Abstract
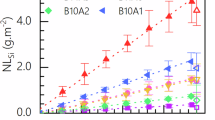
Lanthanide borosilicate (LaBS) glasses are among the most promising waste forms for the immobilization of high-level radioactive waste generated from advanced nuclear fuel cycles. However, the temperature dependence of their dissolution kinetics remains poorly understood and constrained, limiting the integration of these materials into established performance assessment models. Here, we investigate the dissolution behavior of the legacy AmCm2-19 LaBS glass and the benchmark alkali aluminoborosilicate ISG-1 in deionized water between 50 °C and 250 °C using ASTM C1285 (Product Consistency Test-B) protocols. For AmCm2-19 LaBS glass, normalized elemental release rates for boron and silicon increase with temperature before plateauing near 150 °C, consistent with solubility-limited behavior. From data obtained at 50 °C and 100 °C, Arrhenius analysis yields activation energies of Ea(B) = 24.8 ± 0.3 kJ mol⁻¹ and Ea(Si) = 14.4 ± 0.2 kJ mol⁻¹, similar or slightly lower than those previously reported for two other compositions of LaBS glasses. No secondary phases or alteration layers were detected by SEM-EDX or pXRD. These results establish one of the first temperature-dependent kinetic datasets for LaBS glass dissolution, providing quantitative parameters to inform mechanistic corrosion models and predictive simulations of glass degradation in geological disposal environments.
Similar content being viewed by others
Data availability
All data are available in the manuscript and supplementary information file or can be obtained from the corresponding authors upon request.
References
International Atomic Energy Agency. Roadmap for Implementing a Geological Disposal Programme. Report No. NW-T-1.43. (International Atomic Energy Agency, 2024).
King, F. et al. Review of the modelling of corrosion processes and lifetime prediction for HLW/SF containers—part 2: performance assessment models. Corros. Mater. Degrad. 5, 289–339 (2024).
Ruiz-Fresneda, M. A. et al. Impact of microbial processes on the safety of deep geological repositories for radioactive waste. Front. Microbiol. 14, 1134078 (2023).
Morales-Hidalgo, M. et al. Insights into the impact of physicochemical and microbiological parameters on the safety performance of deep geological repositories. Microorganisms 12, 1025 (2024).
Andrews, N. C. et al. Mechanistic Source Term Considerations for Advanced Non-LWRs. Report No. SAND2021-2668. (Sandia National Laboratories, 2021).
Jantzen, C. M. & Ojovan, M. I. On selection of matrix (wasteform) material for higher activity nuclear waste immobilization (review). Russ. J. Inorg. Chem. 64, 1611–1624 (2019).
Thorpe, C. L. et al. Forty years of durability assessment of nuclear waste glass by standard methods. npj Mater. Degrad. 5, 61 (2021).
Frankel, G. S. et al. Recent advances in corrosion science applicable to disposal of high-level nuclear waste. Chem. Rev. 121, 12327–12383 (2021).
Gin, S., Jollivet, P., Tribet, M., Peuget, S. & Schuller, S. Radionuclides containment in nuclear glasses: an overview. Radiochim. Acta 105, 927–959 (2017).
Gin, S. et al. Insights into the mechanisms controlling the residual corrosion rate of borosilicate glasses. npj Mater. Degrad. 4, 41 (2020).
Fournier, M., Gin, S. & Frugier, P. Resumption of nuclear glass alteration: state of the art. J. Nucl. Mater. 448, 348–363 (2014).
Vienna, J. D., Neeway, J. J., Ryan, J. V. & Kerisit, S. N. Impacts of glass composition, pH, and temperature on glass forward dissolution rate. npj Mater. Degrad. 2, 22 (2018).
Grambow, B. A general rate equation for nuclear waste glass corrosion. MRS Online Proc. Libr. 44, 15 (1984).
Oelkers, E. H. General kinetic description of multioxide silicate mineral and glass dissolution. Geochim. Cosmochim. Acta 65, 3703–3719 (2001).
Frugier, P. et al. SON68 nuclear glass dissolution kinetics: current state of knowledge and basis of the new GRAAL model. J. Nucl. Mater. 380, 8–21 (2008).
Frugier, P., Minet, Y., Rajmohan, N., Godon, N. & Gin, S. Modeling glass corrosion with GRAAL. npj Mater. Degrad. 2, 35 (2018).
Minet, Y., Bonin, B., Gin, S. & Frugier, P. Analytic implementation of the GRAAL model: application to a R7T7-type glass package in a geological disposal environment. J. Nucl. Mater. 404, 178–202 (2010).
Delcroix, M., Frugier, P., Geiger, E. & Noiriel, C. The GRAAL2 glass alteration model: initial qualification on a simple chemical system. npj Mater. Degrad. 9, 38 (2025).
Strachan, D. Defense HLW Glass Degradation Model. Report No. ANL-EBS-MD-000016. (U.S. Department of Energy Offi ce of Civilian Radioactive Waste Management, 2004).
Yucca Mountain Repository License Application Safety Analysis Report. Report No. DOE/RW-0573. (U.S. Department of Energy Offi ce of Civilian Radioactive Waste Management, 2008).
Curti, E. Aqueous Corrosion of Vitrified Nuclear Waste: Current Process Understanding, Literature Review and Recommended Rates. Report No. NAB 23-09. (National Cooperative for the Disposal of Radioactive Waste, 2022).
Kelly, J. E. Generation IV International Forum: a decade of progress through international cooperation. Prog. Nucl. Energy 77, 240–246 (2014).
Generation IV Nuclear Reactors - World Nuclear Association. https://world-nuclear.org/information-library/nuclear-fuel-cycle/nuclear-power-reactors/generation-iv-nuclear-reactors (2025).
Bibler, N. E., Ramsey, W. G., Meaker, T. F. & Pareizs, J. M. Durabilities and Microstructures of Radioactive Glasses for Immobilization of Excess Actinides at the Savannah River Site. MRS Online Proc. Libr. (OPL) 412, 65 (1995).
Crawford, C. L., Marra, J. C. & Bibler, N. E. Glass fabrication and product consistency testing of lanthanide borosilicate glass for plutonium disposition. J. Alloy. Compd. 444–445, 569–579 (2007).
Peeler, D. Composition/Property Relationship for the Phase 2 Am-Cm Glass Variability Study. Report No. WSRC-TR-99-00393. (Savannah River Site, 2000).
Peeler, D. K. et al. Composition/Property Relationships for the Phase 1 Am/Cm Glass Variability Study. Report No. WSRC-TR-99-00055. (Savannah River Site, 1999).
Marra, J. C., Peeler, D. K. & Jantzen, C. M. Development of an Alternative Glass Formulation for Vitrification of Excess Plutonium. Report No. WSRC-TR-2006-00031. (Savannah River National Laboratory, 2006).
Peeler, D. K. et al. Results of an Inter-Laboratory Study of Glass Formulation for the Immobilization of Excess Plutonium. Report No. SRT-PUM-97-0017. (Savannah River Site, 1997).
Riley, B. J. Electrochemical salt wasteform development: a review of salt treatment and immobilization options. Ind. Eng. Chem. Res. 59, 9760–9774 (2020).
Riley, B. J. & Chong, S. Glass waste form options for rare-earth fission products from electrochemical reprocessing. J. Non Cryst. Solids 545, 120161 (2020).
Fox, K. M., Marra, J. C., Edwards, T. B., Hoffman, E. N. & Crawford, C. L. Plutonium feed impurity testing in lanthanide borosilicate (LaBS) glass. MRS Online Proc. Libr. 1107, 397 (2008).
Fox, K. M. et al. Variability Study to Determine the Solubility of Impurities in Plutonium-Bearing, Lanthanide Borosilicate Glass. Report No. WSRC-STI-2007-00477. (Savannah River National Laboratory, 2007).
Daniel, W. E. & Best, D. R. Am/Cm Target Glass Durability Dependence on pH (U). Report No. WSRC-MS-96-0165. (Savannah River Site, 1996).
Ebert, W. L. Corrosion Testing of a Plutonium-Loaded Lanthanide Borosilicate Glass Made with Frit B. Report No. ANL-06/35. (Argonne National Laboratory, 2006).
Daniel, W. E. & Best, D. R. Time, Temperature, and Compositional Study of Am/Cm Target Glass Durability. Report No. WSRC-MS-96-0163. (Savannah River Site, 1996).
Deschanels, X., Peuget, S., Cachia, J. N. & Charpentier, T. Plutonium solubility and self-irradiation effects in borosilicate glass. Prog. Nucl. Energy 49, 623–634 (2007).
Fabian, M., Pinakidou, F., Tolnai, I., Czompoly, O. & Osan, J. Lanthanide (Ce, Nd, Eu) environments and leaching behavior in borosilicate glasses. Sci. Rep. 11, 13272 (2021).
Fabian, M. et al. Structural investigation of borosilicate glasses containing lanthanide ions. Sci. Rep. 10, 7835 (2020).
Tolnai, I. et al. Structural characterization of uranium and lanthanide loaded borosilicate glass matrix. Sci. Rep. 15, 28352 (2025).
Mabrouk, A., Vaills, Y., Pellerin, N. & Bachar, A. Structural study of lanthanum sodium aluminoborosilicate glasses by NMR spectroscopy. Mater. Chem. Phys. 254, 123492 (2020).
Kim, M., Ha, M. G., Um, W., Kim, H. G. & Hong, K.-S. Relationship between leaching behavior and glass structure of calcium-aluminoborate waste glasses with various La2O3 contents. J. Nucl. Mater. 539, 152331 (2020).
Malchukova, E., Levitskii, V., Tyurnina, N. & Tyurnina, Z. Structure and optical behavior of mixed lanthanides aluminoborosilicate glasses. Ceram. Int. 51, 9570–9576 (2025).
Nicoleau, E. et al. Rare-earth silicate crystallization in borosilicate glasses: effect on structural and chemical durability properties. J. Non Cryst. Solids 438, 37–48 (2016).
Ryan, J. V. et al. ISG-2: properties of the second International Simple Glass. npj Mater. Degrad. 7, 47 (2023).
Jiménez, J. A., Crawford, C. L., Lascola, R. J., Christian, J. H. & Foley, B. J. Physico-chemical properties of γ-irradiated International Simple Glass (ISG) nuclear waste simulants with low levels of iron impurities. Chem. Phys. Impact 6, 100233 (2023).
Jiménez, J. A., Crawford, C. L. & Lascola, R. J. Physico-chemical properties of international simple glass (ISG) nuclear waste simulants: luminescence baseline study. J. Am. Ceram. Soc. 105, 4009–4026 (2022).
Li, L., Strachan, D. M., Li, H., Davis, L. L. & Qian, M. Crystallization of gadolinium- and lanthanum-containing phases from sodium alumino-borosilicate glasses. J. Non Cryst. Solids 272, 46–56 (2000).
Fortner, J. A., Mertz, C. J., Chamberlain, D. C. & Bates, J. K. Plutonium Alteration Phases from Lanthanide Borosilicate Glass. Report No. ANL/CMT/CP--92880. (Argonne National Laboratory, 1997).
Gaddam, A., Fernandes, H. R., Tulyaganov, D. U. & Ferreira, J. M. F. The structural role of lanthanum oxide in silicate glasses. J. Non Cryst. Solids 505, 18–27 (2019).
Hordieiev, Yu. S. & Zaichuk, A. V. Study of the influence of R2O3 (R = Al, La, Y) on the structure, thermal and some physical properties of magnesium borosilicate glasses. J. Inorg. Organomet Polym. 33, 591–598 (2023).
Angeli, F. et al. Influence of lanthanum on borosilicate glass structure: a multinuclear MAS and MQMAS NMR investigation. J. Non Cryst. Solids 376, 189–198 (2013).
Molières, E. et al. Chemical durability of lanthanum-enriched borosilicate glass. Int. J. Appl. Glass Sci. 4, 383–394 (2013).
Peeler, D. K., Marra, J. E., Reamer, I. A., Vienna, J. D. & Li, H. Development of the Am/Cm Batch Vitrification Process. Report No. WRSC-MS-99-00657. (Westinghouse Savannah River Company, 1999).
Deshpande, V. K. & Taikar, R. N. Effect of cerium oxide addition on electrical and physical properties of alkali borosilicate glasses. Mater. Sci. Eng. B 172, 6–8 (2010).
Mekki, A. X-ray photoelectron spectroscopy of CeO2–Na2O–SiO2 glasses. J. Electron Spectrosc. Relat. Phenom. 142, 75–81 (2005).
Inagaki, Y., Kikunaga, T., Idemitsu, K. & Arima, T. Initial dissolution rate of the international simple glass as a function of pH and temperature measured using microchannel flow-through test method. Int. J. Appl. Glass Sci. 4, 317–327 (2013).
Neeway, J. J., Rieke, P. C., Parruzot, B. P., Ryan, J. V. & Asmussen, R. M. The dissolution behavior of borosilicate glasses in far-from equilibrium conditions. Geochim. Cosmochim. Acta 226, 132–148 (2018).
Gervasio, V. et al. Liquidus Temperature: Assessing Standard Glasses for Furnace Calibration. Report No. PNNL-29312. (Pacifi c Northwest National Laboratory, 2019).
Standard Test Methods for Determining Chemical Durability of Nuclear, Hazardous, and Mixed Waste Glasses and Multiphase Glass Ceramics: The Product Consistency Test (PCT). Report No. ASTM C1285-21. (American Society for Testing and Materials, 2021).
Acknowledgements
This work was supported by the Advanced Research Projects Agency - Energy (ARPA-E) of the U.S. Department of Energy (DOE; ARPA-E Grant No. DE-AR0001621); part of the research was conducted at the Lawrence Berkeley National Laboratory, a U.S. DOE Office of Science national laboratory managed by the University of California (UC) under contract No. DE-AC02-05CH11231. The authors thank Dr. Brian Riley, Dr. Vivianaluxa Gervasio, Dr. Benjamin Parruzot, and Dr. Joelle Reiser of the Radiological Materials group at PNNL for fruitful discussion and for providing samples of ISG-1 and AmCm2-19 glass. The authors thank John Grimsich from the Department of Earth and Planetary Sciences at UC Berkeley for his guidance with SEM imaging and EDX measurements.
Author information
Authors and Affiliations
Contributions
J.R.M. – Conceptualization (equal); formal analysis; investigation; methodology (lead); project administration; supervision; visualization; data curation; writing – original draft. D.A.S. – Investigation; methodology (supporting); writing – review and editing. J.A.G. – Investigation; methodology (supporting). T.W. – Investigation; methodology (supporting). L.G.C. – Investigation. E.B. – Conceptualization (equal); writing – review and editing. S.F. – Conceptualization (equal); writing – review and editing. J.S. – Conceptualization (equal); funding acquisition (equal); writing – review and editing. R.S. – Conceptualization (equal); funding acquisition (equal); writing – review and editing. P.F.P. – Conceptualization (equal); funding acquisition (equal); supervision; writing – review and editing. R.J.A. - Conceptualization (equal); funding acquisition (equal); resources; supervision; writing – review and editing.
Corresponding authors
Ethics declarations
Competing interests
P.F.P. declares financial interest in Deep Isolation Nuclear, Inc, and both he and the company could benefit from commercialization of products, the development of which is supported by this research. All other authors declare no competing financial or non-financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McLachlan, J.R., Stanley, D.A., Garcia, J.A. et al. Toward the performance assessment of advanced nuclear waste forms: temperature dependence of lanthanide borosilicate glass dissolution. npj Mater Degrad (2026). https://doi.org/10.1038/s41529-026-00756-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-026-00756-1