Abstract
Recent studies demonstrate that Parkinson’s disease (PD) is associated with dysregulated metabolic flux through the kynurenine pathway (KP), in which tryptophan is converted to kynurenine (KYN), and KYN is subsequently metabolized to neuroactive compounds quinolinic acid (QA) and kynurenic acid (KA). Here, we used mass-spectrometry to compare blood and cerebral spinal fluid (CSF) KP metabolites between 158 unimpaired older adults and 177 participants with PD. We found increased neuroexcitatory QA/KA ratio in both plasma and CSF of PD participants associated with peripheral and cerebral inflammation and vitamin B6 deficiency. Furthermore, increased QA tracked with CSF tau, CSF soluble TREM2 (sTREM2) and severity of both motor and non-motor PD clinical symptoms. Finally, PD patient subgroups with distinct KP profiles displayed distinct PD clinical features. These data validate the KP as a site of brain and periphery crosstalk, integrating B-vitamin status, inflammation and metabolism to ultimately influence PD clinical manifestation.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative condition characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta and aggregation of misfolded α-synuclein protein in Lewy bodies across the brainstem and other structures1. Striatal dopamine depletion contributes to classical PD motor and non-motor clinical dysfunction, the latter of which may precede appearance of motor symptoms by years or decades. Furthermore, significant heterogeneity exists in the presentation of PD symptoms – which often fluctuate – and disease progression varies greatly between patients2. Given the unpredictable nature in the presentation and progression of PD, there is a pressing need to develop biomarkers capable of predicting PD pathophysiology and clinical presentation3.
Multiple lines of evidence implicate central nervous system (CNS) and peripheral inflammation in the PD initiation and progression4,5,6. Indeed, genetic studies have identified PD risk variants relating to immune function7. Postmortem examinations have demonstrated reactive microglia and incursion of immune cells in PD brain8. Moreover, imaging markers of neuroinflammation9,10 and biofluid biomarkers11,12,13,14 reveal chronic immune activation in PD in vivo. Despite these findings, the mechanisms linking inflammation and PD remain unclear.
The kynurenine pathway (KP) is modified by peripheral and CNS inflammation, and improper KP metabolism leads to the accumulation of proinflammatory and neuroactive intermediate metabolites. Under physiological conditions, tryptophan (TRP) is converted to kynurenine (KYN), which is subsequently metabolized via B vitamin-dependent reactions to neuroactive intermediates, including neuroprotective kynurenic acid (KA) and neuroexcitatory quinolinic acid (QA). From QA, quinolinate phosphoribosyl transferase (QPRT) serves as the rate limiting enzyme in de novo nicotinamide adenine dinucleotide (NAD + ) synthesis15, an essential energy source for many cellular functions16,17. Under inflammatory conditions, interferon (IFN)-γ, IFN-α and tumor necrosis factor (TNF)-α direct TRP metabolism toward KYN by inducing the enzyme indoleamine 2,3-dioxygenase 1 (IDO1)18,19. Vitamin B6 deficiency leads to increased 3-hydroxykynurenine (3-HK) and QA, with high 3-HK promoting oxidative stress and apoptotic neuronal death20 and high QA/KA ratios promoting glutamatergic excitotoxicity and further neuroinflammation21,22. In contrast, KA directly blocks QA excitotoxicity23 and protects against dopaminergic cell death24. KP activation has been demonstrated to occur across various inflammatory conditions, including aging25, multiple sclerosis26, Alzheimer’s disease27,28,29, schizophrenia30, peripartum depression31, lung cancer32, and in children with inflammatory neurological disorders33.
Severe KP disruption has also been reported in PD, involving increases in 3-HK, increases in the immune activation marker neopterin, and decreases in neuroprotective KA27,34,35,36,37,38,39,40,41,42,43. Yet, our understanding of the peripheral and central contributions to KP activation in PD remains incomplete. In addition, it is unclear whether there exists PD clinical subgroups with specific patterns of KP dysmetabolism. This study aimed to identify such endophenotypes to further support the development of individualized therapeutic strategies in PD.
Results
This multicenter study included 335 participants drawn from 8 research centers and consisted of 158 control older adult participants and 177 participants with PD. Demographic information for the study participants is shown in Table 1 and details on the originating research centers for study population participants are presented in Supplementary Fig. 1. Control participants were on average 67.70 years old (SD = 7.58) while the PD participants were 67.41 years old (SD = 8.23). Consistent with disease prevalence44, males were overrepresented in the PD participants compared to the controls (69.4% vs. 50.6%; P < 0.001, Chi-Square test). There were no differences between PD and controls with respect to APOE-ε4 carriage (23.8% vs. 24.7%; P > 0.05, Chi-Square test) or years of education (16.22 vs. 16.53; P > 0.05, two-sided Student’s t test). PD participants had an average time since diagnosis of 5.53 years (range = 0 to 26) and Levodopa equivalent daily dose (LEDD) of 566.15 mg (range = 0 to 2150). PD participants had lower Montreal Cognitive Assessment (MoCA) scores (24.30 ± 3.94) compared to controls (27.57 ± 2.01; P = 0.0002, two-sided Student’s t test). No differences were observed in CSF biomarkers p-tau181 or total tau (P > 0.05, two-sided Student’s t test), although CSF Aβ42/Aβ40 was slightly higher in the PD group (P < 0.05, two-sided Student’s t test).
Blood and CSF KP metabolites and B-vitamin cofactors required for their enzymatic synthesis were measured using a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay with high sensitivity and precision (Fig. 1a, b). Notably, many metabolites are transported from the blood across the blood-brain barrier (BBB) by the large neural amino acid transporter45,46 and basal BBB permeability is susceptible to change in inflammatory disease states47 (Fig. 1c). Accordingly, CSF and plasma pools for many metabolites were often correlated (Supplementary Fig. 2a–c).
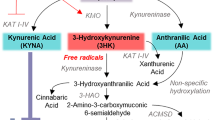
a Parkinson’s disease is a multisystem neurodegenerative disease. Recent data support that peripheral α-synuclein pathology and inflammation are related to the gut microbiome and that misfolded α-synuclein may undergo transneuronal transport via the vagus nerve to the brain. b In the kynurenine pathway, tryptophan is metabolized via IDO and TDO to kynurenine, which is converted to either 3-hydroxykynurenine by KMO, to anthranilic acid by kynureninase, or to kynurenic acid by KAT. 3-hydroxykynurenine is converted to xanthurenic acid by KAT. Kynureninase converts 3-hydroxykynurenine and anthranilic acid to 3-hydroxyanthranlic acid. 3-Hydroxyanthranilic acid is converted by 3-HAO, and further spontaneously converts to quinolinic acid, which is converted by QPRT to NAD+, a major energy source for cells. NAD+ is further converted to nicotinamide and N1-methylnicotinamide or to nicotinic acid and then to the antioxidant trigonelline. Tryptophan is also a precursor for serotonin and melatonin production. Key converting enzymes are indicated in blue. Also shown are enzymes supported by the active form of vitamin B6, PLP and B2 vitamer, FAD, in purple). IDO indoleamine 2,3-dioxygenase, 3-HAO 3-hydroxyanthranilate-3,4-dioxygnase, FAD flavin adenine dinucleotide, KAT kynurenine aminotransferase, KMO kynurenine 3-monooxygenase, PLP pyridoxal 5’-phosphate, QPRT quinolinic acid phosphoribosyltransferase, TDO tryptophan 2,3-dioxygenease. c Several kynurenine pathway metabolites (black) have high blood-brain barrier permeability (green arrows), while others are considered to cross the blood-brain barrier poorly (red X). Enzymes are shown in blue and do not cross the blood-brain barrier. High blood-brain permeability would allow passage of circulating kynurenines into brain kynurenine pools. d Box and whisker plot showing median concentration of plasma 3-hydroxykynurenine in control and PD participants. Whiskers indicate minimum to maximum data range. ***P < 0.001 analyzed by ANCOVA with age and sex included as covariates. e Ratio of plasma QA/KA in control and PD participants. ***P < 0.001 analyzed by ANCOVA with age and sex included as covariates. f Concentration of CSF 3-hydroxykynurenine in control and PD participants. **P < 0.01 analyzed by ANCOVA with age and sex included as covariates. g CSF QA/KA in control and PD participants. ***P < 0.001 analyzed by ANCOVA with age and sex as covariates. h Metabolic pathway maps showing plasma (left) and CSF (right) kynurenine pathway metabolites. Metabolites are colored by the log2 fold-change scale comparing the PD group to the control group. Metabolites that were not measured are shown as black circles. FC fold change. i Linear regression of plasma QA predicting CSF QA. Age and sex were included as covariates in the linear model. Shown are the β-estimates and P-values from the linear model and the 95% confidence band of the line of best fit. j Linear regression of plasma 3-HK predicting CSF 3-HK. Age and sex were included as covariates in the linear model. Shown are the β-estimates and P-values from the linear model and the 95% confidence band of the line of best fit. k Linear regression of plasma 3-HK predicting CSF QA. Age and sex were included as covariates in the linear model. Shown are the β-estimates and P-values from the linear model and the 95% confidence band of the line of best fit.
KP activation in Parkinson’s disease
LC-MS/MS analysis revealed that while plasma concentrations of the essential amino acid and KP precursor TRP and KYN were lower in PD participants compared to control, CSF concentrations did not differ between the two groups (P > 0.05, ANCOVA with age and sex included as covariates), nor did levels of KYN (P > 0.05; Supplementary Fig. 3a, b). In plasma, higher concentrations of the neuroexcitatory 3-HK were found in PD (P < 0.001; Fig. 1d). Similarly, PD participants had higher plasma levels of the neuroexcitatory QA/KA ratio (P < 0.001; Fig. 1e). Conversely, levels of neuroprotective KA were lower in PD plasma (P < 0.001), as were levels of nicotinamide (P < 0.001; Supplementary Fig. 3a). In CSF, 3-HK was higher in PD participants compared to controls (P < 0.01; Fig. 1f), as was the QA/KA ratio (P < 0.001; Fig. 1g). CSF QA was also higher in PD compared to control (P < 0.01; Supplementary Fig. 3b). Lower concentrations of KA (P < 0.001), NAM (P < 0.001) and NMN (P < 0.001) – an anti-inflammatory metabolite48 – were observed in PD. KP metabolic maps demonstrate lower plasma TRP, KYN and KA and higher 3-HK, along with lower CSF KA and higher CSF H-HK, 3-HAA and QA in PD (Fig. 1h). While plasma and CSF pools of QA are positively associated (β = +0.692, P < 0.001; Fig. 1i), QA is not actively transported across the BBB27. However, QA substrate 3-HK is transported from the blood across the BBB by large-neutral amino acid transporters49. Thus, peripheral-CNS communication through 3-HK BBB transport may contribute to the increase in neuroexcitatory CSF QA in PD. In support of this, plasma 3-HK was found to be positively associated with CSF 3-HK (β = +0.290, P < 0.001; Fig. 1i) and with CSF QA (β = +0.388, P < 0.001; Fig. 1k). Notably, when we investigated whether the KP metabolites associated with Levodopa equivalent daily dose (LEDD; Supplementary Table 1), we found that plasma TRP was negatively associated (β = −0.231, P = 0.01), while plasma 3-HAA was positively associated (β = +0.208, P = 0.01. Similarly, CSF QA was positively associated with LEDD (β = +0.192, P < 0.05).
Low B-vitamin status associates with inflammation and KP activation in Parkinson’s disease
Enzymatic conversion of some KP metabolites to their subsequent products requires the active forms of vitamins B2 (flavine adenine dinucleotide, FAD) and B6 (pyridoxal 5’-phosphate; PLP) to serve as enzyme cofactors (Fig. 2a). B-vitamin levels are determined by the balance of intestinal absorption and liver catabolism to impact overall availability. The HK ratio (HKr) is a functional measure of vitamin B6 status, calculated as the ratio of 3-HK to the four kynurenine products of PLP-dependent enzymes KAT and KYNU (i.e., 3-HK/(KA + AA + XA + HAA))50. Similarly, the PAr Index is a blood-based measure of vitamin B6 catabolism, calculated as the ratio of plasma B6 catabolite 4-pyridoxic acid (4-PA) to the sum of its active form PLP and its transport form pyridoxal (PL) (4-PA/(PLP + PL)51. The PAr Index is elevated in inflammatory conditions51,52, cancer53,54, coronary artery disease55, stroke56 and depression57.
a Kynurenine pathway enzymes (blue) require B-vitamin cofactors (green) for their activity. 3-HK: 3-hydroxykynurenine, FAD flavine adenine dinucleotide, KAT kynurenine aminotransferase, KMO kynurenine 3-monooxygenase, KYNU kynureninase, PLP pyridoxal 5’phosphate. b B-vitamins, HKr, and the PAr Index in plasma from control and PD participants. HKr is a validated functional measure of vitamin B6 status. PAr is an index of vitamin B6 catabolism. ***P < 0.001 using ANCOVA including age and sex as covariates. HKr HK ratio. c B-vitamins and HKr in CSF from control and PD participants. HKr is a validated functional measure of vitamin B6 status. **P < 0.01, ***P < 0.001 using ANCOVA including age and sex as covariates. HKr HK ratio. d Plasma neopterin, a validated marker of inflammation, in control and PD participants. ***P < 0.001 using ANCOVA including age and sex as covariates. e Linear regression reveals that plasma PAr is significantly correlated with plasma neopterin concentration. Age and sex were included as covariates in the linear model. Shown are the β-estimates and P-values from the linear model and the 95% confidence band of the line of best fit. PAr PAr Index. f Correlation matrix showing association between plasma metabolites (black text) and B-vitamins (blue text) in PD participants. Age and sex were included as covariates. Significant correlations are shown with the circle size indicating the P-value of the correlation and color indicating size and direction of the β-estimate. 3-HK 3-hydroxykynurenine, HKr HK ratio, Kyn kynurenine, PAr PAr Index, QA quinolinic acid. g Correlation matrix showing association between CSF metabolites (black text) and B-vitamins (blue text) in PD participants. Age and sex were included as covariates. Significant correlations are shown with the circle size indicating the P-value of the correlation and color indicating size and direction of the β-estimate. 3-HK 3-hydroxykynurenine, HKr HK ratio, Kyn kynurenine, QA quinolinic acid.
In examining whether B-vitamin deficiency links proinflammatory responses and KP activation in PD, it was found that plasma flavin mononucleotide (FMN, P < 0.001), PLP (P < 0.001), pyridoxal (P < 0.001) and 4-pyridoxic acid (4-PA, P < 0.001) were all lower in PD participants when compared to controls (Fig. 2b and Supplementary Fig. 4a). Similarly, plasma HKr (P < 0.001) was higher in PD compared to control, confirming lower vitamin B6 status in the PD group. In CSF, PLP (P < 0.001) and pyridoxal (P < 0.001) were both lower in PD compared to control (Fig. 2c; Supplementary Fig. 4b) and the PD group had higher CSF HKr compared to the control group (P < 0.001). When we investigated whether the B-vitamins associated with Levodopa equivalent daily dose (LEDD; Supplementary Table 2), we found that only plasma 4-PA was negatively associated with LEDD (β = −0.262, P < 0.01).
Next, levels of the peripheral inflammatory marker neopterin – an indicator of monocyte/macrophage activation58— were assessed to further investigate the link between inflammatory responses and vitamin B6 catabolism. Indeed, plasma neopterin was higher in PD compared to controls (P < 0.001; Fig. 2d). Moreover, a positive association was demonstrated between plasma neopterin and the PAr Index (β = +0.261, P < 0.01; Fig. 2e). Further correlation analyses examined associations between the kynurenines and B-vitamin derivatives within plasma (Fig. 2f) and CSF (Fig. 2g) pools in the PD group. Notably, 3-HK and QA levels were higher at lower vitamin B6 (PLP) levels, further highlighting the possibility that vitamin B6 deficiency serves as a link between peripheral inflammation and the altered KP profile observed in PD.
CSF QA associates with motor symptom severity and neurodegeneration in Parkinson’s disease
Associations between CSF QA and PD symptoms were next investigated using participant data from the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS59; Fig. 3a). CSF QA was positively associated with MDS-UPDRS Part II total score, which is sensitive to motor aspects of experiences of daily living (β = +0.175, P < 0.035), with Part III OFF, which is a measure of motor severity (β = +0.277, P < 0.042), and Part IV, which is related to motor complications of Levodopa replacement therapy (β = +0.198, P < 0.028).
a Linear regression analysis showing associations between CSF QA with MDS-UPDRS Parts I-IV scores in PD participants. Plotted is the 95% confidence band of the best-fit line from the linear regression. Age and sex were included as covariates. β-estimates and P-values from the linear model are shown. MDS-UPDRS Movement Disorder Society Unified Parkinson’s Disease Rating Scale, QA quinolinic acid. b Linear regression analysis showing associations between CSF QA and core AD biomarkers CSF total tau, p-tau181, and Aβ42/Aβ40 ratio in PD participants. Plotted is the 95% confidence band of the best-fit line from the linear regression. CSF biomarkers were log-transformed in a linear model that included age and sex as covariates. β-estimates and P-values from the linear model are shown. c Linear regression analysis showing associations between CSF QA and biomarker of microglial activation CSF sTREM2 in PD participants. Plotted is the 95% confidence band of the best-fit line from the linear regression. CSF biomarkers were log-transformed in a linear model that included age and sex as covariates. β-estimates and P-values from the linear model are shown.
Additionally, associations between CSF QA with Alzheimer’s disease biomarkers CSF amyloid and tau were examined given the high comorbidity of Alzheimer’s disease neuropathology in PD60,61,62,63,64. This analysis revealed that CSF QA was associated with CSF total tau (β = +0.198, P < 0.0001), but not CSF p-tau181 (P > 0.05) or the proxy for cortical amyloidosis, CSF Aβ42/Aβ40 (P > 0.05; Fig. 3b), suggesting a specific link between QA-induced neurotoxicity and neurodegeneration. We previously found that the marker of CNS microglial activation, CSF sTREM2, was elevated in PD participants and corresponded with comorbid tau neuropathology12. In the current study, we found that CSF sTREM2 was positively associated with CSF QA in PD (β = +0.166, P < 0.006; Fig. 3c). Together, these data highlight an important link between CNS neurotoxic QA with both tau neurodegeneration and microglial activation in PD.
CSF and plasma KP metabolites accurately predict Parkinson’s disease
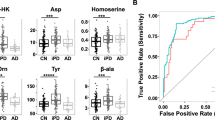
We next evaluated whether CSF and plasma kynurenines and related B-vitamin levels might be useful in distinguishing PD from controls. For this analysis all kynurenines, their ratios and related B vitamins were included in the models and a cross validation strategy was used to simultaneously optimize the integrative model on a subset of metabolite data and then test its performance on previously unseen participants. We found that both CSF (AUC: 0.740) and plasma (AUC: 0.893) metabolites correlated with PD diagnosis (AUC combined model: 0.897; Fig. 4a). Indeed, KP metabolite levels were robust predictors of PD diagnosis, sex, and years since diagnosis, but not age or years of education (Fig. 4b). Correlation-based network analysis was used to evaluate the relationships between plasma and CSF metabolite features in a bottom-up approach. In this scenario, the metabolite features are presented as nodes in the network map and links are drawn between them when a relationship is present. KP metabolite features that effectively predicted PD diagnosis included 3-HK/KA, 3-HKr, 3-HK, PLP, QA/KA, KA, and TRP in plasma and QA/KA, 3-HK/KA – an excitotoxic ratio36 – along with HKr, KA, PL, PLP, 3-HK and 3-HAA in CSF (Fig. 4c). Together, these results demonstrate the utility of the KP, particularly in plasma, to serve as a reliable biofluid biomarker for distinguishing PD from controls.
a ROC curve analysis of a combined model consisting of plasma and CSF KP-related metabolites and their ratios (black curve), and single fluid models with plasma (cyan curve) or CSF (salmon curve) in distinguishing PD participants (n = 149) from control participants (n = 158). All metabolite biomarkers were used in these models. A cross-validation strategy was employed such that half the participants were used to optimize the integrated model, after which the remaining half were presented to the model to test its performance. b Combined model performance in correctly classifying diagnosis, age, sex, years since diagnosis and education. c Network analysis revealing kynurenine pathway metabolites correlating with predicting PD vs control (Spearman correlation P < 0.05). Metabolites are coded according to sample type (plasma: cyan or CSF: salmon). Circle size indicates degree of association between metabolite and predicting PD diagnosis. KA kynurenic acid, 3-HK 3-hydroxykynurenine, 3HAA 3-hydroxyanthranilic acid, QA quinolinic acid, NMN N1-methylnicotinamide.
Parkinson’s disease clinical subgroups display distinct patterns of KP alterations and distinct clinical features
Characterizing the clinical and biochemical heterogeneity of Parkinson’s disease is critical to understanding its origins and devising targeted management strategies3. Therefore, given the size of our multi-center patient cohort, we applied unbiased t-Sne clustering to better define the heterogeneity of our study population using metabolite data (Fig. 5a, Supplementary Fig. 5a). Note that clinical features were not included in the clustering at this stage. Thus, by using metabolite data alone, we preliminarily identified three small yet distinct PD clinical subgroups with specific KP metabolic profiles which clustered separately from the majority of other PD patients and all controls. Further exploratory analyses were undertaken to better define their distinguishing features, and we were able to define these subgroups as: 1) the Dystonia Subgroup (n = 25); 2) the Rigid Subgroup (n = 20); and 3) the Vitamin B1 Subgroup (n = 10; Fig. 5b). These PD clinical subgroups did not show differences in years since diagnosis or age (Supplementary Fig. 5b, c), suggesting they represent separate pathophysiologic trajectories as opposed to distinct stages along the same trajectory. PD participants in the Dystonia Subgroup had high plasma 3-HK, CSF HKr, CSF 3-HK/KA and CSF QA/KA ratio in comparison to PD participants in the other subgroups. In contrast, the Rigid Subgroup had low plasma KYN. The Vitamin B1 Subgroup had higher levels of plasma thiamine and thiamine monophosphate (TMP; Supplementary Fig. 5d).
a Schematic depicting study participant subgrouping by KP metabolite data. b Kynurenine pathway metabolite and B-vitamin markers of the PD clinical subgroups. Increased KP metabolites marking the Dystonia Subgroup included plasma 3-HK, CSF HKr, CSF 3-HK/KA and CSF QA/KA. The Rigid Subgroup was marked by lower levels of plasma KYN. The Vitamin B1 Subgroup was marked by high plasma thiamine levels found to be in the range of vitamin supplementation. Group differences were assessed using one-way ANOVA with Tukey’s post hoc test for pairwise comparisons. ***P < 0.001 and ****P < 0.0001. c Clinical features associating with each of the PD clinical subgroups. The Dystonia Subgroup had higher MDS-UPDRS scores on Dystonia and Impact of Fluctuations compared to the other subgroups. In addition, the Dystonia Subgroup had higher scores for Anxiety, Depression, Insomnia, and Pain. Conversely, the Rigidity Subgroup had lower scores across these domains but did have higher Rigidity LUE and Body Bradykinesia. Group differences were assessed using one-way ANOVA with Tukey’s post hoc test for pairwise comparisons. *P < 0.05. Abbreviations: LUE: left upper extremity.
MDS-UPDRS clinical scores across subgroups were assessed to determine whether the identified PD clinical subgroups were related to specific clinical dysfunction (Fig. 5c). Indeed, the Dystonia Subgroup participants had more severe dystonia and were more likely to report a functional impact of motor fluctuations on their daily activities and social interactions compared to the other subgroups. In terms of non-motor symptoms, they also reported greater anxiety, depression, insomnia, and more painful OFF-state dystonia. In contrast, the Rigid Subgroup reported higher rigidity in the left upper extremities and higher body bradykinesia. The Rigid Subgroup also had a lower mean LEDD compared to the other PD subgroups (Supplementary Fig. 5e). In terms of symptoms, the Vitamin B1 Subgroup reported greater right-sided postural tremor in comparison to the other PD clinical subgroups.
Discussion
The present study revealed KP dysregulation in PD is linked to peripheral and CNS inflammation and low vitamin B6 status. The shift in KYN metabolism was characterized by lower neuroprotective KA and higher 3-HK and QA, which are both neuroexcitatory compounds. KP metabolic maps for plasma and CSF showed that lower KA and higher 3-HK were observed in both plasma and CSF pools in PD, as were higher CSF QA levels. BBB-permeable 3-HK may serve as a conduit for CNS-periphery crosstalk, whereby peripheral 3-HK traffics to increase CNS 3-HK pools and an increase neuroexcitatory QA. While future studies should aim to provide experimental evidence of the BBB transfer of 3-HK directly, we found that 3-HK concentrations in CSF and plasma were significantly correlated. Clinically, high CSF QA associated with more severe PD motor symptoms, neurodegeneration biomarker CSF tau, and biomarker of microglial activation CSF sTREM2. An exploratory analysis of PD patient subgroups suggests the possibility that specific alterations in KP metabolism may be linked to distinct clinical dysfunction.
Results of the current study suggest KP dysfunction might result from vitamin B6 deficiency shifting production away from the neuroprotective KA and AA and towards overproduction of neuroexcitatory 3-HK and QA. As a key cofactor, vitamin B6 is required for the enzymatic activities of KYNU and KAT65, which produce AA and KA, respectively. Consistent with previous work52, the current study demonstrates that the plasma marker of inflammation neopterin was linked to B6 deficiency, directly implicating peripheral inflammation in low vitamin B6 status. The proposed connection between B6 deficiency, KP dysmetabolism, and neuroinflammation may further explain epidemiological studies showing reduced dietary B6 intake is tied to increased PD risk66. Vitamin B6 catabolism (and consequently PAr) rises in inflammatory conditions51 and peripheral inflammation may both drive or potentiate PD-associated B6 deficiency. B6 deficiency might also be related to levodopa administration, as previous work has shown that individuals taking more than 2000 mg LEDD were nearly always found to have B6 deficiency67. In this case, B6 deficiency may be explained by consumption of PLP during levodopa metabolism and deactivation of PLP by carbidopa68,69,70,71. Thus, additional work is required to determine the source of B6 deficiency in PD and data on intake of B6 and the other vitamins would be useful.
The current study also suggest that KP biomarkers may be capable of distinguishing PD clinical subgroups with distinct pathophysiology and clinical profiles. While these findings should be validated in larger studies, interesting preliminary associations were observed. For example, the Dystonia Subgroup was biologically defined by high levels of neuroexcitatory and inflammatory CSF QA/KA. Clinically, this subgroup also showed greater involuntary muscle contractions (dystonia) and reported greater impact of their fluctuations. The Dystonia Subgroup also showed greater anxiety, depression and pain compared to the other subgroups. Interestingly, these symptoms may be consistent with the “Anxious fluctuators” clinical endophenotype previously identified using only clinical variables72. This clinical subgroup was uncovered in the current study using only metabolite data, potentially providing a biological substrate for this previously identified clinical endophenotype. The KP alterations specific to the Dystonia Subgroup were increased neuroexcitatory and neuroinflammatory CSF QA/KA. In contrast, the Rigid Subgroup showed higher rigidity and body bradykinesia. Together with akinesia, these are symptoms commonly defined as motor deficits relating to the akinetic/rigid (AR) type of PD. AR symptoms have been linked to gray matter decline and altered functional connectivity within frontal-parietal networks critical for motor planning and execution73, and as such, the AR motor subtype is associated with poorer prognosis and increased risk of dementia compared to the tremor subtype74,75. The Rigid Subgroup showed lower plasma KYN, which may indicate elevated KMO activity. This subgroup also showed lower LEDD, which is consistent with AR type of PD which is less responsive to L-DOPA and therefore, it is less prescribed. That differences in clinical symptoms correspond to differences in KP profiles, with increased CSF QA/KA and normal 3-HK/Kyn in the Dystonia Subgroup, and normal CSF QA/KA but lower KYN in the Rigid Subgroup, suggests the KP may be sensitive to specific PD endophenotypes.
Distinct KP alterations may provide insight into the clinical trajectory associated with each symptom dimension/profile and inform person-tailored therapeutic strategies. For example, PD patients with altered KP metabolism may benefit from strategies aiming to restore vitamin B6 levels and reducing peripheral inflammation. Indeed, KMO inhibition has been tested as therapeutic strategy for Alzheimer’s and PD, although these drugs are complicated by low BBB permeability. However, recent second generation KMO inhibitors with increased BBB permeability have shown promise in reducing the production of neuroexcitatory KP metabolites and in shifting the pathway towards neuroprotective KA76. An alternative strategy to address KP dysfunction might involve exercise, which can increase KAT expression by directing peripheral KYN metabolism toward KA production77. Combinational therapies incorporating anti-inflammatory agents, KMO inhibitors or KAT enhancers, in addition to lifestyle factors such as exercise, may have synergistic effects.
This study has several strengths, including its multicenter design, analysis of kynurenines and B-vitamins in matched CSF and plasma pools in the same sample, and application of unbiased machine learning approaches. Several opportunities to extend on our findings remain open. First, because of the cross-sectional design, longitudinal studies should be undertaken to examine the changes in KYN metabolism over the course of disease development. For example, it is possible that metabolites fluctuate within different clinical populations or across an individual person’s disease trajectory, and longitudinal study may reveal coupling between metabolites and clinical dysfunction. Second, in keeping with PD prevalence, our study had an overrepresentation of males in the PD cohort, which makes it difficult to assess potential sex differences. Importantly, previous studies have shown sex-specific differences in the KP78,79. Moreover, it would be important to assess associations between ethnic and racial variables with KP levels. Third, given that this is an observational study, it is difficult to determine whether the observed KP disruptions are a cause or consequence of PD pathophysiology. Interventional studies and preclinical experiments will be essential in determining causality. Along the same lines, influence of LEDD on KP metabolites and B-vitamin metabolism should be further investigated. Fourth, PD diagnosis was made based on patient-reported history and neurological exam using the United Kingdom Parkinson’s Disease Society Brain Bank diagnostic criteria, but still may not be fully accurate80. Synuclein seeding assays would be useful to confirm synucleinopathy across the cohort81. Similarly, future studies should further assess the role of individual systemic inflammatory states which may affect metabolite levels. Fifth, the PD clinical subgroup populations were of modest sample size and these findings should be expanded, ideally in a large cohort of de novo PD participants. Nevertheless, that these subgroups emerged in the current multicenter design lends support to the external validity of the subgroup findings. Finally, information on dietary intake of B-vitamins or use of vitamin supplements was not available for all participants. This information would be useful in future studies aimed at directly assessing relationships between vitamin status and KP activation in PD participants.
In conclusion, the current study identifies the KP as a site of peripheral-cerebral crosstalk, integrating inflammation and metabolic dysfunction. Furthermore, this study provides new evidence to suggest that the KP can reflect specific PD endophenotypes. Identification of PD clinical subgroups widens opportunities for developing biomarker candidates, novel avenues for investigating disease pathogenesis and new strategies for person-tailored therapeutic targeting.
Methods
Study participants and study design
Three-hundred and twenty-five participants were included in this multicenter study and were drawn from 8 research centers (Supplementary Fig. 1). This included participants from 1) the Stanford Movement Disorders Clinic (Stanford MDC); 2) the Stanford Alzheimer’s Disease Research Center (Stanford ADRC); 3) the Stanford Aging and Memory Study (SAMS); 4) the VA Puget Sound Health Care System/University of Washington (Seattle VA); 5) the VA Portland Medical Center (Portland VA); 6) the Oregon Health & Science University Layton Aging and Alzheimer’s Disease Research Center (OHSU ADRC); 7) the University of Pennsylvania Alzheimer’s Disease Center (Penn ADC); and 8) the University of Pennsylvania Frontal Temporal Dementia Center (Penn FTDC). Participants from the Stanford MDC and Stanford ADRC were included if they were cognitively normal healthy adults with or if they met United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria for PD. Healthy control participants were recruited from the family of PD participants and from the surrounding community. They had no history of PD, other neurodegenerative diseases, or chronic neuropsychiatric disorders. SAMS participants were clinically unimpaired older adults with Clinical Dementia Rating global score of zero82, had normal or corrected-to-normal vision, right-handed, and were native English speaking. A full neuropsychological battery assessing multiple domains was given to assign a cognitive diagnosis. Participants from Stanford were defined as cognitively impaired if scores were ≥ 1.5 standard deviations below age- and education-matched normative values on at least two separate neuropsychological measures, regardless of domain83. Seattle VA and Portland VA and OHSU ADRC assigned diagnoses at a clinical consensus conference. Control participants from Penn ADC and Penn FTDC underwent cognitive testing and determination of cognitive diagnosis by clinical consensus after the time of sampling. In this study, participants were not excluded on the basis of presence of systemic inflammatory disorders. Levodopa equivalent daily dose (LEDD) was determined as previously described84. Study protocols were approved by the Stanford University Institutional Review Board, the University of Pennsylvania Institutional Review Board, the Oregon Health and Science University Institutional Review Board, or VA Puget Sound Health Care System/University of Washington Institutional Review Board. In accordance with the Declaration of Helsinki, written informed consent was obtained from each study participant or their legally authorized representative.
MDS-UPDRS
The Movement Disorder’s Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) was used to evaluate various aspects of Parkinson’s disease including non-motor and motor experiences of daily living and motor complications59.
Plasma and CSF collection
For the Stanford MDC, Stanford ADRC and SAMS, fasting plasma was collected within 2 weeks of lumbar puncture85. For the remaining cohorts, CSF and plasma blood draws occurred on the same morning. No hemolysis or discoloration was apparent in any of the included CSF or plasma samples (visual inspection). CSF samples were restricted to two freeze-thaw cycles, as recommended by Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry86.
Liquid chromatography-tandem mass spectrometry
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed on CSF and plasma samples by Bevital AS (Bergen, Norway, www.bevital.no)) and measured kynurenine pathway metabolites and related vitamins including tryptophan, kynurenine, kynurenic acid, quinolinic acid, picolinic acid, 3-hydroxykynurenine, anthranilic acid, 3-hydroxanthranillic acid, nicotinamide, N1-methylnicotinamide, pyridoxal, pyridoxal 5’-phosphate, thiamine, riboflavin, flavin mononucleotide, thiamine monophosphate, and neopterin, as previously described87.
Measurement of AD CSF core biomarkers
CSF amyloid and tau were measured by the Stanford ADRC Biomarker Core using the fully automated Lumipulse G assays (Fujirebio Diagnostics, US, Malvern, PA) on the Lumipulse G1200 instrument as previously described12,88. Investigators were blind to clinical and demographic information of the sample.
Measurement of CSF sTREM2
CSF sTREM2 was measured using the MesoScale Discovery platform (Rockville, MD) pas previously described12. Briefly, MSD GOLD 96-Well Streptavidin plates were coated with biotinylated polyclonal goat anti-human TREM2 capture antibody (0.25 µg/ml; BAF1828, R&D Systems, Minneapolis, MN). CSF was diluted 1:4 in 1% BSA. Monoclonal mouse anti-human TREM2 detection antibody (1 µg/ml; B-3, sc373828, Santa Cruz Biotechnology, Santa Cruz, CA) was used followed by SULFO-TAG anti-mouse secondary antibody (1 µg/ml, R32AC-5, MSD). Plates were read using a MESO QuickPlex SQ 120.
Predictive modeling and subgroup analysis
A flexible machine learning method was applied to predict whether CSF and plasma levels of kynurenine pathway metabolites and related B-vitamins might be used to distinguish PD from control individuals. All participants having both plasma and CSF data were included in machine learning analysis, resulting in cohort of PD participants (n = 149) from control participants (n = 158). Metabolites and ratios included were thiamine, thiamine monophosphate, flavin mononucleotide, riboflavin, nicotinamide, N1 methylnicotinamide,pyridoxal 5’-phosphate, pyridoxal, tryptophan, kynurenine, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, quinolinic acid, picolinic acid, 3-HK/KA, HKr, QA/KA in CSF and thiamine, thiamine monophosphate, flavin mononucleotide, riboflavin, nicotinamide, N1-methylnicotinamide, pyridoxal 5’-phosphate, pyridoxal, 4-pyridoxic acid,tryptophan, kynurenine, kynurenic acid, anthranilic acid, 3-hydroxykynurenine, xanthurenic acid, 3-hydroxyanthranilic acid, quinolinic acid, picolinic acid, 3-HK/KA, HKr, QA/KA, Par, and 3-HK/Kyn in plasma. The outcome of interest was PD vs control. Specifically, a two-step procedure was used to train the prediction model. The first step involved building two prediction models using random forest with metabolic features from CSF and plasma as input and the second step combined the predictions from two models built in the first step to generate a final prediction. Prediction models from the first step were also kept for comparison purpose. A cross validation strategy was used to simultaneously build the model on a subset of metabolite data and then to test its performance on previously unseen participants as previously described in ref. 89. The model performance in cross-validation was measured via area under the ROC curve (AUC). Also tested was whether kynurenine pathway metabolite levels were robust predictors of sex, years since diagnosis, age or years of education. Correlation network analysis was used to evaluate the relationships between plasma and CSF metabolite features in a bottom-up approach. Separately, unsupervised dimension-reduction using t-distributed stochastic neighbor embedding (tSNE) algorithm90 was performed on all metabolites to project the individual participants into a two-dimensional plane for potential pattern detection. Based on tSNE projection, multiple PD participant clusters were identified.
Statistical analysis
Differences in demographic variables between PD and control participants were assessed using Student’s t test or Chi-square test. CSF and plasma biomarkers were log-transformed to approximate Gaussian distribution. To evaluate differences between PD and control diagnostic groups, data were analyzed using analysis of covariance (ANCOVA). As metabolites were often found to associate with participant age and sex (Supplementary Table 3), these were included as covariates in statistical models. Similarly, multiple linear regression analyses were used to assess associations between continuous biomarker variables also after adjusting for age and sex. ANOVA was used to assess differences in selected variables between identified PD participant clusters. For metabolite data, data points below first quartile minus 1.5*IQR, or above third quartile plus 1.5*IQR were identified as outliers and removed from analysis. Outliers were not excluded from the subgroup analysis. Statistical analyses were performed using R, version 3.6.1 (R Foundation for Statistical Computing) and GraphPad Prism, version 10 (GraphPad Software, Boston, MA) and figures were prepared using BioRender (Toronto, Ontario, Canada). All statistical tests were two-sided and significance was defined as P < 0.05.
Data availability
Anonymized data are available to qualified researchers upon request to the corresponding author or by contacting the individual cohort studies.
References
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013 (2017).
Lawton, M. et al. Parkinson’s Disease Subtypes in the Oxford Parkinson Disease Centre (OPDC) Discovery Cohort. J. Parkinsons Dis. 5, 269–279 (2015).
Chen-Plotkin, A. S. et al. Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 10, https://doi.org/10.1126/scitranslmed.aam6003 (2018).
Tan, E. K. et al. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat. Rev. Neurol. 16, 303–318 (2020).
Harms, A. S., Yang, Y. T. & Tansey, M. G. Central and peripheral innate and adaptive immunity in Parkinson’s disease. Sci. Transl. Med 15, eadk3225 (2023).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673 (2022).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291 (1988).
Yacoubian, T. A. et al. Brain and Systemic Inflammation in De Novo Parkinson’s Disease. Mov. Disord. 38, 743–754 (2023).
Kouli, A. et al. Neuroinflammation is linked to dementia risk in Parkinson’s disease. Brain. https://doi.org/10.1093/brain/awad322 (2023).
Bartl, M. et al. Blood Markers of Inflammation, Neurodegeneration, and Cardiovascular Risk in Early Parkinson’s Disease. Mov. Disord. 38, 68–81 (2023).
Wilson, E. N. et al. Soluble TREM2 is elevated in Parkinson’s disease subgroups with elevated CSF tau. Brain 143, 932–943 (2020).
Pedersen, C. C. et al. Inflammatory Biomarkers in Newly Diagnosed Patients With Parkinson Disease and Related Neurodegenerative Disorders. Neurol. Neuroimmunol. Neuroinflamm. 10, https://doi.org/10.1212/NXI.0000000000200132 (2023).
Shen, J. et al. Plasma MIA, CRP, and Albumin Predict Cognitive Decline in Parkinson’s Disease. Ann. Neurol. 92, 255–269 (2022).
Nishizuka, Y. & Hayaishi, O. Studies on the Biosynthesis of Nicotinamide Adenine Dinucleotide. I. Enzymic Synthesis of Niacin Ribonucleotides from 3-Hydroxyanthranilic Acid in Mammalian Tissues. J. Biol. Chem. 238, 3369–3377 (1963).
Minhas, P. S. et al. Macrophage de novo NAD(+) synthesis specifies immune function in aging and inflammation. Nat. Immunol. 20, 50–63 (2019).
Katsyuba, E. et al. De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 563, 354–359 (2018).
Yoshida, R., Imanishi, J., Oku, T., Kishida, T. & Hayaishi, O. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc. Natl Acad. Sci. USA 78, 129–132 (1981).
Takikawa, O., Tagawa, Y., Iwakura, Y., Yoshida, R. & Truscott, R. J. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv. Exp. Med. Biol. 467, 553–557 (1999).
Okuda, S., Nishiyama, N., Saito, H. & Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 70, 299–307 (1998).
Jhamandas, K. H., Boegman, R. J., Beninger, R. J., Miranda, A. F. & Lipic, K. A. Excitotoxicity of quinolinic acid: modulation by endogenous antagonists. Neurotox. Res 2, 139–155 (2000).
Ferreira, F. S., Schmitz, F., Marques, E. P., Siebert, C. & Wyse, A. T. S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res 38, 50–58 (2020).
Foster, A. C., Vezzani, A., French, E. D. & Schwarcz, R. Kynurenic acid blocks neurotoxicity and seizures induced in rats by the related brain metabolite quinolinic acid. Neurosci. Lett. 48, 273–278 (1984).
Lee, D. Y. et al. Kynurenic acid attenuates MPP(+)-induced dopaminergic neuronal cell death via a Bax-mediated mitochondrial pathway. Eur. J. Cell Biol. 87, 389–397 (2008).
Solvang, S. H. et al. The kynurenine pathway and cognitive performance in community-dwelling older adults. The Hordaland Health Study. Brain. Behav. Immun. 75, 155–162 (2019).
Lim, C. K. et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 7, 41473 (2017).
Sorgdrager, F. J. H. et al. Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J. Neurochem. 151, 656–668 (2019).
Jacobs, K. R. et al. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid-beta and tau. Neurobiol. Aging 80, 11–20 (2019).
Giil, L. M. et al. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J. Alzheimers Dis. 60, 495–504 (2017).
Kindler, J. et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry https://doi.org/10.1038/s41380-019-0401-9 (2019).
Sha, Q. et al. Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl. Psychiatry 12, 35 (2022).
Midttun, O. et al. A cross-sectional study of inflammatory markers as determinants of circulating kynurenines in the Lung Cancer Cohort Consortium. Sci. Rep. 13, 1011 (2023).
Yan, J. et al. CSF neopterin, quinolinic acid and kynurenine/tryptophan ratio are biomarkers of active neuroinflammation. EBioMedicine 91, 104589 (2023).
Ogawa, T. et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 42, 1702–1706 (1992).
Heilman, P. L. et al. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 35, 2028–2037 (2020).
Lewitt, P. A. et al. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov. Disord. 28, 1653–1660 (2013).
Havelund, J. F. et al. Changes in kynurenine pathway metabolism in Parkinson patients with L-DOPA-induced dyskinesia. J. Neurochem. 142, 756–766 (2017).
Klatt, S. et al. A six-metabolite panel as potential blood-based biomarkers for Parkinson’s disease. NPJ Parkinsons Dis. 7, 94 (2021).
Widner, B., Leblhuber, F. & Fuchs, D. Increased neopterin production and tryptophan degradation in advanced Parkinson’s disease. J. Neural Transm. (Vienna) 109, 181–189 (2002).
Tohgi, H., Abe, T., Takahashi, S., Takahashi, J. & Hamato, H. Concentrations of serotonin and its related substances in the cerebrospinal fluid of parkinsonian patients and their relations to the severity of symptoms. Neurosci. Lett. 150, 71–74 (1993).
Iwaoka, K. et al. Impaired metabolism of kynurenine and its metabolites in CSF of parkinson’s disease. Neurosci. Lett. 714, 134576 (2020).
Chang, K. H. et al. Alternations of Metabolic Profile and Kynurenine Metabolism in the Plasma of Parkinson’s Disease. Mol. Neurobiol. 55, 6319–6328 (2018).
Lim, C. K. et al. Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 155, 76–95 (2017).
Collaborators, G. B. D. P. s. D. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Fukui, S., Schwarcz, R., Rapoport, S. I., Takada, Y. & Smith, Q. R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 56, 2007–2017 (1991).
Kita, T., Morrison, P. F., Heyes, M. P. & Markey, S. P. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. J. Neurochem. 82, 258–268 (2002).
Haruwaka, K. et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 10, 5816 (2019).
Bryniarski, K., Biedron, R., Jakubowski, A., Chlopicki, S. & Marcinkiewicz, J. Anti-inflammatory effect of 1-methylnicotinamide in contact hypersensitivity to oxazolone in mice; involvement of prostacyclin. Eur. J. Pharmacol. 578, 332–338 (2008).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H. Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Ulvik, A. et al. Tryptophan catabolites as metabolic markers of vitamin B-6 status evaluated in cohorts of healthy adults and cardiovascular patients. Am. J. Clin. Nutr. 111, 178–186 (2020).
Ulvik, A. et al. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 100, 250–255 (2014).
Ueland, P. M., McCann, A., Midttun, O. & Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 53, 10–27 (2017).
Zuo, H. et al. Markers of vitamin B6 status and metabolism as predictors of incident cancer: the Hordaland Health Study. Int. J. Cancer 136, 2932–2939 (2015).
Gylling, B. et al. Vitamin B-6 and colorectal cancer risk: a prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am. J. Clin. Nutr. 105, 897–904 (2017).
Ulvik, A. et al. Vitamin B-6 catabolism and long-term mortality risk in patients with coronary artery disease. Am. J. Clin. Nutr. 103, 1417–1425 (2016).
Zuo, H. et al. The PAr index, an indicator reflecting altered vitamin B-6 homeostasis, is associated with long-term risk of stroke in the general population: the Hordaland Health Study (HUSK). Am. J. Clin. Nutr. 107, 105–112 (2018).
Ryan, K. M., Allers, K. A., Harkin, A. & McLoughlin, D. M. Blood plasma B vitamins in depression and the therapeutic response to electroconvulsive therapy. Brain Behav. Immun. Health 4, 100063 (2020).
Murr, C., Widner, B., Wirleitner, B. & Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 3, 175–187 (2002).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Tsuang, D. et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 70, 223–228 (2013).
Dickson, D. W. et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 91, e1182–e1195 (2018).
Robinson, J. L. et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141, 2181–2193 (2018).
Plastini, M. J. et al. Multiple biomarkers improve diagnostic accuracy across Lewy body and Alzheimer’s disease spectra. Ann. Clin. Transl. Neurol. 11, 1197–1210 (2024).
Abdelnour, C. et al. Plasma pTau181 Reveals a Pathological Signature that Predicts Cognitive Outcomes in Lewy Body Disease. Ann. Neurol. 96, 526–538 (2024).
Ogasawara, N., Hagino, Y. & Kotake, Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J. Biochem 52, 162–166 (1962).
Murakami, K. et al. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson’s disease: a case-control study in Japan. Br. J. Nutr. 104, 757–764 (2010).
Loens, S., Chorbadzhieva, E., Kleimann, A., Dressler, D. & Schrader, C. Effects of levodopa/carbidopa intestinal gel versus oral levodopa/carbidopa on B vitamin levels and neuropathy. Brain Behav. 7, e00698 (2017).
Bender, D. A. Effects of benserazide, carbidopa and isoniazid administration on tryptophan-nicotinamide nucleotide metabolism in the rat. Biochem. Pharmacol. 29, 2099–2104 (1980).
Bender, D. A. Inhibition in vitro of the enzymes of the oxidative pathway of tryptophan metabolism and of nicotinamide nucleotide synthesis by benserazide, carbidopa and isoniazid. Biochem. Pharmacol. 29, 707–712 (1980).
Bender, D. A., Earl, C. J. & Lees, A. J. Niacin depletion in Parkinsonian patients treated with L-dopa, benserazide and carbidopa. Clin. Sci. (Lond.) 56, 89–93 (1979).
Cellini, B., Montioli, R., Oppici, E. & Voltattorni, C. B. Biochemical and computational approaches to improve the clinical treatment of dopa decarboxylase-related diseases: an overview. Open Biochem J. 6, 131–138 (2012).
Pontone, G. M. et al. Anxious fluctuators’ a subgroup of Parkinson’s disease with high anxiety and problematic on-off fluctuations. Parkinsonism Relat. Disord. 105, 62–68 (2022).
Kann, S. J., Chang, C., Manza, P. & Leung, H. C. Akinetic rigid symptoms are associated with decline in a cortical motor network in Parkinson’s disease. NPJ Parkinsons Dis. 6, 19 (2020).
Jellinger, K. A., Seppi, K., Wenning, G. K. & Poewe, W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J. Neural Transm. (Vienna) 109, 329–339 (2002).
Paulus, W. & Jellinger, K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 50, 743–755 (1991).
Zhang, S., Collier, M. E. W., Heyes, D. J., Giorgini, F. & Scrutton, N. S. Advantages of brain penetrating inhibitors of kynurenine-3-monooxygenase for treatment of neurodegenerative diseases. Arch. Biochem. Biophys. 697, 108702 (2021).
Agudelo, L. Z. et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014).
Brown, S. J. et al. Sex- and suicide-specific alterations in the kynurenine pathway in the anterior cingulate cortex in major depression. Neuropsychopharmacology. https://doi.org/10.1038/s41386-023-01736-8 (2023).
Meier, T. B. et al. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain. Behav. Immun. 67, 59–64 (2018).
Litvan, I. et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov. Disord. 18, 467–486 (2003).
Arnold, M. R. et al. alpha-Synuclein Seed Amplification in CSF and Brain from Patients with Different Brain Distributions of Pathological alpha-Synuclein in the Context of Co-Pathology and Non-LBD Diagnoses. Ann. Neurol. 92, 650–662 (2022).
Trelle, A. N. et al. Association of CSF Biomarkers With Hippocampal-Dependent Memory in Preclinical Alzheimer Disease. Neurology 96, e1470–e1481 (2021).
Hendershott, T. R. et al. Comparative sensitivity of the MoCA and Mattis Dementia Rating Scale-2 in Parkinson’s disease. Mov. Disord. 34, 285–291 (2019).
Tomlinson, C. L. et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653 (2010).
Trelle, A. N. et al. Plasma Abeta(42)/Abeta(40) is sensitive to early cerebral amyloid accumulation and predicts risk of cognitive decline across the Alzheimer’s disease spectrum. Alzheimers Dement. https://doi.org/10.1002/alz.14442 (2024).
Lewczuk, P. et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: An update of the Consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 19, 244–328 (2018).
Midttun, O., Hustad, S. & Ueland, P. M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1371–1379 (2009).
Wilson, E. N. et al. Performance of a fully-automated Lumipulse plasma phospho-tau181 assay for Alzheimer’s disease. Alzheimer’s Res. Ther. 14, 1–12 (2022).
Jehan, F. et al. Multiomics Characterization of Preterm Birth in Low- and Middle-Income Countries. JAMA Netw. Open 3, e2029655 (2020).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Acknowledgements
We thank contributors who collected samples used in this study, as well as research participants and their families, whose help and participation made this work possible. Funding: This work was supported by NIH/NIA grants RF1AG058047, R01AG048232 to KIA, NS115114, K23 NS075097 and Michael J. Fox Foundation grant 6440.0 to K.L.P., and NIH grant R35GM138353 and the American Heart Association grant (19PABHI34580007) to NA. N.I.H. R01AG048076 (A.D.W.) and R01AG074339 (E.C.M.). NIH/NIA P30 AG072979 (A.C.P. and D.A.W.), NIH/NINDS RO1NS115139 (A.C.P.). A.C.P. is also supported by the Parker Family Chair. NIH grant P30 AG066518 to J.F.Q. and N.E.G.; C.S.F. and plasma samples were obtained from the Stanford ADRC (P30 AG066515), the University of Pennsylvania ADRC (P30 AG072979), the Pacific Udall Center (NINDS) P50 NS062684, and the National Centralized Repository for Alzheimer Disease and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the National Institute on Aging (NIA). This material is the result of work supported with resources and the use of facilities at the VA Puget Sound and VA Portland Health Care Systems. The authors would also like to acknowledge the generous support of the Jean Perkins Foundation and the Scully Research Initiative. KIA is a Chan Zuckerberg Biohub-San Francisco Investigator. E.N.W., T.W.C., K.L.P., and K.I.A. are supported by the Phil & Penny Knight Initiative for Brain Resilience.
Author information
Authors and Affiliations
Contributions
E.N.W. and J.U. performed experimental design, data collection, statistical analysis, and manuscript writing. M.S.S. performed data collection and statistical analysis. P.S.M. performed experimental design and data collection. Ø.M., A.U. and P.M.U. led mass spectrometry experiments. J.H.M., M.S.-B., P.L., S.D.M., Q.W., D.C., N.K.C. performed data collection. L.T. contributed to statistical analysis. C.A.F., G.A.K., E.D.P., B.C., T.J.M., C.P.Z., N.E.G., J.F.Q., S.J.S., V.W.H., T.W-C., F.M.L., D.A.W., A.C-P., A.D.W., E.C.M., K.L.P., and K.I.A. provided samples and clinical data. N.A. led the machine learning analysis. K.L.P. and K.I.A. contributed to experimental design and manuscript writing. All authors revised and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilson, E.N., Umans, J., Swarovski, M.S. et al. Parkinson’s disease is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction. npj Parkinsons Dis. 11, 96 (2025). https://doi.org/10.1038/s41531-025-00964-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-00964-7