Abstract
While conventional deep brain stimulation (cDBS) treatment delivers continuous electrical stimuli, new adaptive DBS (aDBS) technology provides dynamic symptom-related stimulation. Research data are promising, and devices are already available, but are we ready for it? We asked leading DBS experts worldwide (n = 21) to discuss a research agenda for aDBS research in the near future to allow full adoption. A 5-point Likert scale questionnaire, along with a Delphi method, was employed. In the next 10 years, aDBS will be clinical routine, but research is needed to define which patients would benefit more from the treatment; second, implantation and programming procedures should be simplified to allow actual generalized adoption; third, new adaptive algorithms, and the integration of aDBS paradigm with new technologies, will improve control of more complex symptoms. Since the next years will be crucial for aDBS implementation, the research should focus on improving precision and making programming procedures more accessible.
Similar content being viewed by others
Introduction
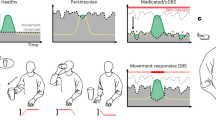
Deep Brain Stimulation (DBS) is a standard neurosurgical therapy to treat selected patients with neurological disorders, including essential tremor (ET), Parkinson’s disease (PD), and dystonia1. Traditionally, DBS has been employed using open-loop stimulation techniques, i.e., delivering continuous, uninterrupted stimulation at the same parameter setting (conventional DBS, cDBS) that is independent of the real-time patient’s functional status or of the side effects induced by intermittent stimulation2. Despite the evident positive results, DBS of the subthalamic nucleus (STN-DBS3) in PD has been prominently associated with stimulation-induced speech impairments4,5, risk of falling6,7, dyskinesia8, stimulation-induced impulsivity9, and, more importantly, only partial control of clinical fluctuations10. Adaptive DBS (aDBS) was conceived to overcome some of the disadvantages of cDBS by facilitating optimized current delivery to improve symptoms and drive improved outcomes11. This technology relies on the principle of on-demand or contingency-based stimulation, where clinically relevant biofeedback signals (e.g., brain signals) can be used to determine and deliver a real-time, more effective stimulation parameters in order to address emerging symptoms or side effects12. Although the aDBS concept is perceived as a natural evolution of current cDBS, in line with the historical development of cardiac pacemakers, the evidence collected on its clinical application needs to be expanded, especially to better understand the emerging limitations, and to boost its adoption and understanding in everyday clinical practice.
The challenges aDBS field is facing might be divided into technical (i.e., the technological open questions) and clinical (i.e., applications to patients). Among the technical challenges, the reliability of the biomarker(s) used to control stimulation (i.e., how precisely the biomarker that drives the stimuli correlates with the patient’s clinical status) is of crucial importance to allow optimal adaptation. Local field potentials (LFPs) recorded directly from the DBS electrodes, while being the most used biomarkers in movement disorders, still have limitations13,14,15, especially with patients presenting different phenotypes (e.g., tremor dominant or akinetic-rigid PD)16. Also, aDBS needs to be integrated with current (e.g., segmented electrodes17) or new (e.g., artificial intelligence, AI18) technologies for a large-scale application. Although preliminary results suggest the successful combinations of technologies19, solid knowledge is still lacking. Similarly, the introduction of aDBS raises questions about the specific expertise required to program the devices, the costs (in terms of economic and time burden) of implantation, and the management of the programming phase with stimulation algorithms that might change according to symptoms and clinical outcomes20,21,22.
Among the clinical challenges, aDBS needs to further prove its safety and tolerability for the patients. Although growing evidence suggests that aDBS can be used safely, with comparable effects to cDBS23,24, the aDBS community has not defined the characteristics of patients who might benefit the most from the adaptive stimulation, yet. First, it is likely that, apart from PD, also ET and epilepsy patients25, likewise psychiatric patients25, could benefit from an adaptive approach. Second, despite PD being the first and most studied use case of aDBS, it is not clear which clinical manifestation (e.g., akinetic-rigid or hyperkinetic PD) or symptoms (e.g., non-motor, stimulation side effects) is more sensible to aDBS, as well as the exact mechanism of interaction between adaptive stimulation and pharmacological therapy25.
In such a challenging scenario, it is difficult to understand whether this innovation will be able to reach all patients, and when. To answer this question, we identified internationally recognized clinical and academic DBS experts to discuss the methodological and clinical challenges of aDBS, and we asked them to participate in a Delphi method-based study26.
Results
Specialists panel
For the Steering Committee (SC), all eight invited authors agreed to participate (SC = 8, response rate: 100%). For the Expert Panel (EP), out of the 20 authors identified, two declined to participate and five did not reply (EP = 13, response rate: 65%). Therefore, the overall number of panelists was 21 (overall response rate: 75%, Supplementary Table 1). Demographic characteristics of the panelists are displayed in Table 1. Briefly, most of them were male (16, 76%), >50 years old (14, 66.6%) and high-experienced in clinical routine (20, 95.5% with >10 years of clinical experience) and research (19, 90.4% and 18, 85.7% with >10 years of experience in, respectively, the DBS field and DBS clinical trials) settings.
Delphi Panel results: technical aspects
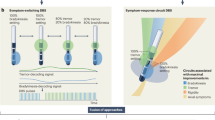
As for the 21 statements on the technical aspects of aDBS, the first round led to no consensus for any of the statements (Supplementary Fig. 1); in the second, the consensus was reached in only one statement (Supplementary Fig. 2); finally, in the third round, consensus was reached in other seven statements, for a total of eight out of 21 statements (see Fig.1). More specifically, in the second round, the panelists agreed that automatic programming would be safe as long as stimulation intensity is constrained by upper and lower limits (90% agreed, median ± IQR: 4 ± 0). After the third round, panelists agreed that aDBS has technological limitations (Statement 1—80% agreed, median ± IQR: 4 ± 0), but that current pacemaker technology might be suitable to implement aDBS algorithms (Statement 20—90% agreed, median ± IQR: 4 ± 0). They strongly agreed that it requires high levels of expertise (Statement 8—80% strongly agreed, median ± IQR: 5 ± 0), but strongly disagreed in its feasibility for patients with not well-positioned electrodes (Statement 3—85% strongly disagreed, median ± IQR: 1 ± 0). Lastly, panelists were undecided on the role of aDBS in spreading segmented electrodes use (Statement 18—85% undecided, median ± IQR: 3 ± 0), or whether fast adaptation methods are superior or inferior than slow adaptation methods (Statement 14 and Statement 15—90% undecided, median ± IQR: 3 ± 0 for both). The secondary analysis performed on the third round of answers revealed an agreement on further statements (Supplementary Fig. 3). Specifically, experts agreed that aDBS is feasible only in centers with neurophysiological expertise (Statement 9—90% agreed), that programming is time-consuming (Statement 11—85% agreed) but automatic programming will allow to save time (Statement 12—90% agreed). Also, they agreed that current pacemaker technology allows aDBS installation (Statement 20—95% agreed), and that AI will spread its use (Statement 19—90% agreed). Lastly, common agreement was reached on the use of signal recording from DBS electrodes as a biomarker for electrical stimuli delivery (Statement 17—80% agreed).
A consensus was reached for Statement 1 (80% of the responses fell in the response label “Agree”), Statement 3 (85% of the responses fell in the response label “Strongly Disagree”), Statement 8 (80% of the responses fell in the response label “Strongly Agree”), Statement 14 (90% of the responses fell in the response label “Undecided”), Statement 15 (90% of the responses fell in the response label “Undecided”), Statement 18 (85% of the responses fell in the response label “Undecided”), and Statement 20 (90% of the responses fell in the response label “Agree”). DBS Deep Brain Stimulation, S statement.
Delphi Panel results: clinical aspects
As for the 21 statements on the clinical aspects of aDBS, no consensus was reached after the first round (Supplementary Fig. 4). After the second, the panelists agreed on one statement (Supplementary Fig. 5), and other eight after the third round, for a total of 9 out of 21 statements (see Fig.2). In particular, in the second round the panelists agreed on the use of aDBS technology also for tremor-dominant PD patients (Statement 28—80% agreed, median ± IQR: 4 ± 0). After the third round, an agreement was reached on the safety of aDBS technology (Statement 25—85% agreed, median ± IQR: 4 ± 0) and that it will enter clinical routine in 10 years (Statement 22—85% agreed, median ± IQR: 4 ± 0), with positive long-term impact for patients (Statement 35—80% agreed, median ± IQR: 4 ± 0), also for those with significant motor fluctuations before surgery (Statement 30— 90% agreed, median ± IQR: 4 ± 0) and on cDBS treatment (Statement 31—95% agreed, median ± IQR: 4 ± 0), and for patients with significant dyskinesias on cDBS treatment (Statement 32—90% agreed, median ± IQR: 4 ± 0). Lastly, panelists agreed that aDBS might lead to a faster stable treatment response after the definition of stimulation settings (Statement 37—80% agreed, median ± IQR: 4 ± 0), but were uncertain if fast adaptation technology could lead to long-term plastic changes (Statement 38—80% undecided, median ± IQR: 3 ± 0). The secondary analysis performed on the third round of answers revealed an agreement on four further statements (Supplementary Fig. 6). Experts disagreed that aDBS is applicable only for PD non-tremor patients (Statement 27—90% disagreed) and that primary clinical indication will be ET patients, more than PD patients (Statement 29—85% disagreed). Conversely, they agreed that aDBS will reduce DBS-induced side effects (Statement 34—95% agreed) and will easily adapt to pharmacological changes (Statement 36—80% agreed).
A consensus was reached for Statement 22 (85% of the responses fell in the response label “Agree”), Statement 25 (85% of the responses fell in the response label “Agree”), Statement 30 (90% of the responses fell in the response label “Agree”), Statement 31 (95% of the responses fell in the response label “Agree”), Statement 32 (90% of the responses fell in the response label “Agree”), Statement 35 (80% of the responses fell in the response label “Agree”), Statement 37 (80% of the responses fell in the response label “Agree”), and Statement 38 (80% of the responses fell in the response label “Undecided”). DBS Deep Brain Stimulation, S statement.
Discussion
In this Delphi consensus study, 21 internationally recognized clinical and scientific DBS experts were asked to discuss current technical and clinical challenges related to aDBS research and technical development in the next few years. The integration of the knowledge derived from clinical data and from the experience of leading experts provided (1) a clear scenario for aDBS advantages and limitations at the current state-of-the-art, (2) guidance on the near-future design of trials, and (3) highlights regarding the most promising directions for aDBS. Interestingly, out of the 42 open questions on aDBS proposed, a consensus was reached for 17, thus underlining the complexity and heterogeneity of the scenario and experiences: experts agreed on a time frame of 10 years for aDBS to reach clinical practice, whereas the time frame of 5 years did not achieve the agreement. Such a time frame should therefore be used to focus research towards the clinical and technological gaps between experimental and clinical aDBS treatment.
-
(I)
First, while the experience and knowledge gained so far sufficed to reach a consensus regarding the safety of the adaptive approach and its potential benefits, personalization (i.e., define which population of patients and/or which clinical phenotype of pathology would benefit more from aDBS) rose as a line of research to be explored. Experts, in fact, agreed that aDBS may lead to faster and more stable treatment responses than cDBS in selected patient populations, including tremor-dominant PD patients and those with motor fluctuations and dyskinesia on cDBS.
-
(II)
Second, an important point highlighted with general agreement, and possibly limiting the future widespread adoption, was the need for a high level of expertise to manage aDBS. Automatic programming based on data can be considered as a valuable and safe support for this complex task, if properly developed.
-
(III)
Third, the expert community remained uncertain regarding specific algorithms and their mechanisms of action, thus suggesting that, in the near future, research and trials need to be directed towards the collection of data relevant both for understanding the neurophysiology of the adaptive approach and for identifying better biomarkers and the related stimulation patterns. A last line of research should consider the integration of aDBS paradigm with sensing technologies. For example, the possible combined benefits of aDBS and segmented electrodes remain unclear, while there is general agreement on the fact that aDBS would not help in patients with electrodes that are not well-positioned.
The panelists believe that, despite the technological limitations of aDBS methodology, current hardware technology is suitable to support aDBS optimization, thanks to the recent development of pulse generators, which are also able to record LFPs27. One of the main limitations of aDBS application in routine clinical care remains the uncertainty about which and how many signals could entirely represent patients’ clinical state and whether many of them need to be used together in multimodal algorithms11,28. Most biomarkers have been identified with patients in “off stimulation”29, but, in the aDBS concepts, signals should be recorded in “on stimulation”. Therefore, the availability of devices able to record during stimulation is crucial to shed light on how to select the optimal personalized biomarker28. While the most used closed-loop design (i.e., STN-LFP beta band as control signal to adjust for DBS amplitude) has been questioned30, there is growing consensus that the beta band is a fairly reliable biomarker31. Several alternative approaches have been proposed (e.g., using cortical-subcortical gamma rhythm32), but no conclusive findings have been obtained yet. The panelist acknowledged that a high level of expertise would be required to use aDBS. Also, the secondary analysis added that aDBS would be feasible only in centers with neurophysiological expertise, and that aDBS programming is currently time-consuming. Indeed, currently, the programming phase of aDBS devices might require familiarity and high technical skills (when compared with cDBS devices28); however, the future algorithms will likely become more automated. A suggestion to industries would be to develop simplified workflows or to provide adequate education to clinicians using aDBS. Automatic programming technologies under investigation might reduce this time burden, provided that clinicians maintain a crucial role in assessing LFP recordings and their relationship to patients’ symptoms. Experts agreed that automatic programming would be safe if stimulation intensity were constrained by combined upper and lower limits. The answer is in line with the need to both avoid unpleasant side effects (upper limit) and inadequate treatment of patients’ symptoms (lower limit). However, many algorithms tested in clinical studies to date allow reduction of stimulation amplitude to zero when beta amplitude falls below a threshold, and this should be modified in future aDBS algorithms21,31,33,34.
From a control algorithm point of view, the experts were uncertain about whether fast adaptation methods would be superior or inferior when compared to slow adaptation methods. In fast adaptation algorithms, beta activity immediately triggers a brief increase in stimulation (threshold-based) to shorten prolonged beta bursts31,35, while in slow algorithms, beta activity is smoothed over many seconds to serve as a medication state biomarker and then be used as feedback to drive stimulation21. As for current research, both fast and slow adaptation algorithms reduced the total electrical energy delivered (TEED) over time, but while the first seemed to reduce adverse effects on speech20 and to achieve a better control of bradykinesia and rigidity21, the latter seemed to be more effective in reducing dyskinesias22. These effects should be interpreted with great caution because of the paucity of cases and lack of independent validation, especially in chronic applications with implanted devices36. Indeed, speech was not systematically assessed for the “slow adaptation”, nor dyskinesias for the “fast adapting” algorithms. However, fast beta aDBS did also show the ability to adjust how often aDBS was triggered according to (slower) medication state, with stimulation becoming less frequent in the medication ON state. This suggests that “fast” aDBS algorithms can operate on both fast and slow timescales, and therefore could theoretically help medication-induced dyskinesias37. Currently, the lack of data does not allow us to conclude differential benefits of both algorithms on side effects. Also, aDBS can possibly allow more TEED to be delivered, but only for small time frames, with improved clinical efficacy and without inducing side effects32,38; therefore, reduced TEED seems to be a less critical outcome for DBS implementation, particularly with the advent of rechargeable devices39. Panelists reached a consensus on the unfeasibility of aDBS for patients with suboptimally positioned, meaning that it will likely not be effective. This expert opinion was in line with the evidence that the peak in beta activity is a specific feature of the motor part of the STN40, and, therefore, suboptimally positioned electrodes will not likely detect the LFPs needed to “adapt” aDBS to patients’ symptoms.
Similarly, the panelists were doubtful about the role of aDBS in facilitating the use of segmented electrodes, which may be used to widen the therapeutic window between efficacy and adverse effects by steering the field of stimulation41. The experts did concede that segmented electrodes share with aDBS the common aim to “personalize” and shape stimulation electrical fields for individual patients. Indeed, this technology increases spatial specificity while aDBS improves temporal specificity through the delivery of a dynamic stimulation that changes over time according to disease-related feedback41. Theoretically, these two approaches could be complementary. Differently, experts expressed an optimistic opinion about the use of AI to accelerate aDBS development. Indeed, in recent years, AI and machine learning (ML) have been applied to neurological treatments, in a way that can be categorized into three key areas: (I) Predicting treatment outcomes: In PD, various ML techniques were used to distinguish between the DBS-on and DBS-off states to predict motor performance42, classify the ON / OFF levodopa states43, predict real-time state or classify behavioral tasks44,45,46,47, improving therapy personalization and diagnostic precision. Similarly, ML-based analyses of neurophysiological biomarkers have enabled accurate tic detection in Tourette syndrome48; (II) Determining treatment parameters: In PD, personalized biomarkers have been applied to classify the ON/OFF levodopa states, enabling precise adjustment of parameters43. Similarly, in ET, linear classifiers49 and linear regression models50 in implanted aDBS systems have shown to be feasible, no energy-consuming and effective in suppressing tremor49 or reducing stimulation times with improved therapeutic efficacy50; (III) Dynamically optimizing treatment over time: In PD, aDBS systems have utilized non-linear dynamical features51 and hierarchical and multiple kernel learning approaches52 to initiate or stop stimulation based on the onset of tremors enabling real-time adjustments of DBS parameters. State estimates, in conjunction with fuzzy control mechanisms, have been employed to dynamically adjust stimulation frequencies, thereby enhancing therapeutic effects53. For ET, aDBS devices have adeptly modulated stimulation voltages in real time according to patient feedback, resulting in effective tremor suppression while reducing superfluous stimulation54. All these experiences will ground the future integration of AI/ML to aDBS.
The panelists shared an optimistic opinion in terms of the development and applications of aDBS in clinical routine, and its potential ability to allow a faster and more stable treatment response in select patients. Indeed, despite the initial skepticism of parts of the medical community, the knowledge and technology in the field of aDBS have been constantly growing55. Also, recent technological advancements (e.g., directional leads56 or multiple stimulation methods57,58) may limit side effects and may serve to optimize the response to individual symptoms11. Another important point related to aDBS adoption is its safety, on which the panelists agreed. In addition to the surgical risks that to date are comparable to those of cDBS59, concerns have been expressed in the literature about the potential side effects of aDBS stimulation60. Although no significant side effects have been reported so far25, rapid changes of amplitude or frequency induced by neurosignals could be unpleasant or even intolerable to patients in chronic stimulation. Therefore, algorithms limiting these rapid changes (like the “linear adaptive” algorithm61) or others that balance ramp rates to avoid side effects62 are preferable. One of the major potential advantages of aDBS is its ability to provide personalized therapy. The panelists agreed that aDBS is suitable both for PD patients experiencing motor fluctuations and dyskinesias before surgery or on cDBS, and for tremor-dominant PD patients. This consensus boosts the need for gaining more insights on the “precision medicine” potential of aDBS, i.e., investigating which patients are likely responders to stimulation, or which technology (e.g., which biomarker) is right for a specific patient63. More experimental studies are needed, in which the efficacy of aDBS can be actually tested through different outcomes on different, larger populations, using different biomarkers. For example, Beta frequency correlates more with rigidity/bradykinesia than with resting tremor64,65, while gamma activity, particularly finely-tuned gamma, has been associated with ON medication states and dyskinesia66,67. Beta-driven aDBS follows the dynamic of the levodopa-ON/OFF medication states29 and hence reduces the likelihood of inducing levodopa-induced dyskinesia. Indeed, studies on aDBS in patients with PD and dyskinesia report good efficacy in reducing such symptoms while guaranteeing a similar or even better control of cardinal symptoms of PD21,33,34. Tremor can be detected from brain signals, either by the presence of lower frequency oscillations (3–7 Hz) or more accurately by combining multiple features from the whole LFP spectrum68,69. Additionally, several computational models have been recently developed to test the feasibility and efficacy of aDBS methods that modulate stimulation to control different biomarkers70,71. In these cases, the best control may be provided by selecting between multiple controllers depending on context or patient symptoms (i.e., tremor or beta oscillations). Recent studies suggest a similar efficacy of aDBS both for tremor and bradykinesia dominant patients72,73. Additionally, peripheral sensors may also be used for aDBS for tremor74,75. Major uncertainties remain on the mechanisms of action of aDBS: the experts were uncertain that fast adaptation technology could lead to long-term plastic changes. Although one might expect an effect close to what has been supposed for cDBS76, whether aDBS might induce neuroplastic changes remains an open question due to the lack of evidence to support any opinion77,78. Similarly, it is still to be determined what impact aDBS will have on the habituation phenomenon (i.e., the progressive loss of DBS benefit in time due to a decreased biological response of the neuronal networks79) that may, in select cases, decrease the effectiveness of cDBS in chronic conditions79. However, some experts believe that habituation of DBS in the setting of PD is rare and that most of the worsening of symptoms is driven by PD progression.
In summary, the panel of experts participating in this study expressed measured optimism on the advancement and implementation of aDBS in clinical practice. However, based on some concepts highlighted by items reaching consensus during the process and on others that emerged from the items that did not reach consensus, it is possible to identify some areas of research that will need to be prioritized soon for aDBS to become a reality in the next 10 years. From a technical point of view:
-
1.
Integration with technologies: future research should focus on optimizing existing technologies. A key area of investigation will be the improvement of the device in terms of reliable sensing technologies, refining current methods, and exploring new approaches to enhance the overall performance of aDBS systems. Also, given the promises of integrating AI and ML technologies in the biomarker discovery pipeline, research should focus on developing robust AI/ML models aimed to provide a solid foundation for the aDBS paradigm. This also underscores the need for ongoing collaboration between researchers and AI/ML companies.
-
2.
Device management and costs: future research should focus on improving the aDBS device management and addressing its associated costs. Studies should develop automated programming algorithms assisting the clinicians, simplified workflows, and comprehensive training programs. Also, unlike DBS80, health economics studies on aDBS cost-effectiveness are not available because of the lack of long-term data on large populations of patients. On the one hand, aDBS is expected to decrease health costs per capita for patients because it should improve patients’ condition and autonomy; on the other hand, it could increase costs related to the production of technology, time consumed by physicians to be trained, and to review the patient’s state.
From a clinical point of view:
-
3.
More solid clinical research: large-scale, multicentric RCTs to evaluate the widespread applicability of aDBS are necessary before moving to routine clinical practice. These studies should assess the long-term safety and efficacy of aDBS across different populations, using different feedback biomarkers to monitor various outcomes. For example, it is to be determined whether aDBS could improve non-motor symptoms in PD. Clinical studies should explore the interaction between aDBS and medication, investigating whether aDBS can adjust stimulation automatically if patients miss or take incorrect doses, or the potential risk due to incorrect stimulation (over- or under-stimulation).
-
4.
Treatment personalization: future research and clinical trials should contemplate the collection and storage of recorded data to both deepen the understanding of aDBS on neurological tissues and to facilitate the identification of personalized biomarkers and stimulation patterns. Indeed, a fundamental characteristic of aDBS is the ability to increase treatment personalization, i.e., identifying patient subgroups or specific clinical phenotypes that are likely responders to aDBS, and determining which technologies (e.g., personalized biomarkers) and therapeutic strategies are best suited for individual patients. This research will be critical in refining the application of aDBS and enhancing its clinical efficacy.
However, although consensus achieved through Delphi methods can offer valuable insights, it neither replaces clinical judgment nor original research, and it is not intended to define standards of practice. Also, the feasibility of the consensus reached should be further debated and scientifically demonstrated—even more when considering stimulation targets commonly used for DBS (e.g., globus pallidus internus) not explored for aDBS. Rather, since our results aggregate the opinion of experts who could count on both personal expertise and scientific knowledge, they appear to be relevant in terms of the current state of knowledge and future directions for research, even more for a field which is still at its infancy. Therefore, although we might expect aDBS to reach clinical adoption in the next 10 years, several uncertainties remain that need to be addressed through solid experimental studies, particularly regarding economic barriers, accessibility, and patient-specific factors.
Methods
The Delphi study methodology is a multistage process designed to combine opinions into group consensus81, where a series of structured questionnaires (rounds) are anonymously completed by experts (panelists) and the responses from each questionnaire are fed back in summarized form to the participants82,83. This allows the panelists to reassess their initial judgments, considering the positive aspects of interacting groups (e.g., inclusion of different backgrounds) without the negative ones (e.g., influence of dominant members)84. For the purpose of our study, a modified Delphi process85,86,87 was designed in three rounds, which are considered as sufficient to collect the needed information and to reach a consensus84,88. The Delphi consensus process does not involve human research participants, and therefore, ethics approval is not required. However, data gathering from the experts and analysis occurred, guaranteeing the compliance with the Declaration of Helsinki and the current legislation on the collection and processing of personal data.
Steering Committee and Delphi Panel selection
An SC of experts (n = 8) based on the collaborative network of the leading authors was selected to define the questionnaire. Then, together with the SC, a larger EP (n = 13) was involved in the Delphi consensus process. Therefore, a total of 21 panelists took part in the consensus, which is a number of experts within the recommended range84,89. Since no exact criterion is currently available on the definition of “expert”90, we considered positional leaders91 in the field according to the number of peer-reviewed publications92,93, as suggested by previous works26.
Questionnaire definition
The SC was in charge of outlining the scope of the research, discussing the topic, defining the questions, and developing the structured questionnaire, including key items pertinent to aDBS using five-point Likert scales (1 = strongly disagree; 2 = disagree; 3 = undecided; 4 = agree; 5 = strongly agree)26.
Delphi process
In rounds one, two, and three, quantitative assessments to reach the consensus were performed by SC and EP members. The panelists were asked to rate 42 statements on several technical (21 statements) and clinical (21 statements) aspects of aDBS (Table 2). In order to maintain the rigor of this method, we considered a response rate of >70% for each round94 to be a minimum, with missing or incomplete responses excluded from the analysis. Electronic questionnaires were used in all steps of the process. In case one item reached a consensus during the first or second round, it was excluded from the following round to avoid confirmation bias; otherwise (i.e., if no consensus was reached), it was included in the following round90. Although no guidelines are available90, consensus was achieved when ≥80% of the responses fell in the same response label26,95.
Data analysis
Data were analyzed and reported by descriptive statistics using JASP (Version 0.19.3) [Computer software]. We opted for median and interquartile range (IQR), as suggested by the literature84,96. We report the results of each round separately in both textual (i.e., with median ± IQR) and graphical representation, to better illustrate the strength of support for each round90. As an additional analysis, we chose to convert the 5-point Likert scale into a 3-point Likert scale, considering the middle point (undecided) and only two points (agree and disagree) as union of the two highest (4 = agree; 5 = strongly agree) and lowest (1 = strongly disagree; 2 = disagree) points, respectively. Only the outcomes of the third round were subjected to this secondary analysis.
Preprint
This work was published as a preprint in the online archive medRxiv97.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DBS:
-
Deep Brain Stimulation
- ET:
-
Essential Tremor
- PD:
-
Parkinson’s disease
- cDBS:
-
conventional DBS
- STN-DBS:
-
Deep Brain Stimulation of the Subthalamic Nucleus
- aDBS:
-
Adaptive Deep Brain Stimulation
- LFPS:
-
Local Field Potentials
- SC:
-
Steering Committee
- EP:
-
Expert Panel
- STN-LFP:
-
Local Field Potentials of the Subthalamic Nucleus
- TEED:
-
total electrical energy delivered
- AI:
-
Artificial Intelligence
- ML:
-
Machine Learning
References
Roy, H. A., Green, A. L. & Aziz, T. Z. State of the art: novel applications for deep brain stimulation. Neuromodulation 21, 126–134 (2018).
Priori, A. et al. Adaptive deep brain stimulation (aDBS). Int. Rev. Neurobiol. 159, 111–127 (2021).
Peng, L. et al. The long-term efficacy of STN vs GPi deep brain stimulation for Parkinson disease: a meta-analysis. Medicine97, e12153 (2018).
Aldridge, D., Theodoros, D., Angwin, A. & Vogel, A. P. Speech outcomes in Parkinson’s disease after subthalamic nucleus deep brain stimulation: a systematic review. Parkinsonism Relat. Disord. 33, 3–11 (2016).
Bernasconi, T. et al. 20 Rehabilitative Approaches and Prognosis for Acquired Motor Speech Disorders: Dysarthria and Dyspraxia. In Phoniatrics III: Acquired Motor Speech and Language Disorders – Dysphagia – Phoniatrics and COVID-19 (eds am Zehnhoff-Dinnesen, A. et al.) 105–179. https://doi.org/10.1007/978-3-031-48091-1_5 (Springer Nature Switzerland, Cham, 2025).
Xie, T. et al. Effect of low versus high frequency stimulation on freezing of gait and other axial symptoms in Parkinson patients with bilateral STN DBS: A mini-review. Transl. Neurodegener. 6, 13 (2017).
Guidetti, M. et al. Is physical therapy recommended for people with parkinson’s disease treated with subthalamic deep brain stimulation? a delphi consensus study. J. NeuroEng. Rehabilitation 22, 80 (2025).
Baizabal-Carvallo, J. F. & Jankovic, J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat. Disord. 25, 1–9 (2016).
Ray, N. J. et al. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia 47, 2828–2834 (2009).
Mahlknecht, P., Foltynie, T., Limousin, P. & Poewe, W. How does deep brain stimulation change the course of Parkinson’s disease?. Mov. Disord. 37, 1581–1592 (2022).
Marceglia, S. et al. Deep brain stimulation: Is it time to change gears by closing the loop? J. Neural Eng. 18, 061001 (2021).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75 (2021).
Leblois, A. et al. Late emergence of synchronized oscillatory activity in the pallidum during progressive Parkinsonism. Eur. J. Neurosci. 26, 1701–1713 (2007).
Özkurt, T. E. et al. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp. Neurol. 229, 324–331 (2011).
Bocci, T. et al. Asymmetries of the subthalamic activity in Parkinson’s disease: phase-amplitude coupling among local field potentials. Brain Commun. 6, fcae201 (2024).
Quinn, E. J. et al. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov. Disord. 30, 1750–1758 (2015).
Steigerwald, F., Matthies, C. & Volkmann, J. Directional deep brain stimulation. Neurotherapeutics 16, 100–104 (2019).
Oliveira, A. M. et al. Machine learning for adaptive deep brain stimulation in Parkinson’s disease: closing the loop. J. Neurol. 270, 5313–5326 (2023).
Fra̧czek, T. M. et al. Closing the loop with cortical sensing: the development of adaptive deep brain stimulation for essential tremor using the Activa PC+S. Front. Neurosci. 15, 749705 (2021).
Little, S. et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J. Neurol. Neurosurg. Psychiatry 87, 1388–1389 (2016).
Arlotti, M. et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90, e971–e976 (2018).
Rosa, M. et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov. Disord. 32, 628–629 (2017).
An, Q. et al. Adaptive deep brain stimulation for Parkinson’s disease: looking back at the past decade on motor outcomes. J. Neurol. 270, 1371–1387 (2022).
Little, S. & Brown, P. Debugging adaptive deep brain stimulation for Parkinson’s disease. Mov. Disord. 35, 555–561 (2020).
Guidetti, M. et al. Clinical perspectives of adaptive deep brain stimulation. Brain Stimul. 14, 1238–1247 (2021).
Hsu, C.-C. & Sandford, B. A. The Delphi technique: making sense of consensus. Pract. Assess. Res. Eval. 12, 10 (2007).
Priori, A., Foffani, G., Rossi, L. & Marceglia, S. Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp. Neurol. 245, 77–86 (2013).
Neumann, W.-J., Gilron, R., Little, S. & Tinkhauser, G. Adaptive deep brain stimulation: from experimental evidence toward practical implementation. Mov. Disord. 38, 937–948 (2023).
Little, S. & Brown, P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease?. Ann. N. Y. Acad. Sci. 1265, 9–24 (2012).
Johnson, L. A. et al. Closed-loop deep brain stimulation effects on Parkinsonian motor symptoms in a non-human primate—Is beta enough?. Brain Stimulation 9, 892–896 (2016).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Oehrn, C. R. et al. Personalized chronic adaptive deep brain stimulation outperforms conventional stimulation in Parkinson’s disease. 2023.08.03.23293450 Preprint at https://doi.org/10.1101/2023.08.03.23293450 (2023).
Rosa, M. et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov. Disord. 30, 1003–1005 (2015).
Bocci, T. et al. Eight-hours conventional versus adaptive deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. npj Parkinsons Dis. 7, 1–6 (2021).
Tinkhauser, G. et al. The modulatory effect of adaptive deep brain stimulation on beta bursts in Parkinson’s disease. Brain 140, 1053–1067 (2017).
Oehrn, C. R. et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat. Med. 30, 3345–3356 (2024).
Little, S. et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol., Neurosurg. Psychiatry 87, 717–721 (2016).
Mameli, F. et al. Energy delivered by subthalamic deep brain stimulation for Parkinson disease correlates with depressive personality trait shift. Neuromodulation Technol. Neural Interface 26, 394–402 (2023).
Beudel, M., Heijmans, M., Habets, J. G. V. & Kubben, P. L. Future perspectives: adaptive deep brain stimulation. in Fundamentals and Clinics of Deep Brain Stimulation: An Interdisciplinary Approach (eds Temel, Y., Leentjens, A. F. G., de Bie, R. M. A., Chabardes, S. & Fasano, A.) 49–65. https://doi.org/10.1007/978-3-030-36346-8_5 (Springer International Publishing, 2020).
Chen, C. C. et al. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’s disease surgery. Exp. Neurol. 198, 214–221 (2006).
Cagnan, H., Denison, T., McIntyre, C. & Brown, P. Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 37, 1024–1033 (2019).
Castaño-Candamil, S. et al. Identifying controllable cortical neural markers with machine learning for adaptive deep brain stimulation in Parkinson’s disease. NeuroImage Clin. 28, 102376 (2020).
Sand, D. et al. Machine learning-based personalized subthalamic biomarkers predict ON-OFF levodopa states in Parkinson patients. J. Neural Eng. 18, 046058 (2021).
Mohammed, A., Zamani, M., Bayford, R. & Demosthenous, A. Toward on-demand deep brain stimulation using online Parkinson’s disease prediction driven by dynamic detection. IEEE Trans. Neural Syst. Rehabil. Eng. 25, 2441–2452 (2017).
Golshan, H. M., Hebb, A. O., Hanrahan, S. J., Nedrud, J. & Mahoor, M. H. A Multiple Kernel Learning approach for human behavioral task classification using STN-LFP signal. In Proc. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1030–1033. https://doi.org/10.1109/EMBC.2016.7590878 (2016).
Golshan, H. M., Hebb, A. O. & Mahoor, M. H. LFP-Net: a deep learning framework to recognize human behavioral activities using brain STN-LFP signals. J. Neurosci. Methods 335, 108621 (2020).
Niketeghad, S., Hebb, A. O., Nedrud, J., Hanrahan, S. J. & Mahoor, M. H. Single trial behavioral task classification using subthalamic nucleus local field potential signals. In Proc. 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 3793–3796. https://doi.org/10.1109/EMBC.2014.6944449 (2014).
Shute, J. B. et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. NeuroImage Clin. 12, 165–172 (2016).
Ferleger, B. I. et al. Fully implanted adaptive deep brain stimulation in freely moving essential tremor patients. J. Neural Eng. 17, 056026 (2020).
Castaño-Candamil, S. et al. A pilot study on data-driven adaptive deep brain stimulation in chronically implanted essential tremor patients. Front. Hum. Neurosci. 14, 541625 (2020).
Camara, C. et al. Non-linear dynamical analysis of resting tremor for demand-driven deep brain stimulation. Sensors 19, 2507 (2019).
Golshan, H. M., Hebb, A. O., Hanrahan, S. J., Nedrud, J. & Mahoor, M. H. A hierarchical structure for human behavior classification using STN local field potentials. J. Neurosci. Methods 293, 254–263 (2018).
Mohammed, A., Bayford, R. & Demosthenous, A. A framework for adapting deep brain stimulation using Parkinsonian state estimates. Front. Neurosci. 14, 499 (2020).
Houston, B., Thompson, M., Ko, A. & Chizeck, H. A machine-learning approach to volitional control of a closed-loop deep brain stimulation system. J. Neural Eng. 16, 016004 (2018).
Cuschieri, A., Borg, N. & Zammit, C. Closed loop deep brain stimulation: a systematic scoping review. Clin. Neurol. Neurosurg. 223, 107516 (2022).
Fricke, P. et al. Directional leads for deep brain stimulation: technical notes and experiences. Stereotact. Funct. Neurosurg. 99, 305–312 (2021).
Marceglia, S. et al. Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease. J. Physiol. 571, 579–591 (2006).
Marceglia, S. et al. Basal ganglia local field potentials: applications in the development of new deep brain stimulation devices for movement disorders. in Expert Review of Medical Devices Vol. 4, 605–614 (Taylor & Francis, 2007).
Arlotti, M., Rosa, M., Marceglia, S., Barbieri, S. & Priori, A. The adaptive deep brain stimulation challenge. Parkinsonism Relat. Disord. 28, 12–17 (2016).
Baker, S. et al. Ethical considerations in closed loop deep brain stimulation. Deep Brain Stimul. 3, 8–15 (2023).
Arlotti, M. et al. A new implantable closed-loop clinical neural interface: first application in Parkinson’s disease. Front. Neurosci. 15, 763235 (2021).
Petrucci, M. N. et al. Ramp rate evaluation and configuration for safe and tolerable closed-loop deep brain stimulation. In Proc. 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER) 959–962. https://doi.org/10.1109/NER49283.2021.9441336 (2021).
Wendt, K. et al. Physiologically informed neuromodulation. J. Neurol. Sci. 434, 120121 (2022).
Beudel, M. & Brown, P. Adaptive deep brain stimulation in Parkinson’s disease. Parkinsonism Relat. Disord. 22, S123–S126 (2016).
Yin, Z. et al. Local field potentials in Parkinson’s disease: a frequency-based review. Neurobiol. Dis. 155, 105372 (2021).
Swann, N. C. et al. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson’s disease. J. Neurosci. 36, 6445–6458 (2016).
Wiest, C. et al. Finely-tuned gamma oscillations: spectral characteristics and links to dyskinesia. Exp. Neurol. 351, 113999 (2022).
Shah, S. A., Tinkhauser, G., Chen, C. C., Little, S. & Brown, P. Parkinsonian tremor detection from subthalamic nucleus local field potentials for closed-loop deep brain stimulation. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2320–2324 (2018).
Hirschmann, J., Schoffelen, J. M., Schnitzler, A. & van Gerven, M.aJ. Parkinsonian rest tremor can be detected accurately based on neuronal oscillations recorded from the subthalamic nucleus. Clin. Neurophysiol. 128, 2029–2036 (2017).
Tinkhauser, G. & Moraud, E. M. Controlling clinical states governed by different temporal dynamics with closed-loop deep brain stimulation: a principled framework. Front Neurosci. 15, 734186 (2021).
Fleming, J. E., Senneff, S. & Lowery, M. M. Multivariable closed-loop control of deep brain stimulation for Parkinson’s disease. J. Neural Eng. 20, 056029 (2023).
Velisar, A. et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 12, 868–876 (2019).
Sarikhani, P. et al. Automated deep brain stimulation programming with safety constraints for tremor suppression in patients with Parkinson’s disease and essential tremor. J. Neural Eng. 19, 046042 (2022).
Yamamoto, T. et al. On-demand control system for deep brain stimulation for treatment of intention tremor. Neuromodulation 16, 230–235 (2013).
Malekmohammadi, M. et al. Kinematic adaptive deep brain stimulation for resting tremor in Parkinson’s disease. Mov. Disord. 31, 426–428 (2016).
Kricheldorff, J. et al. Evidence of neuroplastic changes after transcranial magnetic, electric, and deep brain stimulation. Brain Sci. 12, 929 (2022).
Guidetti, M., et al. Neuroprotection and non-invasive brain stimulation: facts or fiction? Int. J. Mol. Sci. 23, 13775 (2022).
Guidetti, M. et al. Electric fields induced in the brain by transcranial electric stimulation: a review of in vivo recordings. Biomedicines 10, 2333 (2022).
Peters, J. & Tisch, S. Habituation after deep brain stimulation in tremor syndromes: prevalence, risk factors and long-term outcomes. Front. Neurol. 12, 696950 (2021).
Smilowska, K. et al. Cost-effectiveness of device-aided therapies in Parkinson’s disease: a structured review. J. Parkinson’s Dis. 11, 475–489 (2021).
Lynn, M. R., Layman, E. L. & Englebardt, S. P. Nursing administration research priorities. A national Delphi study. J. Nurs. Adm. 28, 7–11 (1998).
Ludwig, B. Predicting the future: Have you considered using the Delphi methodology? J. Ext. 35, 15 (1997).
Adler, M. & Ziglio, E. Gazing into the Oracle: The Delphi Method and Its Application to Social Policy and Public Health. (Jessica Kingsley Publishers, 1996).
Rowe, G. & Wright, G. Expert opinions in forecasting: the role of the Delphi technique. in Principles of Forecasting: A Handbook for Researchers and Practitioners (ed. Armstrong, J. S.) 125–144. https://doi.org/10.1007/978-0-306-47630-3_7 (Springer US, 2001).
Ekhtiari, H. et al. A checklist for assessing the methodological quality of concurrent tES-fMRI studies (ContES checklist): a consensus study and statement. Nat. Protoc. 17, 596–617 (2022).
Ekhtiari, H. et al. A methodological checklist for fMRI drug cue reactivity studies: development and expert consensus. Nat. Protoc. 17, 567–595 (2022).
Brunoni, A. R. et al. Digitalized transcranial electrical stimulation: a consensus statement. Clin. Neurophysiol. 143, 154–165 (2022).
Custer, R. L., Scarcella, J. A. & Stewart, B. R. The modified Delphi technique-A rotational modification. (1999).
Armstrong, J. S. & Forecasting, L.-R. From crystal ball to computer. New York ua 348, 553 (1985).
Hasson, F., Keeney, S. & McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 32, 1008–1015 (2000).
Ludwig, B. G. Internationalizing Extension: An Exploration of the Characteristics Evident in a State University Extension System That Achieves Internationalization (The Ohio State University, 1994).
Meyer, J. H. Rethinking the outlook of colleges whose roots have been in agriculture. (University of California, Davis, 1992).
Miller, G. The development of indicators for sustainable tourism: results of a Delphi survey of tourism researchers. Tour. Manag. 22, 351–362 (2001).
Sumsion, T. The Delphi Technique: an adaptive research tool. Br. J. Occup. Ther. 61, 153–156 (1998).
Green, B., Jones, M., Hughes, D. & Williams, A. Applying the Delphi technique in a study of GPs’ information requirements. Health Soc. Care Community 7, 198–205 (1999).
Jacobs, J. M. Essential Assessment Criteria for Physical Education Teacher Education Programs: A Delphi Study (West Virginia University, 1996).
Guidetti, M. et al. Adaptive Deep Brain Stimulation in Parkinson’s disease: a Delphi Consensus study. https://doi.org/10.1101/2024.08.26.24312580 (2024).
Acknowledgements
Preliminary results were awarded as best scientific contribution “Youth and Research Project” at the LIMPE-DISMOV Congress (Italian Academy for the Study of Parkinson's Disease and Movement Disorders—Milan, April 11th, 2024). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. G.T. received funding from the Swiss National Science Foundation (project number: PZ00P3_202166).
Author information
Authors and Affiliations
Contributions
M.G., A.F., J.K.K., A.A.K., A.L., M.O., A.P., L.T., and J.V. contributed to the design and execution. A.P., J.O., G.D., P.S., S.L., R.M.F., G.T., C.G.S., M.A.P., Y.T., A.S., P.L., C.H., and V.V.V. contributed to execution. M.G., S.M., N.V.M., S.O., T.B., and E.S. contributed to the analysis and writing. All the authors contributed to the editing of the final version of the manuscript and accept responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
M.G., N.V.M., S.O., T.B., E.S., Y.T., C.H., and P.L. declare no conflict of interest. M.A.P is a consultant for Boston Scientific, Insightec, Medtronic, and Abbott. She has received reimbursement of travel expenses to attend scientific meetings by Palex, Boston Scientific, and Medtronic. She has received speaker honoraria from Palex. G.D. has served as a consultant for Boston Scientific and Cavion and as a DSMB member for Functional Neuromodulation. He has received royalties from Thieme Publishers and funding from the German Research Council (SFB 1261, T1). A.F. has received payments as a consultant and/or speaker from Abbott, Boston Scientific, Ceregate, Inbrain Neuroelectronics, Medtronic, and Iota, and has received research support from Boston Scientific, Medtronic. R.M.F. has received speaker honoraria from the Spanish Neurological Society research foundation, Insightec, Palex, Bial, and Zambon; has a consulting agreement with Treefrog Therapeutics; has received reimbursement of travel expenses to attend scientific meetings by Palex, Zambon, the International Parkinson and Movement Disorder Society, the IAPDRD, and the World Parkinson’s Congress; and has received research funding from Instituto de Salud Carlos III, Madrid, Spain for health research projects (PI21 Proyectos de Investigacion en Salud, AES 2021). C.G.S. has received lecture honoraria from Exeltis, Zambon, Palex, Insightec, Fundación ACE, Società Italiana Parkinson e Disordini del Movimento, and Asociación Madrileña de Neurología, and reimbursement of travel expenses to attend scientific conferences from Boston Scientific and Esteve. J.K.K. is a consultant to Medtronic, Boston Scientific, aleva, and Inomed. A.A.K. is a consultant to Medtronic, Boston Scientific, and Teva. S.L. is a consultant for Iota Biosciences and has previously received honorarium from Medtronic. S.L.’s research is supported by NINDS NIH grants R01NS131405, K23NS120037, and Wellcome Discovery Award 226645/Z/22/Z. A.M.L. is a consultant to Abbott, Boston Scientific, Insightec, Medtronic, and Functional Neuromodulation (Scientific Director). M.S.O. serves as Medical Advisor in the Parkinson’s Foundation, and has received research grants from NIH, Parkinson’s Foundation, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. M.S.O.’s research is supported by: R01 NS131342, NIH R01 NR014852, R01NS096008, UH3NS119844, U01NS119562. M.S.O. is PI of the NIH R25NS108939 Training Grant. M.S.O. has received royalties for publications with Hachette Book Group, Demos, Manson, Amazon, Smashwords, Books4Patients, Perseus, Robert Rose, Oxford, and Cambridge (movement disorders books). M.S.O. is an associate editor for the New England Journal of Medicine, Journal Watch Neurology, and JAMA Neurology. M.S.O. has participated in CME and educational activities (past 12–24 months) on movement disorders sponsored by WebMD/Medscape, RMEI Medical Education, American Academy of Neurology, Movement Disorders Society, Mediflix, and by Vanderbilt University. The institution and not M.S.O. receives grants from industry. M.S.O. has participated as a site PI and/or co-I for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria. Research projects at the University of Florida receive device and drug donations. J.L.O. received consulting payments from Abbott, Acorda, Jazz, Adamas, AcureX, and Aspen as well as research or training grants from Biogen, Boston Scientific, Medtronic, Neuroderm, Runelabs, AbbVie, Merz, Amneal, and Acadia. A.S. received consulting fees from Abbott, Zambon, and AbbVie, and speaker honoraria from bsh Medical communication, Abbott, Kyowa Kirin, Novartis, AbbVie, Alexion, and GE Healthcare. The institution of AS, not AS personally, received funding by the Deutsche Forschungsgemeinschaft, the Brunhilde Moll Foundation, and Abbott. P.A.S. is compensated for time spent on the data safety and monitoring board for Neuralink, Inc. L.T. received occasional payments as a consultant for Boston Scientific, L.T. received honoraria as a speaker on symposia sponsored by Boston Scientific, AbbVIE, Novartis, Neuraxpharm, Teva, the Movement Disorders Society, and DIAPLAN. The institution of L.T., not L.T. personally, received funding from Boston Scientific, the German Research Foundation, the German Ministry of Education and Research, the Otto-Loewi-Foundation, and the Deutsche Parkinson Vereinigung. Neither L.T. nor any member of his family holds stocks, stock options, patents, or financial interests in any of the above-mentioned companies or their competitors. L.T. serves as the president of the German Neurological Society without any payment or any income. G.T. received financial support from Boston Scientific and Medtronic; research agreement with RuneLabs and Medtronic not related to the present work. V.V.V. received occasional payments as a consultant or speaker on symposia from Boston Scientific and Medtronic. J.V. reports grants and personal fees from Medtronic, grants and personal fees from Boston Scientific, and personal fees from Abbott outside the submitted work. J.V. was supported by the German Research Foundation (DFG, Project-ID424778381, TRR 295)—J.V. received consulting and lecture fees from Boston Scientific, Medtronic, and Newronika. Research grants from the German Research Foundation, the German Ministry of Research and Education, Boston Scientific, and Medtronic. Lecture Honoraria from UCB, Zambon, and Abbott. A.P. and S.M. are founders and shareholders of Newronika Spa, Italy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guidetti, M., Bocci, T., De Pedro Del Álamo, M. et al. Will adaptive deep brain stimulation for Parkinson’s disease become a real option soon? A Delphi consensus study. npj Parkinsons Dis. 11, 110 (2025). https://doi.org/10.1038/s41531-025-00974-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-00974-5