Abstract
Flexible hybrid systems usually combine soft modules (mechanically matched with skin or clothes) and hard modulus (like rigid circuits). However, the risk of interface failure due to modulus mismatch between flexible components and rigid circuits limits the system’s complexity and durability. The diverse features of flexible components further complicate the development of a universal interface. In this work, we demonstrated a cocoon-mimetic feature-matched interface (CFI) that offers stable electrical contact with flexible surface. It also matches flexible systems features in stretchability (lower than 0.22 Ω cm−1 during 900% elongation), durability (stable resistance after 20000 times 100% elongation), breathability (gas permeability 614 mm S−1) and self-adhesive (0.18 ± 0.01 N mm−1). We developed a direct spray-on-skin sensor and used CFI to form a hand task recognition system. This system, deployable in seconds, has 97.7% accuracy in recognition of eight hand tasks. This research offers a promising solution for flexible hybrid systems interfacing challenges.
Similar content being viewed by others
Introduction
Flexible hybrid systems offer substantial potential in diverse applications, including human-machine interfaces, biomedicine, and soft robotics1,2,3,4,5,6,7. Interconnection plays a critical role in integrating various modules within these systems8,9. Flexible anisotropic conductive film10,11,12, copper tape13, silver paste, and solder paste14 are commonly used interconnection methods. However, these methods are prone to failure during deformation due to modulus mismatch9.
A “universal” interface must not only address the issue of modulus mismatch, but also accommodate the diverse mechanical and functional properties intrinsic to flexible systems, including stretchability7,15,16, durability17,18, and breathability19,20,21 (Supplementary Table 1). Any discrepancy in these features can hinder the system’s usability. Any mismatch among these attributes can compromise system performance. For instance, an interface lacking in durability, when interfaced with a robust, flexible component, may serve as a bottleneck for overall system reliability8.
Various approaches have been attempted to solve these issues. Fully soft electronics, devoid of rigid Si components, have been developed to eliminate the needs of extra interfaces22,23. Yet, Si-based components remain indispensable for signal processing and wireless communication, thereby motivating the development of flexible hybrid systems. Within such systems, mesh-based interfaces have been investigated to address requirements of both stretchability and breathability24; While promising, no current mesh adhesives can provide strong adhesion and high breathability at the same time. In parallel, liquid metals have been utilized to fabricate highly conductive and stretchable interfaces25,26. Despite these advancements, the high surface tension leads to low surface adhesiveness, and the liquid metal may smear to undesired places. Other studies have deposited conductive elements—such as gold (Au) or liquid metal—into self-adhesive polymers like styrene-ethylene-butylene-styrene (SEBS) or pressure-sensitive adhesives (PSA) to create stretchable and self-adhesive interfaces9,27. Nevertheless, these composites generally lack sufficient breathability, and their resistance stability is further limited by particle separation under strain. Considering the unmet requirements for a universal electrical interface that bridges materials with different moduli in flexible hybrid systems, an interface that combines stretchability, durability, self-adhesion, and breathability remains highly desirable (Fig. 1f, Supplementary Table 1).
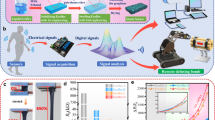
a Inspiration for this work: Insects utilize silk to anchor their cocoons to flexible surfaces (leaves), ensuring structural stability and environmental adaptability. b SEM image of biomimetic design of CFI, using a complex mesh to mount the coil and liquid metal on the skin (bar=500um). c Structure scheme of CFI as interface between a flexible component (e.g., skin or sensor) and rigid circuits in flexible hybrid system. d EDS (energy dispersive spectroscopy) image of the conductive part of CFI which is liquid-metal-in-coil structure (complete elemental analysis is provided in Supplementary Fig. 1); e False-color micro-CT structure of CFI interfaced with deformable surface, liquid metal (LM) is overflowed from coil. f Feature comparison between this work, other interface work and need of flexible components9,19,21,24,25,26,27,40,49,50,51,52,53,54,55,56,57. WVTR refers to water vapor transferring rate. g CFI has an adhesive layer for enhanced contact. h adhesive mesh structure of CFI for breathability. i CFI has 3D-coil structure for stretchability. j CFI has a liquid metal enhanced electrical interface. NOTE: The authors thank Andreas Kay for permission to use the photo of silk cocoon in Fig. 1a.
In this study, inspired by insects that employ silk to construct cocoons on deformable surfaces—with inherent breathability and self-adhesiveness28,29,30 (Fig. 1a). We developed an adhesive yet breathable mesh that mimics these natural silk properties to anchor conductive components on flexible surface (Fig. 1b, c). Furthermore, by incorporating a liquid metal integrated with a coil structure, our interface achieves exceptional stretchability and durability (Fig. 1d, e).
To elaborate on our approach, we developed a melt-spinning process to produce a mesh adhesive with high gas permeability and self-adhesiveness (Fig. 1g, 1h, Supplementary Figs. 1, 2), overcoming the limitations of conventional spreading techniques that yield less permeable layers31. We use a copper coil to meet the extreme stretchability needs of the electrical interface (Fig. 1i) and utilize the low modulus and excellent conductivity of liquid metal to enhance electrical contact32 (Fig. 1j, Supplementary Fig. 3). A liquid-metal-in-coil structure has been designed, the liquid metal is injected and stored in the coil due to surface tension (Fig. 1d, Supplementary Fig. 4, 5). During operation, the liquid metal overflows from the coil under strain and inertial force (Fig. 1e, Supplementary Fig. 6). The overflow threshold is lowered by strain during operation, which effectively prevents liquid metal from smearing onto undesired areas before operation. (Supplementary Fig. 7-8, Supplementary Note 1).
We systematically evaluated CFI’s performance and compared it with previous sensors’ needs together with other interface works. The stretchability, durability, and breathability of CFI can cover most wearable sensors’ performance needs (Fig. 1f, Supplementary Table 1). Therefore, the CFI can be used without concerns about interface mechanical/electrical failure or limiting the system’s performance.
For application demonstration, CFI can form a stable electrical interface with deformable surfaces in a plug-and-play manner. Two main kinds of soft modulus in flexible systems that need electrical interface are sensors and the human body. Taking skin as an example of the human body, we used CFI as dry electrodes to build a wearable biopotential monitoring system and obtain high-quality ECG data. To demonstrate CFI -sensor interface, we developed a direct spray-on-skin sensor. This type of sensor is easily fabricated and conforms to the skin for high signal quality1, but lacks a substrate for predefined electrode. CFI works as an excellent interface between the sensor and rigid circuits, forming a gesture recognition system with 97.7% accuracy.
Results
CFI design and benchmarking
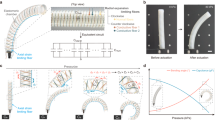
Inspired by caterpillars using silk to anchor their cocoons to leaves, we developed an elastic yet adhesive mesh structure (complex mesh) to firmly mount the coil and liquid metal on a flexible surface (Fig. 2a, Supplementary Fig. 9). The mesh’s tension ensures consistent coil contact with surfaces, even in varying surface curvature (Fig. 2b-i, Supplementary Fig. 10). Due to the adhesive mesh, the peeling force of the complex mesh (0.18 ± 0.01 N mm⁻¹) is two orders of magnitude higher than that of PDMS (1.2 × 10⁻³ N mm⁻¹) and Eco-flex (1.0 × 10−³ N mm⁻¹) (Fig. 2b-ii). Despite its strong adhesive properties, the complex mesh also exhibits durable adhesiveness under various mechanical stimuli commonly occurred under daily use (Supplementary Fig. 11), it remains adhered to the skin for over 24 hours without significant loss of adhesive strength (Fig. 2b-iii).
a schematic view of biomimetic design of CFI, using a complex mesh to mount the coil and liquid metal on the skin. b self-adhesive of CFI: i, FEM simulation of CFI mounted on different surface geometries. ii, 180° peeling test of complex mesh compared with PDMS and Eco-flex. iii, The complex mesh remains sticky after 1 day on the skin; c Breathability of CFI: i, Principle of breathability, the blue arrow shows the potential gas flow. ii, Comparison of gas permeability and water vapor transfer rate (WVTR) between CFI and two kinds of surgical tapes. iii, Infrared thermal imaging of a volunteer with their left arm in contact with 2 kinds of surgical tape (A, B), 300 nm thick PDMS (C), and CFI (D); d Stretchability and durability of CFI: i, Optical image of CFI during 600% strain. iii, Schematic view of CFI at different strains, showing mechanical breaking (complex mesh breaking) and electrical breaking (coil breaking). iv, Electrical stretchability and resistance rate of different interface methods. v, Durability of CFI during 100% strain and 0.25 cm−1 bending. e Biocompatibility of CFI: i, Cell toxicity test of CFI after 24 h, 48 h and 72 h. ii, Cell viability after 24 h, 48 h, and 72 h.
Breathability, including gas permeability and water vapor transmission rate (WVTR), is crucial for wearable systems, which are major kinds of flexible system8,33,34,35. It impacts biocompatibility and wearing comfort10,36, while also preventing moisture-related damage that can affect system durability. The complex mesh, which constitutes most of the CFI’s area, provides more gas transfer pathways than breathable surgical tapes (Fig. 2c-i). Gas permeability tests show that the complex mesh achieves a gas permeability of 614 mm s⁻¹ and a WVTR of 239.6 g m⁻² h⁻¹, surpassing surgical tapes (Fig. 2c-ii, Supplementary Fig. 12). To show the complex mesh’s breathability, we tested the heat accumulation on human skin after sweating. The infrared image shows significant heat accumulation under low-permeability PDMS and surgical tape B (Fig. 2c-iii). This is likely because sweat evaporation is a primary method of heat transfer in humans post-exercise37. The sweat accumulation may influence the wearing comfort and performance of wearable electrical systems. The permeability of the complex mesh remained high after 72 hours of adhesion to human skin (Supplementary Fig. 12). SEM imaging of its microstructure confirmed that gas transfer pathways remained unobstructed throughout the attachment period (Supplementary Fig. 13).
Stretchability is essential for flexible componets38,39. To our knowledge, flexible sensors have achieved stretchability up to 920%40 (Supplementary Table 1). The CFI interface is strain-insensitive due to the highly packed coil structure, which allows a long length of wire to be accommodated in a unit length. CFI maintains less than 2 Ω resistance until it breaks at 939 ± 84% strain (Fig. 2d-i, Fig. 2d-ii). The coil remains electrically conductive, although the mechanical breaking of the complex mesh will cut the conductive path of the liquid metal at around 500%-600% strain (Fig. 2d-iii). The stretchability of CFI is much higher than commonly used interface like silver paste, Cu tape, and carbon tape (Fig. 2d-iv, Supplementary Fig. 14), meeting the stretchability requirements for flexible components in most hybrid systems (Supplementary Table 1).
Despite its stretchability, resistance is also important. Resistance changes in the interface can introduce noise to circuits. The resistance change of CFI during strain until it breaks is less than 1 ohm, which is significantly lower than the typical resistance changes in flexible sensors (which can range from hundreds to thousands of ohms41,42). Therefore, CFI’s resistance change under strain introduces minimal noise. Durability is also crucial. CFI maintains stable resistance (with changes less than 0.2 Ω) after 10,000 curvature cycles (0.25 cm⁻¹) or 20,000 cycles at 100% strain (Fig. 2d-v).
In addition to performance, biocompatibility is a fundamental requirement for wearable devices. We conducted cytotoxicity tests of CFI (both as a whole interface and as separate building blocks) on human skin fibroblasts (HSF-T25) over 24, 48, and 72 hours. The results showed no significant change in cell morphology (Fig. 2e-i) and cell viability (Fig. 2e-ii). Skin irritation tests of the liquid metal and complex mesh used in CFI were also conducted on rabbits (Supplementary Fig. 15), which indicated no significant irritation (Supplementary Tables 2-5).
CFI interfaced with flexible component
A high-quality interface between the skin and rigid circuits is essential for biopotential monitoring, as it requires electrodes to maintain firm, stable contact to reduce motion artifacts during use43,44. To demonstrate that CFI forms a stable electrical interface with the human body, we developed a wearable three-lead ECG system employing CFI as dry electrodes (Fig. 3a). Owing to the liquid metal component in CFI, which directly bridges the coil and the skin, the interface impedance (40 kΩ·cm²) is comparable to that of Ag/AgCl gel electrodes (Supplementary Fig. 16). Furthermore, because the CFI interface is deformable and conforms well to the skin, it reliably captures low-noise PQRST waveforms during various movements (Fig. 3b, Supplementary Fig. 17, 18), with signal quality comparable to commercial gel electrodes (Supplementary Fig. 19).
a Schematic view of CFI working as an electrical interface with the skin in an electrocardiogram (ECG) monitoring system. RA, LA, RR, and LL indicate the CFI electrode placement on the human body. b the ECG signal obtained by CFI during lying down, standing, or walking. c s schematic view of the CFI working as an electrical interface with the conformable sensor. d SEM image of conformable strain sensor on synthetic leather; e CFI-interfaced sensor at different amplitude. f CFI-interfaced sensors at different frequencies of 5% strain; g CFI can protect the sensor from pressure and artificial sweat. h sensing principle of crack-based strain sensor. i FEM simulation of stress distribution in crack-based sensor with and without complex mesh coverage, j Sensing performance comparison between the CFI-interfaced sensor and electrical interconnections without CFI.
As a demonstration of the CFI-sensor interface (Fig. 3c), we fabricated a substrate-less conformable sensor on synthetic leather (Fig. 3d, Supplementary Fig. 20). Such sensors adapt to the curvature and texture of soft, irregular surfaces, ensuring consistent and reliable signal acquisition at resolution of hundreds of microns (Supplementary Fig. 21). However, these devices typically require post-fabrication electrical interconnection. An ideal interface should not only establish robust contact with complex surface textures but also maintain sufficient deformability for high-quality signal acquisition during use. Owing to its high stretchability and self-adhesiveness, the CFI provided high-quality signals under varying amplitudes and frequencies of deformation (Fig. 3e, f). Additionally, the complex mesh in CFI covers the sensor that shields it from external interferences - such as mechanical contact and liquid infiltration—that commonly occur during operation (Fig. 3g, Supplementary Fig. 22, 23).
CFI not only facilitates robust signal transmission and protection but also enhances the durability of stretchable circuits. In stretchable systems, cyclic strain can induce microcrack formation and propagation in the material, potentially leading to device failures45,46. To illustrate how CFI can mitigate this degradation, we examined crack-based strain sensors, which rely on the periodic formation and healing of microcracks during operation47,48 (Fig. 3h). When the sensor surface is covered by CFI, stress at the crack sites is dispersed (Fig. 3i), thereby reducing crack propagation. Both experimental data and theoretical modeling confirm that the performance of these sensors is stabilized by CFI (Fig. 3j, Supplementary Fig. 24, Supplementary Note 2). Furthermore, a substrate-free conformable sensor incorporating CFI achieved three times higher durability than a similar sensor previously reported1 (Fig. 3j).
CFI-Enabled Spray-on-Skin Sensors
CFI enables sensors to be fabricated directly on the skin, eliminating the transfer process and removing the need to integrate electrodes and substrates during sensor fabrication. As a demonstration of a CFI-enabled spray-on-skin sensor, we developed an efficient process using a spray bottle and a sticky mask to print sensors directly onto the skin (Fig. 4a–c, Supplementary Fig. 25). Following printing, the CFI is pressed onto the top of the sensor to complete the system (Fig. 1i, Supplementary Fig. 26), allowing a motion-sensing system to be deployed within minutes and ensuring stable performance for over 24 hours under routine environmental and mechanical interference (Supplementary Fig. 26-27). The unique muscle and skin movements associated with each gesture facilitate the recognition of eight distinct gestures using a single sensor (Fig. 4e, f). A convolutional neural network (CNN) was trained to interpret these signals, achieving 97.7% accuracy on the validation dataset (Fig. 4g, Supplementary Fig. 28). Gesture recognition is critical for human–machine interaction; as a demonstration, gesture signals (Fig. 4h) were mapped to specific actions in three-dimensional space and then either projected into a virtual reality environment (Supplementary Fig. 29) or used to control a robotic hand (Supplementary Fig. 25).
a schematic view of spray-on-skin sensor fabrication. b optical image of spray-on-skin sensor. c SEM view of spray-on-skin sensor on the skin cast, showing the conformability of the sensor. d Eight gestures recognized in this research. Each gesture was mapped to an action of a plane model in VR. e gestures and their signals. f unsupervised clustering result of 8 different gestures. g confusion matrix using a convolutional neural network for recognition. h a piece of gesture signal and its control output.
Discussion
We developed a user-friendly (plug-and-play), simple-to-prepare (no need for complex equipment during the fabrication process), and high-performance interface that ensures strong electrical interconnection and stable operating conditions for flexible systems. CFI’s stretchability, durability, breathability, and skin-mountability match the needs of flexible systems. It effectively shields sensors from external interference, such as sweat or touch. It also increases the durability of stretchable circuits. We proposed a crack growth model to explain this durability enhancement. CFI helps researchers develop their sensing system without worrying about the interface. As a practical application, we built a spray-on-skin conformable sensing system with CFI that can recognize eight different hand tasks with over 97% accuracy. Then we used this system to control a plane model in VR and a robotic hand in the real world. Further development can be investigated, including the reusability of CFI, which may be done by changing the conductive structure. Or further improve skin contact and reduce motion artifact for long-term monitoring of electrophysiological signals43,44. The widespread adoption of CFI will showcase its capability to support more customized, flexible systems and bring these systems closer to real-world applications.
Methods
Fabrication of adhesive mesh
Adhesive mesh: biocompatible press-sensitive adhesive (Dupont, LiveoTM BIO-PSA7-4560) was melted at about 155 °C (Supplementary Fig. 2) and centrifuged to form adhesive fiber using a cotton candy machine (NOSTALGIA ELECTRONICS). Then the adhesive fiber was received by a rotated metal ring to form an adhesive mesh.
Fabrication of PU mesh
PU mesh: PU (15% wt) was dissolved in Dimethylformamide (DMF) and Tetrahydrofuran (THF) solvent (1:1 vol. to vol). The PU solution was electrospun at 12 kV to get PU mesh at 80 um thickness.
Fabrication of complex mesh
The PU mesh was initially supported by aluminum foil. The prepared adhesive mesh was placed over the PU mesh, followed by a layer of release paper. Pressure was then applied to the release paper to ensure effective bonding between the two meshes. Finally, the aluminum foil and release paper backing the PU mesh were carefully removed.
Liquid-metal-in-coil
Coil was custom manufactured using 100 µm diameter copper wire, with an outer spiral diameter of 1 mm and a coil density of 30 turns per centimeter (Shenzhen Haoxin United Industrial Co., Ltd.). Liquid metal (Ga-In alloy, 75.5:24.5 wt.%) was injected into the coil using a 1 ml syringe (Supplementary Fig. 5). The syringe was equipped with a needle of approximately 600 µm in diameter, which was carefully inserted into the coil before initiating the injection process. A precision syringe pump (ISPLab02) was employed to control the injection rate at 0.1 mm/min. To maintain experimental consistency, the syringe needle was cleaned with laboratory paper after each injection to remove residual liquid metal before proceeding with the next coil.
Overflow threshold of liquid-metal-in-coil
CFI at different strains, where the liquid metal inside the coil structure was also subjected to varying strains, was adhered to a glass slide. The sample was then placed in a centrifuge tube and centrifuged (Sorvall ST4 Plus) at a specific acceleration. X-ray imaging (Carl Zeiss Xradia 610 Versa) was performed to examine whether the liquid metal overflowed from the coil.
Fabrication of CFI
Liquid-metal-in-coil is adhered to the adhesive mesh at the desired position. This process is guided by a metal musk.
SEM and EDS image
Both images are taken by scanning electron microscopes (ZEISS GeminiSEM 300). SEM image is taken at a voltage 5–20 kV with a second electron detector. The EDS image was taken at a voltage 15 kV.
Micro-CT image of CFI
The CFI device was adhered to a 100 μm cu film. Then, pat the CFI gently to get the liquid metal form in contact with the cu film. The image was taken by Carl Zeiss Xradia 610 Versa with 140 kV voltage.
Peeling force test
Adhesive mesh, PDMS (300um thickness) and Ecoflex-50 (500um thickness) all at 1 cm width was adhered to stainless steel with 500 g roller press. Then, using a universal testing machine (Shimadzu AGS-X 10kN) to peel the films at a speed of 1 mm/s.
Gas permeability
A gas permeability test was performed a gas permeability tester (TQD-G1 Labthink). Testing condition is 23 °C, 49%RH with 1000 Pa pressure gradient.
Water vapor transfer rate
The water vapor transferring rate is tested in an oven at 30°C. CaCl2 was placed in the oven to remove the water vapor. Using 4 hours weight loss of a bottle covered with a test sample to calculate the water vapor transfer rate.
Infrared thermal imaging
A mobile infrared thermal imager (HIKMICRO-P20Max) was used for infrared thermal imaging. For a demonstration of gas permeability, 4 different kinds of membranes were stuck on the forearm of a volunteer.
Cell viability test
The human skin fibroblasts (HSF-T25) were grown in a sterile CO2 incubator at 37° using Dulbecco’s Modified Eagle Medium (DMEM) with 10% FBS and 1 × penicillin/streptomycin.
Cells were transferred into 12 12-well plate 24 h before the experiment. The density is 8*104 cell/well. Samples were added into each well 24,48, and 72 h before imaging. Was using microscopy. Cells are stained (Hoechst 33342, calcein AM, and ethidium homodimer) before confocal (Agilent BioTek Cytation C10) imaging. Total cell was counted by Hoechst 33342-stained cells, and dead cells are counted by ethidium homodimer-stained cells.
Cell viability= (total cell - dead cell) /total cell nucleus
Skin irritation test
This test is commissioned to a company with ILAC Mutual Recognition Arrangement accreditation. New Zealand White Rabbits were designated as the animal model for evaluating skin irritation. 24 hours before the test, enough hair was removed from both sides of the animal’s spine (approximately 10 cm × 15 cm area) as the test sites. The test material samples were applied directly to the skin on both sides of the rabbit’s spine. The material contact sites were covered with 2.5 cm × 2.5 cm water absorbent gauze, then fixed with bandages for at least 4 hours. After the exposure period, the bandages were removed, and any residual test material was cleaned off appropriately. Observations of each contact site were recorded at (1 ± 0.1) hours, (24 ± 2) hours, (48 ± 2) hours, and (72 ± 2) hours after removing the bandages.
Spray on skin sensor
Few layers graphene was dissolved in ethanol with 49.5 g/L. The solution is fully mixed before use. Customized stickers with a 2 cm*2 cm square hole in the center were used as mask. Commercial spray bottles were used to spray the graphene on skin.
Consent for use of identifiable images
All figures containing potentially identifiable images of individuals (including Fig. 4b–d and Supplementary Figs. 25 and 26) were captured with the participants’ full awareness and cooperation. Written informed consent was obtained from all individuals involved, granting permission to use and publish their images in this research.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Other raw data is available from the corresponding authors upon request.
Code availability
The code supporting the findings of this study is available from the corresponding authors upon request.
References
Kim, K. K. et al. A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition. Nat. Electron. 6, 64–75 (2023).
Liu, Y. et al. Ag–thiolate interactions to enable an ultrasensitive and stretchable MXene strain sensor with high temporospatial resolution. Nat. Commun. 15, 5354 (2024).
Yoon, K. et al. Highly stretchable thermoelectric fiber with embedded Copper(I) Iodide nanoparticles for a multimodal temperature, strain, and pressure sensor in wearable electronics. Adv. Funct. Mater. 35, 2407759 (2024).
Cui, Y. et al. Highly stretchable, sensitive, and multifunctional thermoelectric fabric for synergistic-sensing systems of human signal monitoring. Adv. Fiber Mater. 6, 170–180 (2024).
Oh, J. et al. Air-permeable waterproofing electrocardiogram patch to monitor full-day activities for multiple days. Adv. Healthc. Mater. 11, 1–8 (2022).
Hegde, C. et al. Sensing in Soft Robotics. ACS Nano 17, 15277–15307 (2023).
Arwani, R. T. et al. Stretchable ionic–electronic bilayer hydrogel electronics enable in situ detection of solid-state epidermal biomarkers. Nat. Mater. 23, 1115–1122 (2024).
Luo, Y. et al. Technology Roadmap for Flexible Sensors. ACS Nano 17, 5211–5295 (2023).
Jiang, Y. et al. A universal interface for plug-and-play assembly of stretchable devices. Nature 614, 456–462 (2023).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 12, 907–913 (2017).
Sun, B. et al. Gas-permeable, multifunctional on-skin electronics based on laser-induced porous graphene and sugar-templated elastomer sponges. Adv. Mater. 30, 1–8 (2018).
Fan, J. A. et al. Fractal design concepts for stretchable electronics. Nat. Commun. 5, 3266 (2014).
Yang, Q. et al. Mixed-modality speech recognition and interaction using a wearable artificial throat. Nat. Mach. Intell. 5, 169–180 (2023).
Wang, Y. et al. Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale. Sci. Adv 6, eabd0996 (2020).
Jang, T. M. et al. Stretchable and biodegradable self-healing conductors for multifunctional electronics. Sci. Adv. 10, 1–10 (2024).
Kim, J. H., Cho, K. G., Cho, D. H., Hong, K. & Lee, K. H. Ultra-Sensitive and Stretchable Ionic Skins for High-Precision Motion Monitoring. Adv. Funct. Mater. 31, 1–8 (2021).
Wang, Y. et al. A durable nanomesh on-skin strain gauge for natural skin motion monitoring with minimum mechanical constraints. Sci. Adv. 6, 1–9 (2020).
Hong, J. et al. Transferred laser-scribed graphene-based durable and permeable strain sensor. Adv. Mater. Interfaces 8, 1–10 (2021).
Dong, H. et al. Ultra-flexible, breathable, and robust PAN/MWCNTs/PANI nanofiber networks for high-performance wearable gas sensor application. ACS Sens. 9, 3085–3095 (2024).
Sun, G., Wang, P., Jiang, Y., Sun, H. & Meng, C. Intrinsically flexible and breathable supercapacitive pressure sensor based on mxene and ionic gel decorating textiles for comfortable and ultrasensitive wearable healthcare monitoring. ACS Appl. Electron. Mater. 4, 1958–1967 (2022).
Liu, Z. Highly breathable and stretchable strain sensors with insensitive response to pressure and bending. Adv. Funct. Mater. 31, 2007622 (2021).
Niu, S. et al. A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2, 361–368 (2019).
Zhong, D. et al. High-speed and large-scale intrinsically stretchable integrated circuits. Nature 627, 313–320 (2024).
Zhuang, Q. et al. Permeable, three-dimensional integrated electronic skins with stretchable hybrid liquid metal solders. Nat. Electron. 7, 598–609 (2024).
Li, M. et al. Graded Mxene-doped liquid metal as adhesion interface aiming for conductivity enhancement of hybrid rigid-soft interconnection. ACS Appl. Mater. Interfaces (2022) https://doi.org/10.1021/acsami.2c23002.
Liu, S., Shah, D. S. & Kramer-Bottiglio, R. Highly stretchable multilayer electronic circuits using biphasic gallium-indium. Nat. Mater. 20, 851–858 (2021).
Tang, L., Yang, S., Zhang, K. & Jiang, X. Skin electronics from biocompatible in situ welding enabled by intrinsically sticky conductors. Adv. Sci. 9, 1–11 (2022).
Sun, J. et al. Fabrication and mechanical properties of engineered protein-based adhesives and fibers. Adv. Mater. 32, 1906360(2020).
Kunz, R. I., Brancalhão, R. M. C., Ribeiro, L., de, F. C. & Natali, M. R. M. Silkworm Sericin: Properties and Biomedical Applications. Biomed. Res. Int. 2016, 1–19 (2016).
Reizabal, A., Costa, C. M., Pérez-Álvarez, L., Vilas-Vilela, J. L. & Lanceros-Méndez, S. Silk fibroin as sustainable advanced material: material properties and characteristics, processing, and applications. Adv. Funct. Mater. 33, 2210764 (2023).
Benedek, I. & Feldstein, M. M. Technology of pressure-sensitive adhesives and products. Technology of Pressure-Sensitive Adhesives and Products (CRC Press, 2008). https://doi.org/10.1201/9781420059410.
Park, B., Jeong, C., Ok, J. & Kim, T. Materials and structural designs toward motion artifact-free bioelectronics. Chem. Rev. 124, 6148–6197 (2024).
Liu, X. et al. Organic flexible electronics for innovative applications in electronic skin. Adv. Mater. Technol. 10, 2400661 (2025).
Dickey, M. D. Stretchable and soft electronics using liquid metals. Adv. Mater. 29, 1606425 (2017).
Yang, J. C. et al. Electronic skin: recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 31, 1904765 (2019).
Fan, W. et al. Sweat permeable and ultrahigh strength 3D PVDF piezoelectric nanoyarn fabric strain sensor. Nat. Commun. 15, 3509 (2024).
Khonsary, S. Guyton and Hall: Textbook of Medical Physiology. Surgical Neurology International vol. 8 (Elsevier Health Sciences, 2017).
Shih, B. et al. Electronic skins and machine learning for intelligent soft robots. Sci. Robot. 5, eaaz9239 (2020).
Gao, Z. et al. Advances in wearable strain sensors based on electrospun fibers. Adv. Funct. Mater. 33, 1–42 (2023).
Zhu, C. et al. Breathable ultrathin film sensors based on nanomesh reinforced anti-dehydrating organohydrogels for motion monitoring. Adv. Funct. Mater. 2411725, 1–13 (2024).
Qiao, Y. et al. Multilayer graphene epidermal electronic skin. ACS Nano 12, 8839–8846 (2018).
Zhong, M. et al. A flexible wearable strain sensor based on nano-silver-modified laser-induced graphene for monitoring hand movements. Micromachines 15, 989 (2024).
Park, B. et al. Cuticular pad–inspired selective frequency damper for nearly dynamic noise–free bioelectronics.Science376,624–629 (2022).
Shin, J. H., Choi, J. Y., June, K., Choi, H. & Kim, T. Polymeric conductive adhesive-based ultrathin epidermal electrodes for long-term monitoring of electrophysiological signals. Adv. Mater. 36, 1–15 (2024).
Suresh, S. Fatigue of Materials. Fatigue of Materials (Cambridge University Press, 1998). https://doi.org/10.1017/cbo9780511806575.
Halford, G. & Gallagher, J. Fatigue and Fracture Mechanics: 31st Volume. Fatigue and Fracture Mechanics: 31st Volume vol. 14 (ASTM International100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, 2000).
Kim, Yn., Lee, J. & Kang, S. K. Ultrasensitive crack-based strain sensors: mechanism, performance, and biomedical applications. J. Mech. Sci. Technol. 36, 1059–1077 (2022).
Amjadi, M., Pichitpajongkit, A., Lee, S., Ryu, S. & Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire-elastomer nanocomposite. ACS Nano 8, 5154–5163 (2014).
Chen, J. et al. Breathable strain/temperature sensor based on fibrous networks of ionogels capable of monitoring human motion, respiration, and proximity. ACS Appl. Mater. Interfaces 13, 51567–51577 (2021).
Zhang, X. et al. Breathable and wearable strain sensors based on synergistic conductive carbon nanotubes/cotton fabrics for multi-directional motion detection. ACS Appl. Mater. Interfaces 14, 25753–25762 (2022).
Hao, Y. et al. A Stretchable, breathable, and self-adhesive electronic skin with multimodal sensing capabilities for human-centered healthcare. Adv. Funct. Mater. 33, 1–12 (2023).
Wang, S. et al. Waterproof and breathable graphene-based electronic fabric for wearable sensors. Adv. Mater. Technol. 7, 1–8 (2022).
Ding, Y. R. et al. Flexible superamphiphobic film with a 3D conductive network for wearable strain sensors in humid conditions. ACS Appl. Electron. Mater. 4, 345–355 (2022).
Xu, R. et al. Breathable Kirigami-Shaped Ionotronic e-Textile with Touch/Strain Sensing for Friendly Epidermal Electronics. Adv. Fiber Mater. 4, 1525–1534 (2022).
Liu, X. et al. Recent progress on smart fiber and textile based wearable strain sensors: materials, fabrications and applications. Adv. Fiber Mater. 4, 361–389 (2022).
Ai, L. et al. Tough soldering for stretchable electronics by small-molecule modulated interfacial assemblies. Nat. Commun. 14, 7723 (2023).
Lei, M. et al. Breathable and waterproof electronic skin with three-dimensional architecture for pressure and strain sensing in nonoverlapping mode. ACS Nano 16, 12620–12634 (2022).
Acknowledgements
We thank Andreas Kay’s permission for using photo of cocoon. We thank Xiaoli Ren, Mengli Fu, Jing Li, Yalu Chen, and Xinchen Shen for their help in experiments. We thank Fan Wu, Xiaoshi Li, and Jinming Jian for their help in experiment design. This work was supported by the National Key Research and Development Program of China (2021YFC3002200), the National Natural Science Foundation of China (U20A20168), the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2023C002YG, ZN2023A01), and a grant from the Guoqiang Institute, Tsinghua University.
Author information
Authors and Affiliations
Contributions
S.L. conceived the project, performed experiments, processed data, and drafted as well as revised the manuscript. Z.W. conducted hand-task data processing, contributed to drafting the manuscript, and assisted with revisions. J.Y. processed data for the mathematical modeling of crack generation and contributed to the manuscript writing. J.P. participated in project conception and carried out experimental operations. B.Z. provided support through FEM simulations. X.L. designed experiments and contributed to revising the manuscript. D.L. assisted with experiments and offered valuable insights during concept discussions. Z.L. (affiliated with the School of Pharmacy) performed the biocompatibility and cell experiments. J.R. contributed to the manuscript writing and its revisions. P.G. assisted with manuscript revisions. Q.Z. (from the Basic Industrial Training Center) provided experimental support. Y.Y. contributed to manuscript drafting, revisions, and overall project conception. T.-L.R. contributed to the conceptualization of the project as well as manuscript drafting and revisions. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Wang, Z., Yin, J. et al. Cocoon-mimetic feature-matched interface for flexible system. npj Flex Electron 9, 99 (2025). https://doi.org/10.1038/s41528-025-00462-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41528-025-00462-z