Abstract
Micro-segregation and resulting nanoscale microstructural heterogeneities are unavoidable in wrought 7xxx aluminum alloys produced using current casting and thermomechanical processes. This study demonstrates that these nanoscale heterogeneities significantly impact pit growth in an extruded, age-hardened aluminum alloy 7075-T651. The alloy exhibits a complex microstructure with heterogeneously distributed E-Al18Mg3Cr2 dispersoids, which promote the precipitation of coarse ƞ-Mg(ZnAlCu)2 nanoparticles resulting in the formation of solute-depleted regions both within the grain interior and along the grain boundaries in dispersoid-rich areas. These alterations affect resistance to pit growth at the nanoscale, governing the transition of micro-galvanic sites into large pits with potential for crack initiation. This work underscores the necessity for modifying alloy composition and casting processes to develop superior aluminum alloys for critical applications.
Similar content being viewed by others
Introduction
Age-hardened 7xxx series aluminum alloys are widely used for structural components in aerospace applications due to their high strength-to-weight ratio, achieved through a specific set of thermomechanical and heat treatments that develop unique multiphase microstructures inclusive of the intermetallic particles (IMPs)1,2. These alloys are the primary choice for the critical components including upper wing covers (using rolled products) and wing stringers (using extrusion products) which demand a high resistance to stress corrosion cracking3. The IMPs in 7xxx series aluminum alloys include Cu-Fe-containing constituent particles (Al7Cu2Fe, Al23Fe4Cu, etc.), Si-rich particles (Mg2Si and SiO2), and nano-precipitates (ƞ-MgZn2 and θ-Al2Cu), ultimately governed by the alloy composition and thermal treatment2,4. The aerospace-grade 7xxx series alloys also contain minor amounts of Cr, and Zr, which form nanosized dispersoids (E-Al18Mg3Cr2, and Al3Zr) during the preheating of the as-cast ingot, which in turn inhibit recrystallization following deformation (rolling or extrusion) and promote elongated grain structure known for their higher resistance to crack growth1,2.
The heterogeneous microstructure, however, creates numerous micro-galvanic corrosion sites, which can lead to large pits when exposed to aggressive electrolytes and act as nucleation points for cracking under tensile loading. Given the myriad applications of these alloys in critical settings, the role of IMPs in pitting and subsequent mechanical failure of 7xxx alloys has been extensively studied5,6,7,8,9,10,11. It is well documented that the ‘cathodic’ Fe/Cu-containing constituent particles polarize the matrix phase to its pitting potential, while anodic Mg-rich IMPs (Mg2Si and ƞ-MgZn2) undergo selective dissolution and act as pit nucleation sites in chloride-containing electrolytes4,9,12. The addition of Cu in 7xxx alloys results in ƞ-phase with different stoichiometries including Mg(Zn1−xCux)2, and MgZn2xAl1−xCu1−x5,13. Therefore, it is reasonable to refer ƞ-phase as ‘ƞ-Mg(ZnAlCu)2’. Because the peak-aged alloy exhibits coarse ƞ-Mg(ZnAlCu)2 precipitates along the grain boundaries (GBs), it often suffers from intergranular corrosion. Several researchers have investigated the importance of the morphology of GB precipitates and their effect on stress corrosion cracking of 7xxx alloys13,14,15,16,17,18,19. However, the transition of intergranular dissolution into a three-dimensional pit remains less explored. A critical, unanswered question is why only a few micro-galvanic sites transition into pits with the potential for crack nucleation, while most IMPs and GB sites remain minimally affected or have pits that undergo rapid repassivation. This knowledge gap highlights the need for a comprehensive analysis of chemical and microstructural heterogeneities at the nanoscale, which ultimately govern the corrosion pathways. Such a study is crucial for predicting the most vulnerable sites in complex multiphase microstructures.
Although least explored in terms of corrosion, chemical and associated nanoscale heterogeneities are unavoidable in 7xxx series aluminum alloys produced with current casting processes and thermomechanical treatments20. The typical manufacturing process of extruded alloys involve preheating of ingot, forcing it through a die to create a desirable cross-sectional shape followed by aging heat treatment. Non-equilibrium solidification results in a nonuniform distribution of alloying elements in the as-cast ingot, evident both at the scale of individual dendrites (micro-segregation) and often throughout the entire casting (macro-segregation). The homogenization treatment at 470–480 °C leads to the dissolution or phase transformation of non-equilibrium phases and a uniform distribution of most solutes21. However, the sluggish diffusion kinetics of dispersoid-forming solutes, along with simultaneous precipitation, leads to the formation of dispersoid clusters in the grain interior, with noticeable dispersoid-free regions throughout the microstructure20,22,23,24,25,26,27. The Cr-based dispersoids (E-Al18Mg3Cr2) act as sites for the heterogeneous precipitation of coarse ƞ-Mg(ZnAlCu)2 nanoparticles during artificial aging, thereby further enhancing nanoscale heterogeneity within the grain interior8,28.
The impact of nanosized dispersoids on the corrosion mechanisms of aluminum alloys at the nanoscale has not received significant research attention. This lack of attention may be due to the challenges in preparing sub-micron dispersoids and resolving their nano-galvanic corrosion relationships with the matrix. Additionally, there is a belief that nanosized dispersoids do not affect corrosion kinetics, as they cannot sustain large anodic/cathodic currents4. Relatively recently however, Akuata et al.8 reported that adding 0.1 wt% Cr to 7449 aluminum alloy increased its pitting potential, regardless of aging treatment. This effect was attributed to the depletion of solutes from the matrix due to the precipitation of coarse ƞ-Mg(ZnAlCu)2 around the dispersoids. Although a detailed mechanistic understanding is still lacking, this finding highlights the need for further investigation into the role of dispersoids in the passivity of aerospace-grade aluminum alloys.
The present study focuses on the significance of the heterogeneous distribution of Cr-containing dispersoids on the nanoscale pit growth in an extruded 7075-T651 aluminum alloy. Through detailed electron microscopy, we demonstrate that the dispersoids influence the precipitation kinetics of ƞ-Mg(ZnAlCu)2, thereby modifying the chemical morphology of both the grain interiors and boundaries in dispersoid-rich areas. Our findings reveal that these changes dictate the corrosion pathways at the nanoscale, consequently affecting the susceptibility of micro-galvanic sites.
Results
Microstructural analysis
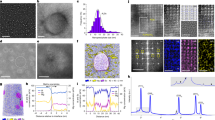
The typical microstructure of 7075-T651 obtained using scanning electron microscopy (SEM) is shown in Fig. 1, illustrating a complex, multiphase microstructure with grains elongated in the extrusion direction of the rod (Fig. 1a, b). Electron backscattered diffraction (EBSD) analysis indicates that the alloy has a random crystallographic orientation and a trimodal grain size distribution (Fig. 1c, d). Large grains, with an equivalent circle diameter (ECD) of 160–180 µm, make up 1.5% of the total. Medium-sized grains, with an ECD of 75–150 µm, constitute 16%, while small grains, with an ECD of 8–75 µm, account for 82%. As shown in Fig. 1e, the minimal fraction of low-angle GBs (2%), mostly partitioning large grains, indicates minimal strain in the alloy.
a Schematic diagram of an extruded 7075-T651 rod indicating the analysis location with a red-dashed box. b Secondary electron micrograph showing stringers of Al₂₃Fe₄Cu constituent particles and globular Mg₂Si and SiO₂ particles. c EBSD map revealing that the alloy exhibits random crystallographic orientation with grains elongated in the extrusion direction. d, e Plots showing a trimodal grain distribution with a minimal fraction of large grains and low-angle GBs.
The dominant constituent phase was identified as the Al23Fe4Cu compound, which appeared in the form of stringers (Figs.1b and 2b, c). Other IMPs, including Mg2Si and amorphous SiO2, were present with globular morphologies (Fig. 2d). Voids were observed near these large particles (Figs. 1b, 2d), indicating mechanical breakdown during extrusion29. High-resolution transmission electron microscopy (TEM) micrographs revealed a high density of uniformly distributed spherical nano-clusters (GP Zones) and a small fraction of precipitates (ƞ’ and ƞ-Mg(ZnAlCu)2) with platelet morphologies within the grain interiors, with sizes ranging from 3 to 10 nm (Fig. 2e, f). Elemental analysis using EDS confirmed the presence of coarse E-Al18Mg3Cr2 dispersoids (Fig. 2g), which were heterogeneously distributed within the grains (Fig. 2b, h). Some grains exhibited interconnected dispersoid-free regions, as seen in Fig. 2h. All E-Al18Mg3Cr2 dispersoids were affiliated with coarse ƞ-Mg(ZnAlCu)2 precipitates (Fig. 2g) and surrounded by a solute-depleted zone (SDZ) of approximately 10 nm (Fig. 2k). A small amount of Cu was also found in the ƞ precipitates (Fig. 2k), similar to previous studies5,8. The ƞ-Mg(ZnAlCu)2 precipitates surrounding the dispersoids were dissolved by exposing the TEM lamellae to 0.6 M NaCl for 30 s. This process revealed that the E-Al18Mg3Cr2 dispersoids exhibit three distinct morphologies: rod-like, triangular, and hexagonal (Supplementary Fig. 1). These shapes represent the face-centered cubic structure from different angles28,30.
a Schematic diagram of an extruded 7075-T651 rod indicating the location of analysis with a red-dashed box. b TEM micrograph showing a constituent particle entangled with grain boundaries, heterogenous distribution of precipitates along the GB and heterogeneously distributed dispersoids in the grain interior. c, d Elemental maps confirming the Cu/Fe-rich constituent particle as Al₂₃Fe₄Cu, GB precipitate as ƞ-Mg(ZnAlCu)2, and the presence of globular Mg₂Si and SiO₂ particles separated by a void. e, f High-resolution micrograph revealing the grain interior densely packed with nano-clusters, likely GP zones, with a few platelet-like η‘ and ƞ-Mg(ZnAlCu)2 precipitates. g Elemental maps confirming the presence of coarse ƞ-Mg(ZnAlCu)2 precipitates at the dispersoid/matrix interface. h TEM micrograph confirming the presence of interconnected dispersoid-free regions within a single grain. i, j TEM micrographs showing the compositional and morphological variations of GB precipitates depending on dispersoid density in the surrounding grains. k Elemental line profile revealing SDZ around a dispersoid and along the nearby GB. Micrographs in (c, g) are taken in STEM-BF mode for better phase contrast, whereas others are in STEM-HAADF mode.
High-resolution micrographs and chemical maps reveal that GBs exhibit ƞ-Mg(ZnAlCu)2 and θ-Al2Cu precipitates, with their fraction and morphology depending on the dispersoid density in the surrounding area (Fig. 2b, i, j). GBs in dispersoid-free regions exhibit continuous ƞ-Mg(ZnAlCu)2 precipitates In contrast, dispersoid-rich areas exhibit no or discontinuous ƞ-Mg(ZnAlCu)2 precipitates along the GBs, influenced by the distance and density of the dispersoids (Fig. 2b, i, j and Supplementary Fig. 2). GBs without ƞ-Mg(ZnAlCu)2 precipitates display coarse θ-Al2Cu precipitates with large solute-depleted intervals (Fig. 2j, k).
Exposure test
To study the mechanism of corrosion at the scale of the dispersoids, a specimen of the alloy surface with a surface area of 1 cm² was exposed to 40 ml of 0.6 M NaCl for 200 hours. The small volume of electrolyte was used to accelerate changes in pH and chemistry during corrosion as would happen in thin electrolyte characteristic of atmospheric conditions. Initially, the open-circuit potential (OCP) of the alloy surface was approximately −0.85 VAg/AgCl, but it rapidly increased to a steady value of approximately −0.74 VAg/AgCl within the first 5 min (Fig. 3a, b). After 15−16 h, the OCP gradually decreased to −0.83 VAg/AgCl, then slightly increased and remained at approximately −0.82 VAg/AgCl for the remainder of the exposure (Fig. 3b). The initial pH of the electrolyte was approximately 5.5, which increased with exposure duration and stabilized at 7 within 24 h (Fig. 3b).
a, b Evolution of OCP with exposure duration: The OCP increases from approximately −0.84 V to a steady value of −0.74 VAg/AgCl within the first 5 min, followed by a decrease to −0.83 VAg/AgCl after 15–16 h of exposure. b Increase in pH of the electrolyte: The pH rises from 5.5 to a steady value of 7 within the first 24 h. c Evolution of pit density and nucleation rate: The pit density sharply increases, reaching a constant value of 3000 pits/cm² within the first 24 h. The nucleation rate initially increases sharply for the first 12 h, followed by an exponential decrease with continued exposure. d−i SEM micrographs: These images show the crystallographic attack morphology of the pit surface during early exposure, transitioning to a fibrous attack morphology as large pits grow with further exposure duration (>24 h).
The evolution of pit morphology and pit density was studied using SEM analysis on samples exposed for various durations (Fig. 3d–i). After 4 h of exposure, a thick corrosion product, likely Al(OH)3/Al2O3, formed on the surface, thickening further with longer exposure times (Supplementary Fig. 3). To prepare for microscopic analysis, the exposed surfaces were ultrasonically cleaned in 70% HNO3 for 20−30 min. As reported by Ferrer and Kelly31, such treatment leads to selective dissolution of corrosion products and does not cause additional corrosion attack in aluminum alloys (Supplementary Fig. 4).
Pit density increased sharply in the first 24 h, reaching a constant value of around 3,000 pits/cm² (Fig. 3c). The pit nucleation rate, estimated from the slope of the pit density versus exposure time plot, showed a sharp increase in the first 12 h, which then decreased rapidly and became negligible after 48 h (Fig. 3c). Initial pit growth was crystallographic for the first 12−15 h, primarily propagating laterally (Fig. 3d–f), with a few spots within the grain interiors allowing vertical pit propagation (deepening) at (Fig. 3e, f). After longer exposure durations (>24 h), the alloy surface exhibited only a few large pits (~3−5 pits/cm²), while most other pits remained small, likely being pits that repassivated soon after their initiation. The bottom surfaces of the large pits displayed a fibrous attack morphology, with damage elongated in the extrusion direction (Fig. 3h, i).
The origin of pit nucleation was investigated in detail using micrographs obtained after the exposure test. Most pits were observed to nucleate around the Al23Fe4Cu particles, followed in frequency of nucleation by Mg2Si particles, with very few pits forming along the GBs or within grain interiors (Fig. 4). The matrix around the Al23Fe4Cu particles dissolved to some extent, creating trenches around the particles (Fig. 4a). Pits nucleated both at the particle/matrix interface and on the trench surface, at a distance from the particle/matrix interface (Fig. 4a). Pits around Mg2Si particles appeared to nucleate and grow from the interior side of the particle/matrix interface, resulting in matrix remnants on top (Fig. 4b, c). Intergranular pits exhibited different morphologies, including continuous intergranular dissolution and discontinuous dissolution spots (Fig. 4d–g). Most pits nucleating at GBs propagated into only one of the adjacent grains (Fig. 4d–f), with very few growing in both directions (Fig. 4g). Trenching around θ-Al2Cu GB precipitates was also observed, with minimal damage along the solute-depleted intervals (Fig. 4h).
To understand the crystallographic attack morphology of pits in the early stages, the alloy surface was exposed to the electrolyte for 4 h after acquiring the EBSD maps. Pre-exposure EBSD maps and micrographs of selected grains with crystallographic pits are shown in Fig. 5. Overlaying the crystal orientation map revealed that the surfaces of the crystallographic pits were aligned with the {001} planes (Fig. 5a–d). As seen in Fig. 3e and supplementary Fig. 5, the lateral development of crystallographic pits followed irregular paths, leaving non-corroded areas behind. Pit growth was generally restricted at most GB fronts, although a few sites allowed pits to develop into adjacent grains (Supplementary Fig. 5). Post-exposure microscopy indicated that crystallographic orientation is not the limiting factor for pit growth within the grain interior, as pits were observed growing in grains with different crystal orientations. Crystallographic pits grew laterally to cover entire grains; however, vertical growth (deepening) was observed only in a few spots.
Therefore, post-exposure TEM analysis was performed on a grown pit to understand the preferred direction of pit growth within the grain interior. The selected spot for the FIB lift-out also included a GB with no visible damage (Fig. 6a). As shown in Fig. 6b, a low-resolution TEM micrograph suggests that the pit has developed around a Al23Fe4Cu particle. Figure 6c shows that the GB was partially dissolved from the top; however, the growth was restricted by the solute-depleted interval. This GB exhibited non-continuous θ-Al2Cu precipitates with solute-depleted intervals (Supplementary Fig. 2). Therefore, the dissolution at the top could be due to nano-galvanic corrosion around an exposed θ-Al2Cu precipitate. The Al23Fe4Cu particle was surrounded by a dispersoid-free/depleted region, within which the pit was observed to grow (Fig. 6b). The high-resolution micrographs (Fig. 6d–g) illustrating the corrosion fronts show that pit growth was restricted by the dispersoids, causing the pit to expand along dispersoid-free areas. As depicted in Fig. 6e, the oxygen enrichment detected around the dispersoids indicates the formation of an oxide layer, likely Al(OH)3 and/or Al₂O₃. The effectiveness of the solute-depleted region around the dispersoids in inhibiting pit growth is clearly established in Fig. 6g.
a Top view SEM micrograph, and (b) cross-sectional STEM-HAADF micrographs showing pit growth hindered in dispersoid-rich areas, forcing pits to grow along dispersoid-free regions. c–g High-resolution micrographs confirming the inhibition of pit growth by solute-depleted intervals along the GB and around dispersoids.
Electrochemical polarization testing
A series of potentiodynamic polarization tests were conducted to elucidate the pit nucleation sites and the evolution of the pit nucleation rate. Anodic potentiodynamic polarization tests, performed after 10 min of OCP stabilization, revealed that the 7075-T651 alloy exhibits a corrosion potential of −0.740 VAg/AgCl in quiescent 0.6 M NaCl, with a small anodic polarizability, a typical electrochemical characteristic of localized corrosion mode (Fig. 7a). The polarization curves for the as-cast alloys, which are present as the IMPs in 7075-T651, are shown in Fig. 7b. The results confirm that, compared to pure Al, the Cu-based IMPs (Al23Fe4Cu and θ-Al2Cu) are cathodic, whereas the Mg-based IMPs (ƞ-MgZn2 and Mg2Si) are anodic, as reflected by their relative corrosion potentials (Fig. 7b). The corrosion potential of θ-Al2Cu, ƞ-MgZn2 and Mg2Si obtained herein are consistent with values reported by others4,32,33. Interestingly, the pitting potential of 7075-T651 is comparable to that of pure Al in 0.6 M NaCl (Fig. 7a, b). It is reasonable to infer that the initial OCP trend, shifting from around −0.84 VAg/AgCl to −0.74 VAg/AgCl (Fig. 7a) reflects the transition from anodic dissolution of Mg-rich precipitates to pit growth within the matrix.
a Potentiodynamic polarization curves of 7075-T651 in 0.6 M NaCl showing a corrosion/pitting potential of −0.74 VAg/AgCl. b Potentiodynamic polarization curves of pure Al and IMPs, confirming that Cu-based IMPs are cathodic and Mg-based IMPs are anodic relative to pure Al in 0.6 M NaCl. c, d Potentiodynamic polarization curves revealing a decrease in the pitting potential of 7075-T651 with an increasing fraction of AlCl₃ until the saturation limit in 0.6 M NaCl, and a slight decrease in cathodic kinetics with pH increasing from 5.5 to 7.
It is known that the cathodic reduction reaction increases the pH in the surrounding region, while excessive anodic dissolution raises the cationic concentration in the pit, which enhances the ingress of Cl− ions into the pit and the lowering of pH by hydrolysis of metal cations12,34. The electrochemical responses of the surrounding area and the pit surface to such electrolytic changes were studied and are presented in Fig. 7c. The increase in pH from 5.5 to 7 does not affect the corrosion or pitting potential but slightly suppresses the cathodic kinetics, as indicated by the decrease in the cathodic current density (Fig. 7c). Conversely, the corrosion or pitting potential of the 7075-T651 surface decreases with increasing concentrations of AlCl3 in 0.6 M NaCl, reaching −0.83 VAg/AgCl at saturation concentration (Fig. 7c, d). This result indicates that the enhanced anodic kinetics on the pit surface, promoted by the increased Cl− concentration in the pit electrolyte, can polarize the surrounding surface down to a potential where shallower or open pits (with relatively lower Cl- concentrations) will be repassivated. Furthermore, this electrochemical modification of the alloy surface, along with the precipitation of a thick Al(OH)3 and/or Al2O3 film, can completely halt the nucleation of new pits (as seen in Fig. 3c), while the growth of champion pits continues.
Discussion
In this study, we explored the heterogenous distribution of Cr-containing dispersoids commonly seen in commercial high-strength Al alloy 7075-T651 and its effects on the growth of corrosion pits during exposure to 0.6 M NaCl. The dispersoids provide interfaces for the precipitation of coarse ƞ-Mg(ZnAlCu)2 particles resulting in the formation of solute-depleted zones in the matrix during artificial aging, making the region more resistant to pit growth compared to the dispersoid-free region (Figs. 2k and 5i). The heterogeneous distribution of dispersoids around the GBs is also notable. The GBs in the dispersoid-free zones exhibit a thick, continuous line of ƞ-Mg(ZnAlCu)2 (Fig. 2i), which is favorable for intergranular corrosion, a damage mode to which this alloy has high susceptibility13,14,18. In contrast, the GBs in the dispersoid-rich zones show either a non-continuous ƞ-Mg(ZnAlCu)2 line or noncontinuous, coarse θ-Al2Cu precipitates with large solute-depleted intervals (Fig. 2b, j, k). Because the kinetics and morphology of GB precipitates are influenced by solute concentrations in the surrounding area, the minimal presence of anodic ƞ-Mg(ZnAlCu)2 precipitates along the GB in the dispersoids-rich areas can be attributed to the local consumption of Mg and Zn in the formation of coarse ƞ-Mg(ZnAlCu)2 precipitates at the dispersoid/matrix interface. In other words, the dispersoids enhance precipitation in the grain interior, decreasing the availability of solutes for continuous precipitation of ƞ-Mg(ZnAlCu)2 along the GBs. Consequently, the precipitation of noncontinuous θ-Al2Cu is promoted along the GBs located in the dispersoid-rich areas. Nevertheless, such morphology does not allow intergranular corrosion to propagate enough in size (or depth) to stabilize a pit (Figs. 4h and 5j).
The alloy surface exhibits a significant number of micro-galvanic corrosion sites associated with constituent particles and GB precipitates. However, the rate and direction of pit growth depend on the compositional and crystallographic structures of the surrounding matrix. In the present study, it is observed that the susceptibility of micro-galvanic sites to result in a stable pit follows the order: Al23Fe4Cu > Mg2Si > GB ƞ-Mg(ZnAlCu)2. As discussed, compositional and morphological variations make most GBs less severely susceptible, but a few GB locations exhibiting coarse continuous ƞ-Mg(ZnAlCu)2 precipitates are still susceptible to pit nucleation. The anodic Mg2Si particles reportedly undergo selective dissolution of Mg, leading to the subsequent formation of an inert SiO2 film on the surface35, thereby decreasing the probability of the nucleated pit transitioning into a stable pit. After the dissolution of ƞ-Mg(ZnAlCu)2 and the passivation of Mg2Si (by SiO2) exposed on the surface, the high cathodic kinetics upon Al23Fe4Cu particles polarize the matrix to its pitting potential (Fig. 7b), as shown by the evolution of OCP over exposure time (Fig. 3a). Consequently, the alkalization-enhanced dissolution of passive film around the Al23Fe4Cu particles makes the surrounding matrix region highly susceptible for pit nucleation.
Irrespective of the pit nucleation site, the initial growth of the pits was crystallographic, with pit surfaces aligned with the {001} planes (Fig. 5a–d). This pit orientation is similar to what has been reported for pure aluminum and other aluminum alloys, including 2024-T3, 5083-O and 6082-T636,37,38. It is generally believed that the fast-dissolving {011} and {111} planes leave the slow-dissolving {001} planes to form the pit walls37,38. However, within a grain (with a single crystal orientation), the growth of the pits with crystallographic surfaces was irregular, with both the lateral and vertical propagation of the pit front following irregular paths (Supplementary Fig. 5). Post-corrosion TEM micrographs reveal that pit growth in the grain interior is restricted around the dispersoids and follows the dispersoid-free regions (Fig. 6). This result confirms that the solute-depleted regions around the dispersoids provide high resistance to pit growth, forcing the pit front to propagate in the dispersoid-free regions, which ultimately govern the shape and size of the pit. It is well established that the addition of Zn decreases the pitting potential of Al alloys. Conversely, Mg has a negligible effect, while Cu and Cr are known to improve the passivity39,40,41,42,43. Muller and Galvele found that Zn destabilizes the passive film and accelerates the dissolution kinetics to hinder repassivation40. Sato and Newman reported similar findings42,43. Therefore, the superior passivity of solute-depleted regions can primary be attributed to depletion of Zn from those areas.
The initial geometry is vital in stabilizing the pit, as limited diffusion of cations from undercutting and deeper pits increases its ability to maintain the aggressiveness of the electrolyte against diffusion. The increase in metal cation concentration promotes acidification and the ingress of Cl- ions inside the pit, which decreases the protectiveness of corrosion products and increases the anodic dissolution kinetics on the pit surface. A pit that finds a long, dispersoid-free region can grow with the least interruption, while the enhanced anodic kinetics on the pit surface polarize the surrounding region cathodically (Fig. 7c), forcing shallower pits to repassivate (Figs. 3b, 3c and 8). Therefore, microstructural heterogeneity at the nanoscale plays a significant role in the pit growth mechanism and in championing a pit to become a crack nucleation site (Fig. 8).
The findings in this study highlight the potential benefits of a homogeneous distribution of dispersoids in inhibiting pit growth in an aerospace-grade aluminum alloy 7075-T651. Achieving a uniform distribution of dispersoids before rolling or extrusion will not only ensure a uniform, un-recrystallized, and elongated grain structure, but will also decorate the grain interiors with almost interconnected solute-depleted regions. This vascular network of the solute-depleted region will decrease the pit growth rate, while the uniform grain structure will ensure superior mechanical properties when stressed in the longitudinal direction. However, achieving a uniform dispersoid distribution during the homogenization of as-cast microstructures is challenging, especially with coarser as-cast grains. The results of this work emphasize the need to modify the casting processes and composition to produce a finer as-cast microstructure and minimize dispersoid-free zones. Ongoing efforts to refine as-cast microstructures include techniques such as electromagnetic stirring, controlling the cooling rate, modifying composition, and adding inoculants44,45,46,47. Our study herein further underscores the importance of such efforts.
Methods
Materials and chemicals
For this study, we used an extruded 7075-T651 rod with a diameter of 20 mm, supplied by Kaiser Aluminum. The alloy composition includes 5.1–6.1 wt% Zn, 2.1-2.9 wt% Mg, 1.2–2.0 wt% Cu, 0.18–0.28 wt% Cr, < 0.50 wt% Fe, < 0.40 wt% Si, < 0.30 wt% Mn, < 0.20 wt% Ti, and < 0.05 wt% Zr, with the remainder being aluminum. The rod exhibits a yield strength of 519–522 MPa, ultimate tensile strength of 767–769 MPa, and elongation of 15.5–16.5% when stressed in the longitudinal (extrusion) direction. Both microstructural and electrochemical studies were conducted on the longitudinal section of the rod. Various alloys, which appear as intermetallic particles (IMPs) in 7075-T651, were synthesized using pure metals (supplied by Alfa-Aesar) via induction melting or arc melting under an argon atmosphere.
All chemicals used in this study, including sodium chloride, anhydrous aluminum chloride, sodium hydroxide, and nitric acid, were reagent-grade (provided by Fisher Chemicals). Electrolytes were prepared using distilled water with a resistivity of 18 MΩ-cm.
Electron microscopy
The specimen surface was initially ground with 4000 grit SiC paper and then polished with cloth using 0.5 µm diamond paste. Secondary electron micrographs and EBSD maps were obtained using a dual-beam Helios TM G4 SEM equipped with an Oxford Aztec EBSD detector. EBSD maps were captured with a scan step of 1 µm and a binning size of 4 × 4. Analysis of EBSD data was conducted using Aztec crystal software.
For post-exposure microscopy, the corrosion film on the surface after exposure was removed by ultrasonic cleaning in 70% HNO3 for 20–30 min, followed by rinsing in deionized water and drying with compressed air. TEM lamellae from both as-polished and post-corrosion specimens were prepared via focused ion beam milling (FIB). High-resolution micrographs and chemical maps were acquired using a Themis TEM (300 keV) equipped with a SuperX EDS detector.
Electrochemical exposure and polarization experiment
Immersion corrosion testing was performed to investigate the evolution of pit density and pit surface morphology. A vertical cylindrical cell with a diameter of 2.5 inches and a height of 2 inches was used for the immersion test. The cell featured a 1 cm2 opening at the bottom to expose the specimen. A specimen surface of 1 cm² was exposed to 40 ml of quiescent 0.6 M NaCl for 200 h under ambient conditions. Continuous measurements of the surface’s open-circuit potential (OCP) and electrolyte pH were conducted. Microstructural analysis at various time intervals was performed using multiple samples exposed for different durations (1, 2, 4, 7, 12, 24, 48, 100, and 200 h).
Potentiodynamic polarization testing was carried out in a three-electrode setup with a Ag/AgCl reference electrode and a Pt mesh counter electrode, using a SP200 Biologic Potentiostat controlled by EC-Lab software (V 11.46). Initially, OCP was stabilized for 10 min. Subsequently, separate anodic and cathodic potentiodynamic polarization curves were acquired at a scan rate of 1 mV/s. To examine the impact of electrolyte chemistry, potentiodynamic polarization tests were conducted in 0.6 M NaCl with varying concentrations of AlCl3 and NaOH. All chemicals (reagent grade) were provided by Fisher chemicals. All solutions were quiescent and were prepared using distilled with the resistivity of 18 MΩ-cm.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Starke, E. A. & Staley, J. T. Application of modern aluminium alloys to aircraft. in Fundamentals of Aluminium Metallurgy: Production, Processing and Applications 747–783 (Woodhead Publishing Limited, 2010). https://doi.org/10.1533/9780857090256.3.747.
Zhou, B., Liu, B. & Zhang, S. The advancement of 7xxx series aluminum alloys for aircraft structures: A review. Metals (Basel). 11, 718 (2021).
Wanhill, R. J. H. & Bray, G. H. Aerostructural Design and Its Application to Aluminum-Lithium Alloys. In Aluminum-Lithium Alloys: Processing, Properties, and Applications 27–58 (Elsevier Inc., 2013). https://doi.org/10.1016/B978-0-12-401698-9.00002-1.
Birbilis, N. & Buchheit, R. G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 152, B140–B151 (2005).
López Freixes, M. et al. Revisiting stress-corrosion cracking and hydrogen embrittlement in 7xxx-Al alloys at the near-atomic-scale. Nat. Commun. 13, 1–9 (2022).
Meng, Q. & Frankel, G. S. Effect of Cu Content on Corrosion Behavior of 7xxx Series Aluminum Alloys. J. Electrochem. Soc. 151, B271 (2004).
Wang, S.-S. et al. Effect of Cu Content and Aging Conditions on Pitting Corrosion Damage of 7xxx Series Aluminum Alloys. J. Electrochem. Soc. 162, C150–C160 (2015).
Akuata, C. K., Gunawan, F. R., Suwanpinij, P. & Zander, D. Microstructure Evolution and Localized Corrosion Susceptibility of an Al-Zn-Mg-Cu-Zr 7xxx Alloy with Minor Cr Addition. Materials (Basel). 16, 946 (2023).
Maitra, S. & English, G. C. Mechanism of Localized Corrosion of 7075 Alloy Plate. Metall. Trans. A 12A, 535–541 (1981).
Wang, S. S. et al. Mechanism of Localized Breakdown of 7000 Series Aluminum Alloys. J. Electrochem. Soc. 160, C493–C502 (2013).
Li, J. F. et al. Mechanical properties, corrosion behaviors and microstructures of 7075 aluminium alloy with various aging treatments. Trans. Nonferrous Met. Soc. China 18, 755–762 (2008).
Foley, R. T. Localized Corrosion of Aluminum Alloys-A Review. Corrosion 42, 277–288 (1986).
Xiong, Y. et al. Mitigation effects of over-aging (T73) induced intergranular corrosion on stress corrosion cracking of AA7075 aluminum alloy and behaviors of η phase grain boundary precipitates during the intergranular corrosion formation. Corros. Sci. 225, 111570 (2023).
Goswami, R., Lynch, S., Holroyd, N. J. H., Knight, S. P. & Holtz, R. L. Evolution of grain boundary precipitates in Al 7075 upon aging and correlation with stress corrosion cracking behavior. Metall. Mater. Trans. A 44, 1268–1278 (2013).
Qiu, Y. et al. Influence of Grain Boundary Precipitates on Intergranular Corrosion Behavior of 7050 Al Alloys. Coatings 12, 1–12 (2022).
Corsetti, L. V. & Duquette, D. J. Effect of Mean Stress and Environment on Corrosion Fatigue Behavior of 7075-T6 Aluminum. Metall. Trans. 5, 1087–1093 (1974).
Liu, X. et al. Effect of grain boundary precipitation on corrosion of heating-aging treated Al-4.47Zn-2.13Mg-1.20Cu alloy. J. Mater. Res. Technol. 9, 5815–5826 (2020).
Zhou, L., Chen, K., Chen, S., Ding, Y. & Fan, S. Correlation between stress corrosion cracking resistance and grain-boundary precipitates of a new generation high Zn-containing 7056 aluminum alloy by non-isothermal aging and re-aging heat treatment. J. Alloy. Compd. 850, 156717 (2021).
Huang, L., Chen, K. & Li, S. Influence of grain-boundary pre-precipitation and corrosion characteristics of inter-granular phases on corrosion behaviors of an Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. B 177, 862–868 (2012).
Microstructure of Alloys. In Aluminum: Properties and Physical Metallurgy (ed. Hatch, J. E.) 58–104 (Americal Society for Metals, 1984).
Xu, D. K., Rometsch, P. A. & Birbilis, N. Improved solution treatment for an as-rolled Al-Zn-Mg-Cu alloy. Part I. Characterisation of constituent particles and overheating. Mater. Sci. Eng. A 534, 234–243 (2012).
Priya, P., Johnson, D. R. & Krane, M. J. M. Modeling phase transformation kinetics during homogenization of aluminum alloy 7050. Comput. Mater. Sci. 138, 277–287 (2017).
Robson, J. D. Dispersoid Precipitation in Aluminum Alloys. in Encyclopedia of Aluminum and Its Alloys 770–777 (CRC Press, 2018). https://doi.org/10.1201/9781351045636.
Saitoh, H. & Ou, B. L. Effect of homogenization conditions on recrystallized structure in Al-Zn-Mg-Cu alloys containing chromium. J. Jpn. Inst. Light Met. 36, 485–490 (1986).
Dong, Y., Ren, B., Wang, K. & Leng, J. Microstructure of 7075 aluminum alloy by homogenization. Mater. Sci. Forum 993, 138–145 (2020).
Zhou, Y., Kang, L., Duan, C., Zhao, G. & Tian, N. Control of dispersolds in 7475 aluminum alloy. Mater. Sci. Forum 941, 896–901 (2018).
Zhou, Y. ran et al. Mechanism of heterogeneous distribution of Cr-containing dispersoids in DC casting 7475 aluminum alloy. Trans. Nonferrous Met. Soc. China (English Ed) 32, 1416–1427 (2022).
Ding, L. et al. Atomic-scale investigation of the heterogeneous precipitation in the E (Al18Mg3Cr2) dispersoid of 7075 aluminum alloy. J. Alloys Compd. 851, 156890 (2021).
Xing, L., Gao, P. F., Zhan, M., Ren, Z. P. & Fan, X. G. A micromechanics-based damage constitutive model considering microstructure for aluminum alloys. Int. J. Plast. 157, 103390 (2022).
Ayer, R., Koo, J. Y., Steeds, J. W. & Park, B. K. Microanalytical study of the heterogeneous phases in commercial Al-Zn-Mg-Cu alloys. Metall. Trans. A 16, 1925–1936 (1985).
Ferrer, K. S. & Kelly, R. G. Comparison of methods for removal of corrosion product from AA2024-T3. Corrosion 57, 110–117 (2001).
Gharbi, O. & Birbilis, N. Clarifying the Dissolution Mechanisms and Electrochemistry of Mg2Si as a Function of Solution pH. J. Electrochem. Soc. 165, C497–C501 (2018).
Gupta, R. K., Sukiman, N. L., Fleming, K. M., Gibson, M. A. & Birbilis, N. Electrochemical Behavior and Localized Corrosion Associated with Mg2Si Particles in Al and Mg Alloys. ECS Electrochem. Lett. 1, C1–C3 (2012).
Frankel, G. Pitting Corrosion of Metals: A Review of the Critical Factors. J. Electrochem. Soc. 145, 2186–2198 (1998).
Kairy, S. K. & Birbilis, N. Clarifying the Role of Mg2Si and Si in Localized Corrosion of Aluminum Alloys by Quasi In Situ Transmission Electron Microscopy. Corrosion 76, 464–475 (2020).
Donatus, U. et al. Corrosion pathways in aluminium alloys. Trans. Nonferrous Met. Soc. China (English Ed.) 27, 55–62 (2017).
Seo, J. H., Ryu, J.-H. & Lee, D. N. Formation of Crystallographic Etch Pits during AC Etching of Aluminum. J. Electrochem. Soc. 150, B433 (2003).
Zhang, X., Zhou, X., Hashimoto, T. & Liu, B. Localized corrosion in AA2024-T351 aluminium alloy: Transition from intergranular corrosion to crystallographic pitting. Mater. Charact. 130, 230–236 (2017).
Szklarska-Smialowska, Z. Insight into the pitting corrosion behvaior of aluminum alloys. Corros. Sci. 33, 1193–1202 (1992).
Muller, I. L. & Galvele, J. R. Pitting potential of high purity binary aluminium alloys-II. Al-Mg and Al-Zn alloys. Corros. Sci. 17, 995–1007 (1977).
Muller, I. L. & Galvele, J. R. Pitting potential of high purity binary aluminium alloys-I. Al-Cu alloys. Pitting and intergranular corrosion. Corros. Sci. 17, 179–193 (1977).
Sato, F. & Newman, R. C. Mechanism of Activation of Aluminum by Low Melting Point Elements: Part 1 — Effect of Zinc on Activation of Aluminum in Metastable Pitting. Corrosion 54, 955–963 (1998).
Sato, F. & Newman, R. C. Mechanism of Activation of Aluminum by Low-Melting Point Elements: Part 2 — Effect of Zinc on Activation of Aluminum in Pitting Corrosion. Corrosion 55, 3–9 (1999).
Leng, Jinfeng, Ren, B., Wu, H., Wang, R. & Dong, Y. Effect of Minor Sc and Zr Addition on the Microstructure of 7075 Aluminum Alloy during Homogenization Treatment Process. Phys. Met. Metallogr. 122, 1482–1491 (2021).
Zuo, M. et al. Microstructure Control and Performance Evolution of Aluminum Alloy 7075 by. Nano-Treat. Sci. Rep. 9, 1–11 (2019).
Gao, Z., Xu, J., Zhang, Z. & Tang, M. Effect of annular electromagnetic stirring process on solidification microstructure of 7075 aluminium alloy. Adv. Mater. Res. 652–654, 2418–2426 (2013).
Quested, T. E. & Greer, A. L. Grain refinement of Al alloys: Mechanisms determining as-cast grain size in directional solidification. Acta Mater. 53, 4643–4653 (2005).
Acknowledgements
We acknowledge the use of electron microscopes in the Nanoscale Materials Characterization Facility (NMCF) at the University of Virginia. We thank Dr. Helge Henrich and Mr. Richard R. White for assisting with TEM and EBSD data acquisition. We thank Dr. Martin M. Morra (General Electric Research) for providing the as-cast alloy Al23Fe4Cu. The financial support of the Defense Advanced Research Projects Agency (DARPA) Morphogenic Interfaces (MINT) program (V. Sundaresan, Program Manager) through the General Electric Company (Contract HR001122C0106, S. Prochaska, PI) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
S. Choudhary designed and performed the experiments, and wrote the manuscript. R.G. Kelly supervised the project, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choudhary, S., Kelly, R.G. Nanoscale heterogeneities dictate corrosion pathways in a high-strength aluminum alloy. npj Mater Degrad 8, 103 (2024). https://doi.org/10.1038/s41529-024-00520-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-024-00520-3