 × B), an external driving magnetic field is applied to excite the MMR to contract and compress the bladder. When the wearable magnetic field sensor detected that the bladder was full (400 ml), the actual injected volume was 420 ml, resulting in a monitoring error of 4.8%. Figure 3e shows the real-time pressure variation of the silicone bladder during the MMR contraction phase. The MMR increased the internal pressure of the silicone bladder by 36.7 cmH2O, and discharged 398 ml of water within 100 s, achieving a voiding efficiency of 94.8% (as shown in Fig. 3f). Our system detects bladder filling with high accuracy and urodynamic data that consistent with normal urination is verified on the silicone bladder. This result shows that our intelligent bladder volume control system has the potential to help UAB patients achieve autonomous and controllable urination behavior.
Biocompatibility improvement and in vivo tests of the MMR
The ferromagnetic particles in MMR are directly exposed to the peritoneal environment and are prone to cause rejection or tissue damage28,29. Figure 4a shows our idea of improving the biocompatibility of ferromagnetic particles. We first treated the surface of the ferromagnetic particles with a PDMS coating, followed by a hydrogel covering. This is due to the high volume fraction of NdFeB magnetic powder in the ferromagnetic particles, which makes it difficult for the hydrophilic initiator to access their surface. The PDMS coating ensures the integrity of the hydrogel coverage. The hydrogel skin has good biocompatibility and can improve the compatibility of implantable MMR with human organs30,31,32. In addition, the hydrogel skin also physically blocks the direct contact between magnetic materials and the human peritoneal cavity, reducing friction with organs in the body and potential rejection reactions. We further performed the dead-live staining assay33 to evaluate the biocompatibility of the ferromagnetic particles covered with hydrogel. The operation process of the dead-live staining assay is detailed in the Experimental Section. Figure 4b and c shows the microscopic imaging of ferromagnetic particles covered with hydrogel skin and ferromagnetic particles without hydrogel skin co-cultured with L929 cells, respectively. The dead and live staining microscopy images show that, compared to the blank control, the number of live cells is significantly lower in the bare particle group and significantly higher in the hydrogel-covered group, indicating that the hydrogel coating effectively reduces the toxicity of ferromagnetic particles and enhances their biocompatibility (Supplementary Fig. 12).
a Comparison of ferromagnetic particles without PDMS coating and ferromagnetic particles with PDMS coating under a microscope. b Microscopic imaging of hydrogel skin covering ferromagnetic particles co-cultured with L929 cells. c Microscopic imaging of co-culture of ferromagnetic particles and L929 cells without hydrogel skin covering. d Schematic of MMR implanted in a UAB porcine model, sutured to the bladder. e Photo of MMR implantation and assisted urination in a UAB porcine model. f Representative urodynamic curves of bladder pressure change (ΔP), urine flow rate and bladder injection volume (stopped when bladder pressure reaches 30 cmH2O) as a function of time during four cycles of MMR-assisted voiding process. g Magnified view of ΔP and ΔFlowmax of the round 2 urodynamic test in (f). h Pressure increase ΔP on MMR and UAB. i The bladder voiding efficiency over four cycles during the MMR-assisted voiding process.
To validate the MMR in vivo, we constructed a neurogenic UAB pig model and performed urodynamic tests 7 days after implantation. First, we selected female pigs to perform sacral nerve transection34,35 to construct UAB pig models. As the physiological characteristics and urodynamics of the female pig bladder are similar to the human bladder, it can provide an environment that closely resembles human physiological conditions36,37. We compared the urodynamic data of the pigs before and after surgery (Supplementary Fig. 13) to validate the constructed UAB model. Before surgery, when 300 ml of physiological saline was infused into the bladder, the intravesical pressure rose rapidly, and spontaneous urination occurred after reaching 370 ml. Postoperative data showed that despite increasing physiological saline infusion, the pressure increased slowly and failed to trigger spontaneous urination, confirming the successful construction of the neurogenic UAB pig model.
The MMR was implanted into UAB pigs by cutting open the skin and abdomen to fully expose the bladder, followed by tightly suturing the MMR to the bladder’s outer surface with surgical sutures to complete the implantation process (Fig. 4d). One week later, to verify whether the implanted MMR could assist UAB pigs in urination, we performed urodynamic tests. A catheter and pressure sensor were inserted into the bladder, with the other end connected to a urodynamic testing device. Physiological saline was infused into the bladder at a rate of 50 mL/min until the bladder was full, followed by the application of a driving magnetic field. The expelled urine was collected using a beaker (Fig. 4e and Supplementary Video 1). We repeated this procedure four times for each experimental pig. Fig. 4f shows the urodynamic data of one pig over four urination cycles. The data indicate that after the bladder gradually filled, when we applied the driving magnetic field, the meshed magnetic soft robot rapidly generated a peak ΔP of up to 33 cmH2O for the UAB pig, with a maximum urine flow rate reaching 17 mL/s (Fig. 4g), expelling 108.32 ml of urine within 14 s and achieving a urine emptying efficiency of 83%. In all four trials, the ΔP generated by the meshed magnetic soft robot exceeded 20 cmH2O (Fig. 4h), with peak urine flow rates ranging from 14 mL/s to 17 mL/s, completing urination within 14 to 18 s (Supplementary Fig. 14). Also, the urine emptying efficiency ranged from a minimum of 83% to a maximum of 90% (Fig. 4i). These urodynamic data are consistent with the normal urination of pigs, demonstrating the excellent performance and potential of MMR as an implantable artificial detrusor.
Discussion
Facing challenges of the technical complexity of integrating mechanical emptying with non-invasive/minimally invasive sensing functions, here we present an intelligent bladder volume control system. The implantable artificial detrusor muscle works in coordination with the external wearable sensor, offering a biocompatible solution for wireless and continuous bladder volume management. The wearable sensor monitors bladder volume in real time by measuring the magnetic flux density, which is highly linearly related to bladder volume. The MMR achieved a bladder voiding efficiency of 83%-90% in the UAB pig model. The system’s bladder volume control capability was validated, demonstrating the ability to achieve both highly accurate monitoring and high bladder voiding efficiency.
The MMR integrates hernia mesh technology with magnetic soft robot technology, improving the lightweight degree while ensuring high compressive capability, paving a new direction for implantable soft robot designs. The MMR closely adheres to the bladder, changing its shape as the bladder gradually fills. Through five real-time measurements, a database correlating magnetic flux density with bladder volume was established. Linear fitting of the data revealed a strong linear relationship between the magnetic flux density and bladder volume. The soft and stretchable wearable magnetic field sensor is suitable for daily use, allowing real-time monitoring of bladder volume by measuring magnetic flux density. It is worth mentioning that combining wireless communication technologies such as NFC, Bluetooth or Wi-Fi with wearable sensors could truly enable the full wireless design of robotic systems and show great potential in various applications38,39. In this study, the proposed IBCS has the potential to achieve wireless, continuous monitoring of bladder volume during free walking in humans, as well as facilitate bladder voiding when bladder filling is detected. The wearable magnetic field sensor, connected to an FPGA board that integrates a Wi-Fi module, achieved wirelessly data transmission regarding bladder volume. Once detecting bladder filling, patients could approach driving magnets installed in public locations (e.g., toilets) and assume a seated posture for urination.
In summary, we propose a wireless and continuous bladder volume management solution for UAB patients, which integrates sensing and actuation, demonstrating the potential to help achieve normal bladder function in UAB patients. In the future, we plan to further optimize the configuration of the external driving magnetic field to enhance its convenience in daily use. Additionally, we will expand the database correlating magnetic flux density with bladder volume and introduce algorithms to improve the accuracy of bladder volume monitoring by the sensor.
Methods
Materials
The composite mesh was fabricated using thermoplastic polyurethane rubber (PolyFlex TPU95) via fused deposition modeling 3D printing (RAISE 3D E2). The filament diameter of the mesh is 0.4 mm, with a 2 mm height difference between the vertical and horizontal filaments. The mesh was designed as a square (180 mm × 180 mm) with rectangular cutouts at each corner (60 mm × 30 mm). Neodymium-iron-boron (NdFeB) magnetic powder with a particle size of 5 μm was purchased from Xinnuo Co., Ltd., under the full product name “Bonded NdFeB Rare Earth Magnetic Powder (LW-BA (16-7 A)-2000 mesh/5 μm).” Ferromagnetic particles were prepared by mixing bonded NdFeB magnetic powder with polydimethylsiloxane (PDMS) resin (Sylgard 184, Dow Corning). The dimensions of each ferromagnetic particle were 14 mm × 6.5 mm × 5 mm. A hydrophobic initiator solution, containing 10 wt.% benzophenone (Aladdin®), was prepared with anhydrous ethanol as the solvent. The pre-gel hydrogel solution contained 30 wt.% acrylamide monomer (Aladdin) and 1 wt.% Irgacure 2959 (2-hydroxy-4’-(2-hydroxyethyl)-2-methylpropiophenone, Aladdin), with sterile water (autoclave treated) as the solvent. The silicone bladder model used for ex vivo testing of the meshed magnetic soft robot was fabricated from Ecoflex 00-30 to simulate the human bladder’s physical properties. The magnetoresistive chip used in the wearable magnetic sensor was a TMR2901, encapsulated in polydimethylsiloxane (PDMS) with a curing agent-to-base ratio of 1:40. All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee (IACUC), protocol number WDRM(F) 202403003. The experimental animals were female Bama miniature pigs, aged six months, with an average weight of approximately 35 kg.
Fabrication process of ferromagnetic particles
The ferromagnetic particle curing molds were designed using SolidWorks and printed with PLA material via a 3D printer. Bonded NdFeB rare-earth magnetic powder, with an average particle size of 5 μm, was mixed with polydimethylsiloxane (PDMS) resin (Sylgard 184, Dow Corning) at predetermined volume ratios to prepare the uncured ferromagnetic mixture. In this study, four different NdFeB magnetic powder volume fractions were designed: 30%, 35%, 40%, and 45%. It is important to note that for ferromagnetic particles with higher NdFeB magnetic powder volume fractions (e.g., 40% and 45%), the NdFeB powder and PDMS tend to become difficult to mix. In such cases, an appropriate amount of Glossy agent was added to dilute the ferromagnetic mixture. Since most of the Glossy agent evaporates during the curing stage, it does not affect the final NdFeB magnetic powder volume fraction.
The ferromagnetic mixture was then placed into a planetary mixer and stirred at a speed of 3000 rpm for 2 min, followed by 1 min of degassing. Afterward, a mold release agent (Ease Release 200) was sprayed onto the ferromagnetic particle curing molds. The prepared ferromagnetic mixture was poured into a syringe and injected into the curing molds. The molds were placed in an oven at 40 °C for 24 h to cure. Once the curing process was complete, the molds were opened, and any excess material was trimmed off with a blade. The ferromagnetic particles were then placed into a DPM1 pulse magnetizer powered by 230 V, with a rated power of 300 W. The particles were uniformly magnetized in a 3850 mT pulsed magnetic field, resulting in the final ferromagnetic particles.
MMR fabrication
The mesh material is thermoplastic polyurethane rubber (model: PolyFlex TPU95), which is printed by a fused deposition 3D printer (RAISE 3D E2). The diameter of the mesh wire is 0.4 mm, and there is a height difference of 2 mm between the vertical and horizontal wires, forming a woven print. The shape of the mesh is a square with a size of 180 mm, and rectangular grids with a size of 60 mm × 30 mm are cut off at the four corners. The mesh is printed with a TPU grid that carries ferromagnetic particles. According to the force of the bladder under the compression of the detrusor muscle, a large grid is placed in the middle of the composite mesh, and a small grid is placed on both sides of the mesh. The size of the small grid is 14 mm × 6.5 mm, and the outer wall thickness is 0.5 mm; the large grid is composed of multiple small grids, the inner wall thickness between each small grid is 1 mm, and the large grid is composed of small grids arranged horizontally. For example, the size of a large grid composed of 4 small grids is 30 mm × 15 mm × 5 mm. A gap of 5 mm is set between each adjacent grid. Sil-Poxy silicone adhesive is injected into the TPU grid on the mesh, and finally, the ferromagnetic particles are respectively pasted into each TPU small grid. Wait for 1 h at room temperature 20 °C until the adhesive is completely cured to obtain MMR.
Live/dead staining assay
L929 cells were cultured in a 5% CO2, 37 °C incubator with DMEM complete medium, where DMEM complete medium consisted of DMEM medium, 10% fetal bovine serum, and 1% penicillin or streptomycin. The staining test was divided into 4 groups, namely ferromagnetic particles, hydrogel-covered ferromagnetic particles, blank group, and negative control group (90% ethanol culture medium). Before live-death staining, L929 cells were inoculated into a 24-well cell culture plate and cultured for 16 h. L929 cells in the logarithmic growth phase were selected for counting and the cell concentration was adjusted to 20,000 cells/well. The complete medium was removed, 100 μL of fresh complete medium and samples were added, and the cells were cultured in a constant temperature incubator for 72 h. The medium was then removed again, each well was washed with PBS, and PBS containing 0.1% Calcein-AM and 0.3% PI was added for staining culture. Finally, the mixed dye solution was removed, PBS was added for washing, and images were collected using a laser confocal (Leica LASX) at excitation waves of 490 nm and 545 nm.
Preparation of silicone bladder membrane
The curing mold of the silicone bladder was designed by Solidworks and printed by a fused deposition 3D printer (RAISE 3D E2) using PLA material. Part A and Part B of Ecoflex 00-30 were mixed in a ratio of 1:1. The above mixture was added to a planetary mixer and stirred at a speed of 3000 rpm for 5 min. The stirred Ecoflex 00-30 mixture was placed in a vacuum-degassing box, and the vacuum pump was turned on to evacuate the mixture. It was allowed to stand for 5 min in a vacuum state until all the bubbles in the mixture rose or burst and disappeared. The Ecoflex 00-30 mixed liquid with bubbles removed was slowly poured into the printed silicone bladder curing mold. The Ecoflex 00-30 mixed liquid had good fluidity before curing and could slowly level itself. The mold needed to be placed vertically to prevent the thickness of the cured silicone bladder from being uneven. The mold was placed in a 40 °C oven and left to stand for 10 h before demolding to obtain a silicone bladder with a thickness of 2 mm.
Animal test
A six-month-old female Bama pig (35 kg) was used in the in vivo experiments. Bama pigs were housed under controlled temperature (25 °C) conditions and fed with standard food and water. All animal experiments were conducted by protocols approved by the Committee on Animal Care of Hubei Yizhicheng Biotechnology Co. Ltd. (Approval No. WDRM-202403003).
Sacral Nerve Transection Surgery: Sacral laminectomy and sacral nerve transection were performed to establish the neurogenic UAB porcine model. A Female Bama pig weighing 35 kg was anesthetized by intramuscular injection of 2 ml of Shutai 50. A longitudinal incision was made in the skin and subcutaneous tissue at the S2-S5 pyramid. After completely exposing the lateral edge of the erector spinae muscle, all muscles on the surface of the sacrum were separated below S1. The posterior wall of the sacrum was opened with laminectomy forceps, and the residual bone fragments and nucleus pulposus tissue were removed to completely expose the cauda equina bundle. The electrical stimulation generator was used to expose the S1-S5 nerves. The S2 and S3 nerves were separated with a nerve dissector and clamped and cut with long curved forceps. After hemostasis, the muscles, subcutaneous tissue, and skin were sutured.
Urodynamic tests: Urodynamic tests were performed one day before and one week after the establishment of the neurogenic UAB pig model, and the baseline urination data were compared to determine whether the modeling surgery was successful. Anesthesia was as described above. The catheter and the pressure gauge were inserted into the bladder, with their other ends connected to the urodynamic analysis machine (Andromeda). Warm saline was infused at a rate of 50 mL/min. And the measurement was continued until urination. Urodynamic tests were performed again after MMR implantation, and the preparations were as described above. When the urodynamic analysis machine (Andromeda) measured the intravesical pressure to reach 30 cmH2O (the bladder was considered to be just full at this time), an external driving magnetic field was applied (Fig. S5), and urodynamic data were recorded. Four MMR-assisted urination experiments were performed on each animal.
Implantation of the MMR: After 1 mL intramuscular injection of Sumianning anesthesia behind the ear of the neurogenic UAB Bama pig, the pig was placed in a supine position with its head secured with an anesthesia mask put on the mouth. A longitudinal incision was made in the skin and subcutaneous tissue. The abdominal skin, subcutaneous tissue, and fat were incised with a scalpel to expose the bladder. The MMR was tightly sutured to the outer surface of the bladder with surgical sutures, and the pig’s abdomen was closed and the wound was sutured to complete the operation.
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Santos-Pereira, M. & Charrua, A. Understanding underactive bladder: a review of the contemporary literature. Porto Biomed. J. 5, e070 (2020).
Chang, Y.-H., Siu, J. J.-Y., Hsiao, P.-J., Chang, C.-H. & Chou, E. C.-L. Review of underactive bladder. J. Formos. Med. Assoc. 117, 178–184 (2018).
Miyazato, M., Yoshimura, N. & Chancellor, M. B. The other bladder syndrome: underactive bladder. Rev. Urol. 15, 11 (2013).
Fowler, C. J., Griffiths, D. & De Groat, W. C. The neural control of micturition. Nat. Rev. Neurosci. 9, 453–466 (2008).
Wyndaele, J. Intermittent catheterization: which is the optimal technique? Spinal Cord. 40, 432–437 (2002).
Wyndaele, J. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord. 40, 536–541 (2002).
Coolen, R., Groen, J. & Blok, B. Electrical stimulation in the treatment of bladder dysfunction: technology update. Med. Dev. 12, 337–345 (2019).
Drossaerts, J., Jairam, R., van Koeveringe, G. & van Kerrebroeck, P. Neurostimulation and neuromodulation for the treatment for the underactive bladder. In Underactive Bladder, 57–61 (Springer, 2017).
Yang, Y. et al. Magnetic soft robotic bladder for assisted urination. Sci. Adv. 8, eabq1456 (2022).
Yang, X. et al. Soft artificial bladder detrusor. Adv. Healthc. Mater. 7, 1701014 (2018).
Hassani, F. A. et al. A 3D printed implantable device for voiding the bladder using shape memory alloy (SMA) actuators. Adv. Sci. 4, 1700143 (2017).
Arab Hassani, F., Jin, H., Yokota, T., Someya, T. & Thakor, N. Soft sensors for a sensing-actuation system with high bladder voiding efficiency. Sci. Adv. 6, eaba0412 (2020).
Arab Hassani, F. et al. Toward self-control systems for neurogenic underactive bladder: a triboelectric nanogenerator sensor integrated with a bistable micro-actuator. ACS Nano 12, 3487–3501 (2018).
Yan, D. et al. Ultracompliant carbon nanotube direct bladder device. Adv. Healthc. Mater. 8, 1900477 (2019).
Wyndaele, J. J. Normality in urodynamics studied in healthy adults. J. Urol. 161, 899–902 (1999).
Mahfouz, W., Al Afraa, T., Campeau, L. & Corcos, J. Normal urodynamic parameters in women: part II—invasive urodynamics. Int. Urogynecology J. 23, 269–277 (2012).
Elango, S., Perumalsamy, S., Ramachandran, K. & Vadodaria, K. Mesh materials and hernia repair. BioMedicine 7, 16 (2017).
See, C. W., Kim, T. & Zhu, D. Hernia mesh and hernia repair: a review. Engineered Regeneration 1, 19–33 (2020).
Pergal, M. V. et al. Structure and properties of thermoplastic polyurethanes based on poly (dimethylsiloxane): assessment of biocompatibility. J. Biomed. Mater. Res. Part A 102, 3951–3964 (2014).
Qi, H. J. & Boyce, M. C. Stress–strain behavior of thermoplastic polyurethanes. Mech. Mater. 37, 817–839 (2005).
Jiang, Y.-H. et al. Observation of whole body CT tomography of Guangxi bama mini-pigs. J. Southern Agric. 51, 677–685 (2020).
Heim, J. W. & Vander Wal, R. L. NdFeB permanent magnet uses, projected growth rates and Nd plus Dy demands across end-use sectors through 2050: a review. Minerals 13, 1274 (2023).
Yang, S. Mechanics and applications of stretchable serpentine structures Ph.D. thesis. Austin, USA: University of Texas at Austin; (2016).
Xu, S. et al. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 4, 1543 (2013).
Victor, A., Ribeiro, J. & Araújo, F. F. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 4, 1–9 (2019).
Bélanger, M. C. & Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low‐density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 58, 467–477 (2001).
Schober, P., Boer, C. & Schwarte, L. A. Correlation coefficients: appropriate use and interpretation. Anesthesia Analgesia 126, 1763–1768 (2018).
Iacovacci, V. et al. Stability and in vivo safety of gold, titanium nitride and parylene C coatings on NdFeB magnets implanted in muscles towards a new generation of myokinetic prosthetic limbs. RSC Adv. 11, 6766–6775 (2021).
Iacovacci, V. et al. Polydimethylsiloxane films doped with NdFeB powder: magnetic characterization and potential applications in biomedical engineering and microrobotics. Biomed. Microdevices 17, 1–7 (2015).
Naahidi, S. et al. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 35, 530–544 (2017).
Saroia, J. et al. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 1, 265–279 (2018).
Spencer, K. C. et al. Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci. Rep. 7, 1952 (2017).
Boulos, L., Prevost, M., Barbeau, B., Coallier, J. & Desjardins, R. LIVE/DEAD® BacLight™: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiological Methods 37, 77–86 (1999).
Zhang, H.-Y., Thongtrangan, I., Balabhadra, R. S., Murovic, J. A. & Kim, D. H. Surgical techniques for total sacrectomy and spinopelvic reconstruction. Neurosurgical Focus 15, 1–10 (2003).
Guiho, T. et al. Validation of a methodology for neuro-urological and lumbosacral stimulation studies in domestic pigs: a humanlike animal model. J. Neurosurg.: Spine 30, 644–654 (2019).
Dittrich, R. et al. The extracorporeal perfusion of the female pig detrusor as an experimental model for the study of bladder contractility. Neurourol. Urodynamics: Off. J. Int. Cont. Soc. 26, 1024–1029 (2007).
Kumar, V., Chapple, C. C. & Chess-Williams, R. Characteristics of adenosine triphosphatase release from porcine and human normal bladder. J. Urol. 172, 744–747 (2004).
Li, D. et al. Battery-free, wireless, and electricity-driven soft swimmer for water quality and virus monitoring. Sci. Adv. 10, eadk6301 (2024).
Li, D. et al. Touch IoT enabled by wireless self-sensing and haptic-reproducing electronic skin. Sci. Adv. 8, eade2450 (2022).
Acknowledgements
We thank the Technology Analytical & Testing Center at Huazhong University of Science and Technology for SEM measurements. This work is supported by the National Natural Science Foundation of China (nos. T2350001, 52173280), the China Postdoctoral Science Foundation (no. 2022M711256), the HUST Interdisciplinary Research Project (no. 2023JCYJ044), and the Taihu Lake Innovation Fund for Future Technology, HUST (no. 2023A3). All animal experiments were conducted by protocols approved by the Committee on Animal Care of Hubei Yizhicheng Biotechnology Co. Ltd. (Approval No. WDRM-202403003).
Author information
Authors and Affiliations
Contributions
J.Z. and H.T. conceived the concept of the experiment. Z.W. and Y.T. designed the experiment. Q.H., Z.W., Y.T., J.W., Z.P., Yang Yu, Y.C., and Yueying Yang performed the experiments. Q.H., Z.W., and Y.T. analyzed the data. Q.H., Y.T., H.T., and J.Z. prepared the manuscript with input from all authors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.Z., Z.W., and Y.C. are named as inventors on a patent (CN117379055A) that covers the design and fabrication of a wearable magnetic field sensor.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, Q., Wu, Z., Tian, Y. et al. A magnetic soft robotic system for intelligent bladder volume control. npj Flex Electron 9, 33 (2025). https://doi.org/10.1038/s41528-025-00401-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00401-y
This article is cited by
-
Chitosan and polycaprolactone blended PDMS coatings improve biocompatibility of magnetic elastomers
Scientific Reports (2026)