Abstract
The Mg–9Gd–3Y–2Zn–0.5Zr alloy was studied for its long-term corrosion behavior in a simulated coastal storage environment. The results show that the Mg12 (Y, Gd) Zn phase in the Mg–9Gd–3Y–2Zn–0.5Zr alloy forms a galvanic couple with α-Mg, and the Mg12 (Y, Gd) Zn phase acts as a cathode to accelerate α-Mg during the corrosion initiation period. The corrosion of the anode is subsequently transformed into corrosive dissolution of the anode. With the dissolution of the Mg12 (Y, Gd) Zn phase, elements such as Gd and Y are gradually distributed into the entire corrosion product layer, improving the protective performance of the corrosion product layer by forming dense Gd2O3 and Y2O3.
Similar content being viewed by others
Introduction
Magnesium alloys incorporating rare earth (RE) elements have attracted significant interest in the aerospace, automotive, electronics, and other industries due to their exceptional mechanical properties and advantages in terms of lightweight design. By incorporating rare earth elements such as Gd, Y, and Nd, solid solution strengthening, precipitation strengthening, and grain refinement effects can be achieved, thus enhancing the strength of magnesium alloys1,2,3. As the understanding of alloy design deepens, a series of rare earth magnesium alloys such as Mg–Y, Mg–Gd, Mg–Nd, and Mg–Ce have gradually emerged4. Among them, the Mg–RE–Zn–Zr alloy with superior high-temperature strength and creep resistance has been widely researched5,6,7. Nevertheless, there still exists an inherent contradiction between high strength and corrosion resistance, which hinders its further industrial applications.
In magnesium alloys, the second-phase structure and impurities are considered to be decisive factors influencing their corrosion behavior8,9. Impurity elements, including Fe, Cu, Si, and Al have a tendency to form intermetallic compounds within the magnesium matrix. When the impurity content exceeds tolerance limits, it significantly diminishes the corrosion resistance of magnesium alloys10. With advancements in high-purity magnesium purification techniques, effective control over impurity elements in magnesium alloys has been achieved11,12. The corrosion effect on magnesium alloys by the second phase is dependent on the potential. When the second phase acts as a cathode participating in corrosion, it exhibits a dual mechanism affecting the corrosion of magnesium alloys13. Research conducted by Dai et al.14 investigated the impact of the reticulated 18R-LPSO structure on the corrosion of magnesium alloys. The 18R-LPSO structure acted as a cathode, accelerating the corrosion of α-Mg, while the net-like distribution of the second phase also hindered the penetration of the corrosive medium. In the Mg–Zn–Gd–Zr alloy, Yin et al.15 identified three distinct cathodic second phases, namely X–Mg12GdZn, W–Mg3Gd2Zn3, and I–Mg3Zn6Gd. Due to the maximum potential difference between the W–Mg3Gd2Zn3 and the substrate, it has the highest accelerating effect on the corrosion of the substrate, followed by the I phase. Some studies have also reported the presence of anodic second phases in magnesium alloys. Research by Song et al.16 investigated the corrosion process of GW93 alloy in NaCl solution, demonstrating that the second phase Mg24Y5 in the alloy primarily dissolves as an anode during corrosion progression, leading to subsequent pit formation. In recent years, research on rare earth magnesium alloys has predominantly focused on mechanical properties and corrosion resistance mechanisms, while there has been limited exploration of corrosion in actual service environments.
Understanding the corrosion characteristics of magnesium alloys in atmospheric environments is crucial for their practical applications. Research by Zhang et al.17 indicated that the corrosion rates of Mg–7Gd–5Y–1Nd–0.5Zr alloy after six months of exposure in Beijing and Qingdao were 25.6 g/m2 and 34.7 g/m2, respectively. Jiang et al.18 investigated the corrosion characteristics of Mg–5Y–1.5Nd–xZn–0.5Zr alloy in marine environments and found Mg–5Y–1.5Nd–4Zn–0.5Zr alloy to possess outstanding corrosion resistance, with an annual average corrosion weight loss of 285 g/m2 on the KEXUE research vessel. As the ideal candidate material for extremely lightweight aerospace equipment, rare earth magnesium alloys face challenges in marine atmospheric environments. Fortunately, they are mostly stored in environments with stable temperatures and humidity and lower chloride ion content. However, there are no reports available on the corrosion of magnesium alloys in coastal storage environments.
In this study, an accelerated test method simulating coastal storage environments was designed. The corrosion evolution of wrought Mg–9Gd–3Y–2Zn–0.5Zr alloy in coastal storage environments was investigated, and the roles of the second phase in the early and long-term corrosion were discussed.
Results
Microstructure
The OM and SEM images of Mg–9Gd–3Y–2Zn–0.5Zr alloy are presented in Fig. 1. The OM results reveal that the sample consists of equiaxed and columnar crystals within an α-Mg matrix. The backscattered electron morphology distinctly shows a second-phase microstructure in the alloy, with brighter regions indicating enrichment of heavier elements arranged in a banded pattern on the sample surface. From Fig. 1b, the proportion of the second phase is calculated to be about 16%. Figure 1c shows the grain size distribution of the samples with an average grain size of 30–40 μm.
Elemental compositions of the α-Mg matrix phase and the secondary phase were detected using EDS, as shown in Fig. 2. It is evident that besides magnesium, the α-Mg matrix phase contains 8.45 wt% of Gd. Gd and Mg both possess a close-packed hexagonal crystal structure. As a result, Gd demonstrates a remarkable solubility in Mg, reaching a maximum of 23.5 wt% at 819 K. This indicates a substantial solid solution strengthening effect19. The results in Fig. 2b indicate the occurrence of element segregation of Y, Gd, and Zn in the second phase.
XRD spectrum of the samples is presented in Fig. 3, revealing the presence of α-Mg matrix and Mg12(Y, Gd)Zn. The low content of Zr element in the alloy resulted in its undetectable presence in the XRD pattern. The second phases play a vital role in refining the grain size of magnesium alloys during the deformation process, as they can interact with dislocations to hinder basal dislocation slip, thus serving as crucial strengthening phases in magnesium alloys.
Corrosion morphology
In Fig. 4, the corrosion macro morphology of the test specimen after different durations of exposure in a simulated coastal storage environment is presented. After 284 h, the surface of the specimen accumulated a significant amount of white corrosion products, which were distributed in a fragmented manner. As the test duration increased, the specimen surface was entirely corroded, and the corrosion products increased and thickened.
After simulating coastal storage environmental tests, the surface corrosion products of samples were studied using SEM. The results are shown in Fig. 5, and Table 1 lists the chemical compositions at different locations analyzed by EDS. Following the 284-h test, a blocky corrosion product layer covered the surface of the samples, with cracks and localized damage on the blocky corrosion product layer revealing granular corrosion products beneath. The corrosion products were primarily composed of C, O, Mg, and Gd, with minimal amounts of Cl detected. After 568 h of testing, the blocky corrosion product layer on the sample surface exhibited severe damage, with needle-like corrosion products appearing in localized areas. EDS measurements indicated a higher concentration of chlorine in the needle-like corrosion products, which are commonly observed in outdoor atmospheric exposure and laboratory immersion studies and are typically attributed to residual MgCl220,21. Subsequently, the damaged areas on the sample surface increased, with needle-like corrosion products containing high chlorine content found at each point of damage.
The micrograph and elemental distribution of samples after exposure to a simulated coastal storage environment for varying durations are illustrated in Fig. 6. As seen in Fig. 6a, after 284 h of testing, the thickness of the corrosion product layer is 29 μm, exhibiting overall compactness with numerous cracks near the substrate, potentially serving as pathways for corrosive media penetration into the substrate. Subsequently, the corrosion product layer increases to 54 μm after 568 h of testing. Here, the region near to the substrate is relatively dense but contains longitudinal cracks, while the layer farther from the substrate is more porous. Over time, the corrosion product layer increases to approximately 100 μm and stabilizes. After 1136 h of testing, a significant enrichment of Cl is observed in the rust layer. In addition, it can be seen from the cross-sectional morphology of the specimen at 284 h and 568 h that the second phase seems to hinder the development of corrosion, which may be related to the galvanic effect of the second phase22.
The morphology of the corrosion pits, as depicted in Fig. 7, clearly illustrates the evolution of the corrosion process. After 284 h of testing, the sample surface showed numerous pits with a certain orientation, resembling the distribution characteristics of the second phase within the sample. By 562 h of testing, the sample surface is covered with large and deep corrosion pits, indicating that the early pits are still continuously developing in-depth and around them. As the experiment progresses, after 1136 h of corrosion, river-like corrosion pits can be observed, indicating severe corrosion on the sample surface.
Electrochemical measurements
Figure 8 illustrates the polarization curves obtained from experiments in a simulated coastal storage environment at different intervals, along with the electrochemical parameters determined through Tafel fitting. After the 284-h test, the activation–passivation transition zone of the anode branch exhibited characteristics of oxide film dissolution and local corrosion23. As the experiment progresses, the anodic polarization curves in each period demonstrate typical active dissolution characteristics.
The self-corrosion potential (Ecorr) and corrosion current density (icorr) of the sample after different experimental periods were obtained through Tafel fitting, as shown in Fig. 8b. The Ecorr gradually shifted positively from −1.703 V to −1.685 V between 284 h and 568 h of the experiment and increased to −1.536 V after 852 h. A higher corrosion potential indicates a lower corrosion tendency as it reveals the material’s thermodynamic stability24. As the experiment progressed, the oxide layer on the samples was gradually destroyed, transitioning the sample from localized corrosion to general corrosion. A significant quantity of corrosion products can serve as a protective barrier in corrosive environments, thereby augmenting the thermodynamic stability of the specimen against corrosion. After 852 h to 1136 h of testing, a slight negative shift in Ecorr was observed, possibly attributed to fluctuations caused by the damage to the corrosion product film on the sample surface.
EIS measurements were conducted on samples to delve deeper into the degradation of the protective film and the development of corrosion product films during testing, as illustrated in Fig. 9. It is evident that after 284 h of testing, the Nyquist plot of the sample exhibits high-frequency capacitive loops and low-frequency inductive loops. The capacitive loop in the high-frequency range can be attributed to the charge transfer process at the corrosion product film/electrolyte interface, while the inductive loop in the low-frequency range is associated with the relaxation process of intermediate species adsorbed on the surface during the anodic reaction25.
Figure 9d illustrates the equivalent circuit models resulting from various experiments. Specifically, Fig. 9d1 corresponds to the equivalent circuit after 284 h, while Fig. 9d2 corresponds to the equivalent circuit from 568 to 1136 h. In these diagrams, Rs represents the electrolyte resistance, Rf and Rct correspond to the resistance of the corrosion product film and charge transfer resistance, respectively, and L and RL reflect the inductance and inductive impedance. Due to the non-uniformity of the surface corrosion product film, a constant phase element CPEf is employed to represent the double-layer capacitance of the corrosion product layer. The impedance (ZCPE) and capacitance (CCPE) of CPE are described according to the following formula26:
The symbol ω represents the angular frequency (rad/s), and i denotes the imaginary unit. When n equals 1, the CPE is regarded as an ideal double-layer capacitor; whereas when 0.5 < n < 1, CPE is viewed as a non-ideal capacitor. By considering the distinctions in the equivalent circuit, the polarization resistance Rp can be determined using the provided formula:
The fitting parameters are shown in Table 2 (see Table 2 for details). It is evident that the value of Rf remained relatively stable during the first two experimental cycles, but increased after the third cycle with the prolongation of the experimental duration, indicating the presence of a loosely packed and defective corrosion product film within the 568-hour test period. On the other hand, the polarization resistance RP increased gradually as the experiment progressed, suggesting a decrease in the sample’s dissolution rate, which aligns with the trend observed in the corrosion current density.
Composition of corrosion product
Figure 10 shows the composition of corrosion products in the sample. The corrosion products of the samples after simulated coastal storage environment testing primarily consist of Mg(OH)2, MgO, MgCl2, Gd2O3, and Y2O3. MgO and Mg(OH)2 are common corrosion products in magnesium alloys, while the presence of Gd2O3 and Y2O3 indicates the involvement of Gd and Y in the corrosion process of the samples. The Pilling–Bedworth (P–B) ratio is commonly used to evaluate the density of oxide films. The P–B ratio between 1 and 2 signifies a relatively dense film, which can be calculated using Eq. (3)27,28:
In the given formula, M represents the relative molecular mass of the oxide, ρ denotes the density, and n signifies the quantity of metal atoms within the oxide molecule.
According to Eq. (3), the Pilling–Bedworth ratios for MgO, Gd2O3, and Y2O3 are calculated to be 0.806, 1.751, and 1.613, respectively. The Pilling-Bedworth ratios of Gd2O3 and Y2O3 being greater than 1 suggest that corrosion product films containing these oxides can effectively enhance the compactness of the films. Additionally, as the experiments progress, the diffraction peaks of Gd2O3 and Y2O3 significantly intensify, indicating an enhanced protective effect of the corrosion product layer on the substrate.
Corrosion kinetics
In Fig. 11, the corrosion weight loss and calculated instantaneous corrosion rate of samples in simulated coastal storage environment are presented. It can be observed that throughout the experimental duration, there is a well-fitted power-law relationship between the corrosion weight loss and the experimental time. The power value of the fitting function is less than 1, indicating that the corrosion product layer can mitigate the corrosion of the substrate.
Since there are few natural exposure tests for rare earth magnesium alloys, the test results are compared with the corrosion behavior of AZ31 alloys exposed in the marine environment. After one cycle of accelerated testing, the corrosion weight loss of the specimen was 17 g/m2, which was about 1/6 of the exposure of the dynamic shipping route in the western Pacific and 1/2 of the exposure of the Xisha Islands in 1 year20,29. In addition, we also found that the n value of the corrosion kinetics of AZ31 alloys is greater than 1 in the marine atmosphere, indicating that the corrosion product layer is less protective. Our test results show that the corrosion product layer of the sample has good protection, which may be related to the lower Cl concentration in the storage environment.
Discussion
The galvanic effect between the α-Mg and the second phase has been identified as a crucial factor influencing the corrosion resistance of magnesium alloys30,31,32. In this study, the presence of a second phase structure in the Mg–9Gd–3Y–2Zn–0.5Zr alloy was noted. To elucidate the role of the second phase during the corrosion process, the potential difference between the α-Mg matrix and the second phase was measured using SKPFM, as depicted in Fig. 12. In Fig. 12a, it can be clearly seen that the bright area is the second phase, while the dark area is α-Mg. The surface potential difference between the two is about 36 mV. Generally speaking, a lower surface potential means more activity33,34. Therefore, in the galvanic couple composed of the matrix and the second phase, α-Mg acts as the anode and corrodes preferentially, while the second phase acts as the cathode to accelerate the corrosion of α-Mg.
The second phase, acting as the cathode has been reported to function as a corrosion barrier35,36,37. In this study, the presence of the second phase was not observed in the corrosion product layer, indicating that corrosion dissolution of the second phase also occurred during the corrosion process, which may also be driven by the potential difference. Therefore, the polished sample was etched with 4% nitric acid alcohol solution, and SKPFM was used to measure the surface potential of the etched sample. The results are shown in Fig. 13. The bright areas in the figure can see the characteristics of grain boundaries and are therefore identified as α-Mg, while the dark areas are identified as the second phase. It can be seen that the surface potential of α-Mg at this time is about 1.71 V, which has not changed significantly compared with before etching, while the surface potential of the second phase decreased from 1.75 V to 1.57 V. This causes the second phase to change from cathode to anode, and corrosion will change from the dissolution of α-Mg to the dissolution of the second phase. Although there are differences between the corrosion method and the actual corrosion process of the sample, this result also well explains the reason why the second phase is not found in the corrosion products and provides a reference for the corrosion process of the sample.
The SKPFM results revealed the thermodynamic corrosion stability of α-Mg and the second phase. In order to observe the corrosion process of the sample more intuitively, an immersion experiment was conducted in 1 wt% NaCl solution. The results are shown in Fig. 14. It can be seen that after immersion for 30 min, slight corrosion occurred in the α-Mg around the second phase. After immersion for 2 h, the corrosion of α-Mg increased, and slight cracks could be seen. After 24 h of immersion, more corrosion products adhered to the surface of the sample, and it was observed that the second phase also began to corrode. This result is consistent with the test results of SKPFM.
Based on the analysis above, a corrosion development model for Mg–9Gd-3Y–2Zn–0.5Zr alloy is proposed, as shown in Fig. 15.
As corrosion occurs, a corrosion product film gradually forms on the surface of the sample, which will have a certain protective effect on the substrate. As mentioned earlier, there is a sequence between α-Mg and the second phase in the corrosion process. Here we will discuss the transformation process of the corrosion product film under this influence.
First, corrosion occurs preferentially at α-Mg around the second phase. It can be seen from Fig. 3a that there is a certain amount of Gd dissolved in the matrix, so the corrosion products generated by α-Mg should contain hydroxides and oxides of Mg and Gd. As corrosion products are generated, the electrochemical activity of α-Mg around the second phase gradually decreases, and the second phase will develop into new corrosion sites. The second phase gradually dissolves, and Gd, Y, and Zn will exist in the liquid film on the surface of the sample in an ionic state, and diffuse to the entire sample surface, gradually generating hydroxides and oxides of Gd, Y, and Zn during the subsequent corrosion process. According to the P–B theory, Gd2O3, Y2O3, and ZnO have good thermodynamic stability, so the protective ability of the corrosion product film on the sample surface can be continuously improved during this process.
Methods
Materials
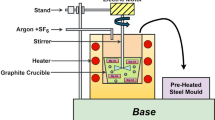
The experimental material is wrought Mg–9Gd–3Y–2Zn–0.5Zr alloy produced by Luoyang Special Materials Research Institute, and its chemical composition is shown in Table 3.
Characterization of microstructure
The samples have dimensions of 10 mm × 10 mm × 2 mm. They were sequentially polished using 400#, 800#, 2000#, and 3000# sandpapers, followed by mechanical polishing with Al2O3 suspension, etched in 4% nitric acid ethanol solution, and examined for metallographic structure using an optical microscope (OM). The grain size of the samples was counted using Image J software.
Surface potential distribution of the samples was measured in a scanning Kelvin probe force microscope (SKPFM, Dimension ICON, Bruker). The probe model used was PFQNE-AL, with a probe cantilever made of silicon nitride and a tip made of single-crystal silicon. The probe had a rated resonant frequency of 300 kHz and a rated spring constant of 0.8 N/m. Surface potential analysis was performed using the dual-scan mode, where topography data was acquired in the first line scan using the tapping mode, and Volta potential data was recorded with the tip lifted up to 50 nm in the second line scan. The results were processed using NanoScope analysis software.
Design of simulated coastal storage acceleration tests
The coastal storage environment in southern China is characterized by an average temperature of 25 °C throughout the year, reaching up to 35 °C in summer. The average humidity stands at 70% RH. Additionally, due to the shielding effect of buildings, the concentration of salt mist in the environment is approximately 155 µg/m3 38. Based on these environmental features, a method for accelerated testing to simulate the coastal storage environment is depicted in Fig. 16. The acceleration test is divided into three steps. The first and third steps are both salt spray tests at 25 °C for 137 h, while the second step is a salt spray test at 35 °C for 10 h. After completing one cycle of testing, we hope that the test results are equivalent to one year of outdoor exposure. The samples for the accelerated testing of the coastal storage environment are sized at 100 mm × 75 mm × 2 mm and processed using wire cutting.
Electrochemical measurements
To investigate the electrochemical properties, samples were processed into dimensions of 20 × 20 × 2 mm, with only one working surface exposed while the backside was connected to a copper wire, and the remaining five surfaces were sealed with epoxy resin. The open-circuit potential (OCP), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization curves of samples at different test cycles were tested by an electrochemical workstation (PARSTAT 4000, Princeton). The electrolyte used for testing was 1 wt% NaCl solution. The OCP was initially tested for 1800 s to assess system stability. Electrochemical impedance measurements were conducted within a frequency range of 100 kHz to 10 mHz, using a 5 mV sine wave excitation signal. Potentiodynamic polarization curves were measured within a range of ±0.5 V relative to the open circuit at a scan rate of 0.5 mV/s. All electrochemical tests were performed at 25 °C and repeated three times to ensure the reproducibility of results. In order to investigate the characteristics of the rust layer during the corrosion of the specimens, the EIS results were fitted using ZsimpWin.
Corrosion morphology and characterization of corrosion products
Surface and cross-section analyses of the specimens after testing were conducted using a scanning electron microscope (SEM, Quanta 250, FEI), combined with energy dispersive X-ray spectroscopy (EDS) for elemental analysis. A laser confocal scanning microscope (LCSM, VK-X200K, Keyence) was used to obtain the morphology of corrosion pits on the surface of the specimen after rust removal (by chromic acid). To analyze the composition of the corrosion product layer post-experiment, the corrosion products on the specimen surface were scraped with a blade and then ground to a fine powder using an agate mortar to remove any visible particles. X-ray diffraction (XRD, Ultima IV, Rigku) was employed to determine the phase composition of the corrosion products, with a scanning range from 10° to 90°, and MDI Jade 9.0 software was used to analyze the obtained XRD patterns.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Stanford, N., Atwell, D. & Barnett, M. R. The effect of Gd on the recrystallisation, texture and deformation behaviour of magnesium-based alloys. Acta Mater. 58, 6773–6783 (2010).
Kubásek, J. et al. Novel magnesium alloy containing Y, Gd and Ca with enhanced ignition temperature and mechanical properties for aviation applications. J. Alloy. Compd. 877, 160089 (2021).
Wang, L., Jalar, A. & Dan, L. Dynamic precipitation and dynamic recrystallization behaviors of Mg-Gd-Nd-Zr magnesium alloy during thermal compression deformation. J. Mater. Res. Technol. 26, 7634–7648 (2023).
Bai, J. et al. Applications of magnesium alloys for aerospace: a review. J. Magnes. Alloy. 11, 3609–3619 (2023).
Tong, L. B. et al. Achieving an ultra-high strength and moderate ductility in Mg–Gd–Y–Zn–Zr alloy via a decreased-temperature multi-directional forging. Mater. Charact. 171, 110804 (2021).
Bazhenov, V. E. et al. Development of Mg–Zn–Y–Zr casting magnesium alloy with high thermal conductivity. J. Magnes. Alloy. 9, 1567–1577 (2021).
Zheng, J. et al. Preparation of ultra-high strength Mg-Gd-Y-Zn-Zr alloy by pre-ageing treatment prior to extrusion. J. Alloy. Compd. 894, 162490 (2022).
Zhang, J. et al. Role of second phases on the corrosion resistance of Mg-Nd-Zr alloys. J. Alloy. Compd. 849, 156619 (2020).
Song, G.-L. & Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloy. 11, 3948–3991 (2023).
Yang, L. et al. Effect of iron content on the corrosion of pure magnesium: critical factor for iron tolerance limit. Corros. Sci. 139, 421–429 (2018).
Liang, D. et al. Preparation of high purity magnesium by vacuum gasification-directional condensation technology. Sep. Purif. Technol. 327, 124878 (2023).
Liang, D. et al. One-step preparation of high purity magnesium by vacuum distillation technology. Vacuum 192, 110464 (2021).
Song, G. Recent progress in corrosion and protection of magnesium alloys. Adv. Eng. Mater. 7, 563–586 (2005).
Dai, J. et al. Insight into the role of Y addition in the microstructures, mechanical and corrosion properties of as-cast Mg-Gd-Y-Zn-Ca-Zr alloys. Mater. Des. 221, 110980 (2022).
Yin, S. et al. Influence of specific second phases on corrosion behaviors of Mg-Zn-Gd-Zr alloys. Corros. Sci. 166, 108419 (2020).
Song, Y., Shan, D. & Han, E.-H. Pitting corrosion of a Rare Earth Mg alloy GW93. J. Mater. Sci. Technol. 33, 954–960 (2017).
Jiang, Q. T. et al. Atmospheric corrosion of Mg–rare earth alloy in typical inland and marine environments. Corros. Eng. Sci. Technol. 49, 651–655 (2014).
Jiang, Q., Lu, D., Cheng, L., Liu, N. & Hou, B. The corrosion characteristic and mechanism of Mg-5Y-1.5Nd-xZn-0.5Zr (x = 0, 2, 4, 6 wt.%) alloys in marine atmospheric environment. J. Magnes. Alloy. 12, 139–158 (2024).
Gao, L., Chen, R. S. & Han, E. H. Effects of rare-earth elements Gd and Y on the solid solution strengthening of Mg alloys. J. Alloy. Compd. 481, 379–384 (2009).
Cui, Z., Li, X., Xiao, K. & Dong, C. Atmospheric corrosion of field-exposed AZ31 magnesium in a tropical marine environment. Corros. Sci. 76, 243–256 (2013).
Liao, J. & Hotta, M. Corrosion products of field-exposed Mg-Al series magnesium alloys. Corros. Sci. 112, 276–288 (2016).
Sun, L. et al. Influence of corrosion products on the corrosion behaviors of Mg-Nd-Zn alloys. Mater. Today Commun. 33, 104891 (2022).
Cao, F., Song, G.-L. & Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 111, 835–845 (2016).
Ci, W. et al. Effect of minor Gd addition on microstructure, mechanical performance, and corrosion behavior of Mg–Y–Gd alloys. J. Mater. Res. Technol. 26, 4107–4120 (2023).
Liu, Q. et al. Enhanced corrosion resistance of AZ91 magnesium alloy through refinement and homogenization of surface microstructure by friction stir processing. Corros. Sci. 138, 284–296 (2018).
Man, C., Dong, C., Wang, L., Kong, D. & Li, X. Long-term corrosion kinetics and mechanism of magnesium alloy AZ31 exposed to a dry tropical desert environment. Corros. Sci. 163, 108274 (2020).
Liu, J. et al. Comparative in vitro study on binary Mg-RE (Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) alloy systems. Acta Biomater. 102, 508–528 (2020).
Wu, J. et al. The oxidation behavior and reaction thermodynamics and kinetics of the Mg-X (X = Ca/Gd/Y) binary alloys. Corros. Sci. 225, 111609 (2023).
Wang, Y. et al. Research on dynamic marine atmospheric corrosion behavior of AZ31 magnesium alloy. Metals 12, 1886 (2022).
Wang, J. et al. Effects of RE (RE = Sc, Y and Nd) concentration on galvanic corrosion of Mg-Al alloy: a theoretical insight from work function and surface energy. J. Mater. Res. Technol. 24, 6958–6967 (2023).
Zhang, K. et al. Effects of solute redistribution during heat treatment on micro-galvanic corrosion behavior of dilute Mg-Al-Ca-Mn alloy. Corros. Sci. 213, 110971 (2023).
Feng, B. et al. Different role of second phase in the micro-galvanic corrosion of WE43 Mg alloy in NaCl and Na2SO4 solution. J. Magnes. Alloy. 10, 1598–1608 (2022).
Zhang, Y. et al. A comparative study of the role of Ag in microstructures and mechanical properties of Mg-Gd and Mg-Y alloys. Mater. Sci. Eng. A 731, 609–622 (2018).
Liu, J. et al. The special role of anodic second phases in the micro-galvanic corrosion of EW75 Mg alloy. Electrochim. Acta 189, 190–195 (2016).
Shao, Z., Nishimoto, M., Muto, I. & Sugawara, Y. Fabrication of a model specimen for understanding micro-galvanic corrosion at the boundary of α-Mg and β-Mg17Al12. J. Magnes. Alloy. 11, 137–153 (2023).
Liu, M., Schmutz, P., Uggowitzer, P. J., Song, G. & Atrens, A. The influence of yttrium (Y) on the corrosion of Mg–Y binary alloys. Corros. Sci. 52, 3687–3701 (2010).
Wang, J. H. et al. Effects of Y substituting Gd on the microstructure evolution, mechanical properties and dissolution behaviors in the Mg-Gd-Ni alloys used as fracturing plugging tools. J. Mater. Res. Technol. 28, 4262–4274 (2024).
Tang, Y., Meng, Q. & Ren, P. Spatial distribution and concentrations of salt fogs in a coastal urban environment: a case study in Zhuhai city. Build. Environ. 234, 110156 (2023).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
J.C. and C.Z. contributed equally to this work. J.C.: Investigation, methodology, formal analysis, Writing—original draft. C.Z.: Resources, investigation. Z.H.: Investigation, formal analysis. Y.T.: Investigation, formal analysis. S.Z.: Resources, investigation. J.G.: Investigation, methodology. X.Z.: Resources. K.X.: Investigation, formal analysis, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, J., Zhang, C., Hu, Z. et al. Corrosion behavior of the second phase in Mg–9Gd–3Y–2Zn–0.5Zr alloy under simulated coastal storage environment. npj Mater Degrad 8, 69 (2024). https://doi.org/10.1038/s41529-024-00492-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-024-00492-4
This article is cited by
-
Controllable degradation behavior, mechanical properties and biocompatibility of Mg-Sr-Mn alloys for the orthopedic medical applications
npj Materials Degradation (2025)
-
New frontiers in marine corrosion research
npj Materials Degradation (2025)