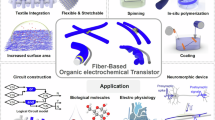
Abstract
Organic electrochemical transistors (OECTs) are emerging organic semiconducting devices intensively used in biological detection, environmental monitoring, biomimetic electronics, and computing circuits, due to the high transconductance, low working voltage, and exceptional biocompatibility. Most reported OECTs are based on planar structures built by two dimensional (2D) semiconducting materials, which have found great challenges of rigid architecture, complicated fabrication, and small-scale production. To improve overall performance and extend the use of OECTs into wearables, integralization, miniaturization, and intellectualization, researchers have made intensive efforts to use 1D conducting polymer fibres as active channel for building new breed of fibre-based OECTs, namely F-OECTs. Here we present the research progress of F-OECTs along three lines: working principles, evaluation methods, and applications. Covering from P-type polymer to N-type polymer, various kinds of conducting polymers have been processed into channel materials of F-OECTs through mainstream wet spinning methods. The prepared F-OECTs have been widely used in in vivo recording, in vitro detection, neuromorphic sensing, and logical circuits. To conclude this review, we summarized current challenges in terms of performance optimization and material innovation, further suggesting possible solutions. This review could provide guidance for understanding the working principles of F-OECTs, designing high-performance F-OECTs, and fabricating advanced electronics.

Similar content being viewed by others
Introduction
Organic transistors, including organic field-effect transistor (OFET), electrolyte-gated organic field-effect transistor (EGOFET) and organic electrochemical transistor (OECT), have a wide range of applications in flexible display technology, wearable sensors, and other cutting-edge fields due to their flexibility and biocompatibility1. As shown in Fig. 1a, OFET is a transistor that uses an organic semiconductor to modulate charge carriers via an electric field without involving ions or electrochemical processes2. EGOFET uses an electrolyte as the gate dielectric, creating an electric double layer to modulate the organic semiconductor at low operating voltages3. OECT operates by modulating the mobility of mixed charge carriers in an organic semiconductor via electrochemical doping or de-doping. Unlike OFETs, in OECTs, ions from an electrolyte penetrate the organic semiconductor, altering its doping state and, consequently, its conductivity4,5. Among the organic transistors, OECTs have received particular attentions as the combination of electronic manipulation and electrochemical response to control current flow. Due to their unique electrochemical behaviors, OECTs are able to amplify electrical signals by electrochemically modulating the active channel, where a small change in the voltage between the gate electrode and the drain-source electrode can cause a significant change in the conductivity of the active channel, permitting an intrinsically amplified of the electrical signal. As a result, OECTs have shown exceptional potential for bioelectronics and chemical sensing, further attracting widespread attention6,7.
a The mechanism of OFET, EGOFET and OECT; b the research publications and citations data in Web of Science for the terms “Organic Electrochemical Transistors”; c the key milestones in the evolution of OECT and F-OECT in the past 40 years8,9,13,14,26,32,107,119,128,135,136. Reprinted with permission8. Copyright 1984, American Chemical Society. Reprinted with permission135. Copyright 1984, American Chemical Society. Reprinted with permission136. Copyright 1990, Springer Nature Limited. Reprinted with permission26. Copyright 2007, Springer Nature Limited. Reprinted with permission9. Copyright 2007, WILEY-VCH Verlag GmbH & Co. KGaA. Reprinted with permission13. Copyright 2011, American Physical Society. Reprinted with permission14. Copyright 2011, American Chemical Society. Reprinted with permission119. Copyright 2024, The Royal Society of Chemistry. Reprinted with permission107. Copyright 2023, Springer Nature. Reprinted with permission32. Copyright 2021, The Authers. Published by American Association for the Advancement of Science. Reprinted with permission128. Copyright 2023, The Royal Society of Chemistry.
OECTs were invented by Mark S. Wrighton and his colleagues for functionalizing microelectrode array in contact with electrolyte solution in 1984, which propelled the development of microelectronics from solid devices to solution-based surroundings8. As an electrochemically active and ion-driven device, OECT mainly used conducting polymers, such as poly(3,4-ethylenedioxythiophene): poly(styrene sulfonic acid) (PEDOT:PSS), as the channel materials. In 2007, Bernards, D. A., and Malliaras, G. G. provided a comprehensive analysis of the steady-state and transient behavior of OECTs, contributing to the understanding of their operation and performance9. Starting from the early 2000s, researchers have proposed different theoretical models, such as Equivalent Circuit module10, Drift-Diffusion module11, and Poisson-Nernst-Planck module12, to understand the fundamental principles of OECTs, further enhancing device performance, and finding potential applications. Based on this, Rivnay, J., et al. explored the use of OECTs for biological applications, particularly in cell-based impedance sensing, highlighting their potential in bioelectronics furthering optimizing OECTs by tuning the channel thickness of the devices13,14,15. To meet the requirements for various applications, the development of high-transconductance OECTs with high sensitivity and regulation ability became necessary. Ever since the invention of OECTs over 40 years ago, they have received a great deal of attention among the researchers and industry (Fig. 1b, c). These endeavors, including micro-nanofabrication techniques, developing N-type polymers, and innovated geometry of OECTs, have solidified the role of OECT in in vivo recording16,17,18, in vitro detection19,20,21,22, biomimetic electronics17,23,24,25, and logic circuits26,27,28.
OECTs have emerged as promising components in bioelectronics due to their inherent advantages, including biocompatibility, ion-to-electron transduction efficiency, and low operating voltages. However, conventional OECTs predominantly adopt planar architectures based on two-dimensional thin-film channel semiconductors29,30,31, which impose significant constraints. Specifically, their rigid planar geometry make them less adaptable to dynamic, irregular, or curved surfaces, limiting their integration and effectiveness in implantable biosensors, wearable health monitoring devices, neuromorphic sensing platforms, and flexible logic circuits. F-OECTs, utilizing the intrinsic advantages of fibre substrates, such as lightweight construction, exceptional flexibility, conformability to complex geometries, ease of fabrication, and low cost, offer a compelling solution to these challenges. By combining one-dimensional fibre morphology with favorable bio-interfacing properties, F-OECTs effectively overcome the geometric limitations and electrolyte patterning constraints associated with planar devices, facilitating integration into textiles, wearable bioelectronics, and implantable systems16,26,32,33. The first F-OECT was demonstrated by Olle Inganäs in 2007, utilizing fibres coated with PEDOT:PSS to fabricate wire electrochemical transistor devices16,26. This pioneering work demonstrated the feasibility of integrating fibres with organic semiconductors, sparking significant interest in the research community. Since then, diverse fibre types and fabrication techniques have been explored, significantly broadening the functional capabilities and application scope of F-OECTs. This review aims to critically examine the operating principles, performance evaluation methodologies, and recent advancements in F-OECT technologies, highlighting their unique benefits and potential applications in flexible and wearable bioelectronics.
As the developing of OECTs and F-OECTs, we collected most inspiring findings and new avenues in this domain in this review. We highlighted the fundamental mechanisms and evaluation methods to understand the working principle of F-OECTs, as well as recent optimization strategies through material design and innovated fabrication. The optimized devices, with high sensitivity, fast response, good biocompatibility, have found extensive uses in biosensors, wearable electronics, electrochemical devices, soft robots and human-machine interfaces (HMI). Finally, we prospected new trends and outlooked the future of F-OECTs.
Overview of F-OECTs
Working principle of F-OECTs
F-OECTs are based on the traditional three-electrode system of gate, source, and drain, in which electrolyte solution is immersed in the active channel layer formed between the source and the drain electrodes, as shown in Fig. 2a. By adjusting the voltage of gate/electrolyte interface and electrolyte/channel interface, the biochemical signal can be amplified to the electrochemical signal. Driven by the gate voltage, the ions in the electrolyte inject into or out of the channel, resulting in the oxidation or reduction of the doped organic semiconductor layer34. This leads to the variation of the current between the source and drain electrode so that the modulation of current can be regulated by gate voltage signal as shown in Fig. 2b. To use the most frequently used conductive polymers materials, PEDOT: PSS, as an example, when applying forward a positive voltage, cations in the electrolyte will penetrate into PEDOT: PSS, forming the non-conductive reduction state PEDOT:PSS0 together with conductive oxidation state PEDOT:PSS+35. Owing to this process, the resistance of the organic semiconductor increases, resulting in the drop in the current between the channels. When the applied voltage is removed, the cations injected into the conducting polymers will extract back to electrolyte, and the reduction state will also reverse to oxidation state PEDOT: PSS+. Both the injection and extraction process of cations as well as the conductive change of PEDOT: PSS are reversible, which can be described as following chemical equation:
a, b cross-section; the diagram of F-OECTs’ operation modes, c depletion mode and d accumulation mode; diagram of a typical; e the schematic of conventional ionic transport (ions penetrate into the channel manly dominated by vertical pathway) and LI-assisted ionic transport (ion transports through a synergistic effect of the lateral and vertical pathways); f subthreshold137, g transfer curve137, and h output curve137. Reprinted with permission137. Copyright 2022, Springer Nature Limited.
This reversible redox reaction serves as the foundation for the electrochemical modulation observed in OECTs34,35,36,37.
F-OECTs can operate in two distinct modes, i.e., depletion mode and accumulation mode, depending on the intrinsic doping state of the channel material and its response to the gate voltage. In depletion-mode shown in the Fig. 2c, the channel is intrinsically doped and conductive without gate voltage. For instance, PEDOT:PSS-based F-OECTs typically operate in depletion mode because PEDOT:PSS is naturally in a doped, conductive state38,39. When a positive gate voltage is applied, cations from the electrolyte are injected into the channel, pairing with PSS- anions. This causes holes to be extracted at the drain electrode, leading to the de-doping of the channel and a reduction in the drain current (ID)40. In contrast, accumulation-mode F-OECTs, shown in Fig. 2d, are based on intrinsically undoped channel materials, which are non-conductive in their natural state. For N-type accumulation-mode F-OECTs, negative gate voltage drives anions from the electrolyte into the channel, stabilizing holes along the polymer backbone and increasing the channel conductivity. This process turns the device from the OFF state to the ON state. Depletion-mode F-OECTs are naturally ON in the absence of a gate voltage, while accumulation-mode F-OECTs remain OFF until a gate voltage is applied and are particularly advantageous for low-power applications, offering high ON/OFF ratios that make them suitable for in vivo bioelectronics and neuromorphic computing.
F-OECTs introduce additional complexity to the ion transport mechanism due to their unique 3D geometry. Unlike traditional planar OECTs, where ions primarily diffuse vertically through the electrolyte and channel, F-OECTs exhibit a synergistic effect known as lateral insertion-assisted ion transport (LI-assisted Ionic Transport). This mechanism enables ions to diffuse both vertically and laterally along the fibre surface and within its porous structure as shown in Fig. 2e41. Experimental evidence from patterned P3HT films with striped microstructures (2–100 μm width) demonstrates that lateral intercalation reduces ion diffusion time (tDiff) from 4.7 s (100 μm stripes) to 0.2 s (2 μm stripes), while response time (τ) improves from 21.3 s to 1.9 s41. GIWAXS analysis confirms that lateral TFSI⁻ anion intercalation preserves the polymer backbone orientation, reducing π–π stacking distances and enhancing lamellar packing41. TOF-SIMS depth profiling further validates homogeneous doping in 2 μm-patterned films, with TFSI⁻ content increasing from 7.5 to 17.4%, correlating with a 600% rise in Gm,max/τ (2.2 to 16.2 mS s−1)41. What’s more, the microstructure and crystallinity of the fibre material significantly influence ion diffusion behavior. For example, polymers with high crystallinity and edge-on stacking (e.g., PBTTT) hinder lateral ion transport due to densely packed layers, whereas face-on stacking (e.g., P3HT) or disordered polymers (e.g., p(g2T-TT)) facilitate lateral diffusion by providing accessible pathways42,43,44. Electrochemical impedance spectroscopy reveals that lateral channels reduce channel resistance (Rch) from 1330 Ω to 408 Ω, enhancing ion mobility (diffusion coefficient increases from 9.9 × 10−11 to 2.7 × 10−10 m² s−1))42. Amorphous regions in polymers like p(g2T-TT) further lower energy barriers for ion transport, achieving ultrafast response (τ = 0.028 s)42. Structural modulation of fibres, such as tuning porosity or crystallinity via stripe patterns (Fig. 1b), allows precise control over ion injection rates, optimizing device performance for applications like ECG monitoring (SNR improves from 14.5 to 19.6 dB)42. Additionally, fibres with alkoxy side chains in their organic mixed ionic-electronic conductors (OMIECs) demonstrated faster ion transport in aqueous electrolytes45. Amorphous phases within the fibre facilitate ion movement but increasing the energy barrier for ion transport, further decreasing response time4. As discussed above, the additional lateral surface area of F-OECT enhances ion transport efficiency, leading to faster response times and higher sensitivity. By adjusting porosity or crystallinity of fibers, it’s possible to precisely control ion injection and release rates in fibers, optimizing applications in neuromorphic computing and biosensing. Furthermore, increased crystallinity can trap ions within ordered side chains, ensuring stable switching under sufficient potential.
Transfer characteristic reveals the dependance of drain current (ID) on gate voltage (VG) at a constant drain-source voltage (VDS) while output characteristic reflects the relationship of ID with VDS at different VG27,46. As shown in Fig. 2e–g, transfer and output characteristic curves can be measured by I–V test via a semiconductor parameter analyzer. Through a series of derivations, various metrics evaluating devices performance including threshold voltage, Ion/Ioff ratio, charge carrier mobility and subthreshold swing can be calculated, which will be discussed in the “Device Physics and Performance Evaluation”. In terms of charge carriers among device-OECTs, there are categories, i.e., P-type holes and N-type holes. As the majority of F-OECTs is made of P-type conductive polymers, we focus on P-type OECTs to describe the corresponding transfer and output curves. During the output characteristic curves, there are normally three operation regions, i.e., pinch-off, linear and saturation region. Among the linear region, the value of ID increases as the rise of VDS and VG, when the VG is above threshold voltage (VT) and value of VDS is relatively small. Assumed that the charge carrier’s mobility is constant, ID will increase linearly as the induced charge increases. As the VDS reaches to pinch-off voltage, ID will no longer increase. Furthermore, ID starts to saturate in the saturation region, during which the saturation ID is only increasing quadratically as VG rises. This current is normally regarded as the output current for devices. For transfer characteristic curves, variation of ID versus VG at a constant VDS is reflected. During transfer curves, VT, gm and Ion/Ioff ratio can be extracted, which will be discussed in the following sessions.
Device physics and performance evaluation
Bernards–Malliaras model
The performance of F-OECTs is evaluated based on theoretical models and measurable parameters, which together provide a comprehensive understanding of working principles and device optimization. This section introduces the Bernards-Malliaras model as the foundational framework for F-OECT operation, followed by a discussion of key performance metrics such as transconductance (gm), carrier mobility (μ), volumetric capacitance (C∗), and other auxiliary parameters.
The Bernards-Malliaras model, proposed by Bernards et al. in 2007, is widely regarded as the main theoretical model for describing the operation of OECTs9. This model assumes that electron transport in OECTs is primarily a drift process, like the mechanism in conventional thin-film transistors. It divides the OECT into two coupled circuits: the ionic circuit and the electronic circuit. The ionic circuit describes the movement of ions through the electrolyte and into the channel, which behaves like a volume capacitor capable of storing charge without chemical reactions. The electronic circuit describes the flow of electronic charge through the source, channel, and drain, which is modeled as a resistor. At steady state, the ionic capacitor is fully charged (or discharged), and the gate current approaches zero9. For OECTs based on PEDOT: PSS, the Bernards-Malliaras model provides a mathematical framework to describe the ID as a function of VG and VD. For example, the channel current can be expressed as:
where VT is the pinch-off voltage, μ is the hole mobility, C∗ is the bulk capacitance, ρ is the intrinsic charge carrier density, e is the elementary charge, and t, W, and L represent the channel thickness, width, and length, respectively. However, this model features certain limitations, including assumptions of purely capacitive processes and neglecting ion-film charge exchange reactions, thus reducing its accuracy in describing the complex, interdependent ionic and electronic dynamics occurring within modern F-OECTs. Recent advancements have addressed these limitations by incorporating non-uniform carrier mobility, diffusion-dominated transport, and detailed ion-electron interactions into refined theoretical models28,47,48,49. These refinements provide a more precise and comprehensive representation of the charge transport mechanisms in F-OECTs, enabling improved device design and performance evaluation.
Transconductance (gm)
gm is one of the most important parameters for evaluating F-OECT, as it reflects the ability to amplify signals. gm is defined as the change in ID in response to a change in VG at a constant VD. In the saturation region, transconductance can be calculated as the first derivative of the transfer curve. According to the Bernards-Malliaras model, gm can be further expressed as:
Where W, d, and L denote channel width, thickness and length, respectively. \(\frac{{Wd}}{L}\) describes the device geometry, \({\rm{\mu }}{{\rm{C}}}^{* }\) reflects the material and electrochemical performance and \({V}_{G}-{V}_{T}\) represents the bias conditions50.
Larger devices typically exhibit higher gm, so area-normalized transconductance (gm,A) is often used for fair comparisons across devices of different sizes. For F-OECTs, gm,A is defined as transconductance per unit cross-sectional area (A)5:
This normalization is especially critical for F-OECTs, as it accounts for both device geometry and transconductance, providing a more comprehensive evaluation of device performance28.
We summarized recent reported F-OECTs in Table 1, showing that the highest gm achieved in F-OECTs was up to 40 mS51. Investigating conducting polymers with higher ion mobility and charge storage capacity would fabricate F-OECTs with higher gm52,53. Stavrinidou et al. proposed that ion mobility in PEDOT:PSS depends on water uptake: pristine PEDOT:PSS films in contact with an aqueous electrolyte swell and support the transport of small ions with the same mobility as in water54. There is no dielectric between the channel and the electrolyte in F-OECTs, and so ions can be injected into the former and produce a response determined by the channel volume55. For example, making the channel thinner can lead to a faster response as fewer ions are required for de-doping, which will be further discussed in “Geometry Design”. Finally, in addition to materials and geometry, applying higher VD increases gm, but the voltage that can be applied in an aqueous electrolyte before electrolysis occurs is limited to ~1 V. The use of ionic liquids or gel electrolyte could achieve higher voltage operation and therefore higher performance56.
Charge carrier mobility (µ) and capacitance (C*)
μ and C* are two additional key parameters that determine the charge transport and ion storage capabilities of F-OECTs. Both μ and C* influence critical aspects of device performance, such as response time and signal amplification. μ can be extracted from transfer characteristics in either the linear or saturation regions. In the linear region, ID is given by:
showing that ID is proportional to μ, C∗, and VG − VT. When VD is much smaller than VG − VT, μ can be calculated using the simplified equation:
In the saturation region, μ can be determined by plotting the square root of ID against VG:
Notably, μ in the saturation region is often higher than that in the linear region. The choice of contact materials significantly impacts carrier mobility, as carrier injection/extraction is determined by the work function (WF) of the electrode and the energy levels of the organic semiconductor. For example, electrodes with well-matched WF values (e.g., silver or graphite) yield higher μ. To provide a geometry-independent performance metric, Sahika Inal et al. introduced the product of μ and C∗ as figure of merit (μC∗)57. μC∗ is particularly useful for comparing materials across different device architectures. Table 1 summarizes the μC∗ values for various materials used in F-OECTs, highlighting their suitability for applications where high transconductance and fast response times are required. In contrast to planar OECTs, F-OECTs exploit their three-dimensional fiber geometry and porous microstructure to enhance both μ and C*. For instance, the aligned polymer chains in strain-engineered PEDOT:PSS microfibers reduce interchain hopping barriers, increasing μ to 12.9 cm² V−1 s−1 significantly higher than planar PEDOT:PSS films (μ ≈ 1–3 cm² V−1 s−1)58. The porosity of fibers also amplifies C* by enabling volumetric ion penetration via lateral pathways, similar to the striped microstructures in planar OECTs where C* increases from 120 F cm⁻³ (non-patterned) to 280 F cm⁻³ (2 μm stripes)42. This results in F-OECTs achieving a μC* of 1500 F cm−1 V−1 s−158, surpassing planar devices (typically <300 F cm−1 V−1 s−1)59. The synergistic effect of fiber alignment and porosity thus bridges the ionic-electronic coupling gap, enabling faster switching and higher gain in bioelectronic applications. Strain engineering via uniaxial tension during the drying process induces anisotropic crystallite alignment along the fibre direction, optimizing charge transport and setting a benchmark for substrate-free, fibrillar bioelectronics58.
Others
To guide the fabrication of high-performance F-OECTs, hereby we outline the key metrics and techniques parameters requiring comprehensive understanding. VT is the lowest voltage to turn on F-OECTs. Normally, the ideal VT should be as much lower as possible, which means devices can operate at lower voltage. The value of VT is related to the density of trapping charges and quality of contract between source and drain. Ion/Ioff ratio, the ratio of the maximum on-state current (Ion) to the off-state current (Ioff), reflects the switching performance of devices at certain gate voltage. In output curves, the values of Ioff and Ion correspond to ID at VT and saturation ID under given VG. It is noteworthy that the conductivity of channel is proportional to the quantity of electronic charge which is in proportion to VG. Hence the conductivity of transistor devices rises as the VG grows. As for F-OECTs, the higher value of Ion/Ioff ratio, the higher stability, and the lower driving loads. Through there are many ways to reduce the response time of F-OECTs, it takes much longer time for turn-on state compared to turn-off state in accumulation mode. To understand this asymmetry, turn-off times are generally much faster than turn-on times in accumulation mode F-OECTs. Subthreshold swing (SS) represents the voltage value by which the subthreshold current must be reduced in order of 10. Apparently, the smaller the value of SS, the faster the device will switch (i.e., transition between the on and off states). Therefore, the size of SS value reflects the switching performance in the subthreshold region. In early research of OECTs, the contract resistance is rarely mentioned due to the low efficiency of devices, where the current between source and drain is mainly limited by channel resistance. Since the rise of µ, the influence of contract resistance gradually becomes a major factor in device performance. It turns out that the contract resistance is associated with the electrode characteristic (e.g., work function). Besides, the contract resistance is also related to the value of VG. The rise of VG result in the drop of contract resistance.
In general, a F-OECT with excellent performance should meet the following requirements: a high ID at a low VD and VG, high Ion/Ioff ratio, high µ as well as µC*, and low VT. For conventional OECTs devices, normally, Ion/Ioff ratio and µ are required to larger than 106 and 0.1 cm−1 V−1 s−139. For current F-OECTs devices, µ of the P-type one has surpassed over 10 cm−1 V−1 s−158, while µ of the N-type one is still under 0.1 cm−1 V−1 s−122. In terms of gm, it is highlighted that the value of P-type devices has reached over 0.1 mS59, while for N-type devices, it remains near to zero22.
Material and fabrication
To fabricate superb F-OECTs, the channel fibre should have good electrical conductivity to ensure effective carrier transport. The consistency of fibre diameter and length is also crucial for controlling uniform electrical properties throughout area of the device22,50,60. In addition, the fibres must have sufficient mechanical strength and flexibility to maintain structural integrity, especially in bending or stretching applications61. They should also be easy to process for on-demand shaping and alignment for F-OECT integration62. The chemical and physical properties of the fibre surface must be adapted to surroundings and provide good environmental stability and biocompatibility, which are essential for the stable operation and wide application of F-OECTs63. We discussed the impacts of material selection and manufacturing techniques in device performance to gain a deep understanding of F-OECTs.
Material design
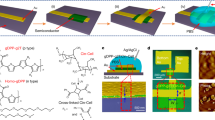
Selecting suitable conjugated polymers is crucial for the fabrication and optimization of F-OECTs. The arrangement of polymer chains and the presence of specific functional groups can influence charge mobility and stability under various environmental conditions. For instance, strong π–π stacking interactions between polymer chains can enhance charge transport by providing continuous pathways for charge carriers64. These interactions depend on the distance and orientation between adjacent chains. Functional groups that enable crosslinking can enhance the mechanical stability of the polymer network, which can be beneficial under varying environmental conditions65. Furthermore, the introduction of electron-donating groups (such as alkyl chains) or electron-withdrawing groups (EWGs, such as fluorine atoms) can modify the electronic properties of the polymer by adjusting the energy levels, improving charge injection and transport66,67,68. By designing the polymer structure and selecting appropriate functional groups, researchers can optimize both charge mobility and environmental stability, tailoring materials for specific applications in F-OECTs69. In 2001, Alan J. Heeger, a prominent researcher in the field of conjugated polymers, discussed the development of conjugated polymers, including both P-type and N-type polymers70. Figure 3 illustrates several common conducting polymer structures, including P-type polymer, N-type polymer, ambipolar conjugated polymers. In this section, we provide a further summary of recent advances in conjugated polymer materials, emphasizing the importance of material selection in high-performance F-OECTs.
P-type polymers transport current primarily through the movement of holes (positive charge carriers)71. When current is applied, electrons are excited from the valence band (VB) to the conduction band (CB), allowing holes to move within the VB72. The widespread use of P-type polymers in the early OECT was mainly due to their stability in water electrolytes, which is a key requirement for bioelectronic applications73. For example, PEDOT:PSS has demonstrated reliable electrochemical performance under physiological conditions, which allows reliable operation of the device. In addition, P-type polymers have a high electrical conductivity and are compatible with scalable fabrication processes67,74. When incorporated in devices, they allow low operating voltages < 1 V and high signal intensities which are useful for biosensing and wearables applications30,75,76,77. Although flexibility and stretchability are possible in P-type and N-type optimized materials, the historical dominance of P-type polymers is due to their early maturity in synthesis and fabrication78. The combined advantages have enabled P-type polymers to show promising applications in biosensing, neural interfaces79, environmental monitoring80, and other fields81. Lan et al. developed glycolated conjugated polymers based on a cyclopentadithiophene (CPDT) framework that exhibited record-high capacities for P-type OECT materials and demonstrated their potential for powerful and flexible OECTs through a facile binary solvent strategy, significantly advancing the field organic mixed ionic−electronic conductor82. As a typical P-type material, PEDOT:PSS has been widely used due to their excellent conductivity and biocompatibility83,84. A recent study highlighted the successful transformation of PEDOT:PSS into an active material for enhancement-mode OECTs through the use of aliphatic amines as de-doping agents. This innovative process not only lowers the turn-on voltage but also significantly reduces the operating current while maintaining the high electron mobility along channels. PEDOT:PSS can be doped with secondary dopants (e.g., dimethyl sulfoxide, ethylene glycol) to improve its electrical conductivity from 3.52 Ω·cm−2 to 0.07 Ω·cm−2 85,86. Similarly, incorporating carbon-based materials, such as carbon nanotubes or graphene, can further enhance charge transport properties51,87. Conjugated polyelectrolytes (CPEs) have emerged as a representative P-type materials for OECTs, combining ionic functionality with electronic conductivity. Schmode et al. demonstrated gradient copolymers (PTHS-TMA+-co-P3HT) achieving high gm (70.49 S·cm-1) and low VT (−0.15 V) through balanced ionic/electronic transport pathways88. Chae et al. optimized self-doped CPE-K by molecular weight engineering, achieving a record μC* (14.7 F·cm−1 V−1 s−1) via enhanced molecular packing and edge-on orientation89. These studies highlight how polyelectrolytes leverage both side-chain ionic groups and backbone conjugation for high-performance OECT operation. Such findings provide a novel strategy for the development of energy-saving and highly stable F-OECTs.
N-type conducting polymers typically use negative charge carriers to transport electrons. This feature makes it possible to develop circuits or devices that require electron conduction90. Unlike P-type materials featuring in higher highest occupied molecular orbital (HOMO) energy levels and higher susceptibilities to oxidation, particularly in humid or aerobic environments, N-type materials are typically characterized by lower HOMO and lower lowest unoccupied molecular orbital (LUMO) energy levels, manifesting the majority of which, such as P(NDI2OD-T2), good oxidation resistance and thus good stability in the air64,91,92. Recent advances in molecular semiconductors further highlight the potential of small-molecule-based OMIECs for N-type OECTs. For instance, glycolated fullerene derivatives (e.g., C60-TEG) achieve volumetric doping through hydrophilic side chains, enabling a competitive μC value of 7.0 F cm−1 V−1 s−1, surpassing many polymer counterparts93. Moreover, helical perylene diimide trimers (hPDI[3]-H) exhibit enhanced air stability (LUMO < −4.5 eV) and high transconductance (49 mS) after thermal annealing to remove solubilizing alkyl chains, demonstrating over 5-week operational stability and applications in biosensing94. Despite these promising developments, challenges remain for both polymer and molecular systems. For polymers, complex synthesis, high costs, and interfacial compatibility issues limit widespread adoption95. Therefore, to obtain the optimal performances and reliability of F-OECTs based on N-type conjugated polymers, it is specifically crucial to designing polymers with appropriate side chains that enhance compatibility with common solvents and enable uniform fabrication techniques, such as wet spinning22. Moreover, focusing on enhancing charge carrier mobility and conductivity by optimizing the polymer’s molecular structure, such as incorporating EWGs to lower the LUMO energy level, facilitating efficient electron transport2. In order to boost the performance and stability of N-type polymers, researchers have focused on designing electron-deficient backbones that incorporate additional electron-withdrawing units (e.g., imide or diimide moieties) and carefully selected side chains for improved solubility and interfacial compatibility. This approach not only lowers the LUMO level to facilitate electron transport but also helps stabilize the charged states in aqueous environments. Moreover, tuning the molecular weight, adopting ring-fusion strategies to reduce torsional disorder, and introducing glycolated side chains can further enhance volumetric doping and mitigate oxidation-induced degradation.
In addition to the materials discussed above, a growing number of studies have focused on ambipolar conjugated polymers, which are capable of transporting both electrons (N-type conduction) and holes (P-type conduction) in a single material system96. For example, emerging ambipolar conjugated polymers such as PIDT-BT and PIL-2T have demonstrated balanced electron and hole mobility, enabling more efficient electrochemical doping/de-doping and enhanced sensitivity in F-OECTs97,98. By fine-tuning the HOMO and LUMO levels of these materials, researchers can achieve near symmetric ambipolar transport, thus simplifying device architectures and improving overall performance. In F-OECTs, the implementation of ambipolar conjugated polymers simplifies the device structure by avoiding the separate utilization of N-type and P-type materials. Since both types of carriers are transported during electrochemical doping and de-doping, the carrier mobility and switching ratio are upgraded, thus enhancing the response speeds and sensitivities99. Besides, relying on its suitable HOMO and LUMO energy levels, it allows ambipolar conjugated polymers to conduct electrons and holes under various conditions in an efficient fashion92.
Fabrication techniques
Wet spinning is a mainstream method to fabricate conducting polymer fibres with high crystallinity and well alignment, working as channel fibres in F-OECTs. A high crystallinity in polymer chains often leads to high charge mobility. This is because ordered structures facilitate more efficient pathways for charge carriers to move. Techniques such as stretching or using alignment-inducing substrates can improve chain alignment100,101. A schematic of the wet spinning process is shown in Fig. 4a. The process involves extruding a polymer solution through a spinneret into a coagulation bath, where solvent exchange induces polymer solidification. Key steps include spinning solution extrusion, solvent exchange in the coagulation bath, and fiber collection. This method enables precise control over fiber diameter and crystallinity.
Schematic illustration of a traditional wet spinning process, and novel wet spinning ways: b good solvent exchange method103, c the FLEX method104, d scalable one-step wet-spinning method105. The SEM images of wet spinning fibres: e PEDOT:PSS126; f composite fibre111; g hollow PEDOT fibre130; h PEDOT:PSS58; i n-PBDF fibre109. Reprinted with permission103. Copyright 2022, Springer Nature Limited. Reprinted with permission104. Copyright 2024, The Authors, exclusive licensee American Association for the Advancement of Science. Reprinted with permission105. Copyright 2019, The Royal Society of Chemistry. Reprinted with permission126. Copyright 2024, Wiley-VCH GmbH. Reprinted with permission111. Copyright 2020, Springer Nature. Reprinted with permission130. Copyright 2021, Wiley-VCH GmbH. Reprinted with permission58. Copyright 2021, Wiley-VCH GmbH. Reprinted with permission109. Copyright 2024, Wiley-VCH GmbH.
In a typical wet spinning process, the solutions of conducting polymer and/or their composites are extruded into a coagulation bath. Solvent exchange induces subsequent aggregation of conducting polymer chains and fibre formation. Wet spinning is particularly suitable for scalable manufacturing, providing consistent yet uniform fibre quality and reproducibility102. With the increasing needs, researchers have developed new approaches to wet spin high-performance fibres. For instance, to prepare ultrafine PANi fibres at a large scale, Tao et al.103. proposed a wet spinning technique based on good solvent exchange shown in Fig. 4b. Different from the traditional wet spinning method, the coagulation bath was replaced with good solvent rather than poor solvent, which reduced the viscosity of the precipitated polymer. These protofibres form refined fibres with diameters less than 5 μm. The ultrafine PANi fibres exhibited a modulus of 29.89 ± 5.6 GPa, a strength of 1080 ± 71 MPa, and an area capacitance measured between 1008 and 1666 mF·cm-2 at current densities between 0.32 and 3.18 mA·cm-2. As the good energy and charge storage properties of fine PANi fibres, researchers prepared an all-solid-state F-OECT with a thickness of less than 300 μm and a transmittance of more than 80% in the visible region. This device demonstrated good amplification performance and high switching current ratios (>103) at low voltages (<1 V) and functioned as a haptic sensor responding to various magnitude of pressure and friction forces. Similarly, Zhang et al.104 introduced an innovative flow-enhanced crystallization (FLEX) method shown in Fig. 4c, which enabled the continuous production of ultra-tough semiconducting polymer fibres with exceptional electronic performance, as well as exhibited precise control over fibre diameter and morphology. FLEX modifies the conventional fast bidirectional solvent-resistant flow to a slower unidirectional flow, aiding in the control of the polymer’s crystallization behavior and chain alignment. Fibres produced by the FLEX method exhibit excellent mechanical properties, including high tensile strength (>200 MPa), toughness (>80 MJ m−3), and enhanced strain-to-failure >50%, attributed to their crystallinity and chain alignment. Zhang et al.105 introduces a fast, scalable one-step wet-spinning method for producing highly conductive PEDOT:PSS fibres (up to 3828 S cm−1) by using concentrated sulfuric acid (H₂SO₄) as a coagulation bath, which removes insulating PSS during fibre formation and enhances PEDOT chain alignment. The approach shown in Fig. 4d eliminates the need for post-treatment and demonstrates applications in touch sensors, humidity sensors, and long fibre-shaped supercapacitors, offering a platform for advanced textiles and flexible devices. By precisely manipulating the fibre diameter and morphology, these advanced wet spinning methods can fabricate homogeneous fibres with optimized mechanical, conductive and electrochemical properties, shown in Fig. 4e–i, thereby improving the reproducibility and reliability of F-OECT devices106. Beyond wet spinning, other techniques such as electrospinning and solution spinning have also been explored. Electrospinning, for instance, allows the production of nanofibres with high surface area, which can enhance ion exchange and improve device performance107,108. Electrode printing, on the other hand, offers finer control over fibre morphology and alignment, enabling the fabrication of highly ordered fibre arrays for OECT integration25,101. Beyond wet spinning, electrospinning and solution spinning are critical for F-OECT fabrication. For example, electrospun P3HT/PEO nanofibers exhibit a high surface-to-volume ratio, enhancing ion exchange and achieving a transconductance of 15 mS in OECTs109. Solution spinning, such as the dry-jet wet spinning of polyaniline (PANI) in chloroform, produces fibers with aligned molecular chains and conductivity exceeding 1000 S cm−1, ideal for high-speed bioelectronic sensing110. The properties of fibres, including conductivity, mechanical strength, and biocompatibility, can be fine-tuned in the fabrication process by adjusting various polymers, solvents, and additives to meet advanced applications of F-OECTs67,106,111.
Geometry design
The geometry of F-OECTs, including fibre diameter, length, and topology, determines electrical, mechanical, and electrochemical performance of devices. For example, small fibre diameters will increase the surface-to-volume ratio, which enhances ion exchange and improves gm. However, excessively thin fibres may suffer from reduced mechanical strength and increased fabrication challenges110,112. As the increasing length of channel fibre, F-OECTs can reduce ID and improve stability with higher resistance and slower ion transport113. In this section, we discuss the relationship between geometry and performance, explore advanced design strategies, and highlight recent progress in geometric optimization.
The geometry design could be conducted from single fibre optimization, network and array design, and composite. By tailoring the cross-sectional shape, surface roughness, and porosity of single fibres, it is possible to enhance ionic and electronic interactions. For example, porous fibre structures can increase ion storage capacity, while smooth surfaces can reduce contact resistance14,15,82. Moreover, network and array design is also a solution. Random networks are suitable for applications requiring high flexibility and mechanical durability, such as wearable electronics. Aligned fibre arrays could improve charge transport efficiency and enable directional signal propagation, making them ideal for high-performance sensors and transistors17,25,37. Multi-scale designs, such as combining thick and thin fibres or integrating porous and solid fibres, can optimize both ionic and electronic properties49,60,108. Multi-layer or coaxial fibre designs, where the core and shell materials serve different functions (e.g., electronic conduction and ionic storage), can further enhance performance107,114. These designs are particularly useful for achieving multifunctionality in F-OECTs.
F-OECTs have two kinds of configurations, i.e., vertical and horizontal structures. In a horizontal structure shown in Fig. 5a, the components of the F-OECTs (channel, gate, and electrolyte) are arranged side by side along the longitudinal axis115. The conducting polymer channel and the gate electrode are positioned longitudinally, with the electrolyte separating them. The electrolyte surrounds or bridges the channel and gate on the surface of channel fibre or within its structure78,116. At the same time, the ionic and electronic interactions occur laterally along the surface, and the current flows along the length. In a vertical structure shown in Fig. 5b, the components of the OECT are stacked radially or concentrically around the core of channel fibre, creating a multilayer cylindrical geometry22. The channel is typically deposited on the core fiber, while the gate electrode is placed as a surrounding layer or concentric shell. The electrolyte is sandwiched between them. The electrolyte forms a thin layer between the channel and the gate, enabling ionic transport and current flow in the radial direction50. The choice between a horizontal or vertical structure for F-OECTs depends on the specific application. For instance, compared to vertical structures, horizontal structure can be fabricated more easily and allows for longer channel lengths, which is well-suited for wearable and textile-integrated devices, where flexibility and distributed sensing are prioritized. There is a summary about advantages and disadvantages of horizontal and vertical structures in Table 2. Figure 5c–e summarized recent work about horizontal F-OECTs, which used in waving fabric sensor, in vivo implantable probe, on‐skin bioelectronics16,115,117. On the other hand, the vertically stacked design in vertical F-OECTs produces short ion diffusion paths between the gate and the channel, enable faster switching and responding and feasible uses in miniaturized and implantable devices, as shown in Fig. 5f–h. Other factors, such as ionic transport, mechanical stability, and integration requirements also affect the structure design118.
a vertical structure and b planar structure; c design of flexible fabric OECT sensors by weaving fibre-based devices with cotton yarns115; d schematic illustration and circuit diagram of the PF-OECT16; e the schematic illustration of the ionic migration between fibre electrolytes for the doping/ de-doping process117; f schematic illustration of coaxial F-OECTs107; g schematic illustration and circuit diagram depicting the configuration of the F-OECT119; h schematic illustration of a fibre-shaped all-in-one F-OECTs111. Reprinted with permission115. Copyright 2018, Wiley-VCH GmbH. Reprinted with permission16. Copyright 2023, Wiley-VCH GmbH. Reprinted with permission117. Copyright 2023, Wiley-VCH GmbH. Reprinted with permission107. Copyright 2023, Springer Nature. Reprinted with permission119. Copyright 2024, The Royal Society of Chemistry. Reprinted with permission111. Copyright 2020, Springer Nature.
Applications of F-OECTs
Combining high gm, low operating voltage, and ionic-electronic coupling with the mechanical flexibility and durability of channel fibres, F-OECTs have found massive opportunities across in vivo probes, in vitro electronics, biomimetic devices, and logic circuits.
Biomedical devices/health monitoring
In vivo
F-OECTs offer significant advantages for in vivo applications due to their biocompatibility, flexibility, sensitivity, low power consumption, and stability in biological environments. These properties allow F-OECTs to detect and monitor physiological signals and biomarkers in complex biological fluids and organs, such as blood and brain. The combination of high transconductance and efficient ion-to-electron coupling enables real-time monitoring of biomarkers like sodium, potassium, and ascorbic acid with high sensitivity and selectivity. Additionally, the use of conductive polymers and biocompatible elastomers minimizes inflammatory responses, enabling long-term in vivo operation.
Recent advancements have suggested the versatility of F-OECTs in in vivo biological applications for long-term neural and cardiac monitoring. For instance, Jiawei Chen et al. reported a F-OECTs featuring a micrometer-scale vertical channel (F-vOECT) to significantly reduce the response time, shown in Fig. 6a, b119. The design of F-vOECT allows for a short channel length controlled by the thickness of the insulating layer between the coaxial source and drain electrodes. The F-vOECT achieved a response time of 12 mS and a maximum transconductance of 16 mS at zero gate bias, making it suitable for electrophysiological signal monitoring. In addition, the F-vOECT showed stable performance under cyclic switching and dynamic deformation, retaining 93.4% of initial drain current after 500 cycles and enabled real-time ECG monitoring for 7 days and successfully identifying atrioventricular block. Jianyou Feng et al. introduced an all-polymer F-OECT (PF-OECT) with a Young’s modulus (3.15 ± 0.05 kPa) similar to soft brain tissue(3.02 ± 0.13 kPa), achieving stable ascorbic acid monitoring in mouse brains for 14 days. This design highlighted the compatibility with neural tissues under dynamic deformation, as shown in Fig. 6c16. Furthermore, Yuan Fang et al. developed a coaxial yet implantable F-OECT with micro-scale channel lengths, which were used for ascorbic acid detection in mouse brains for 7 days107. The coaxial F-OECTs achieved the highest gm of 135 mS, which is one to two orders of magnitude higher than state-of-the-art F-OECTs, which was implanted in the brain of mouse and also showed high sensitivity of 12.78 mA/decade to ascorbic acid.
a Detailed schematic illustrations of the longitudinal section and cross-section of the F-OECT119. b Schematic illustration alongside photographs of a rat implanted with the F-OECT, with the working circuit diagram illustrated119. c Schematic diagram of in vivo detection of AA with the PF-OECT. Real-time sensing of AA for 14 days toward locally injecting 2 µL of 5 mm AA at a rate of 0.4 µL min−1. Normalized sensitivity of PF-OECT for AA before and after implantation in mouse brain for 14 days16. d Photographs of a mouse implanted with the coaxial fibre OECT at low and high magnifications. And the sensitivity study for AA detection of fibre OECT in vivo107. Reprinted with permission119. Copyright 2024, The Royal Society of Chemistry. Reprinted with permission16. Copyright 2023, Wiley-VCH GmbH. Reprinted with permission107. Copyright 2023, Springer Nature.
Despite these innovations, critical challenges hinder the long-term reliability of F-OECTs in biological environments. Protein and cellular adhesion on device surfaces can degrade sensitivity. For instance, while the F-vOECT demonstrated stability in controlled settings, prolonged exposure to cerebrospinal fluid may lead to signal drift due to biofouling. Enzymatic activity and oxidative stress in biological fluids can erode conductive polymers. The PF-OECT’s 14-day performance, while impressive, does not account for potential polymer breakdown over extended periods (>30 days). The F-vOECT’s vertical architecture requires precise coaxial electrode alignment, complicating mass production119. While anti-fouling coatings, encapsulation strategies, stable materials, and modular fabrication showed potential to address these problems, scalability and reproducibility remain impediment. For example, the F-vOECT’s vertical architecture requires precise alignment of coaxial electrodes, complicating mass production119. Similarly, the PF-OECT’s ultrathin polymer layers (<1 μm) are prone to delamination under chronic mechanical stress16. Future efforts could integrate self-healing polymers or modular fabrication, like 3D-printed interconnects, to address these limitations. Each of these work presents innovative approaches to enhancing the performance of F-OECTs for in vivo applications, including chronic chemical detection in the brain, fast-response electrophysiological monitoring, and high-sensitivity biochemical detection.
In vitro
Fibrous channels in F-OECTs provides superior mechanical compatibility conformable to complicated textures like human skin and textiles, performing a feasible platform for in vitro detection and monitoring. In 2022, Andreas B. Fall et al. fabricated water-based PEDOT and cellulose nanofibre yarns with excellent conductivity (150 S cm-1) and mechanical stiffness (20 GPa), demonstrating their robustness under varying environmental conditions78,85,115.
Recent material innovations have further demonstrated the potential of F-OECTs in wearable electronics. For example, Minhu Huang et al. developed PEDOT:PSS and TEMPO-oxidized cellulose nanofibre (CNF) composites, significantly enhancing the mechanical strength of fibres while achieving high carrier mobility (4.0 ± 0.2 cm² V−1 s−1)102. These studies underline the potential of F-OECTs for in-vitro biosensing. As shown in Fig. 7a, Yuan Fang et al. integrated prepared coaxial F-OECTs into fabrics for glucose monitoring in sweat during exercise107. In 2023, Xing Qing et al. developed a thermoelectrically driven fibre-integrated monitoring device (FPMD), enabling self-powered physiological monitoring without external accessories, which marked a step forward in creating lightweight and autonomous wearable biosensors shown in Fig. 7b33. Xing Qing’ group also designed whole-filament integrated fabrics based on PVA-co-PE nanofibres (NFs) and PPy nanofibre networks for highly sensitive dopamine (DA) monitoringin Fig. 7c120. Yao Wang et al. prepared F-OECTs based on multiwalled carbon nanotube functionalized PEDOT nanowires for the detection of ultra-low concentrations of potassium ions in the human body shown in Fig. 7d. K+ was detected in the range of 1–1000 nM, with a fast response and good reproducibility. The detection of K+ in artificial saliva verified the potential of the sensor for practical applications. The sensor can be hand-woven and integrated into fabrics, providing a promising platform for wearable health monitoring116.
a Photograph of a female volunteer wearing the fibre OECT while cycling. The insert shows the fibre OECT woven into the wrister. The interference study for glucose sensing of fibre OECT107. b Optical image of cotton/PDG electrode. Enlarged view of the cotton/PDG-based F-OECT and the electrical circuit diagram of FPMD, where the terminals between the TEF and F-OECT are connected by flexible electric wire (black line). Enlarged view of TE unit in TEF. Sensing application of the FPMD33. c Assembly of the woven F-OECTs and wearable DA sensors, woven series arrayed FECT circuit with PPy/NFs/PA6 fibre in the wearable point-of-care application120. d Schematic illustration of K+ Sensors based on fibre organic electrochemical transistors. Woven FECTs devices. Woven fabric with PEDOT/MWCNT/cotton fibre. Woven series arrayed with PEDOT/MWCNT/cotton fibre in the human wrist116. Reprinted with permission107. Copyright 2023, Springer Nature. Reprinted with permission33. Copyright 2023, Springer Nature. Reprinted with permission120. Copyright 2019, American Chemical Society. Reprinted with permission116. Copyright 2022, Elsevier Ltd.
Key advantages of F-OECTs for in vitro biological electronics include their ability to integrate seamlessly with textiles, enabling the creation of smart clothing for health monitoring, motion tracking, and energy harvesting. Their environmental stability ensures reliable operation under sweat, humidity, and temperature changes, while their low power consumption makes them ideal for portable systems121,122,123,124. To fully realize the potential of F-OECTs in ex-vivo biological electronics, several challenges must be addressed. Improving durability and washability is critical for practical applications, which could be achieved through encapsulation or self-healing materials. Similarly, Sozan Darabi et al. (2020) developed wood-derived PEDOT:PSS-coated conductive yarns with high conductivity and durability, retaining functionality after 10 washing cycles125. Future research should also focus on miniaturization and multifunctionality, enabling compact systems that integrate sensing, data processing, and wireless communication. Self-powered systems, such as those using thermoelectric fabrics or energy-harvesting technologies, are another area of focus to ensure autonomous operation. Expanding biosensing capabilities to detect additional biomarkers, like cortisol or lactate, will broaden their applicability in health monitoring. F-OECTs are well-positioned to revolutionize the in-vitro biological electronics industry.
Biomimetic
F-OECTs emulate neural functions through ion-to-electron coupling, offering a unique platform for neuromorphic systems. Compared to memristors, which rely on resistive switching in rigid dielectric materials, F-OECTs leverage the dynamic interplay between ions and electrons in flexible polymer fibers, enabling analog memory, tunability, and biocompatibility at low operating voltages (<1 V)126,127. Recent progress has demonstrated the potential of F-OECTs in neuromorphic sensing. For instance, as show in Fig. 8a, wet-spun PEDOT:PSS fibers achieve a µC product of 1570.5 F cm−1 V−1 s−1 and hole mobility exceeding 45 cm² V−1 s−1, enabling efficient synaptic signal modulation126. Innovations like 3D dendritic PEDOT:PSS fibers grown via bipolar electropolymerization mimic structural plasticity by tuning conductivity through frequency-dependent assembly (40–320 Hz), while PPy-based F-OECTs demonstrate synaptic features at ultra-low power consumption (0.85 pJ per spike) and exceptional cyclic durability about 4000 cycles128,129,which have been shown in Fig. 8b-c. Biofouling, such as protein and cellular adhesion on fiber surfaces, degrades sensitivity over time, particularly in dynamic biological environments like cerebrospinal fluid. Mechanical mismatch between fiber electrodes and neural tissues risks chronic inflammation, while oxidative stress accelerates material degradation, limiting device lifespan beyond 30 days. To overcome these barriers, future efforts must prioritize scalable solutions, including anti-fouling coatings, encapsulation strategies, and material innovations to enhance environmental resilience. Besides, aligning the mechanical properties of F-OECT with neural tissues could bridge the gap between synthetic devices and biological systems.
a An artificial bio-hybrid synapse with dopamine-dependent plasticity126. b Formation of a third dendrite using the electro polymerization process with fp = 80 Hz. Schematic of the read operation with gate terminal disconnected. Schematic of the programming of the dendritic OECTs with positive/negative sweep applied on the gate. Successive program/read sequences with Vsweep increased from (±) 0.1 to 0.4 V (step of 0.1 V) in between each sequence. Long term potentiation (depression) is obtained at negative (positive) bias. Time interval in between two successive programming was around 30 s129. c Schematic illustration of a biological synapse128. Reprinted with permission126. Copyright 2024, Wiley-VCH GmbH. Reprinted with permission129. Copyright 2021, Wiley-VCH GmbH. Reprinted with permission128. Copyright 2023, The Royal Society of Chemistry.
Logic circuits
Fibre-based organic electrochemical transistors (F-OECTs), with their high transconductance, large ON/OFF ratios, and low-voltage operation, are well suited for complementary logic circuits integrating both P- and N-type devices to perform fundamental operations like NAND and NOR gates. Their inherent flexibility and stretchability further support integration into wearable systems. Zhong et al. demonstrated vertically structured F-OECTs patterned on fibre surfaces via surface lithography as shown in Fig. 9d, enabling high-performance complementary logic gates embedded in knitted and woven fabrics, with gm values up to 41.10 mS (P-type) and 2.25 mS (N-type), as well as excellent cycling stability50. Recent material innovations have enhanced device robustness. Chen et al. developed highly conductive (3573 S cm−1), stretchable PEDOT:PSS fibres, while Wang et al. achieved record area-normalized gm (2.40 μS μm⁻²) in N-type BBL-based fibres, both showing stable performance over extended cycling, which are respectively shown in Fig. 9b–c22,130. Yu et al. further advanced integration by demonstrating F-OECTs with mechanosensitive logic behavior for smart textile applications, shown in Fig. 9a32. However, the integration of complementary logic circuits remains constrained by the limited performance of N-type F-OECTs, particularly in terms of stability and transconductance. Future progress requires the development of balanced P- and N-type materials, along with scalable fabrication techniques that ensure uniformity, alignment, and device yield. Addressing these challenges is crucial for advancing F-OECT logic systems from proof-of-concept stages to practical, scalable platforms suitable for intelligent textiles and fiber-based computing.
a Circuit diagram and optical image of the logic inverter based on the triboiontronic fibre-shaped OECT. Typical voltage/current transfer characteristics of the inverter32. b Schematic of the complementary-type inverter and chemical structures of P-type P(lgDPP-MeOT2) and N-type BBL polymers. Voltage transfer characteristics and voltage gains22. c The schematic diagram, image, and equivalent circuit130. d Woven logic gate using vertical F-OECTs50. Reprinted with permission32. Copyright 2021, The Authers. Published by American Association for the Advancement of Science. Reprinted with permission22. Copyright 2024, Wiley-VCH GmbH. Reprinted with permission130. Copyright 2021, Wiley-VCH GmbH. Reprinted with permission50. Copyright 2023, American Chemical Society.
Others
Pressure sensors are devices that convert pressure signals into electrical signals to measure the magnitude of pressure, and are widely used in industries such as industrial, medical, automotive, aerospace, and consumer electronics to monitor changes in gas or liquid pressure80. F-OECTs enable efficient perception and amplification of external pressure signals, and are suitable for applications in wearable devices, biomedical monitoring, and flexible electronics. Their working principle involves combining flexible sensitive materials with F-OECTs to regulate device performance through pressure, including the integration of pressure sensing elements, amplification effects of ion-electron coupling mechanisms, and flexibility and stretchability16.
Fang et al.103 proposed a type of all-solid-state F-OECT pressure sensor based on ultrafine PANi fibres (UFPFs) shown in Fig. 10. UFPF fibres serve as the source-drain channel material, possessing high electrochemical activity and mechanical strength, with silver wires embedded as the gate electrodes. Pressure-induced enhancement of the electric field and changes in ion permeation regulate the flowing current within the channel to achieve pressure sensing. After pressure is applied, the electric field between the gate and source is enhanced, leading to ion redistribution and an increase in the source-drain current. Within the pressure range of 0–40 kPa, the current can be amplified by up to 92%, with a sensitivity range of 0.01–0.1 kPa−1. Horizontal friction causes displacement of the polyurethane (PU) layer and silver gate electrode, altering the distance between the gate and the UFPF channel, thereby changing the current response. In cyclic tests with different friction forces (1.84–5.55 kPa) and friction speeds (4–20 mm/s), the sensor exhibits a stable bimodal current response. The ultrafine fibre structure of UFPFs (diameter <5 µm) provides high electrochemical activity and a larger specific surface area, enhancing ion transport and signal amplification capabilities. The mechanical strength of UFPFs reaches 1080 ± 71 MPa, ensuring the stability and durability of the sensor during use. Additionally, PU and polyvinyl alcohol (PVA) materials endow the sensor with flexibility and a visible light transmittance of over 80%.
a Schematic of the mechanism explaining the response of ultrafine PANi fiber-based OECT to the action of external pressure. b Relative drain-source change (ΔIDS/IDS0) and sensitivity as a function of pressure. c Response time of the all-solid F-OECT when pressing (rising edge) and releasing (falling part) under the instantaneous pressure of 17.8 kPa. d Cyclic response at three different pressure levels (0.92, 6.8, and 22.2 kPa). e Schematic of the working principle of the response to friction. f Cyclic response at three different frictions (1.84, 4.69, and 5.55 kPa). g An enlarged curve of the marked part in (f). h Cyclic response at different friction speeds from 4, 6, 8, 10, 15, to 20 mm s−1103. Reprinted with permission103. Copyright 2022, Springer Nature Limited.
Challenges and prospects
Combined merits, wearable integration, biocompatibility in aqueous environments, high surface-area-to-volume ratio, and programmable structures, make F-OECTs exhibit tremendous application potential in healthcare, wearable electronics, neuromorphic sensing, and logic circuits due to their high gm, low operating voltage, flexibility, and biocompatibility36,129,131 However, despite their promising prospects, F-OECTs still face a series of challenges in practical applications.
A critical issue is the imbalance between P-type and N-type material performance. P-type materials, such as PEDOT:PSS, are relatively mature, demonstrating high transconductance and good operational stability67,70. In contrast, N-type materials, including ladder-type polymers such as BBL, currently lag behind in terms of transconductance, stability, and cycling durability90,91. This performance disparity limits the development of complementary logic circuits essential for advanced applications. To bridge this gap, specific strategies such as the design of novel N-type donor–acceptor (D–A) copolymers, hybrid polymer–inorganic semiconductor composites, and molecular doping approaches have been proposed to enhance electron mobility, stability, and ionic transport capabilities47,48,49. Additionally, introducing self-healing materials or anti-biofouling coatings can further enhance device durability and resistance to biofouling and corrosion in biological environments. Moreover, combining molecular design with nano-structuring techniques could yield hybrid materials with improved mechanical flexibility and conductivity, enhancing F-OECT stability in dynamic environments.
Device design and fabrication present additional critical challenges. Achieving high-precision micro- and nanoscale patterning on fiber surfaces remains technically demanding, and maintaining consistent device performance and reliability during large-scale production is challenging. Interface irregularities between the material and electrolyte can lead to inconsistent device performance due to the sensitivity of ion-electron coupling mechanisms. Emerging fabrication techniques such as high-resolution 3D printing99,104, inkjet printing, and advanced lithographic methods offer promising solutions to enhance patterning precision and allow the realization of complex device architectures. In particular, 3D printing provides a versatile platform for rapid prototyping, precise control of material deposition, and scalability, addressing key challenges in reproducibility and pattern resolution99,104. Furthermore, improving the engineering of the material–electrolyte interface through nanostructured designs could enhance ion-electron coupling efficiency, ensuring consistent and stable device operation. Continuous wet-spinning processes also represent promising scalable manufacturing routes that balance cost-effectiveness and reproducible device performance.
For wearable and implantable devices, long-term operational stability and low energy consumption are indispensable. In practical application scenarios, such as changes in humidity, extreme temperatures, or dynamic mechanical stress, the performance of F-OECTs may be significantly affected. While F-OECTs demonstrate high initial performance, long-term operation may result in performance degradation, such as reduced gm or increased ID. Moreover, the ionic electrolytes in the devices may degrade or fail during extended use, impacting overall performance and reliability. Therefore, optimizing the chemical stability of ionic electrolytes and developing more durable solid-state electrolytes are urgent tasks to extend device lifespan and improve environmental adaptability. Further reductions in the power consumption of F-OECTs are also necessary to meet the demands of low-power computing and sensing applications. Most current F-OECTs are single-function devices, and achieving highly integrated multifunctional sensing, computation, and signal processing remains an important research direction131,132. Exploring the large-scale application of F-OECTs in textiles could enable the development of smart fabrics capable of real-time monitoring of health parameters and environmental changes132,133. Further research into the application of F-OECTs in low-power neuromorphic computing, combined with artificial intelligent (AI) algorithms, could enable real-time neural signal processing and emulation of learning behaviors133,134.
In conclusion, while F-OECTs have demonstrated significant application potential across multiple fields, breakthroughs in material design, device optimization, fabrication processes, and system integration are still needed. By overcoming these challenges, F-OECTs are expected to find widespread use in smart health monitoring, neural interfaces, low-power computing, and smart textiles, promising a cornerstone of flexible electronics.
Data availability
No datasets were generated or analysed during the current study.
References
Sun, H., Gerasimov, J., Berggren, M. & Fabiano, S. N-type organic electrochemical transistors: materials and challenges. J. Mater. Chem. C. 6, 11778–11784 (2018).
Facchetti, A. Semiconductors for organic transistors. Mater. Today 10, 28–37 (2007).
Kim, S. H. et al. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 25, 1822–1846 (2013).
Inal, S., Malliaras, G. G. & Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 8, 1767 (2017).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 86 (2018).
Marks, A., Griggs, S., Gasparini, N. & Moser, M. Organic electrochemical transistors: an emerging technology for biosensing. Adv. Mater. Interfaces 9, 2102039 (2022).
Picca, R. A. et al. Ultimately sensitive organic bioelectronic transistor sensors by materials and device structure design. Adv. Funct. Mater. 30, 1904513 (2020).
White, H. S., Kittlesen, G. P. & Wrighton, M. S. Chemical derivatization of an array of three gold microelectrodes with polypyrrole: fabrication of a molecule-based transistor. J. Am. Chem. Soc. 106, 5375–5377 (1984).
Bernards, D. A. & Malliaras, G. G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 17, 3538–3544 (2007).
Lee, C. H. & Hsu, S. H. Prediction of equivalent-circuit parameters for double-layer capacitors module. IEEE Trans. Energy Convers. 28, 913–920 (2013).
Shinn, M., Lam, N. H. & Murray, J. D. A flexible framework for simulating and fitting generalized drift-diffusion models. eLife 9, e56938 (2020).
Eisenberg, B. & Liu, W. Poisson–nernst–planck systems for ion channels with permanent charges. SIAM J. Math. Anal. 38, 1932–1966 (2007).
Rivnay, J., Noriega, R., Kline, R. J., Salleo, A. & Toney, M. F. Quantitative analysis of lattice disorder and crystallite size in organic semiconductor thin films. Phys. Rev. B 84, 045203 (2011).
Rivnay, J. et al. Drastic control of texture in a high performance n-type polymeric semiconductor and implications for charge transport. Macromolecules 44, 5246–5255 (2011).
Rivnay, J., Mannsfeld, S. C. B., Miller, C. E., Salleo, A. & Toney, M. F. Quantitative determination of organic semiconductor microstructure from the molecular to device scale. Chem. Rev. 112, 5488–5519 (2012).
Feng, J. Y. et al. All-polymer fiber organic electrochemical transistor for chronic chemical detection in the brain. Adv. Funct. Mater. 33, 2214945 (2023).
Kim, S. J. et al. Dendritic network implementable organic neurofiber transistors with enhanced memory cyclic endurance for spatiotemporal iterative learning. Adv. Mater. 33, 2100475 (2021).
Rivnay, J. et al. Organic electrochemical transistors for cell-based impedance sensing. Appl. Phys. Lett. 106, 043301 (2015).
Lu, M. J. et al. Regulating light-sensitive gate of organic photoelectrochemical transistor toward sensitive biodetection at zero gate bias. Small Struct. 2, 2100087 (2021).
Tang, H., Yan, F., Lin, P., Xu, J. B. & Chan, H. L. W. Highly sensitive glucose biosensors based on organic electrochemical transistors using platinum gate electrodes modified with enzyme and nanomaterials. Adv. Funct. Mater. 21, 2264–2272 (2011).
Lin, P., Yan, F. & Chan, H. L. W. Ion-sensitive properties of organic electrochemical transistors. Acs Appl. Mater. Interfaces 2, 1637–1641 (2010).
Wang, X. et al. Ultrastable N-type semiconducting fiber organic electrochemical transistors for highly sensitive biosensors. Adv. Mater. 36, 2400287 (2024).
Gkoupidenis, P., Koutsouras, D. A. & Malliaras, G. G. Neuromorphic device architectures with global connectivity through electrolyte gating. Nat. Commun. 8, 15448 (2017).
Gkoupidenis, P., Schaefer, N., Garlan, B. & Malliaras, G. G. Neuromorphic functions in PEDOT:PSS organic electrochemical transistors. Adv. Mater. 27, 7176–7180 (2015).
Xu, W., Min, S. Y., Hwang, H. & Lee, T. W. Organic core-sheath nanowire artificial synapses with femtojoule energy consumption. Sci. Adv. 2, e1501326 (2016).
Hamedi, M., Forchheimer, R. & Inganäs, O. Towards woven logic from organic electronic fibres. Nat. Mater. 6, 357–362 (2007).
Jo, Y. J. et al. Fibrillary gelation and dedoping of PEDOT:PSS fibers for interdigitated organic electrochemical transistors and circuits. npj Flex. Electron. 6, 31 (2022).
Zhang, H. Z. et al. Recent progress of fiber-based transistors: materials, structures and applications. Front. Optoelectron 15, 2 (2022).
Wang, N., Yang, A., Fu, Y., Li, Y. & Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 52, 277–287 (2019).
Lin, P. & Yan, F. Organic thin-film transistors for chemical and biological sensing. Adv. Mater. 24, 34–51 (2012).
Mabeck, J. T. & Malliaras, G. G. Chemical and biological sensors based on organic thin-film transistors. Anal. Bioanal. Chem. 384, 343–353 (2006).
Yu, J. et al. Fiber-shaped triboiontronic electrochemical transistor. Research 2021, 9840918 (2021).
Qing, X. et al. All-fiber integrated thermoelectrically powered physiological monitoring biosensor. Adv. Fiber Mater. 5, 1025–1036 (2023).
Friedlein, J. T., McLeod, R. R. & Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 63, 398–414 (2018).
Bharti, R., Gupta, J., Rajamani, P., Moulick, R. G. & Bhattacharya, J. Iron oxide nanoparticles/PEDOT: PSS nanocomposite-based modification of both glassy carbon electrode and flexible cotton fiber OECT for highly sensitive multi-analytes detection. Appl. Nanosci. 12, 3823–3833 (2022).
Raza, A. et al. A focused review on organic electrochemical transistors: a potential futuristic technological application in microelectronics. Microchem. J. 207, 111737 (2024).
Tian, Z., Zhao, Z. & Yan, F. Organic electrochemical transistor in wearable bioelectronics: profiles, applications, and integration. Wearable Electron. 1, 1–25 (2024).
Ilyassov, B., Zavgorodniy, A., Alekseev, A. & Aldasheva, L. Rectifying behavior of organic electrochemical transistors. Phys. B Condens. Matter 696, 416620 (2025).
Ohayon, D., Druet, V. & Inal, S. A guide for the characterization of organic electrochemical transistors and channel materials. Chem. Soc. Rev. 52, 1001–1023 (2023).
Gao, Y. et al. A hybrid transistor with transcriptionally controlled computation and plasticity. Nat. Commun. 15, 1598 (2024).
Yan, C. et al. Lateral intercalation-assisted ionic transport towards high-performance organic electrochemical transistor. Nat. Commun. 15, 10118 (2024).
Gregory, S. A. et al. Quantifying charge carrier localization in pbttt using thermoelectric and spectroscopic techniques. J. Phys. Chem. C. 127, 12206–12217 (2023).
Holliday, S. et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7, 11585 (2016).
Cendra, C. et al. Role of the anion on the transport and structure of organic mixed conductors. Adv. Funct. Mater. 29, 1807034 (2019).
Paulsen, B.D., Tybrandt, K., Stavrinidou, E. & Rivnay, J. Organic mixed ionic–electronic conductors progress at pace. Nat. Mater. 23, 577–577 (2024).
Tao, Y. et al. Fiber based organic electrochemical transistor integrated with molecularly imprinted membrane for uric acid detection. Talanta 238, 123055 (2022).
Bitton, S., Alarcon-Espejo, P., Paterson, A. F. & Tessler, N. Experimentally verified organic electrochemical transistor model. J. Appl. Phys. 136, 125501 (2024).
Friedlein, J. T., Shaheen, S. E., Malliaras, G. G. & McLeod, R. R. Optical measurements revealing nonuniform hole mobility in organic electrochemical transistors. Adv. Electron. Mater. 1, 1500189 (2015).
Paudel, P. R., Tropp, J., Kaphle, V., Azoulay, J. D. & Lüssem, B. Organic electrochemical transistors – from device models to a targeted design of materials. J. Mater. Chem. C. 9, 9761–9790 (2021).
Zhong, Y. H. et al. High-performance fiber-shaped vertical organic electrochemical transistors patterned by surface photolithography. Chem. Mater. 35, 9739–9746 (2023).
Qing, X. et al. High gain fiber-shaped transistor based on rGO-mediated hierarchical polypyrrole for ultrasensitive sweat sensor. Sens. Actuat A Phys. 354, 114297 (2023).
Abruña, H. D., Kiya, Y. & Henderson, J. C. Batteries and electrochemical capacitors. Phys. Today 61, 43–47 (2008).
Inal, S. et al. A high transconductance accumulation mode electrochemical transistor. Adv. Mater. 26, 7450–7455 (2014).
Stavrinidou, E. et al. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 25, 4488–4493 (2013).
Khodagholy, D. et al. High transconductance organic electrochemical transistors. Nat. Commun. 4, 2133 (2013).
Khodagholy, D. et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 4, 1575 (2013).
Sharbati, M. T. et al. Low-power, electrochemically tunable graphene synapses for neuromorphic computing. Adv. Mater. 30, e1802353 (2018).
Kim, Y. et al. Strain-engineering induced anisotropic crystallite orientation and maximized carrier mobility for high-performance microfiber-based organic bioelectronic devices. Adv. Mater. 33, 2007550 (2021).
Zhang, L. S. & Andrew, T. Vapor-coated monofilament fibers for embroidered electrochemical transistor arrays on fabrics. Adv. Electron. Mater. 4, 1800271 (2018).
Kushida, S. et al. Fast response organic supramolecular transistors utilizing in-situ pi-ion gels. Adv. Mater. 33, 2006061 (2021).
Xi, X. et al. Manipulating the sensitivity and selectivity of oect-based biosensors via the surface engineering of carbon cloth gate electrodes. Adv. Funct. Mater. 30, 1905361 (2020).
Yu, S. & Ratcliff, E. L. Tuning organic electrochemical transistor (oect) transconductance toward zero gate voltage in the faradaic mode. Acs Appl. Mater. Interfaces 13, 50176–50186 (2021).
Xie, M. et al. Cycling stability of organic electrochemical transistors. Org. Electron. 117, 106777 (2023).
Wang, Y. & Liu, Y. Insight into conjugated polymers for organic electrochemical transistors. Trends Chem. 5, 279–294 (2023).
Li, P. & Lei, T. Molecular design strategies for high-performance organic electrochemical transistors. J. Polym. Sci. 60, 377–392 (2022).
Saleh, A., Koklu, A., Uguz, I., Pappa, A.-M. & Inal, S. Bioelectronic interfaces of organic electrochemical transistors. Nat. Rev. Bioeng. 2, 559–574 (2024).
Wang, Y. et al. Designing organic mixed conductors for electrochemical transistor applications. Nat. Rev. Mater. 9, 249–265 (2024).
Romele, P., Ghittorelli, M., Kovács-Vajna, Z. M. & Torricelli, F. Ion buffering and interface charge enable high performance electronics with organic electrochemical transistors. Nat. Commun. 10, 3044 (2019).
Rimmele, M., Glocklhofer, F. & Heeney, M. Post-polymerisation approaches for the rapid modification of conjugated polymer properties. Mater. Horiz. 9, 2678–2697 (2022).
Heeger, A. J. Semiconducting and metallic polymers: the fourth generation of polymeric materials. J. Phys. Chem. B 105, 8475–8491 (2001).
Sirringhaus, H., Tessler, N. & Friend, R. H. Integrated optoelectronic devices based on conjugated polymers. Sci 280, 1741–1744 (1998).
Brédas, J.-L., Beljonne, D., Coropceanu, V. & Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and polymers: a molecular picture. Chem. Rev. 104, 4971–5004 (2004).
Dimitrakopoulos, C. D. & Malenfant, P. R. L. Organic thin film transistors for large area electronics. Adv. Mater. 14, 99–117 (2002).
Yu, Z.-D. et al. High n-type and p-type conductivities and power factors achieved in a single conjugated polymer. Sci. Adv. 9, eadf3495 (2023).
Lin, P., Luo, X., Hsing, I.-M. & Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free dna sensing. Adv. Mater. 23, 4035–4040 (2011).
Zhang, S. et al. Patterning of stretchable organic electrochemical transistors. Chem. Mater. 29, 3126–3132 (2017).
Majak, D., Fan, J. & Gupta, M. Fully 3D printed OECT based logic gate for detection of cation type and concentration. Sens. Actuators, B 286, 111–118 (2019).
Gualandi, I. et al. Textile organic electrochemical transistors as a platform for wearable biosensors. Sci. Rep. 6, 33637 (2016).
Lin, H. et al. Conjugated polymers for biomedical applications. Chem. Commun. 58, 7232–7244 (2022).
Hussain, S. et al. Rational design of signal amplifying fluorescent conjugated polymers for environmental monitoring applications: Recent advances and perspectives. Coord. Chem. Rev. 499, 215480 (2024).
Shen, Q. et al. Sensing, imaging, and therapeutic strategies endowing by conjugate polymers for precision medicine. Adv. Mater. 36, 2310032 (2024).
Lan, L. et al. Facilely accessible porous conjugated polymers toward high-performance and flexible organic electrochemical transistors. Chem. Mater. 34, 1666–1676 (2022).
Yi, Y., An, H.-W. & Wang, H. Intelligent biomaterialomics: molecular design, manufacturing, and biomedical applications. Adv. Mater. 36, 2305099 (2024).
Keene, S. T. et al. Enhancement-mode PEDOT:PSS organic electrochemical transistors using molecular de-doping. Adv. Mater. 32, 2000270 (2020).
Glaudell, A. M., Cochran, J. E., Patel, S. N. & Chabinyc, M. L. Impact of the doping method on conductivity and thermopower in semiconducting polythiophenes. Adv. Energy Mater. 5, 1401072 (2015).
Fall, A. B. et al. Spinning of stiff and conductive filaments from cellulose nanofibrils and pedot:pss nanocomplexes. ACS Appl. Polym. Mater. 4, 4119–4130 (2022).
Paul, E. W., Ricco, A. J. & Wrighton, M. S. Resistance of polyaniline films as a function of electrochemical potential and the fabrication of polyaniline-based microelectronic devices. J. Phys. Chem. 89, 1441–1447 (1985).
Schmode, P. et al. High-performance organic electrochemical transistors based on conjugated polyelectrolyte copolymers. Chem. Mat. 31, 5286–5295 (2019).
Chae, S. et al. Impact of molecular weight on the ionic and electronic transport of self-doped conjugated polyelectrolytes relevant to organic electrochemical transistors. Adv. Funct. Mater. 34, 2310852 (2024).
Yang, C.-Y. et al. A high-conductivity n-type polymeric ink for printed electronics. Nat. Commun. 12, 2354 (2021).
Griggs, S., Marks, A., Bristow, H. & McCulloch, I. N-Type organic semiconducting polymers: stability limitations, design considerations and applications. J. Mater. Chem. C. 9, 8099–8128 (2021).
Sworakowski, J. How accurate are energies of HOMO and LUMO levels in small-molecule organic semiconductors determined from cyclic voltammetry or optical spectroscopy? Synth. Met. 235, 125–130 (2018).
Bischak, C. G. et al. Active layers for n-Type organic electrochemical transistors. Acs Appl. Mater. Inter. 11, 28138–28144 (2019).
Tung, N. et al. Air-stable perylene diimide trimer material for n-type organic electrochemical transistors. Adv. Mater. 36, 2312254 (2024).
Tang, H. et al. A solution-processed N-type conducting polymer with ultrahigh conductivity. Nature 611, 271–277 (2022).
Li, D. et al. Unveiling organic electrode materials in aqueous zinc-ion batteries: from structural design to electrochemical performance. Nano-Micro Lett. 16, 194 (2024).
Sun, Y. et al. Indacenodithiophene-based single-component ambipolar polymer for high-performance vertical organic electrochemical transistors and inverters. Aggregate 5, e577 (2024).
Mohammadi, E. et al. Ion gel dynamic templates for large modulation of morphology and charge ntransport properties of solution-coated conjugated polymer thin films. Acs Appl. Mater. Inter. 11, 22561–22574 (2019).
Keene, S. T. et al. Hole-limited electrochemical doping in conjugated polymers. Nat. Mater. 22, 1121–1127 (2023).
Lu, D. & Chen, H. Solid-state organic electrochemical transistors (OECTs) based on gel electrolytes for biosensors and bioelectronics. J. Mater. Chem. A 13, 136–157 (2025).
Lerond, M., Subramanian, A., Skene, W. G. & Cicoira, F. Combining electrospinning and electrode printing for the fabrication of stretchable organic electrochemical transistors. Front. Phys. 9, 708914 (2021).
Huang, M. et al. One-step wet-spinning of conducting polymer and cellulose nanofiber composites for fiber-type organic electrochemical transistors. Carbohydr. Polym. 324, 121559 (2024).
Fang, B. et al. Scalable production of ultrafine polyaniline fibres for tactile organic electrochemical transistors. Nat. Commun. 13, 2101 (2022).
Zhang, Z. et al. Continuous production of ultratough semiconducting polymer fibers with high electronic performance. Sci. Adv. 10, eadk0647 (2024).
Zhang, J. et al. Fast and scalable wet-spinning of highly conductive PEDOT:PSS fibers enables versatile applications. J. Mater. Chem. A 7, 6401–6410 (2019).
Panicker, P. S., Kim, H. C. & Kim, J. An integrated wet-spinning system for continuous fabrication of high-strength nanocellulose long filaments. Sci. Rep. 13, 13137 (2023).
Lee, S. K. et al. Nanofiber channel organic electrochemical transistors for low-power neuromorphic computing and wide-bandwidth sensing platforms. Adv. Sci. 8, 2001544 (2021).
Oh, S. H. et al. Fast and durable nanofiber mat channel organic electrochemical transistors. Acs Appl. Mater. Interfaces 15, 39614–39624 (2023).
Sarabia-Riquelme, R. et al. Highly conductive n-type polymer fibers from the wet-spinning of n-doped pbdf and their application in thermoelectric textiles. Adv. Funct. Mater. 34, 2311379 (2024).
Rivnay, J. et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 1, e1400251 (2015).
Wu, X. et al. Fiber-shaped organic electrochemical transistors for biochemical detections with high sensitivity and stability. Sci. China Chem. 63, 1281–1288 (2020).
Kim, Y. et al. Organic electrochemical transistor-based channel dimension-independent single-strand wearable sweat sensors. Npg Asia Mater. 10, 1086–1095 (2018).
Huang, W. et al. Vertical organic electrochemical transistors for complementary circuits. Nature 613, 496–502 (2023).
Leopold, C. Faces of Geometry: II edn (eds Magnaghi-Delfino, P., Mele, G. & Norando, T) 169–178 (Springer International Publishing, 2021).
Yang, A. et al. Fabric organic electrochemical transistors for biosensors. Adv. Mater. 30, 1800051 (2018).
Wang, Y. et al. Woven fiber organic electrochemical transistors based on multiwalled carbon nanotube functionalized PEDOT nanowires for nondestructive detection of potassium ions. Mater. Sci. Eng. B Adv. 278, 115657 (2022).
Chen, S. et al. Ultra-lightweight, highly permeable, and waterproof fibrous organic electrochemical transistors for on-skin bioelectronics. Adv. Mater. Technol. 8, 2200611 (2023).
Zhao, C., Yang, J. & Ma, W. Transient response and ionic dynamics in organic electrochemical transistors. Nano-Micro Lett. 16, 233 (2024).
Chen, J. et al. Fast-response fiber organic electrochemical transistor with vertical channel design for electrophysiological monitoring. J. Mater. Chem. B 12, 9206–9212 (2024).
Qing, X. et al. Wearable fiber-based organic electrochemical transistors as a platform for highly sensitive dopamine monitoring. Acs Appl. Mater. Inter. 11, 13105–13113 (2019).
Wang, Y. D. et al. Ion sensors based on novel fiber organic electrochemical transistors for lead ion detection. Anal. Bioanal. Chem. 408, 5779–5787 (2016).
Luo, B. et al. Organic electrochemical transistors for monitoring dissolved oxygen in aqueous electrolytes of zinc ion batteries. Sens. Actuators, B 409, 135601 (2024).
Mattana, G. et al. Organic electronics on natural cotton fibres. Org. Electron. 12, 2033–2039 (2011).
Tarabella, G. et al. A single cotton fiber organic electrochemical transistor for liquid electrolyte saline sensing. J. Mater. Chem. 22, 23830–23834 (2012).
Darabi, S. et al. Green conducting cellulose yarns for machine-sewn electronic textiles. Acs Appl. Mater. Inter. 12, 56403–56412 (2020).
Alarcon-Espejo, P. et al. High-hole-mobility fiber organic electrochemical transistors for next-generation adaptive neuromorphic bio-hybrid technologies. Adv. Mater. 36, 2305371 (2024).
van de Burgt, Y. et al. A non-volatile organic electrochemical device as a low-voltage artificial synapse for neuromorphic computing. Nat. Mater. 16, 414–418 (2017).
Li, M. et al. Boron nitride-mediated semiconductor nanonetwork for an ultralow-power fibrous synaptic transistor and C-reactive protein sensing. J. Mater. Chem. C. 11, 5208–5216 (2023).
Janzakova, K. et al. Dendritic organic electrochemical transistors grown by electropolymerization for 3d neuromorphic engineering. Adv. Sci. 8, 2102973 (2021).
Chen, Y. P. et al. Integrated ionic-additive assisted wet-spinning of highly conductive and stretchable pedot:pss fiber for fibrous organic electrochemical transistors. Adv. Electron. Mater. 7, 2100231 (2021).
Conti, S. A new path for organic electrochemical transistors. Nat. Rev. Electr. Eng. 1, 148–148 (2024).
Song, M., Seo, J., Kim, H. & Kim, Y. Ultrasensitive multi-functional flexible sensors based on organic field-effect transistors with polymer-dispersed liquid crystal sensing layers. Sci. Rep. 7, 2630 (2017).
Choi, H. W. et al. Smart textile lighting/display system with multifunctional fibre devices for large scale smart home and IoT applications. Nat. Commun. 13, 814 (2022).
Darbandy, G. et al. Charge-based compact modeling of oects for neuromorphic applications. IEEE J. Electron. Devices Soc. 13, 34–40 (2025).
Kittlesen, G. P., White, H. S. & Wrighton, M. S. Chemical derivatization of microelectrode arrays by oxidation of pyrrole and N-methylpyrrole: fabrication of molecule-based electronic devices. J. Am. Chem. Soc. 106, 7389–7396 (1984).
Burroughes, J. H. et al. Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990).
Cheng, Z. H. et al. How to report and benchmark emerging field-effect transistors. Nat. Electron. 5, 416–423 (2022).
Zhu, R. F., Wang, Y., Tao, Y., Chen, Y. L. & Wang, Y. D. Organic electrochemical transistor based on polypyrrole/crosslinked chitosan/nylon fibers. J. Wuhan. Univ. Technol. 37, 1080–1086 (2022).
Zhu, R. F. et al. Layer-by-layer assembly of composite conductive fiber-based organic electrochemical transistor for highly sensitive detection of sialic acid. Electrochim. Acta 425, 140716 (2022).
Author information
Authors and Affiliations
Contributions
Y.H.: Investigation, Writing, Editing. Z.H.: Investigation, Writing. S.Z.: Investigation. B.F.: Conceptualization, Writing, Review, Editing, Supervision, Funding Acquisition. All authors have reviewed and approved the final version of this paper for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethic approval
All the authors declare that this review article does not include any live vertebrates and/or higher invertebrates experiments.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Hu, Z., Zhu, S. et al. Fibre-based organic electrochemical transistors: principle, evaluation, and application. npj Flex Electron 9, 43 (2025). https://doi.org/10.1038/s41528-025-00417-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00417-4
This article is cited by
-
Integrated Circuits on Fiber Substrates: State-of-the-Art System-on-Fiber Technologies for Smart Textiles and Wearables
Nano-Micro Letters (2026)
-
Schottky-gated transistors using chitosan extracted from marine crab
Scientific Reports (2025)