Abstract
Pure magnesium (Mg) has been widely used in orthopedic medical materials due to its biodegradability and excellent biocompatibility. However, it is difficult to realize pure Mg in clinical procedures due to its poor mechanical properties and non-uniform degradation. Grain refinement is the main strengthening mechanism of pure Mg, and uniform degradation can be achieved by fine-grained microstructure. In this work, the Mg with the average grain size of 3.62 μm was prepared by the low-temperature secondary extrusion in a well-controlled Zener-Hollomon parameter of \({e}^{34.9}\). The fine-grained Mg shows high strength (215 MPa), ductility (20.4%), and low degradation rate (0.18 mm/y), satisfying the requirements of bone load-bearing regions. The degradation mechanisms of different grain-sizes Mg in physiological solutions were also investigated. This work demonstrates that fine-grained Mg exhibits uniform degradation due to less matrix contact with the solution and compact product layers, thus providing a new approach to developing highly corrosion-resistant Mg alloys.

Similar content being viewed by others
Introduction
Mg is widely used in orthopedic implants, such as bone plates and screws1,2. In orthopedic applications, Mg can effectively alleviate the stress shielding effect due to Young’s modulus being similar to that of human bone and promote the reconstruction of bone tissue by interacting with integrins on the osteoblasts to facilitate osteoblast proliferation3,4. In addition, Mg is an essential metal element concerned with all physiological reactions in the body5. As a cofactor for physiological reactions, Mg2+ plays a crucial role in numerous intracellular and extracellular physiological processes6,7. Intracellularly, Mg²⁺ is an essential cofactor for enzymatic reactions and nucleic acid synthesis, maintaining cellular membrane potential and ion transport. Extracellularly, Mg²⁺ contributes to bone mineralization, neuromuscular function, and the regulation of vascular tone8. Approximately 24 g of Mg is present in an adult and more than 50% in the bone9. Therefore, Mg has natural advantages in orthopedic implants.
Nowadays, many Mg alloys have been used in the preparation of orthopedic implants. Mg-Y-RE-Zr alloy screws developed by Syntellix AG (Germany) were the first small bones and fragments in 201310. RE elements may be accumulated in the organ to influence the metabolic process. To solve this problem, the pure Mg screws produced by Ion Technology (China) were certified by Conformite Europeenne and China State Drug Administration in 2020, thus being the first non-toxic screws in the world11. However, the current bio-Mg alloys are mostly designed to improve mechanical and corrosion properties by alloying due to the poor mechanical properties of pure Mg. Zhang et al.12 investigated the bone regeneration of Mg-Zn implants with TYS of 279 MPa and EL of 18.8%. The results showed that the gap between the implant and bone tissue inhibits regeneration owing to the rapid degradation rate (2.33 mm/y). Avey et al.13 identified the Zn/Ca region with the best combination of mechanical properties (TYS: ~300 MPa) and degradation rate (0.25 ~ 0.3 \({\rm{m}}{\rm{g}}/{{{\rm{cm}}}}^{2}\,{\rm{day}}\)) is between 1.18 and 1.8. Li et al.14 implanted Mg-2Zn-1Mn wires into the femur of rats, demonstrating excellent histocompatibility, osteogenesis, and medium tensile strength of 150 MPa. However, most studies for non-toxic alloying elements have been conducted by repetitive testing for alloying, resulting in high costs and inconsistent properties. In addition, rare earth elements that significantly improve corrosion resistance and mechanical properties are toxic and accumulate in the liver, regardless of the contents6.
For pure Mg, it is sufficient to consider the effect of different processes and extrusion parameters on the properties15. Moreover, High-purity Mg (Mg ≥99.99 wt.%) has a lower degradation rate (0.3∼0.5 mm/y) and cytotoxicity compared with most Mg alloys, making it suitable for orthopedic implants16. The mechanical properties of pure Mg can be used in the load-bearing regions of bone repair if they can be significantly improved. The strengthening of grain refinement can enhance the mechanical properties of the matrix significantly, and there are many processing methods to achieve this purpose17. Zheng et al.18 manufactured the fully recrystallized pure Mg with 1.57 μm grain sizes via high-pressure torsion at 200 °C. However, the ultimate tensile strength (UTS) does not meet the minimum requirements for implantation (200 MPa). Fan et al.19 prepared pure Mg with high UTS (>200 MPa) by the equal channel angular pressing process at 350 °C, but the elongation (EL) was only ~8%. It is difficult to achieve a synergistic enhancement of the strength and ductility of pure Mg due to the presence of high internal stresses. In addition, the grain size also affects the degradation behavior of the matrix. Until now, most research has concentrated on severe plastic deformation to induce strain to promote recrystallization, but it is unsuitable for pure Mg20. Excessive strain can reduce grain size while causing internal stress and deteriorating corrosion resistance. Song et al.21 refined the grain size of pure Mg (200 to 50 μm) with the equal channel angular pressing process, and the icorr value was elevated from 0.05 to 0.27 mA/cm2 with the increase in passes. Synergistic enhancement of mechanical properties and corrosion resistance of pure Mg is still a challenge.
In this work, the low-temperature secondary extrusion was designed to prepare fine-grained Mg by modulating the Zener-Hollomon parameter, thus significantly improving the strength and elongation. Different from earlier studies, rational strain, and thermal energy can be introduced by minimizing the extrusion ratio and temperature to obtain fine-grained Mg with a uniform microstructure. Subsequently, the degradation behaviors were also investigated, highlighting the effects of different grain sizes of the product layer and the degradation process.
Results
Mechanical properties
Fine-grained Mg with high strength at room temperature can be obtained through secondary extrusion at or below 150 °C. Samples in the temperature range over 150 °C exhibited poor strength at room temperature. The plastic deformation of samples at high extrusion temperatures happened when tensioned by 50–60 MPa, consistent with previous work. However, Mg via secondary extrusion at 150 °C did not have a yielding point until the strength reached ~147 MPa. Samples of the first extrusion at 150 °C exhibited high strength (UTS: 218 MPa) and poor ductility (12.5%) due to the massive stresses within the microstructure. As shown in Fig. 1 and Table 1, the strength of SE-1 significantly improved without decreasing elongation. The strength and elongation were exhibited in the best combination by decreasing the temperature during the extrusion process.
The mechanical properties of Mg in different processes were also summarized in Fig. 1b. Gan et al.22 introduced a large strain to refine the grain size of Mg with the rotary swaging process. The UTS can be significantly improved (~230 MPa), but there is a decrease in elongation, only 7%. Through the high-pressure torsion process, Zheng et al.18 prepared different grain sizes of pure Mg, and the size of 1.57 μm achieved the maximum properties (TYS: 172 MPa, EL: 27%). Equal channel angular pressing was also performed on the pure Mg, reaching 180 MPa of UTS, but EL showed 10%23. While the grains can be refined through large-strain machining processes to improve mechanical performance, the residual internal stresses lead to poor ductility without synergistic enhancement of strength and elongation24. Compared with grain sizes of pure Mg using different processes, SE-1 showed the best mechanical properties25,26,27,28,29.
The microstructure and texture evolution
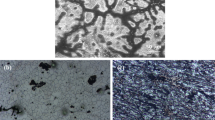
The OM of the samples is shown in Fig. 2. The FE-1 was composed of refined grains and un-recrystallized regions. The microstructure mainly consisted of coarse grains without un-recrystallized regions and other substructures for the high-temperature samples. After the secondary extrusion, many refined equiaxed grains were uniformly distributed in the SE-1. However, when the temperature increased, the SE samples exhibited complete recrystallized regions with coarse grains. The sizes rapidly climbed, and the maximum reached 23.24 ± 4.71 μm at 350 °C. The grain sizes of SE-1 and SE-2 were 3.62 ± 0.04 μm and 16.81 ± 4.72 μm, respectively. The contribution of grain size (\({\sigma }_{{GS}}={{kd}}^{-1/2}\)) to strength can be held as a Hall-Petch relationship with the k value of 260 MPa μm1/230.
In the pole figures (Fig. 3), FE samples exhibited non-basal texture, and most grain orientations belonged to \(\{10\bar{1}1\}\& \{11\bar{2}0\}//{ED}\). Due to low extrusion temperature and extrusion ratio, the grains of FE-1 were difficult to rotate, and the composition of grain orientations was relatively random. Therefore, the texture intensity tended to increment, increasing from 3.4 at 150 °C to 10.0 at 250 °C. After secondary extrusion, the texture evolution depends on the transition from the un-recrystallized region to recrystallized grains. The coarse grains with \(\{10\bar{1}1\}\& \{11\bar{2}0\}\) and recrystallized region formed a strong texture. All SE samples showed the typical \(\left[10\bar{1}1\right]-[11\bar{2}0]\) fiber texture and the maximum texture intensity increased after secondary extrusion. To further investigate the strength contribution (\({\sigma }_{\tau }\)) of texture, it can be described the relationship between the Schmid Factor and the strength31:
where \(\tau\) is the critical shear stress of basal <a> slip and \({m}_{s}\) is the Schmid Factor of basal <a> slip. The magnitude of the Schmidt factor for strength and basal slip was inversely proportional, with low Schmidt factors implying grain orientations detrimental to basal slip activation. As shown in Fig. 4, the Schmid Factors of SE samples were 0.33, 0.32, and 0.36, respectively. The fraction of recrystallized grains in samples was measured through the first and secondary extrusion processes.
For the first extrusion, samples at 150 °C mainly consisted of deformed grains, which reached 52%. With the increase in temperature, the proportion of recrystallized grains increased to 91.5% because the dynamic recrystallization process was promoted by energy introduced to higher extrusion temperature (Fig. 5). Large amounts of dislocations have accumulated on the deformed grain and sub-grain boundaries, and the continuous dynamic recrystallization can be influenced by suitable strain and temperature. After secondary extrusion, the microstructure of SE-1 exhibited a completely recrystallized region consisting of fine-grained grains. As for SE-2 and SE-3, higher extrusion temperatures made recrystallized grains grow, leading to the appearance of many large grains.
To further figure out the dislocation density and strain distribution of samples, the kernel average misorientation (KAM) distributions were measured in Fig. 6. All samples exhibited low KAM values (<0.3) except for the FE-1 sample, and the KAM value of FE-1 was 0.81. Meanwhile, dislocation density can be shown clearly through the geometrically necessary dislocation (GND). The GND density can be calculated as follows32:
where b is Burger’s vector for <a> dislocation (0.32 nm), μ is the step size, and \(\theta\) is the average local misorientation.
As shown in Supplementary Fig. 1, FE-1 exhibited the highest value of 0.16 × 1014 m−2, and the others retained the low value (≤0.03 × 1014 m−2). Such a high value was mainly attributed to numerous dislocations introduced by extrusion at low temperatures, and subsequent extrusion promoted the transition of low-angle grain boundaries to high-angle grain boundaries to eliminate the defects and dislocations, leading to the low-density value33. This was consistent with the recrystallization proportion, where less deformed grains can be presented due to the transition of deformed grains with high dislocation density into strain-free grains34. However, not all of the dislocations are eliminated in the dynamic recrystallization process, and the strength can be improved by residual dislocations, which can be calculated as35:
where the a is the geometric constant (0.2), G is the shear modulus of Mg (~17 GPa), and b is the Burger vector (0.32 nm).
Therefore, the TYS of SE samples can be estimated as follows:
The contributions of different strengthening mechanisms are shown in Fig. 7a. \({\sigma }_{0}\) is 11 MPa for lattice friction strength of pure Mg. The proportion of \({\sigma }_{\tau }+{\sigma }_{{dis}}\) is relatively low, and the maximum contribution is only 4 MPa. While the \({\sigma }_{{dis}}\) had a low contribution to strength, it indicated less internal stress and a more uniform distribution of residual dislocations. The effect of grain size strengthening was more important than other strengthening mechanisms in pure Mg. The contribution of grain refinement to the TYS was 136, 63, and 53 MPa, respectively. It is demonstrated that grain size can be refined without introducing large amounts of strain. The conditions required for pure Mg to achieve refined grains can be quantified by the Zener-Hollomon parameter36. The Zener-Hollomon parameter appears useful in predicting the resulting grain size and controlling the hot extrusion working parameters. According to the previous work, the hot extrusion parameter can be controlled by this value.
where Q is the activation energy of Mg (135 kJ/mol), T is the extrusion temperature, and R is the gas constant (8.314 J/K mol)37. ε is the strain rate, which can be calculated by this equation32:
where ER is the extrusion ratio, V is the ram speed (1 mm/s), and D is the diameter of the billet and extruded rod. Due to billets extruded by two extrusions, the ε needs to be summed twice, and the result was 0.45. When the Zener-Hollomon parameter reaches e31.6, the microstructure of pure Mg is shifted to the fine-grained microstructure (<10 μm). At an extrusion temperature of 285 °C, there is an intersection of the grain size of pure Mg with lnZ, corresponding to a grain size of 18.6 μm (Fig. 7b).
The degradation behavior
Four methods were used for samples to measure the degradation rate in m-SBF specifically. Figure 8a shows the weight loss and Pw value after immersion for 10 days. The weight loss results of SE samples were 0.6%, 1.2%, and 1.2%, respectively. The corrosion resistance can be effectively improved as the grain size reaches the fine-grained range. The SE-1 samples maintained a high value of 99.2% of the initial volume. The concentration of Mg2+ ions released from pure Mg was analyzed in m-SBF, and the SE-1 sample rose more steadily, peaking at 10 days with values of 2.27 × 10−3 mol/L (Fig. 8b). The SE-2 and SE-3 samples exhibited ion concentrations of ~2.5 × 10−3 mol/L.
Supplementary Fig. 2 shows the change of pH value in m-SBF. The pH of Mg climbed rapidly within 24 h and then gradually increased steadily. In particular, SE-1 showed good corrosion resistance, with the pH climbing to ~8.0 after 24 h and then to ~8.6 steadily over 9 days. The hydrogen evolution volumes and rates are shown in Fig. 8c. In the initial period of 7 h, samples were reacted drastically with the solution to generate much hydrogen gas, leading to a rapid climb in the curve of volumes. For the SE-1 sample, the curve increased steadily after 12 h. However, there are two hydrogen stages for other samples, including the steady and rapid release stage. A steady stage from 12 to 120 h with little increase in hydrogen volume and a rapid stage generated ~4 mL hydrogen within the next 120 h, which is about 60% of the total volume. The hydrogen evolution rates (Supplementary Fig. 3) of samples reached the peak value after being immersed for 7 h and then gradually stabilized to 6 × 10−3\({\rm{mL}}/{{{\rm{cm}}}}^{2}\,{\rm{h}}\). The minimum of SE-1 was only 3 × 10−3\({\rm{mL}}/{{{\rm{cm}}}}^{2}\, {\rm{h}}\). Based on the two immersion times, 3 and 120 h, the curves of hydrogen evolution rates can be divided into three zones, A, B, and C. Zone A was the rapid corrosion stage, zone B was the corrosion platform stage, and zone C was the stabilizing stage. In zone B, a continuous decrease in curves from 3 to 48 h illustrated a period of incubation of corrosion pits and precipitation of products. During subsequent immersion time (48–120 h), the products developed the degradation film on the matrix, leading to a smoothing of the curve.
The Nyquist diagrams and polarization (PDP) curves of SE samples are shown in Fig. 9. For the SE-1 and SE-2 samples, high-frequency loops with the change-transfer response and middle/low-frequency loops with the conductivity of product films were presented in the Nyquist diagram. Due to the deficiency of rapid dissolution layer formation on the matrix, the middle/low-frequency loops tended to be more scattered and disorganized. The equivalent circuit (Fig. 9g, h) was used to fit curves, and the fitting components and results are shown in Table 2. As shown in Fig. 9i, sum resistance (Rsum = Rf + Rct) tends to increase with increasing immersion time. The Rsum of SE-1 and SE-2 reached the maximum on day 5 and the maximum is 3253 Ω cm2 of the SE-1 sample. The curve of the SE-3 was relatively stable, reaching the maximum value at 1 day (2190 Ω cm2). Meanwhile, the corrosion potential also decreased from −1.58 to −1.88. All the anode branches except SE-3 exhibited breakdown potential after 5 days of immersion. The corrosion current of the fitting results were 0.10, 0.17, and 0.34 μA cm−2. Considering the data measured by four methods, the fine-grained Mg had better corrosion resistance.
The product layers
According to the results of HER and PDP, there was a turning point and breakdown potential after 5 days of immersion, indicating the presence of the product layer. Therefore, the layer morphology of samples with different immersion times is shown in Supplementary Fig. 4. The layers of all three samples consisted of cracked film layers and flocculent precipitates on the surface. More products were deposited on the microstructure with large grain sizes. It can demonstrate that more matrix is dissolved to form precipitates and is associated with more severe degradation than the other samples. After removing the products, the main corrosion types were pits and filiform corrosion. The proportion of corrosion pits in SE-1 accounted for more on the surface, and filamentary corrosion gradually increased with grain sizes. This was consistent with the results of optical microscopy; SE-3 samples showed the length of pits 0.5 mm.
The 5-day immersion SE-1 sample was selected for compositional testing because the matrix and solutions of the three samples were identical. The XPS and XRD spectra are shown in Fig. 10 to detect the composition of product layers. The Mg 1s spectrum displayed the MgO peak at 1304.5 eV and the MgCO3 peak at 1305 eV. Three bonds were expressed in the C 1s spectrum: C-C, O-C=C, and C-O-C. It is possible to observe that the O 1s binding energy of metal oxides and carbonates falls within a range of 529–530 eV and 531.5–532 eV. The P 2p and Ca 2p peaks were also identified as the formation of calcium phosphate. The atomic percentage can be calculated from the corrected signal of the sensitivity factor, and the content of Mg and Ca was less than 4%. Further measurements by XRD results (Fig. 10b) show that the layers on the surface of samples mainly consist of (Ca, Mg)O, (Ca, Mg)CO3, and (Ca, Mg)3(PO4)2. These oxides may be transformed from the corresponding hydroxides (i.e., Mg(OH)2 and Ca(OH)2).
The layer structure was still a lack of understanding. Therefore, EPMA analysis was used to measure the elemental distribution on the profiles of different grain size samples (Fig. 11). The elements Ca, P, C, and O were examined, with color intensity indicating the amount of elemental concentration. For fine-grained Mg, the layer exhibited a sandwich structure. In combination with XPS and XRD results, the (Ca, Mg)3(PO4)2 was concentrated on the outer layer, (Ca, Mg)CO3 was centralized on the middle layer, and (Ca, Mg)O was distributed on the inner layer. In addition, O element was present in the crack propagation region, and the micro-cracks tended to propagate along the grain boundaries. In the case of coarse-grained Mg, the distribution of elements was the same as the fine-grained Mg, and the order from outside to inside was Ca and C. The oxidized regions occupied the entire layer, and the layer was much thicker than the fine-grained samples. However, the layer with many micro-cracks showed different corrosion depths, indicating the uneven degradation behavior of coarse-grained Mg. In addition, the Ca salts of coarse-grained Mg precipitated less than fine-grained Mg. The product formation of coarse-grained Mg can exceed their stabilization, resulting in an insufficiently dense or even film layer. An uneven film layer cannot effectively adsorb Ca ions, reducing the precipitation of Ca salts38,39. Moreover, excessive degradation may lead to high local pH, where Ca ions preferentially combine with OH− to form Ca hydroxide, reducing the concentration of free Ca ions. The solubility of hydroxyapatite also increased under highly alkaline environments, making it difficult for Ca salts to precipitate40,41. These resulted in a dense and even film of products generated by the fine-grained Mg.
There is a common denominator in the EPMA results of pure Mg. As shown by the elliptical dashed line in EPMA (Supplementary Fig. 5), the micro-cracks can be observed around the grains and have a tendency to expand toward grain boundaries. To observe macroscopically, the degradation trend of pure Mg, OM, and SEM results of samples after 5 days of immersion were detected. Figure 12 illustrates the overall cross-section appearance of the degradation and the microscopic cross-section features. The propagation direction of micro-cracks was parallel to the grain boundary direction, along with the H2 release channel of the layer. The dissolution process of the Mg matrix and hydrogen evolution process was accomplished through these channels on the layer. Therefore, the degradation behavior of pure Mg took grain boundaries as channels for degradation, leading to grain corrosion.
Discussion
Extensive research has been conducted on the degradation properties of pure Mg. The degradation process of Mg can be divided into two parts, including the cathodic hydrogen evolution reaction and the anodic dissolution process. According to the report of Sun42, the cathodic corrosion rate follows the sequence of (\(10\bar{1}0\))B > (\(11\bar{2}0\)) > \((10\bar{1}0)\)A > (0001), and the order of computed work function with cathodic reaction is (\(11\bar{2}0\)) > \((10\bar{1}0)\)A = (0001) > (\(10\bar{1}0\))B. This indicates that the degradation rate of basal orientation is slower than prismatic and pyramidal-oriented grains. To avoid the influence of the basal texture on the degradation rate, samples perpendicular to the extrusion direction were selected for observation and immersion. As shown in Fig. 4, the pole figure exhibited the \(\left[10\bar{1}1\right]-[11\bar{2}0]\) fiber texture, and the effect of the texture on degradation behavior is relatively low.
Therefore, the grain size of Mg is the main impact factor of the degradation process in this text. However, few researches are available to assess the effect of grain size on Mg degradation accurately. Ren et al.43 demonstrated that refining the grain size of pure Mg could reduce the degradation rate in physiological solutions. Meanwhile, Wang et al.39 reported that the grain size of pure Mg affects the degradation rate minimally and stays within a relatively low range. The degradation mechanism of pure Mg has not been studied in detail. So, we briefly describe this degradation process with a schematic diagram (Fig. 13). The grains on both sides of the grain boundaries are mostly oriented differently, hence the atoms on the boundaries are arranged abnormally, leading to higher energies at the boundaries than within the grains44. Thus, degradation is preferred at the grain boundaries with high energy, consistent with our results45,46. While fine grains lead to more grain boundaries and corrosion sites, uniform degradation of fine grains in pure Mg can reduce the exposure to the matrix, slowing down the degradation process. For coarse grains, grain corrosion leads to the exposure of large matrix parts, and these fresh matrices can accelerate degradation by coming into contact with the solution. Excessive degradation leads to thicker product layers than fine grains; however, the layer contains many corrosion channels, causing the layer to be unprotected by the matrix47. Hydrogen release and ion exchange processes are accomplished through these channels. In addition, the product layer is composed of three product layers, including phosphates, carbonates, and hydroxides. Ca2+ and Mg2+ in the solution can be easily reacted with PO43− and CO32− to form phosphates and carbonates deposited in the outer and middle layers48. Large amounts of Mg2+ and OH− from corrosion sites rapidly generate Mg(OH)2 precipitates39. These products decrease part of the corrosion sites, improving corrosion resistance.
Overall, the current study focuses on the enhancement of strength, ductility, and degradation of pure Mg by grain refinement. Through establishing the relationship between the Zener-Hollomon parameter and grain size, fine-grained Mg with low internal stress can be prepared by low-temperature secondary extrusion (Zener-Hollomon parameter of \({e}^{34.9}\)). The samples exhibit excellent mechanical (UTS of 215 MPa and EL of 20.4%) and degradation properties (0.18 mm/y), demonstrating the potential for use in bone load-bearing regions. Meanwhile, the degradation behavior changed when the grain size was reduced from 20 μm to 3 μm. Fine-grained Mg is degraded more uniformly during the degradation process and has fewer corrosion channels in the product layer than coarse-grained Mg. Accordingly, an optimum grain size for pure Mg might be considered for appropriate properties from the standpoints of mechanical and degradation properties. In this regard, the sum of UTS and degradation rate vs. average grain size are shown in Fig. 14. The results show that UTS decreases and degradation rate increases with increasing the grain size, where this difference in much more pronounced after the grain size of ∼10 μm. In addition, the low-temperature secondary extrusion method of Mg is convenient for producing large workpieces to meet the processing requirements of different implantation site devices. However, the limitation of this work is that degradation mechanisms of nano-sized pure Mg with low internal stress have not yet been explored.
Methods
Materials preparation
The high-purity Mg (composition: 0.12 wt.% of Si, 0.09 wt.% of Al, 0.17 wt.% of Mn, 0.19 wt.% of Fe, 0.01 wt.% of Cu, 0.01 wt.% of Ni, 0.01 wt.% of Pb,0.12 wt.% of Ca, 0.23 wt.% of Zn, 0.01 wt.% of Ti and 0.01 wt.% of Sn) were produced by the Shanxi Bada Mg Industry Company. In a protective atmosphere (CO2:SF6 = 99:1), high-purity Mg was heated to melt completely. Then the temperature was increased to 720 °C and kept for 20 min to remove the surface impurities, and finally Mg ingots were obtained by water cooling and machining process. The ingots were directly secondary extruded with different extrusion ratios of 4.5 (the first extrusion: extruder diameter of 85 mm to bar diameter of 40 mm) and 8 (the secondary extrusion: extruder diameter of 40 mm to bar diameter of 14 mm) at different temperatures.
Mechanical tests and microstructure analysis
Samples for tension test were machined to cross-section with the dimension of 6 × 3 mm in diameter and 25 mm in length. All tension tests were measured at room temperature in the CMT5105 mechanical test with a 1.5 mm/min strain rate. To ensure reproducibility, three samples were measured. The grain distribution and texture evolution of samples were investigated by optical microscopy (OM; Nikon Eclipse LV 150N, Japan)), scanning electron microscope (SEM; JEOL-7800F, Japan), and electron backscatter diffraction (EBSD). The sample observation surfaces are all perpendicular to the ED direction, and the specific machining diagram is shown in Supplementary Fig. 6.
Degradation tests
The degradation rate was measured via weight loss, hydrogen evolution, ions release, and electrochemical test at 37.0 ± 0.5 °C. The modified-stimulated body fluid (m-SBF) with a pH of ~7.4 was used as the solution. The composition mainly consists of NaCl (Na⁺, 142.0 mM), KCl (K⁺, 5.0 mM), MgCl2·6H₂O (Mg²⁺, 1.5 mM), CaCl2(Ca²⁺, 2.5 mM), chloride (Cl⁻, 147.8 mM), NaHCO3 (HCO₃⁻, 4.2 mM), K2HPO4·3H2O (HPO₄²⁻, 1.0 mM) and Na2SO4 (SO₄²⁻, 0.5 mM). All samples for the degradation test were 10 × 10 × 3 mm. The solution volume for ions release and hydrogen test was 250 mL. Data of hydrogen evolution test were recorded every 3 h for the first 12 h and every 24 h after that. The hydrogen evolution is an easy method for measuring the degradation rate of Mg alloys. This work measured the hydrogen evolution volumes and rate for 240 h in m-SBF. The volume change \((V)\) is the value at the initial time (\({V}_{{{\rm{Initial}}}}\)) minus the value at the observation time point (\({V}_{{{\rm{observe}}}}\)), as shown below:
where \(V\) is the difference value of hydrogen volume, and s is the surface area.
The corresponding hydrogen evolution rate (Hv) is obtained from the equation:
where t is the immersion time.
The weight loss test of samples needed to be weighed and then exposed to 56 ml of m-SBF at different time intervals. The immersion solution is required to be exchanged every 3 days. By the end of the experiment, samples were ultrasonically cleaned in chromic acid solution (200 g/L CrO3, 10 g/L AgNO3, 20 g/L BaNO3, distilled water) for 3 min, followed by washing with ethanol. The weight loss and degradation rate (Pw) was calculated using the ASTM-G31-72 standard5.
where m0 is the mass before immersion, m1 is the mass after immersion, s is the surface area, t is the immersion time, and ρ is the density of samples.
Degradation products analysis
The morphology was evaluated using OM and SEM, while the element distribution was analyzed with electron probe micro-analyzer (EPMA; JEOL JXA8900R, Japan). Products were probed with X-ray photoelectron spectroscopy (XPS) for the compound composition and elemental information of the external lay; the products were investigated through X-ray diffraction (XRD) to check out the constituents. All the observations were carried out on the cross-section of the bar. The electrochemical test of pure Mg was conducted using an electrochemical workstation (Gamry, Interface 1010E) in m-SBF. Specific test parameters and characterization procedures can be found in our previous works5,49. The samples from different processes are named in Table 3.
Data availability
No datasets were generated or analyzed during the current study.
References
Chaya, A. et al. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 18, 262–269 (2015).
Henderson, S. E. et al. Magnesium alloys as a biomaterial for degradable craniofacial screws. Acta Biomater. 10, 2323–2332 (2014).
Xie, J. et al. Developing new Mg alloy as potential bone repair material via constructing weak anode nano-lamellar structure. J. Magnes. Alloy. 11, 154–175 (2023).
Li, Y. et al. Controllable degradation behavior of Mg-Sr-Y alloys for the bio-applications. npj Mater. Degrad. 7, 45 (2023).
Wang, S. et al. Advancing biodegradable Mg-Gd-Y alloy for cardiovascular stent applications. Mater. Today Commun. 41, 110893 (2024).
Weng, W., Biesiekierski, A., Li, Y., Dargusch, M. & Wen, C. A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater. 130, 80–97 (2021).
Zhou, H., Liang, B., Jiang, H., Deng, Z. & Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: from mechanism to application. J. Magnes. Alloy. 9, 779–804 (2021).
Zhao, D. et al. Current status on clinical applications of magnesium-based orthopaedic implants: a review from clinical translational perspective. Biomaterials 112, 287–302 (2017).
Wang, S. et al. Rational design, synthesis and prospect of biodegradable magnesium alloy vascular stents. J. Magnes. Alloy. 11, 3012–3037 (2023).
Sun, Q. et al. The immunomodulatory effects and mechanisms of magnesium-containing implants in bone regeneration: a review. J. Magnes. Alloy. 12, 2695–2710 (2024).
She, J. et al. Research advances of magnesium and magnesium alloys globally in 2023. J. Magnes. Alloy. 12, 3441–3475 (2024).
Zhang, S. et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 6, 626–640 (2010).
Avey, T. et al. Determining critical Zn/Ca atomic ratio and its role in mechanical and corrosion properties of biodegradable Mg-Ca-Zn-Mn alloys. Materialia 37, 102203 (2024).
Li, D. et al. In vitro and in vivo assessment of the effect of biodegradable magnesium alloys on osteogenesis. Acta Biomater. 141, 454–465 (2022).
Xie, W. et al. Increasing the high-temperature mechanical properties of Mg-Gd-Y-Zn alloys by adding AlN/Al particles. J. Mater. Res. Technol. 33, 2377–2390 (2024).
Zheng, Y. F., Gu, X. N. & Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 77, 1–34 (2014).
Peng, P. et al. Bimodal grained Mg–0.5 Gd–xMn alloys with high strength and low-cost fabricated by low-temperature extrusion. J. Alloy. Compd. 935, 168008 (2023).
Zheng, R. et al. Transition of dominant deformation mode in bulk polycrystalline pure Mg by ultra-grain refinement down to sub-micrometer. Acta Mater. 198, 35–46 (2020).
Fan, G. D. et al. Improved mechanical property and internal friction of pure Mg processed by ECAP. Mater. Sci. Eng. A 556, 588–594 (2012).
Guo, X. et al. Effect of Ca on hot tearing susceptibility of WE43 alloy. J. Alloy. Compd. 1002, 175451 (2024).
Song, D. et al. Corrosion behavior of equal-channel-angular-pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 52, 481–490 (2010).
Gan, W. M. et al. Microstructures and mechanical properties of pure Mg processed by rotary swaging. Mater. Des. 63, 83–88 (2014).
Biswas, S., Dhinwal, S. S. & Suwas, S. Room-temperature equal channel angular extrusion of pure magnesium. Acta Mater. 58, 3247–3261 (2010).
Noell, P. J. et al. The evolution of pit morphology and growth kinetics in aluminum during atmospheric corrosion. npj Mater. Degrad. 7, 12 (2023).
Moghanian, A., Paidar, M., Seyedafghahi, S. S. & Ojo, O. O. Friction stir welding of pure magnesium and polypropylene in a lap-joint configuration: microstructure and mechanical properties. Int. J. Miner. Metall. Mater. 26, 766–774 (2019).
Chang, H. et al. Microstructure and mechanical properties of the accumulative roll bonded (ARBed) pure magnesium sheet. Mater. Sci. Eng. A 527, 7176–7183 (2010).
Diez, M., Kim, H., Serebryany, V., Dobatkin, S. & Estrin, Y. Improving the mechanical properties of pure magnesium by three-roll planetary milling. Mater. Sci. Eng. A 612, 287–292 (2014).
Savaedi, Z., Mirzadeh, H., Aghdam, R. M. & Mahmudi, R. Effect of grain size on the mechanical properties and bio-corrosion resistance of pure magnesium. J. Mater. Res. Technol. 19, 3100–3109 (2022).
Qiao, Y., Wang, X., Liu, Z. & Wang, E. Effects of grain size, texture and twinning on mechanical properties and work-hardening behaviors of pure Mg. Mater. Sci. Eng. A 578, 240–246 (2013).
Xie, W. et al. Microstructural evolution and increased high-temperature tensile strength of extruded and aged AlN/Al reinforced Mg-10Gd-3Y-1Zn composites. J. Alloy. Compd. 1010, 177152 (2025).
Zhou, S. et al. Designing Mg alloys with high strength and ductility by reducing the strength difference between the basal and non-basal slips. Mater. Des. 225, 111476 (2023).
Zhao, D. et al. A high-strength binary Mg-1.2 Ce alloy with ultra-fine grains achieved by conventional one-step extrusion during 300–400° C. J. Mater. Res. Technol. 30, 7697–7708 (2024).
Harjo, S., Wu, G., Aizawa, K., Kawasaki, T. & Yamasaki, M. Strengthening of αMg and long-period stacking ordered phases in a Mg-Zn-Y alloy by hot-extrusion with low extrusion ratio. Acta Mater. 255, 119029 (2023).
Fan, J. et al. Achieving enhanced strength-ductility synergy in Mg-6Zn-1Mn alloy by introducing deformable Ti particles. Mater. Sci. Eng. A 917, 147412 (2024).
Wei, J. et al. Delayed fracture behavior of ultra-high-strength mooring chain steel evaluated by potentiostatic hydrogen-charging combined with SSRT. npj Mater. Degrad. 8, 57 (2024).
Li, Y. S., Zhang, Y., Tao, N. R. & Lu, K. Effect of the Zener–Hollomon parameter on the microstructures and mechanical properties of Cu subjected to plastic deformation. Acta Mater. 57, 761–772 (2009).
Wang, L. et al. Influence of non-rare earth elements on basal stacking fault energy of Mg binary alloys in solid solution. Scr. Mater. 257, 116479 (2025).
Zeng, Z., Nie, J., Xu, S., HJ Davies, C. & Birbilis, N. Super-formable pure magnesium at room temperature. Nat. Commun. 8, 972 (2017).
Wang, C. et al. Degradation behavior of pure Mg in the physiological medium and growth mechanism of surface corrosion product films. J. Magnes. Alloys 13, 1523–1535 (2024).
Zhang, H. et al. Obtaining high-purity struvite from anaerobically digested wastewater: effects of pH, Mg/P, and Ca2+ interactions. Environ. Eng. Sci. 36, 102–113 (2019).
Hao, X. D., Wang, C. C., Lan, L. & Van Loosdrecht, M. C. M. Struvite formation, analytical methods and effects of pH and Ca2+,. Water Sci. Technol. 58, 1687–1692 (2008).
Sun, H. et al. First-principles modeling of the anodic and cathodic polarization to predict the corrosion behavior of Mg and its alloys. Acta Mater. 244, 118562 (2023).
Ren, Y. et al. Study of bio-corrosion of pure magnesium. Acta Metall. Sin. Engl. Lett. 41, 1228–1232 (2005).
Wang, Y., Li, M. & Ren, H. Interfacial structure and energy determine the heterogeneity in the electrochemical metal dissolution activity at grain boundary. Chem. Mater. 35, 4243–4249 (2023).
Singh, A. P. & Srivastava, C. Effect of selective atomic clustering, surface texture and grain boundary constitution on the corrosion behaviour of electrodeposited Sn-xBi coatings. Corros. Sci. 215, 111039 (2023).
Li, Z. et al. Role of segregation behavior of Cu and Sb in the region of inclusions on initial corrosion. npj Mater. Degrad. 7, 29 (2023).
Song, G. & Atrens, A. Recently deepened insights regarding Mg corrosion and advanced engineering applications of Mg alloys. J. Magnes. Alloy. 11, 3948–3991 (2023).
Jiang, J., Geng, X. & Zhang, X. Stress corrosion cracking of magnesium alloys: a review. J. Magnes. Alloy. 11, 1906–1930 (2023).
Wang, S. et al. Grain refinement and mechanical properties of AZ31 alloy processed by pre-die forging extrusion at different temperatures. J. Mater. Eng. Perform. 32, 10877–10884 (2023).
Acknowledgements
This research was funded by the National Natural Science Foundation of China (52471117 and 52225101), Chongqing Academician Special Fund (2022YSZX-JCX0014CSTB), Natural Science Foundation of Hunan Province (2023JJ70048).
Author information
Authors and Affiliations
Contributions
S.W.: conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft; J.S.: conceptualization, funding acquisition, project administration, supervision, writing—review and editing; T.C.: investigation, methodology; J.L.: investigation; Q.Z.: investigation; C.Z.: writing—review and editing; X.C.: conceptualization, funding acquisition; B.Q.: writing—review and editing; F.P.: resources, funding acquisition, project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Cai, T., Liu, J. et al. Synergistic enhancement: realizing superior strength, ductility and degradation performance in pure magnesium. npj Mater Degrad 9, 64 (2025). https://doi.org/10.1038/s41529-025-00609-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-025-00609-3