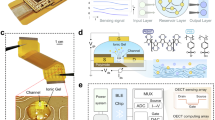
Abstract
We demonstrate an enhancement in the figure of merit (μC*) of a flexible organic electrochemical transistor (OECT) and its dopamine (DA) biosensor by blending various open-shell, non-conjugated radical polymers featuring nitroxide radical active sites as pendant groups with closed-shell, ethylene glycol (EG)-functionalized conjugated polymers as a macromolecular active layer system. The precisely controlled ionic transport of the OECT by the radical polymer modulated the doping level of the EGylated polymer, ensuring well-regulated redox activity and resulting in μC* values exceeding 192 F V-¹ cm-¹ s-¹, along with an on/off ratio of 104. Additionally, we achieved an ultrasensitive detection limit for DA at the clinically relevant level of 1 pM, along with exceptional specificity, effectively distinguishing DA even in the presence of a substantial excess of interfering substances. These findings underscore the potential of a systematic design approach for developing an advanced, flexible OECT-based biosensor platform through the strategic selection and processing of open- and closed-shell macromolecules.
Similar content being viewed by others
Introduction
Organic electrochemical transistors (OECTs) have attracted significant attention in recent times because of their capacity to monitor biological functions, which makes them highly sought-after bioelectronics devices1,2,3,4,5. The advancements in electrolyte-gated transistors have greatly benefited biosensing, especially in the field of ion-rich aqueous media6,7,8. Almost all forms of biological analyte detection and electrophysiology sensing occur within such environments. In these scenarios, a biological occurrence alters the effective gate bias, and the inherent amplification capability of the transistor can be utilized as a transducer to amplify the measured signal, thereby enhancing it9. Leveraging these mechanisms, the cost-effective and easily fabricated OECT-based biosensors effectively detect dopamine (DA), a crucial neurotransmitter10,11. However, achieving clinical-level sensitivity and rapid response in complex biological fluids remains challenging8. While significant emphasis is placed on enhancing the charge carrier mobility through the crystallinity of materials for organic field effect transistor (OFET)12,13,14, the key factor for improving the performance of OECT-based biosensors lies in the volumetric capacitance of the film to facilitate the injection of dopant ions15,16.
Recent advancements in OECT materials, particularly ethylene glycol (EG)-functionalized organic semiconductors, have demonstrated significant improvements in device performance and functionality17,18. These materials excel among various channel types due to their numerous advantages such as high OECT performance, excellent enzymes compatibility for sensing, simplified fabrication process, large volumetric capacitance, and synthetic tunability17,18,19,20. For example, diethylene-tetraethyleneglycol polymer and its derivative for OECT shows a figure-of-merit (μC*) of 500 F V-1 cm-1 s-1 (μ: charge mobility, and C*: volumetric capacitance)21. Considerable advancements have been accomplished in comprehending the essential operational mechanism and exploring various application possibilities using an EGylated conjugated polymer. However, it is important to note that the range of chemistries evaluated for these design motifs is relatively limited.
Radical polymers, a distinct category of flexible organic materials often referred to as “open-shell” materials, are defined by their partially filled frontier molecular orbitals, setting them apart from traditional “closed-shell” materials with fully occupied orbitals22. These unique macromolecules, featuring non-conjugated backbones and stable open-shell pendant active sites, have attracted growing interest in the field of organic electronics research23,24,25,26,27,28,29,30. The self-exchange oxidation and reduction reaction of the nitroxide pendant group in radical polymer enables the charge transfer used as solid-state electronics26,31,32,33,34,35. The considerable ideal capacities above 100 mAh g-1 in theory and rapid redox electrochemical reactions enable them to be utilized in organic batteries36,37,38,39,40,41. However, radical polymers are rarely studied in electronic applications except for batteries due to their low electrical conductivity. To overcome this, Kim et al. have explored the study of blending systems of conjugated and non-conjugated OECTs42. By integrating an open-shell polymer of poly(4-glycidyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl) (PTEO) into the conventional conjugated polymer poly(3-hexylthiophene) (P3HT) enabled a significant increase in μC* > 150 F cm-1V-1s-1, while allowing precise control over ion penetration at a specific operating voltage42. However, for the enhancement of the device performance in this blending system of conjugated semiconducting polymer and the ionically conducting radical polymer, an understanding of the structure-property relationship is still required with the conjugated polymer and non-conjugated radical polymers.
In this study, we fabricated active layer films composed of poly(4-ethylenedioxythiophene-co-ethylene oxide) (PTEO) and poly(4-episulfide-2,2,6,6-tetramethylpiperidine-1-oxyl) (PTES), which function as primary ion conductors, alongside poly(3-(methoxyethoxyethoxy)thiophene) (P3MEET) as the main charge-conducting medium. These components were integrated via solution blending and utilized as the active channel material in OECT-based biosensors specifically engineered for DA detection. The OECT device configuration is presented in Fig. 1a-(i, ii). In this system, P3MEET serves as a closed-shell polymeric semiconductor, designed to enhance ion transport, carrier mobility, stability, and adjustable doping levels (Fig. 1a-iii)43,44,45. The open-shell PTES and PTEO polymers have distinct, hydrophilic backbones resembling poly(ethylene oxide)-like and poly(ethylene sulfide) structures, respectively, with pendant 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) radicals. These polymers function as ionic conductors, facilitating the transport of hydrated ions that act as dopants for the P3MEET segment (Fig. 1a-iv). The chemical structures of all synthesized polymers are provided in Fig. 1b.
The OECT structure functioned in a-i the typical source configuration, employing the Ag/AgCl electrode as the gate with a-ii applied bias. Molecular structure and schematic of interactions of ions with a-iii P3MEET, and a-iv blending of P3MEET with PTES radical polymer. The white, dark blue, and gray circles represent the cation in solution, the anion, and the doped cation, respectively. The gray and red curved lines represent the P3MEET and PTES, respectively. b Chemical structure of P3MEET, PTES, PTEO, and c corresponding radical density of each radical polymer characterized by electron paramagnetic resonance (EPR) compared to the 4-hyroxy TEMPO (hTEMPO). Cyclic voltammetry of the d P3MEET (black), PTES (dark red), and their blends (P3MEET: PTES) films of selected blend ratios of 1:0.5, 1:1, 1:2; and f PTEO (blue) and in blend with P3MEET at a weight ratio of 1:0.5, 1:1, 1:2; acquired at a scan rate of 10 mV s–1 in 0.1 M solution of NaCl. The UV–Vis spectra of e P3MEET-PTES and g P3MEET-PTEO thin films of equal weight fraction of P3MEET and radical polymers (1:0.5).
Electrochemical characterization of the composite layers revealed that TEMPO redox sites effectively regulated ion injection into the film, crucial for tuning electrochemical response. Among the synthesized radical polymers, PTES exhibited the greatest enhancement in doping efficiency due to the strong entanglement between its ethylene sulfide backbone and the EG functional group in P3MEET. This finding underscores the influence of the structural features of the radical polymer backbone and its interaction with both the conjugated polymer and the electrolyte solution. Additionally, the incorporation of TEMPO groups enabled precise tuning of the device’s threshold voltage. By optimizing the blend composition, we achieved a high μC* value of 192 F V-¹ cm-¹ s-¹ with a 50 wt% PTES blend, marking the highest value recorded yet for OECTs using non-conjugated radical polymers and comparable to leading materials in OECT performance.
Compared to previous studies, the optimized OECT-based DA biosensors achieved an impressive DA detection limit of 1 pM and a broad dynamic range from 1 pM to 1 μM. The biosensor demonstrated remarkable selectivity, distinguishing DA even in the presence of high concentrations of potential interferents such as uric acid (UA) and ascorbic acid (AA). These results underscore the role of radical polymer design and the mixed open- and closed-shell polymer matrix in advancing high-performance organic mixed conductors. The blending strategy introduced here highlights significant potential for the development of next-generation DA monitoring platforms.
Results
Synthesis and radical density control in organic radicals
The unpaired electrons are responsible for the unique electronic properties of organic radical conductors. To do so, synthesis techniques allow for the controlled generation of these unpaired electrons, providing necessary building blocks for the organic radical conductor. By introducing specific functional groups or modifications during the synthesis, it becomes possible to fine-tune the electronic properties of the resulting materials. Compared to the 4-hydroxy TEMPO (hTEMPO), a well-studied nitroxyl radical monomer with a radical density of 100%46, the optimization of synthetic methods, reaction conditions, and precursor molecules allows for the modulation of radical density to as high as 99% in both PTES and PTEO (Fig. 1c). This control over radical density directly impacts the conductivity, hence, the structure-function relationship in the organic radical polymer is crucial. PTEO can be readily synthesized from the 4-glycidyloxy-2,2,6,6-tetramethylpiperidine 1-oxyl (GTEMPO, M1) following a reported procedure (Supplementary Scheme 1a)47. An anionic ring-opening polymerization (AROP) initiated by potassium tert-butoxide at 45 °C could provide the polymer with a molecular weight of Mn ~ 7.0 kg.mol-1. The thiirane monomer M2 was prepared by a simple and mild sulfuration protocol from the epoxide M1 (Supplementary Scheme 1b). Due to the stronger nucleophilicity of thiolate compared to alkoxide, the AROP of M2 could be carried out at a milder condition and offered the poly-(TEMPO ethylene sulfide) (PTES) with higher molecular weight Mn ~ 19.0 kg.mol-1 30. Owning to the structural diversity of aziridines compared to their epoxide and thiirane analogs, we expect the synthesis protocol could provide a distinct class of TEMPO-substituted polysulfonamide by functional group engineering. The synthesis of P3MEET involved: (1) alkoxylation by diethylene glycol monomethyl ether from 3-bromothiophene, (2) bromination by NBS, and (3) Grignard metathesis polymerization (GRIM) (Supplementary Scheme 2). The detailed synthesis protocol is provided in the Supporting Information (Supplementary Schemes 1 and 2; Supplementary Fig. 1). The PTEO and PTES synthesis protocols offer the benefit of preserving a substantial radical density. Besides, the mixture solution of conjugated polymer P3MEET and the radical polymer was mixed, and the desired composite was listed in Supplementary Table 1.
Electrochemical properties and mixed conduction in blended polymer systems
The electrochemical properties of each conjugated polymer, blending system of open-shell and closed-shell polymers were analyzed using UV–Vis spectroscopy, spectroelectrochemistry, and cyclic voltammetry (CV), as presented in Fig. 1d–f & Supplementary Fig. 2. First of all, the CV of pristine P3MEET, PTEO, and PTES were measured to compare the electrochemical activity in aqueous media (Fig. 1d, f and Supplementary Fig. 2). The weight ratio conjugated:non-conjugated polymer was varied in a large range from 1:0.5 to 1:2 to monitor the impact of radical polymer on electrochemical behavior in blend system. P3MEET film (black curve) exhibits a relatively low electrochemical response, showing the onset of oxidation at approximately 0.2 V (Supplementary Fig. 2). Its low oxidation potential is influenced by the electronegativity of the O atom in the EG chains next to the π-system, influencing the electronic-rich nature of the polymer backbone. These factors could hinder the infiltration of high levels of chloride anions into the film. Conversely, thin films of PTEO and PTES exhibit distinct redox peaks that correspond to radical TEMPO, occurring with an oxidation peak at +0.74 V compared to the Ag/AgCl reference electrode (Fig. 1d, f)48,49. This notable electrochemical response from radical polymer films can be attributed to the amorphous hydrophilic backbone resembling the PTEO and PTES backbone structure, which facilitates the infiltration of chloride anions into the active semiconducting mixed regime when an external bias is applied.
Subsequently, the interaction between two redox sites in a blending system was investigated, revealing characteristic oxidation peaks at +0.78 V relative to the reference Ag/AgCl electrode. The distinctive peaks associated with TEMPO radicals appeared at low PTES loadings of 4 wt% and PTEO loadings of 4 wt%. With increasing PTES or PTEO content, an additional oxidation peak at +0.78 V (vs. Ag/AgCl), attributed to the oxidation of P3MEET, appeared in films containing over 50 wt% radical polymers. In addition, the TEMPO oxidation peaks exhibited a minor shift towards a lower potential of approximately +0.72 V. The minor reduction in the oxidation potential of TEMPO can be ascribed to the charge transfer occurring between TEMPO radicals and the conjugated polymer backbone, which is influenced by the high packing density of the radical polymer within the mixed matrix42,50,51. Our findings demonstrate that the redox processes of TEMPO, occurring specifically in the ion-conducting domains within the radical polymer phase, can potentially initiate ion penetration, thereby enabling the doping mechanism in the organic active layer for mixed-conduction applications. These results align with previous studies that have demonstrated a mixed-conduction mechanism of blending conjugated and non-conjugated system42. Notably, PTES results in a larger peak growth (oxidation peak of P3MEET), suggesting a higher doping level compared to PTEO, which can affect the advancement of mixed conduction for applications.
Subsequent spectroelectrochemical measurements conducted on blend films of P3MEET and radical polymers (PTES and PTEO) provide additional evidence of the influence of radical polymers and their backbone on the electrochemical doping of the conjugated polymer. Since the UV–Vis light of radical polymer is relatively small, the absorption profile of P3MEET is dominant. The P3MEET exhibits a peak of maximum absorbance at 580 nm (λmax), corresponding to the previous report45,52. Fig. 1e, g present the thin-film UV–Vis spectra under varying doping potential (0 to −1 V in increments of −0.2 V, with the unbiased state at 0 V, in proximity to the open circuit potential, VOC). In all blending systems, the intensity of the primary π–π* associated absorption diminishes, while the polaron peak undergoes a simultaneous red shift and increases in magnitude upon the application of doping potential. The presence of polar side chains like PTEO and PTES induces planarity in the P3MEET polymer chain and strengthens intrachain exciton coupling. This effect is evident in the red-shifted absorbance observed. Among the various blending systems, the PTES blend exhibits the most significant peak shift. This observation can be attributed to the robust intermolecular interaction of the glycol side chain of P3MEET and the sulfide backbone of PTES. The intra- or intermolecular sulfur-oxygen interaction is a recognized parameter to trigger the backbone coplanarity and enhances the active conjugated length of the macromolecules6,53,54. This aspect promotes efficient charge transport while simultaneously increasing ionization energy6,53,54.
The electrochemical analysis indicates that within the measurement range determined by polymer operation in an aqueous chloride electrolyte, two blending systems exhibit distinct operating regimes. As a result, the transistor performance is expected to vary with the influence of the structural characteristics of the backbone of the radical polymers and their interaction with both the conjugated polymer and the electrolyte solution. Figure 2a, b and Supplementary Fig. 3 show the typical transfer and transconductance of electrolyte-gated OECT on a blend system at a ratio of 1:0.5 (P3MEET: radical polymer). The representative output characteristics of IDS/VDS on OECT devices based on polymer blend films are shown in Fig. 2c-i, c-ii and Supplementary Fig. 4. The forward sweep of the threshold voltage (VT) aligns with the point at which the TEMPO radical undergoes oxidation, leading to a remarkably sharp current modulation, showing a high on/off ratio of ~104 (Supplementary Fig. 5).
a Transfer curves and b corresponding transconductance curves of the P3MEET-radical polymer films (PTES and PTEO) of selected blend ratio (P3MEET: radical polymer=1:0.5) with the sweep rate of 10 mV s-1 (channel dimension: W = 5,000 µm, L = 50 µm, and 810 nm of thickness). Representative output curve of c-i P3MEET-PTES and c-ii P3MEET-PTEO films. d Determined μC* as a function of radical polymer concentration, derived from individual transfer curves recorded at VDS = -0.2 V, 0.1 M NaCl electrolyte, sweep rate of 10 mV s-1. e Threshold voltage VT (V) as a function of radical polymer loading. f-i Figure of merit (μC*), f-ii volumetric capacitance, and f-iii mobility of the P3MEET, P3MEET-PTES, and P3MEET-PTEO composites.
The performance of the open-shell and closed-shell blending system in terms of OECT was characterized by the figure of merit (µC*) with variations in the polymer blend composition. The µC* is an intrinsic characteristic of the OECT and can be used to assess the steady-state efficiency of mixed electronic/ionic conductor systems55. The value of µC* was extrapolated as the following equation:
where L, W, and d are the length, width, and thickness of the active channel size, and gm is the transconductance of OECT56. The Vg and VT represented the voltage operating and the threshold voltage, respectively. The µC* of pristine P3MEET was extracted to be 1.23 F V-1 cm-1 s-1, and the µC* as a function of blend composition was calculated and plotted (Fig. 2d). As a higher content of the radical polymer was added, the magnitude of μC* gradually increased until a significant rise at a loading of 50 wt% for PTES, and 200 wt% for PTEO. In our study, the highest μC* was achieved at the 50 wt% PTES in blend composite, with a value of up to 192 F V–1 cm–1 s–1, which is a notably high recorded value for P3MEET and TEMPO-species-containing OECTs42,44. A fast decay in the value of μC* was observed by further increasing the radical polymer’s loading content to the blend. This could be attributed to the less conductive intrinsic property of the radical polymer, where the amorphous region was dominant in the blend, leading to the degradation of OECT’s steady-state performance. This behavior corresponds to the variation in VT (Fig. 2e and Supplementary Fig. 6). The introduction of radical polymers into the P3MEET active layer leads to a shift in VT from 0.83 to ~0.03 V in PTES, and ~0.1 V in PTEO, respectively, indicating effective ion doping with the order of PTES > PTEO.
We observe a significant increase in μC* attributed to enhancements in both mobility (μ) and volumetric capacitance (C*), particularly in the case of the blending of P3MEET-PTES, as evident in Supplementary Table 2 and Fig. 2f-(i-iii). It can be explained by the main factor of when PTES was blended with P3MEET, it expanded the amorphous region and blocked the crystallinity of P3MEET. The amorphous region was expected for better water uptake and ion injection under voltage applied compared to its higher crystallinity packaging, benefiting volumetric capacitance and giving rise to the figure of merit43. Furthermore, the intra- or intermolecular sulfur-oxygen interaction due to the PTES in P3MEET could induce the backbone planarity of the thiophene ring (main charge carrier) with the enhancement of effective conjugated length of the polymer, which should favor efficient charge transport6,53,54. In contrast, a much higher content of radical polymer caused the disruption in the overall blend polymer in terms of electronic conductivity due to the dominant amorphous domain of the lower ION at a higher blend content of radical. This probably decreased mobility, leading to the gradual decay of µC* value with further radical polymer loading content.
Morphological and molecular structural changes in blended polymer films
To understand the morphology, change upon the insertion of radical polymer in EGlyated-conjugated polymer, topographies of the blend films were characterized with the combination of X-ray diffraction (XRD) (Fig. 3a, b) and high-resolution transmission electronic microscopy (TEM) (Supplementary Fig. 7). Notably, we focused on the blending of P3MEET-PTES, which shows the highest μC*. P3MEET exhibits the preferential orientation of lamellar spacing (1.9 nm) along the out-of-plane direction and π-π stacking (0.38 nm) occurring in the in-plane direction45,57. Figure 3a, b show how the lamellar spacing and the π-π stacking of P3MMET varied upon the addition of PTES. We find a shift of the (100) peak of P3MEET to a lower level 2θ, representing ~4% lattice expansion in the blending system. The π-π stacking distance does not change, however, the peak intensity was decreased by disrupting the crystallinity of P3MEET upon the addition of PTES (Fig. 3c). In addition, high-resolution TEM exhibits that the overall domain size of conjugated thiophene rings was slightly decreased in the blending system.
XRD pattern on a blend of P3MEET and PTES at a weight ratio of 1:0.5 at a low (2θ: 3.5–6) and b at high (2θ: 10–60) 2θ range. c Schematic illustration of the morphological change of P3MEET and blend system upon doping (NaCl 0.1 M). P3MEET lattice expands upon the addition of P3MEET-PTES composite, showing its ability to create sufficient space for ion infiltration. d Optimized geometries of P3MEET polymer (left) and the blending of P3MEET-PTES (right) after 1 ns MD simulations. e The molecular orientation angles in degrees of P3MEET polymer (left) and P3MEET-PTES (right) in each geometry.
Molecular dynamics (MD) simulations were conducted to gain deeper insights into how the molecular arrangement changes with the blending of radical polymers (Fig. 3d, e and Supplementary Fig. 8). The initial structure of the P3MEET and PTES blend was established by introducing PTES molecules between 40 aligned P3MEET polymers (Supplementary Fig. 8a). The 1 ns MD simulations were conducted with a time-step of 1.0 fs for each initial structure under NPT ensemble (Supplementary Fig. 8b). It was verified that in the absence of any other radical polymers (as depicted on the left in Fig. 3d), the π–π stacking arrangement of the polythiophene backbone (emphasized by the thick black line) experiences misalignment due to the entanglement of ethylene glycol function groups in P3MEET. The inclusion of PTES appears to exacerbate the π–π stacking, stemming from the creation of amorphous domains through PTES aggregation (the right in Fig. 3d and Supplementary Fig. 8c). Moreover, we quantified the molecular orientation of P3MEET in each geometry obtained from MD simulations as the azimuth and zenith angles with respect to the reference direction (denoted by the red arrow, which corresponds to the x-axis in Fig. 3e. For the P3MEET assembly (the left in Fig. 3e), it was discovered that the P3MEET polymers exhibited a strong orientation within the azimuth angles ranging from 0° to 15° and 165° to 180°, along with zenith angles between 90° to 120°. On the other hand, in the case of the blending of P3MEET-PTES (the right in Fig. 3e), the azimuth and zenith angles of P3MEET polymers were widely scattered rather than concentrated within a specific range. MD simulation corresponds to morphological analysis using XRD, revealing an increase in amorphous regions caused by the incorporation of radical polymers in mixed conditions in a rational manner.
Development and performance evaluation of OECT-based DA sensors with polymer matrix blends
The potential of polymer matrices with both open- and closed-structures as active channel materials in OECTs has been demonstrated for advanced biosensing, especially for dopamine (DA) detection. This capability is highlighted through real-time monitoring of the channel current (IDS-time) as DA is sequentially introduced at different concentrations into a phosphate-buffered saline (PBS) solution (pH = 7.4) (Fig. 4 and Supplementary Figs. 9–13). When constant VGS and VDS are applied, DA undergoes an oxidation reaction at the Ag/AgCl gate electrode as soon as it is introduced to the sensor system. This process generates protons (hydrated H+ ions), two electrons, and o-DA quinone as the primary product, leading to a measurable change in the IDS and producing the corresponding IDS-time response curve (Fig. 4a-i)4,58,59.
a-i Schematic of the flexible OECT-based biosensing mechanism for DA detection, relying on DA oxidation at the Ag/AgCl gate electrode in PBS buffer. a-ii Photographs of the biosensor in flat and bent states. a-iii Normalized IDS trace for P3MEET-PTES blended systems as active channel materials, under sequential DA additions from 1 pM to 1 μM. b Plot of normalized current response (NCR) vs. log[DA], with the curve showing an exponential fit to DA concentration. c Normalized IDS trace of the flexible OECT biosensor with alternating additions of DA (1 nM) and interferents-AA (10 nM), UA (10 nM), and UA (100 nM)-in PBS buffer. The upper graph shows AA and UA followed by DA, and the lower graph shows DA followed by AA and UA. Measurements were conducted at VGS = +0.5 V and VDS = -0.2 V. d Features include a comparison of flexible OECT-based DA detection limits for DA concentration relative to previously reported values. e IDS/IDS,max transient response at a continuous VDS with applied VGS of −0.5 V at 8 s for 50 s of composites. f The switching delay of pristine P3MEET and blend P3MEET-PTES. g Working stabilities of P3MEET and P3MEET-PTES film over 200 min, constant chemical cycling.
To leverage the high μC* characteristic of the P3MEET-PTES OECT, initial DA sensing experiments were conducted at VGS = −0.2 V, the gate bias corresponding to peak transconductance (gm) in our study (Supplementary Fig. 9). However, compared to the control condition (no DA added), the device exhibited negligible changes in IDS trace, even at DA concentrations as high as 10 nM. This lack of response can be attributed to the elevated baseline current at VGS = −0.2 V, which significantly impairs the detection of small variations in current caused by DA addition. Furthermore, a positive gate bias is essential to facilitate the oxidative conversion of DA into o-DA quinone at the gate electrode5. To address this requirement, we maintained a positive VGS at +0.5 V—an optimized setting that balances sensitivity, selectivity, and stability for DA detection in our OECT-based biosensor5,60.
Supplementary Figs. 10–12 demonstrate the conversion of biochemical to electronic signals in rigid OECT-based DA sensors using pristine P3MEET and P3MEET blended with PTES and PTEO at optimal loadings. These figures compare normalized IDS responses for sequential DA additions (1 pM to 1 μM) at 100 s intervals, calculated by taking the absolute value of the real-time IDS data and dividing each value by the minimum value in the dataset. The observed increase in the IDS during DA sensing is attributed to the doping of the P3MEET-PTES active layer by protons61, along with the synergistic interaction between P3MEET and PTES, resulting in a net enhancement of the channel current (details provided in the Supporting Information). Both pristine and blended films exhibit a characteristic IDS shift from baseline, showing a staircase pattern in response to DA oxidation at the gate electrode (Supplementary Figs. 10a, 11a, and 12a). The OECT sensor with PTES and PTEO shows a rapid and distinct channel current response to DA at ultralow concentrations, achieving a detection limit of 1 pM, ideal for biological DA monitoring. As DA concentration increases, the IDS step size consistently rises, shifting the IDS staircase to lower absolute values. Notably, the IDS step size in P3MEET-PTES surpasses that in P3MEET-PTEO, indicating improved DA detection (Supplementary Figs. 10a and 12a). In pristine P3MEET, IDS increase with DA but shows minimal response peaks compared to PTES and PTEO devices, with IDS saturation at 500 pM DA (Supplementary Figs. 11a and 12a). Supplementary Figs. 10b and 11b show a linear relationship between the logarithm of [CDA] and normalized current response (NCR), calculated as (I – I0)/I0, where I0 represents the current value when DA was not added and I represents the response current when DA was added to the OECT-based sensors58. The PTES-loaded blend demonstrated the highest correlation coefficient (R2), highlighting its potential for DA sensing applications. The selectivity of our OECT-based DA sensors was evaluated in the presence of substantial excesses of interferents, including ascorbic acid (AA) and uric acid (UA) (Supplementary Fig. 10c). In clinical DA analysis, these substances can interfere due to their high concentrations and similar oxidation potentials, complicating DA detection62,63. In the lower graph, adding 1 nM DA results in a significant IDS response that surpasses the minimal signals from 10-fold AA/UA (10 nM) and 100-fold UA (100 nM), primarily due to low oxidation currents from the interferents. The upper graph confirms that DA addition continues to produce a distinct IDS shift, regardless of prior AA/UA additions. These findings indicate minimal impact from AA and UA on DA detection, regardless of the order of introduction.
To assess sensor flexibility and reliability for practical use, PET was used as a flexible substrate for OECT-based DA sensors instead of rigid glass. Based on the results obtained from rigid OECT-based DA sensors, the P3MEET-PTES, demonstrating superior performance, was chosen for conducting the measurements of the flexible OECT-based DA biosensor. Figure 4a-ii illustrates the sensor’s ability to bend from 0° to 180°. Due to the exceptional cycling stability of the flexible P3MEET-PTES, the sensor retains the capability to detect DA at clinically relevant concentrations as low as 1 pM, displaying a characteristic IDS staircase with consistent IDS step size increases in response to DA oxidation, even after 500 bending cycles at a radius of curvature (R) of 21 mm (Fig. 4a-iii and Supplementary Fig. 12b). Additionally, an exponential relationship was observed between the logarithm of [CDA] and the NCR of the flexible biosensor, indicating that current variation amplitude becomes more pronounced as DA concentrations increase, with minimal reduction in the R² coefficient under bending conditions (Fig. 4b). The biosensor maintained high selectivity after 500 bending cycles, underscoring its suitability and reliability for practical applications (Fig. 4c and Supplementary Fig. 12c). In addition, the flexible OECT-based biosensor demonstrates a distinct IDS shift even when DA is added in a random sequence, regardless of prior AA or UA additions (Supplementary Fig. 13). These results indicate that the influence of AA and UA on DA detection is minimal, irrespective of the order of introduction, and confirm the reversibility of this sensing method. Our OECT-based DA sensors, utilizing open- and closed-polymer matrices as active electrode materials, demonstrate an outstanding clinical detection limit of 1 pM for DA along with remarkable ultraspecificity. These values meet or exceed those reported in comparable studies for DA detection (Fig. 4d)1,5,8,58,64,65,66,67,68.
In comparison to our previous work4, which utilized conjugated radical polymers resulting in excellent gm, this approach, employing non-conjugated radical polymers, achieves impressive μC* values. While both studies achieved a comparable DA detection limit of 1 pM in OECT-based biosensors, this study demonstrates advancements in applications by exploring the use of flexible substrates, emphasizing their practical advantages. We posit that precise control over the weight ratio of mixed-conduction polymers to TEMPO radical polymers across a broad compositional range, coupled with structural optimizations, active channel adjustments, and refined thin-film formation techniques, can enhance device performance even when employing mixed-conduction polymers with inherently good performance characteristics. Furthermore, the unique structural and chemical attributes of TEMPO radical polymers facilitate the formation of stable and synergistic blends with complementary materials. This synergy can augment the properties of moderately performing materials, thereby more effectively leveraging their contributions within the blend. Therefore, we believe that integrating TEMPO radical polymers with other mixed-conduction materials exhibiting moderate performance can lead to the development of high-performance OECT devices.
The time-dependent current response of the mixture OECTs was monitored by applying pulsed gate voltage and comparing the resulting transient response to the normalized IDS response. The kinetics of the response were enhanced with higher PTES loading (Fig. 4e). Specifically, the incorporation of PTES resulted in a substantial decrease in the switching delay, decreasing from 11.8 s for pristine P3MEET to approximately 2.9 s for P3MEET-PTES (channel thickness of 810 nm) and 1.8 s P3MEET-PTES (channel thickness of 150 nm) at a 1:0.5 weight ratio of P3MEET-PTES (Fig. 4f). Significantly, the thin films exhibited a considerably more pronounced reduction in the switching delay within the identical composite matrix. The improved response kinetics can be attributed to the increased presence of an ion-percolating system, facilitating a higher infiltration of ions to dope the P3MEET main chain42. Furthermore, while the P3MEET retained 0.2 of its initial current, P3MEET:PTES blend film exhibited a significantly improved value of 0.95, indicating that it retained 95% of its initial current (Fig. 4g). This stability is comparable to the highly recorded values of 75%, 87%, and 98% observed in benchmark pristine EGylated conjugated polymers such as p(g2T-TT), p(g2T2-g4T2), p(g1T2-g5T2), and p(g0T2-g6T2)21. We speculate that the increases in hydrophilicity upon the addition of PTES affect the ability to uptake water and stabilize the water molecules in the doped state21,69. Given that the morphology changes in the blending regime affect the volumetric capacitance, the strong interaction with the entanglement between the conjugated polymer and non-conjugated radical polymer could significantly influence the device stability of OECT in a rational manner.
Discussion
In our study, the inclusion of non-conjugated open-shell radical polymers in an EGylated-conjugated polymer has demonstrated the potential benefits of integrating the stable open-shell active units of radical into flexible OECT-based DA biosensor devices alongside conjugated polymer. In addition, the comparative study of PTES and PTEO highlights the importance of the design of polymer backbone chains in numerous features of OECT operation. The VT of the mixed composite, where the current is controlled within specific loading ranges, is determined by the redox-active nitroxide groups. The morphological characterization reveals the possibility of the precise control of ionic dopant infiltration at a specific working voltage, leading to a champion steady-state performance, at which the μC* exceeds 192 F V-1 cm-1 s-1, with the crystallinity of the conjugated polymers being effectively controlled. The inherent amorphous nature of radical polymers, along with their non-conjugated, hydrophilic backbone and redox-active TEMPO active units, makes it an appealing candidate for the macromolecule blend. Importantly, PTES, which has an ethylene sulfide backbone, stands out among other radical polymers for its superior performance in OECT. The key factor behind this achievement is the significant interaction between the sulfur atoms in the polymer backbone and the oxygen atoms in the EGylated-conjugated polymer side chain. The interaction plays a crucial role in optimizing the figure of merit by precisely adjusting the crystallinity of the conjugated polymer’s main chain.
The fabricated flexible OECT-based biosensor achieves an ultralow detection limit of 1 pM for DA, with significant current response amplitude and a strong correlation coefficient, demonstrating high selectivity even in the presence of excess UA and AA interferents, underscoring its potential for DA sensing applications. Consequently, we propose the design principle for highly efficient flexible OECT-based DA biosensor devices. The materials with these characteristics will be extremely valuable for the increasing range of applications that depend on effective mixed electronic and ionic conduction.
Methods
Materials
All reagents were purchased and used in their original states without further purification. The 4-glycidyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl (GTEMPO), 1-butanethiol (C4H9SH, nBuSH), 1,8-diazabicyclo (5.4.0) undec-7-ene (DBU), were obtained from TCI Tokyo. Iodomethane (CH3I), p-toluenesulfonamide (TsNH2), methanesulfonyl chloride (MsCl), benzyl amine (BnNH2) and potassium bis(trimethylsilyl)amide (KHMDS, 0.5 M in toluene), 3-bromothiophene, N-bromosuccinimide (NBS), methylmagnisium chloride (CH3MgCl, 3M in ether), phosphate-buffered saline (PBS, pH = 7.4), uric acid ( ≥ 99%, crystalline), L-ascorbic acid (99%) were purchased from Sigma-Aldrich. The solvents of diethyl ether ((C2H5)2O), tetrahydrofuran (THF), hexane (HPLC grade), 1,4-dioxane, and acetonitrile were purchased from Daejung Co., Korea. Dopamine hydrochloride (DA, 99%) was purchased from Thermo Fisher Scientific Korea Ltd., Seoul, South Korea.
Radical polymer preparation
Preparation of PTEO
The synthesis of PTEO was followed by our previous work (Supplementary Scheme 1a)43. To a solution of TEO (300 mg, 1.31 mmol) and 18-crown-6 (48 mg, 0.18 mmol) in diglymer (0.2 mL) was added 1 M t-BuOK solution in THF (61 μL, 0.061 mmol) under an argon atmosphere to initiate the polymerization. The mixture was stirred at 40 °C for 20 h. The resulting viscous mixture was dissolved in chloroform and slowly poured into hexane. The precipitate was collected by centrifugation and dried under vacuum at room temperature to yield PTEO as a red, viscous solid. The 1H NMR spectrum of PTEO is available in our previous work.
Preparation of PTES
The synthesis of PTES was followed by our previous work (Supplementary Scheme 1b)26. In brief, the polymerization was conducted at room temperature in an argon-filled glove box to avoid water and oxygen. First, 1,8-diazabicyclo(5.4.0) undec-7-ene (DBU) (0.21 mmol) and C4H9SH (0.21 mmol) were dissolved in THF (1 mL) and stirred for 1 hour to obtain the initiator solution. After dissolving the monomer 1 (525 mg, 2.15 mmol) in 2 mL THF, the initiator solution (0.2 mL, [monomer]/[initiator] = 50/1) was added to initiate polymerization. After stirring for 2 h at room temperature, 100 equivalent amounts of iodomethane (against the initiator) were added for quenching. The mixture was purified by precipitation from diethyl ether to obtain the desired polymer PTES. The 1H NMR spectrum of PTES is available in our previous work.
Synthesis of 3-[2-(2-methoxyethoxy)ethoxy]thiophene (Supplementary Scheme 2, compound 2)
In a 500 mL three-neck round bottom flask under an inert atmosphere, 0.74 g of NaH (0.018 mol, 60% in mineral oil) was suspended in 20 mL of anhydrous DMF. After cooling to 0 °C, diethylene glycol monomethyl ether (10 mL) was added dropwise, and the reaction was allowed to proceed for an additional hour at room temperature to ensure the completion of the reaction. Then, 3-bromothiophene (2.0 g, 0.012 mol) and CuBr (175.9 mg, 1.22 mmol) were added to the reaction, and the resulting mixture was then heated to 110 °C in an oil bath for 12 h. After completion, the reaction was extracted with a saturated solution of NH4Cl by ether (x3 times). The organic layers were collected, dried with MgSO4, and concentrated. The residue was purified by flask chromatography (EA:nHex = 1:3) to obtain the glycolated product 2 as a colorless liquid.
Representative synthesis of 2,5-dibromo-3-[2-(2-methoxyethoxy)ethoxy]-thiophene (Supplementary Scheme 2, compound M3)
Compound 2 (1.0 g, 4.94 mmol) was dissolved in 20 mL mixture of THF/AcOH (1:1) under an inert atmosphere. The resulting solution was cooled in an ice-bath, and N-bromosuccinimide (NBS) (1.93 g, 10.87 mmol) was added portion-wise. The reaction mixture was stirred for 6 h at room temperature. After completion, the reaction was washed by saturated NaHCO3 by ether (x3 times). The organic layers were collected, dried with MgSO4, and concentrated. The residue was purified by flask chromatography (EA:nHex = 1:3) to obtain dibromo-monomer M3 as a pale yellow liquid.
Synthesis of P3MEET
The preparation of P3MEET followed the previous work with modification (Supplementary Scheme 2). Under argon atmosphere, 500 mg of M3 (1.39 mmol) was dissolved in 12 mL anhydrous THF in a 50 mL vial. Subsequently, 0.44 mL of 3 M CH3MgCl (1.33 mmol) was then added to the vial, and the resulting mixture was allowed to stir for 1 h at room temperature. 3.8 mg of Ni(dppp)Cl2 (0.007 mmol) dissolved in 2 mL of anhydrous THF was then added to the reaction mixture via a syringe. The polymerization was allowed to proceed for 12 h at 70 °C. The reaction mixture was then allowed to cool down to room temperature and precipitated by adding 100 mL of methanol. The polymers were filtered and purified by Soxhlet extraction in the sequence of methanol, hexane, and CHCl3. Chloroform was later removed under reduced pressure. Residues were dried under vacuum to yield the regioregular P3MEET.
Polymer characterization
The 1H NMR spectra were recorded on an Agilent (600 MHz) spectrometer with TMS as an internal standard. Electron paramagnetic resonance (EPR) spectroscopy experiments were measured using a Bruker EMX-EPR spectrometer. For the solution EPR experiments, the PTES was dissolved in chloroform and transferred into the EPR tubes before placing them in the holder. The set-up experiment was kept under a given temperature for 10 min to allow for thermal equilibration. TEM images were collected on a transmission electron microscope (Titan G2, FEI) with an accelerating voltage of 200 kV. X-ray diffraction (XRD) measurements were made using a Rigaku X-ray imaging system with Cu Kα radiation, λ = 0.15418 nm, 30 mA, and 40 kV (energy step size in the narrow scan of 0.1 eV).
Electrochemical characterization
The cyclic voltammetry was carried out using the three-electrode set-up configuration, where a platinum coil and Ag/AgCl were used as the counter electrode and reference electrode, respectively. The working electrode was in an active area of 1 cm2 on an Interface 1010E, Potentiostat/Galvanostat/ZRA, 27188 Workstation. The scan voltage was swept from −0.2 V to +1 V relative to the reference electrode. All electrochemical assessments were conducted in ultrapure water with a conductivity of 18.2 MΩ·cm and a 0.1 M NaCl solution. Cyclic voltammetry (CV) measurements employed a three-electrode configuration, featuring Ag/AgCl as the reference electrode, a platinum coil as the counter electrode, and a gold working electrode with an active area of 10 mm², utilizing a VersaSTAT 3 Workstation. Voltage scanning ranged from −0.1 V to +0.9 V relative to the reference electrode. Spectroelectrochemical readings were obtained using a customized set-up that combined a Biologic SP-150 potentiostat and an Agilent Cary 5000 UV/Vis-NIR spectrophotometer, with the working electrode being tin-doped indium oxide-coated (ITO-coated) glass. Capacitance was measured using polymer-coated electrodes with electrochemical impedance spectroscopy (EIS) at a frequency range of 100 kHz to 0.1 Hz. The extracted capacitance values were normalized by the measured film volume to determine volumetric capacitance (C*). The Randle’s circuit was used for fitting, Relectrotlye(Rpolymer‖Cpolymer).
Organic electrochemical transistor (OECT) device fabrication and characterization
The microscope glass slide (26 × 76 mm) was used as the substrate and underwent the cleaning process by bath sonication in acetone, isopropyl alcohol, and DI water. After that, the substrates were baked on a hot template at 100 °C for 60 s. The source/drain contacts were patterned by using a metal evaporator deposition of Chromium (Cr, 10 nm) and gold (Au, 50 nm) at a pressure of 5 × 10-6 bar at a speed of 0.1 nm/s to create the bottom contact electrodes on the glass substrates. Herein, the Cr layer served as an adhesion of the Au electrodes and substrate glass. Arrays of transistors were fabricated on the glass substrates with a channel length of 5000 µm and a channel width of 50 µm.
The mixture solution of conjugated polymer P3MEET and the radical polymer was mixed, and the desired composite was listed in Supplementary Table 1. The mixed conductor polymer system was stirred overnight at 250 rpm at 40 °C. The polymer thin films were deposited in a channel size by the drop-casting method, following the hot-baked step at 80 °C for 60 min in a vacuum oven. The electrolyte (100 µL, NaCl 0.1 M) was confined with the help of a PDMS mold. The OECT performance was measured in the three-point geometry on a Keithley 4200-SCS (Tecktronics) source meter with a gate electrode of pellet-type Ag/AgCl (E205, Warner Instrument). The thickness of the film on the active channel size was defined by using the microfigure measuring instrument (Surface profiler, ET-200). The threshold voltage was defined by extracting the x-intercept of the linear fit of the sqrt(IDS) vs. VGS plots at measuring the condition of VDS = −0.2 V and sweep rate voltage of 10 mV s-1. The gm was defined as the first derivative of the IDS/VGS curve.
Fabrication and characterization of a flexible OECT-based dopamine biosensor
The flexible OECT was fabricated using the standard OECT set-up, substituting the glass substrate with a commercial PET flexible substrate. Dopamine (DA) sensing performance was evaluated by measuring the channel current (IDS) in response to sequential 35 µL additions of various DA concentrations, diluted in PBS buffer (pH = 7.4). For selectivity testing, a 35 µL volume of 10 nM DA solution, along with interferents (10 nM AA, 10 nM UA, and 100 nM UA), was introduced sequentially at regular intervals. The experiments were conducted at a constant gate voltage (VGS) of +0.5 V and a source-drain voltage (VDS) of −0.2 V.
Topological analysis
The topographies of the blend were characterized by atomic force microscope (AFM). The prepared solution of all blends was spin-coated on the silicon wafer (1 × 1 cm) at 1000 rpm for 60 s. The thin film was dried in a vacuum oven at 80 °C for 30 min before undergoing the measurement. Transmission electron microscopy (TEM) images were obtained using a Titan G2 electron microscope manufactured by FEI, operating at an acceleration voltage of 200 kV.
Simulation details
The Gromacs packagesS1 was employed with the general AMBER force field (GAFF). P3MEET and PTES polymers were built with 6 repeat units each. For the initial structure of the P3MEET-only assembly, a model was created using 40 well-aligned P3MEET polymers. The initial structure of the P3MEET and PTES blend was established by introducing PTES molecules between 40 aligned P3MEET polymers (Supplementary Fig. 8a). 1 ns MD simulations were conducted with a time-step of 1.0 fs for each initial structure under NPT ensemble (Supplementary Fig. 8b). A constant pressure of 1 atm was maintained and controlled using the Parrinello-Rahman barostat, while the temperature was held at 25 °C through the Berendsen thermostat. Nonbonding interactions were subjected to neighbor searching every 10 fs within a 1.2 nm cut-off sphere. Electrostatic interactions were calculated using the particle mesh Ewald (PME) algorithm with a spatial cut-off of 0.9 nm, and a Fourier grid spacing of 0.09 nm was employed. To explore the molecular orientation of P3MEET polymers after MD simulations, the rotation matrix for the polymers was computed relative to the initial alignment direction, and the coordinates of each molecule were then transformed into the spherical coordinate system. The average spherical coordinates of atoms within each molecule were normalized with respect to the radial distance, “r” and the molecular orientation was represented with azimuthal (θ) and zenith (ϕ) angles shown in Fig. 3e.
Data availability
No datasets were generated or analyzed during the current study.
References
Tseng, H.-S., Chen, Y.-L., Zhang, P.-Y. & Hsiao, Y.-S. Additive blending effects on PEDOT:PSS composite films for wearable organic electrochemical transistors. ACS Appl. Mater. Interfaces 16, 13384–13398 (2024).
Ohayon, D., Druet, V. & Inal, S. A guide for the characterization of organic electrochemical transistors and channel materials. Chem. Soc. Rev. 52, 1001–1023 (2023).
LeCroy, G. et al. Role of aggregates and microstructure of mixed-ionic–electronic-conductors on charge transport in electrochemical transistors. Mater. Horiz. 10, 2568–2578 (2023).
Nguyen, D. C. T. et al. Conjugated radical polymer-based organic electrochemical transistors for biosensing devices. Chem. Mater. 36, 7897–7908 (2024).
Tang, K. et al. Organic electrochemical transistor with molecularly imprinted polymer-modified gate for the real-time selective detection of dopamine. ACS Appl. Polym. Mater. 4, 2337–2345 (2022).
Giovannitti, A. et al. Controlling the mode of operation of organic transistors through side-chain engineering. Proc. Natl Acad. Sci. 113, 12017–12022 (2016).
Li, N. et al. A universal and facile approach for building multifunctional conjugated polymers for human-integrated electronics. Matter 4, 3015–3029 (2021).
Li, W., Jin, J., Xiong, T., Yu, P. & Mao, L. Fast‐scanning potential‐gated organic electrochemical transistors for highly sensitive sensing of dopamine in living rat brain. Angew. Chem. 134, e202204134 (2022).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Zhao, Z., Tian, Z. & Yan, F. Flexible organic electrochemical transistors for bioelectronics. Cell Rep. Phys. Sci. 4, 101673 (2023).
Kim, H. et al. Organic mixed ionic–electronic conductors for bioelectronic sensors: materials and operation mechanisms. Adv. Sci. 11, 2306191 (2023).
McCulloch, I. et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 5, 328–333 (2006).
Funahashi, M., Zhang, F. & Tamaoki, N. High ambipolar mobility in a highly ordered smectic phase of a dialkylphenylterthiophene derivative that can be applied to solution-processed organic field-effect transistors. Adv. Mater. 19, 353–358 (2007).
Pisula, W. et al. A zone-casting technique for device fabrication of field-effect transistors based on discotic hexa-peri-hexabenzocoronene. Adv. Mater. 17, 684–689 (2005).
Di, C.-a, Zhang, F. & Zhu, D. Multi-functional integration of organic field-effect transistors (OFETs): advances and perspectives. Adv. Mater. 25, 313–330 (2013).
Tropp, J., Meli, D. & Rivnay, J. Organic mixed conductors for electrochemical transistors. Matter 6, 3132–3164 (2023).
Wang, Y. et al. Designing organic mixed conductors for electrochemical transistor applications. Nat. Rev. Mater. 9, 249–265 (2024).
Wang, Y. et al. An optoelectrochemical synapse based on a single-component n-type mixed conductor. Nat. Commun. 16, 1–14 (2025).
Wang, Y. et al. Acceptor functionalization via green chemistry enables high‐performance n‐type organic electrochemical transistors for biosensing, memory applications. Adv. Funct. Mater. 34, 2304103 (2024).
Wang, Y. et al. Green synthesis of lactone‐based conjugated polymers for n‐type organic electrochemical transistors. Adv. Funct. Mater. 32, 2111439 (2022).
Moser, M. et al. Side chain redistribution as a strategy to boost organic electrochemical transistor performance and stability. Adv. Mater. 32, 2002748 (2020).
Wilcox, D. A. et al. Tuning the interfacial and energetic interactions between a photoexcited conjugated polymer and open-shell small molecules. Soft Matter 15, 1413–1422 (2019).
Lutkenhaus, J. A radical advance for conducting polymers. Science 359, 1334–1335 (2018).
Wang, S., Li, F., Easley, A. D. & Lutkenhaus, J. L. Real-time insight into the doping mechanism of redox-active organic radical polymers. Nat. Mater. 18, 69–75 (2019).
Wang, S., Easley, A. D. & Lutkenhaus, J. L. 100th anniversary of macromolecular science viewpoint: fundamentals for the future of macromolecular nitroxide radicals. ACS Macro Lett. 9, 358–370 (2020).
Sato, K. et al. Diffusion-cooperative model for charge transport by redox-active nonconjugated polymers. J. Am. Chem. Soc. 140, 1049–1056 (2018).
Rostro, L., Baradwaj, A. G. & Boudouris, B. W. Controlled radical polymerization and quantification of solid state electrical conductivities of macromolecules bearing pendant stable radical groups. ACS Appl. Mater. Interfaces 5, 9896–9901 (2013).
Joo, Y., Agarkar, V., Sung, S. H., Savoie, B. M. & Boudouris, B. W. A nonconjugated radical polymer glass with high electrical conductivity. Science 359, 1391–1395 (2018).
Yu, I., Jeon, D., Boudouris, B. & Joo, Y. Mixed Ionic and electronic conduction in radical polymers. Macromolecules 53, 4435–4441 (2020).
Thi, Q. V. et al. Conductive glassy nonconjugated open-shell radical polymer with organosulfur backbone for macroscopic conductivity. JACS Au 4, 690–696 (2024).
Hay, M. E., Hui Wong, S., Mukherjee, S. & Boudouris, B. W. Controlling open‐shell loading in norbornene‐based radical polymers modulates the solid‐state charge transport exponentially. J. Polym. Sci. Part B Polym. Phys. 55, 1516–1525 (2017).
Tomlinson, E. P., Hay, M. E. & Boudouris, B. W. Radical polymers and their application to organic electronic devices. Macromolecules 47, 6145–6158 (2014).
Ko, J., Nguyen, Q. H., Thi, Q. V. & Joo, Y. Recent advances in open-shell mixed conductors—From molecular radicals to polymers. Chem. Phys. Rev. 4, 041310 (2023).
Ko, J. et al. Mapping out the nonconjugated organic radical conductors via chemical or physical pathways. JACS Au 2, 2089–2097 (2022).
Jo, Y., Yu, I., Ko, J., Kwon, J. E. & Joo, Y. Sequential codoping making nonconjugated organic radicals conduct ionically electronically. Small Sci. 2, 2100081 (2022).
Suga, T., Pu, Y.-J., Oyaizu, K. & Nishide, H. Electron-transfer kinetics of nitroxide radicals as an electrode-active material. Bull. Chem. Soc. Jpn. 77, 2203–2204 (2004).
Oyaizu, K., Ando, Y., Konishi, H. & Nishide, H. Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage purposes. J. Am. Chem. Soc. 130, 14459–14461 (2008).
Xie, Y., Zhang, K., Yamauchi, Y., Oyaizu, K. & Jia, Z. Nitroxide radical polymers for emerging plastic energy storage and organic electronics: fundamentals, materials, and applications. Mater. Horiz. 8, 803–829 (2021).
Tokue, H., Murata, T., Agatsuma, H., Nishide, H. & Oyaizu, K. Charge–discharge with rocking-chair-type Li+ migration characteristics in a zwitterionic radical copolymer composed of TEMPO and trifluoromethanesulfonylimide with carbonate electrolytes for a high-rate li-ion battery. Macromolecules 50, 1950–1958 (2017).
Ma, T. et al. Nonconjugated redox-active polymers: electron transfer mechanisms, energy storage, and chemical versatility. Annu. Rev. Chem. Biomol. Eng. 14, 187–216 (2023).
Ma, T., Li, C.-H., Thakur, R. M., Tabor, D. P. & Lutkenhaus, J. L. The role of the electrolyte in non-conjugated radical polymers for metal-free aqueous energy storage electrodes. Nat. Mater. 22, 495–502 (2023).
Kim, H. J. et al. Radical polymer-based organic electrochemical transistors. ACS Macro Lett. 11, 243–250 (2022).
Flagg, L. Q. et al. Polymer crystallinity controls water uptake in glycol side-chain polymer organic electrochemical transistors. J. Am. Chem. Soc. 141, 4345–4354 (2019).
Schmode, P. et al. The key role of side chain linkage in structure formation and mixed conduction of ethylene glycol substituted polythiophenes. ACS Appl. Mater. Interfaces 12, 13029–13039 (2020).
Dong, B. X. et al. Influence of side-chain chemistry on structure and ionic conduction characteristics of polythiophene derivatives: a computational and experimental study. Chem. Mater. 31, 1418–1429 (2019).
Mahapatro, S. N. et al. TEMPO synthesis, characterization and catalysis: an integrated upper-division laboratory. J. Chem. Educ. 101, 5449–5459 (2024).
Ko, J. et al. A nonconjugated radical polymer enables bimodal memory and in-sensor computing operation. Sci. Adv. 10, eadp0778 (2024).
Li, Y. et al. Novel membrane-free hybrid capacitive deionization with a radical polymer anode for stable desalination. Desalination 481, 114379 (2020).
Liu, T., Wei, X., Nie, Z., Sprenkle, V. & Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte. Adv. Energy Mater. 6, 1501449 (2016).
Xie, Y., Zhang, K., Monteiro, M. J. & Jia, Z. Conjugated nitroxide radical polymers: synthesis and application in flexible energy storage devices. ACS Appl. Mater. Interfaces 11, 7096–7103 (2019).
Li, F., Gore, D. N., Wang, S. & Lutkenhaus, J. L. Unusual internal electron transfer in conjugated radical polymers. Angew. Chem. 129, 9988–9991 (2017).
Zhao, H., Zhu, B., Sekine, J., Luo, S.-C. & Yu, H.-h Oligoethylene-glycol-functionalized polyoxythiophenes for cell engineering: syntheses, characterizations, and cell compatibilities. ACS Appl. Mater. Interfaces 4, 680–686 (2012).
Zhang, X., Gong, Z., Li, J. & Lu, T. Intermolecular sulfur··· oxygen interactions: theoretical and statistical investigations. J. Chem. Inf. Model. 55, 2138–2153 (2015).
Guo, X. et al. Dialkoxybithiazole: a new building block for head-to-head polymer semiconductors. J. Am. Chem. Soc. 135, 1986–1996 (2013).
Zeglio, E. & Inganäs, O. Active materials for organic electrochemical transistors. Adv. Mater. 30, 1800941 (2018).
Bernards, D. A. & Malliaras, G. G. Steady‐state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 17, 3538–3544 (2007).
Dong, B. X. et al. Structure control of a π-conjugated oligothiophene-based liquid crystal for enhanced mixed ion/electron transport characteristics. ACS Nano 13, 7665–7675 (2019).
Qing, X. et al. Wearable fiber-based organic electrochemical transistors as a platform for highly sensitive dopamine monitoring. ACS Appl. Mater. Interfaces 11, 13105–13113 (2019).
Lin, P., Yan, F. & Chan, H. L. Ion-sensitive properties of organic electrochemical transistors. ACS Appl. Mater. Interfaces 2, 1637–1641 (2010).
Tang, H., Lin, P., Chan, H. L. & Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 26, 4559–4563 (2011).
Zhang, L. et al. Proton-penetrable Nafion-induced phase separation in organic semiconductors for high-performance organic electrochemical transistors. J. Mater. Chem. C. 11, 7272–7282 (2023).
Gualandi, I. et al. Selective detection of dopamine with an all PEDOT: PSS organic electrochemical transistor. Sci. Rep. 6, 35419 (2016).
Liao, C., Zhang, M., Niu, L., Zheng, Z. & Yan, F. Organic electrochemical transistors with graphene-modified gate electrodes for highly sensitive and selective dopamine sensors. J. Mater. Chem. B 2, 191–200 (2014).
Ji, W. et al. Carbonized silk fabric-based flexible organic electrochemical transistors for highly sensitive and selective dopamine detection. Sens. Actuators, B 304, 127414 (2020).
Park, S. J. et al. High-performance conducting polymer nanotube-based liquid-ion gated field-effect transistor aptasensor for dopamine exocytosis. Sci. Rep. 10, 3772 (2020).
Zhang, Y. et al. Adaptive biosensing and neuromorphic classification based on an ambipolar organic mixed ionic–electronic conductor. Adv. Mater. 34, 2200393 (2022).
Qiu, J. et al. A flexible organic electrochemical synaptic transistor with dopamine-mediated plasticity. IEEE Electron Device Lett. 44, 176–179 (2022).
Gamboa, J. et al. Carbon quantum dots composite for enhanced selective detection of dopamine with organic electrochemical transistors. Microchim. Acta 191, 639 (2024).
Moser, M. et al. Ethylene glycol-based side chain length engineering in polythiophenes and its impact on organic electrochemical transistor performance. Chem. Mater. 32, 6618–6628 (2020).
Acknowledgements
This work was supported by the Korea Institute of Science and Technology (KIST, Korea) Institutional Program. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2023-00208313 and 2022K1A3A1A91093969). This work was supported by the Glocal University 30 Project Fund of Gyeongsang National University in 2024.
Author information
Authors and Affiliations
Contributions
D.C.T.N., Q.V.T., and Q.H.N. contributed equally to this work. D.C.T.N. and Q.V.T. conceived and designed the experiments, drafted the original manuscript, and revised subsequent drafts. Q.H.N. carried out the material synthesis and contributed to both the original manuscript preparation and its revision. J.K. was responsible for device and system preparation. H.L. performed supplementary tests for the revised draft. B.W.B. and S.-Y.J. conducted simulations, device testing, and characterization. Y.J. provided supervision, conceptualization, visualization, validation, and contributed to both the original manuscript preparation and its revision. All authors contributed to the analysis and discussion of the results and have approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nguyen, D.C.T., Vu Thi, Q., Nguyen, Q.H. et al. Engineering flexible dopamine biosensors: blended EGylated conjugated and radical polymers in organic electrochemical transistors. npj Flex Electron 9, 35 (2025). https://doi.org/10.1038/s41528-025-00412-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41528-025-00412-9